Abstract
Haemophilus parasuis is the agent responsible for causing Glässer's disease, which is characterized by fibrinous polyserositis, polyarthritis, and meningitis in pigs. In this study, we have characterized native outer membrane proteins with affinity to porcine transferrin (NPAPT) from H. parasuis serovar 5, Nagasaki strain. This pool of proteins was used as antigen to developed two vaccine formulations: one was adjuvanted with a mineral oil (Montanide IMS 2215 VG PR), while the other was potentiated with a bacterial neuraminidase from Clostridium perfringens. The potential protective effect conferred by these two vaccines was compared to that afforded by two other vaccines, consisting of recombinant transferrin-binding protein (rTbp) A or B fragments from H. parasuis, Nagasaki strain, and by a commercially available inactivated vaccine. Five groups of colostrum-deprived piglets immunized with the vaccines described above, one group per each vaccine, and a group of nonvaccinated control animals were challenged intratracheally with a lethal dose (3 × 108 CFU) of H. parasuis, Nagasaki strain. The two vaccines containing rTbps yielded similar results with minimal protection against death, clinical signs, gross and microscopic lesions, and H. parasuis invasion. In contrast, the two vaccines composed of NPAPT antigen and commercial bacterin resulted in a strong protection against challenge (without deaths and clinical signs), mild histopathological changes, and no recovery of H. parasuis, thus suggesting their effectiveness in preventing Glässer's disease outbreaks caused by serovar 5.
Respiratory disorders induced by bacterial pathogens are one of the major problems in intensive production systems. Among them, Haemophilus parasuis has emerged in the last few years as one of the main causes of nursery mortality in modern swine husbandry, causing significant financial losses worldwide (12). This organism, a Gram-negative bacillus classified in the Pasteurellaceae family, is commonly found in the upper respiratory tract of healthy conventional pigs, preferentially colonizing the nasal mucosa and/or the tonsillar area (2). Some strains can migrate into the lungs, causing pneumonia, and disseminate to produce a severe systemic disease, characterized by fibrinous polyserositis, polyarthritis, meningitis, and more rarely myositis of the masseter muscles, known as Glässer's disease (3, 29, 30). Fifteen serovars of H. parasuis have been recognized thus far by means of an immunodiffusion test (15); however, there are often a large number of nontypeable strains reported depending on geographic region and typing method (8, 32). Although there is not a strong correlation between serovars and degree of pathogenicity, serovars 1, 5, 10, and 12 to 14 are classified as highly virulent; serovars 2, 4, and 15 showed moderate virulence; and serovars 3, 6 to 9, and 11 are considered nonvirulent (27). It is believed that stress factors, such as transport, unfavorable environment (41), and some practices, such as early weaning, may have influenced the epidemiology of H. parasuis within herds, especially regarding the early colonization of pigs by virulent strains and the spread of them throughout a swine population (27).
Because most swine herds are colonized by H. parasuis and therefore have a degree of protective immunity, reproduction of systemic infection in conventional pigs is often difficult. Nevertheless, both caesarian-derived, colostrum-deprived pigs and naturally farrowed, colostrum-deprived pigs have been used successfully to study this disease experimentally (7, 26, 42). Control of Glässer's disease outbreaks has traditionally been achieved by use of commercial or autogenous bacterins. These vaccines usually give strong protection against challenge with the homologous serovar (5, 13, 40), but more inconsistent results have been described when testing the development of cross-protection, depending on strains and serovars of H. parasuis (5, 16, 28, 33, 38). With regard to modern vaccines based on molecular techniques, outer membrane proteins (Omps) have rendered a high immunogenicity. In this respect, an Omp formulation has resulted in partial protection against the homologous serovar (21), while the purified recombinant OmpA has showed a good antigenicity (44), and four other Omps (PalA, Omp2, D15, and HPS 06257) have also yielded a strong potential to be vaccine candidates (45).
We examined here the immunoprotective effect of two vaccines based on Omps with affinity to porcine transferrin from H. parasuis serovar 5 (a highly virulent serovar of worldwide prevalence [27]) and compared them to other subunit vaccines, also designed in our laboratory, and one commercially available inactivated vaccine. As an animal model, colostrum-deprived piglets (6) challenged with a lethal dose of H. parasuis serovar 5, were used.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. parasuis Nagasaki strain (reference strain of serovar 5) was used in the present study. It was cultured on chocolate blood agar for 24 h at 37°C under 5% CO2. Overexpression of proteins with affinity to porcine transferrin was carried out by culturing H. parasuis in iron restrictive conditions, as described previously (10), but adding the iron chelator 2.2-dipyridyl at a final concentration of 200 μM to 0.025% NAD-supplemented pleuropneumonia-like organisms (PPLO) broth, instead of the chelator Na3CaDTPA. Escherichia coli TOP10 and LMG194 cells were grown in Luria-Bertani medium supplemented with 100 μg of ampicillin/ml.
Antigen preparation.
rTbpA antigen consisted of an H. parasuis TbpA fragment, cloned in E. coli TOP10 and expressed in E. coli LMG194 (22). rTbpB antigen consisted of a N-terminal fragment from H. parasuis TbpB, cloned and expressed in E. coli TOP10 (9).
NPAPT antigen consisted of native outer membrane proteins (Omps) from H. parasuis exhibiting affinity to porcine transferrin, which were purified by gel filtration on a fast-protein liquid chromatography (FPLC)-CNBr-activated Sepharose 4B (GE Healthcare) column, according to the manufacturer's instructions. Briefly, 20 mg of iron-loaded porcine transferrin (First Link, Ltd.) was dissolved in coupling buffer (0.1 M NaHCO3 containing 0.5 M NaCl), the mixture were gently rotated for 2 h at room temperature, and excess ligand was washed with 5 volumes of coupling buffer. Then, any remaining active group was blocked with 0.1 M Tris-HCl buffer (pH 8.0) for 2 h, and the medium was washed with three cycles of alternating pH (0.1 M acetic acid-sodium acetate [pH 4.0] containing 0.5 M NaCl, followed by 0.1 M Tris-HCl [pH 8] containing 0.5 M NaCl). Omps were obtained from liquid cultures of H. parasuis grown in iron restrictive conditions as described previously (10). The cell-free supernatants were dialyzed against coupling buffer (10 mM Tris-HCl, 50 mM NaCl, 0.5% Sarkosyl) in an Amicon Ultra 30K centrifugal filter. The dialyzed sample was then adjusted to 60 mg of proteins/g of Sepharose in a 20-ml volume, and the packed column was connected to the FPLC apparatus (Äkta Prime; GE Healthcare) with a flow rate of 0.2 ml/min. After the sample was passed through the column, it was washed with 50 ml of coupling buffer (flow rate, 0.5 ml/min), and the proteins were eluted with 2 M guanidine-HCl, with monitoring of the elution peak at 280 nm. The eluted proteins were collected in tubes containing 1 mM Tris (pH 9.0), immediately dialyzed against 0.1% Sarkosyl-PBS (pH 7.5), divided into aliquots, and frozen at −20°C until use.
Identification of Tbps in NPAPT antigen.
Tbp identification was carried out at the Proteomic Service Facility from the University Complutense of Madrid (Spain), a member of ProteoRed Network. The protein spots of interest were manually excised from gels by biopsy punches, placed in Eppendorf tubes, and washed twice with double-distilled water. Proteins for analysis were in-gel reduced, alkylated, and digested with bovine trypsin (12.5 ng/μl, sequencing grade; Roche), as described previously (37). After digestion, the supernatant was collected, and 1 μl was spotted on a matrix-assisted laser desorption ionization (MALDI) target plate and allowed to air dry for 10 min at room temperature. Subsequently, 0.4 μl of matrix (3 mg of α-cyano-4-hydroxycinnamic acid [CHCA; Sigma]/ml diluted in 0.1% trifluoroacetic acid-acetonitrile [TFA-ACN]/H2O [1:1, vol/vol]) was added to the dried peptide digest spots and allowed to air dry for another 5 min at room temperature (23).
The samples were analyzed in a 4700 Proteomic Analyzer MALDI-time-of-flight (MALDI-TOF) mass spectrometer (Applied Biosystems). All mass spectra were internally calibrated by using peptides from the autodigestion of trypsin. The analysis by MALDI-TOF mass spectrometry (MS) produced peptide mass fingerprints, and the peptides observed can be collated and represented as a list of monoisotopic molecular weights. For the Tbp identification, the mass spectra were automatically searched by using a local license of Mascot 1.9 from Matrix Science through the Protein Global Server (Applied Biosystems). Sequence database used was the NCBI protein database for H. parasuis.
Vaccines.
Five vaccines were compared. rTbpA vaccine contained 400 μg of rTbpA antigen, adjuvanted with Montanide IMS 2215 VG PR (Seppic, Inc., Paris, France) in a 1:4 ratio. rTbpB vaccine contained 400 μg of rTbpB antigen, also adjuvanted as described for the rTbpA vaccine. NPAPTM vaccine contained 400 μg of NPAPT antigen prepared as described above, adjuvanted with Montanide IMS 2215 VG PR (Seppic, Inc., Paris, France) in a 1:4 ratio. NPAPTCp vaccine also contained 400 μg of NPAPT antigen, potentiated with neuraminidase from Clostridium perfringens (type VI) at a concentration of 100 mU/ml of vaccine. Finally, PG vaccine consisted of a commercially available vaccine (Porcilis Glässer; Intervet), composed of inactivated H. parasuis cells belonging to serovar 5, strain 4800.
The first four vaccines were developed following high aseptic conditions and strict sterility controls. In this way, a 1-ml aliquot of each of these four vaccines was grown in tryptic soy broth and fluid thioglycolate medium at 37 and 24°C, respectively, for 14 days in order to confirm absence of growth after these incubation conditions.
Animals and immunization schedule.
A total of 33 colostrum-deprived Large White × Pietrain piglets, coming from a farm with an excellent sanitary status and no previous clinical history of infections by H. parasuis and Actinobacillus pleuropneumoniae, were housed in isolation rooms designed for biosecurity requirement level II (Isolation Unit, University of León, León, Spain). Nasal and tonsillar swabs were obtained from each piglet and determined to be negative for H. parasuis by PCR (4); in addition, the animals were found to be serologically negative for porcine reproductive and respiratory syndrome virus (PRRSV) and A. pleuropneumoniae by enzyme-linked immunosorbent assay and for circovirus by PCR. The piglets were hand reared and fed a pasteurized bovine colostrum for 7 days, a porridge mixture (bovine colostrum plus Startrite 100 [SCA, Ibérica, Spain]) from 7 to 14 days, and a piglet dry meal formula (Startrite 100) for the rest of the study. At 4 weeks of age, piglets were randomly assigned to one control group and five test groups. The rTbpA (n = 6), rTbpB (n = 5), or NPAPTM (n = 6) test groups received 2 ml of rTbpA, rTbpB, or NPAPTM vaccines, respectively, by intramuscular injection at 28 and 49 days of age. The NPAPTCp test group (n = 6) received 2 ml of NPAPTCp vaccine by intratracheal injection, also at 28 and 49 days of age. The PG test group (n = 6) received 2 ml of PG vaccine intramuscularly at 28 and 36 days of age, as recommended by the vaccine's manufacturer. Finally, the control group (n = 4) received 2 ml of phosphate-buffered saline (pH 7.4) intramuscularly at 28 and 49 days of age.
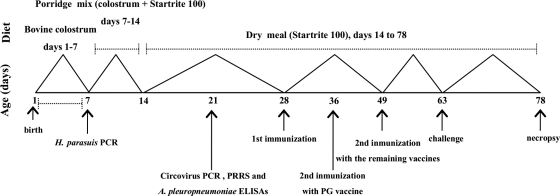
At 63 days of age, all groups were challenged intratracheally as described previously (40) with a lethal dose (3 × 108 CFU) of H. parasuis Nagasaki strain, suspended in 2 ml of RPMI 1640. Piglets with severe signs of distress were immediately euthanized for necropsy. Those that survived challenge were humanely euthanized at 78 days of age with an intracardiac sodium pentobarbital overdose. The immunization and infection schedule is shown in Fig. 1. All animal experiments were conducted in accordance with the guidelines of the University of León Ethical Committee and the Spanish Government.
FIG. 1.
Immunization, infection, and necropsy schedule for colostrum-deprived piglets before and after challenge with H. parasuis.
Clinical and pathological examinations.
Rectal temperatures and other clinical signs (such as weakness, apathy, coughing, limping, dyspnea, lack of coordination, and/or loss of appetite) were monitored every 12 h during the first 7 days postchallenge (dpc) and, after that, once a day until the end of the study. All animals were subjected to necropsy, and gross lesions were recorded, with special attention paid to the pleural, pericardial and peritoneal cavities, joints, lungs and central nervous system. For histopathological examination, representative tissue samples were collected, fixed in 10% neutral buffered formalin, embedded in paraffin by conventional methods, sectioned at 3 to 5 μm, and stained with hematoxylin and eosin (H&E). The severity of the pathological changes was scored blindly as follows for the vaccinated and control groups: −, no changes; ±, minimal to mild changes; +, moderate changes; and ++, severe changes.
Bacterial isolations and H. parasuis confirmation by PCR.
Swabs were collected aseptically from (i) lung, liver, spleen, kidney, and brain parenchymas, (ii) heart blood, (iii) pleural, pericardial, and peritoneal cavities, and (iv) hock and carpal joints and then cultured on chocolate blood agar for 24 h at 37°C under 5% CO2. Suspicious colonies were tested for NAD requirement by observing satellitism on blood agar cross-streaked with a nurse strain of Staphylococcus intermedius and were confirmed by PCR (4).
Statistical analysis.
The one-way analysis of variance was used for comparison of rectal temperatures at various times after challenge in each group and between groups at each time until 48 h, while the Tukey's multiple comparison test was used for comparison between groups at 72 and 144 h. The GraphPad Prism statistical software, version 5.0, was used for comparison between groups in survival studies. P values of <0.05 were considered significant.
RESULTS
Characterization of rTbpA, rTbpB, and NPAPT antigens.
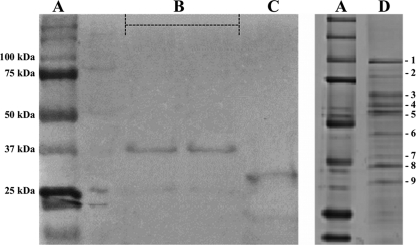
rTbpA antigen was composed of 200 amino acids located in an intermediate region of complete H. parasuis TbpA. The specific location of this rTbpA fragment has been recently published by us (22). The rTbpB antigen contained the first 102 amino acids of N-terminal domain of TbpB. Two bands of 38.5 and 27.0 kDa (Fig. 2B and C, respectively) were revealed by SDS-PAGE for these recombinant proteins, respectively. The purified NPAPT antigen was further analyzed by SDS-PAGE, and seven other proteins able to bind to iron-loaded porcine transferrin could be observed, together with the well-characterized TbpA (100-kDa band, lane 1, in Fig. 2D) and TbpB (75-kDa band, lane 2, in Fig. 2D). The molecular masses of these seven transferrin-binding proteins were lower than those of TbpA and TbpB and comprised between 75 and 25 kDa (Fig. 2D). These native proteins were then characterized by using a MALDI-TOF/TOF mass spectrometer and compared to the sequences previously included in the NCBI protein database. In addition to TbpA and TbpB, transferrin sus scrofa, ABC transporter, periplasmic binding protein, catalase, elongation factor Tu, Omp2, periplasmic iron-binding protein, and chelated ABC transporter, periplasmic binding protein could be identified. Their molecular masses, theoretical isoelectric points, number of matched peptides, protein coverage, and scores are shown in Table 1.
FIG. 2.
SDS-PAGE (Coomassie blue colloidal stain) analysis. (A) Molecular mass marker (Precision Plus Protein standards; Bio-Rad). (B and C) rTbpA (B) and rTbpB (C) eluted from nickel affinity columns. (D) Native proteins (1 to 9) with affinity to porcine transferrin eluted from a CNBr-transferrin affinity column. These nine proteins were further identified by MALDI-TOF/TOF mass spectrometry (see Table 1).
TABLE 1.
Proteins identified in the extract containing NPAPT antigen
| Band | Access no.a | Protein | Mol wt | Theoretical isoelectric point | No. of matched/searched peptides | Protein coverage (%) | Score |
|---|---|---|---|---|---|---|---|
| 1 | gi/33305754 | Transferrin binding protein A | 106,614 | 9.11 | 18/32 | 21 | 152 |
| 2 | sp/P09571 | Transferrin sus scrofa | 78,971 | 6.93 | 8/20 | 14 | 77 |
| 3 | gi/161408008 | Transferrin binding protein B | 59,835 | 6.40 | 16/37 | 31 | 157 |
| 4 | gi/167855391 | ABC transporter, periplasmic binding protein | 57,908 | 8.23 | 15/30 | 30 | 156 |
| 5 | gi/219692447 | Catalase | 54,954 | 6.50 | 14/22 | 33 | 180 |
| 6 | gi/219692224 | Elongation factor Tu | 43,501 | 5.23 | 11/17 | 32 | 140 |
| 7 | gi/209968888 | Outer membrane protein 2 | 38,552 | 9.20 | 5/13 | 17 | 60 |
| 8 | gi/219691519 | Periplasmic iron-binding protein | 37,755 | 8.80 | 15/39 | 50 | 174 |
| 9 | gi/219691491 | Chelated ABC transporter, periplasmic binding protein | 32,632 | 7.74 | 7/22 | 32 | 84 |
As listed in the NCBI protein database.
Clinical signs.
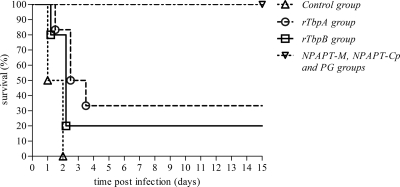
All of the animals belonging to the control group died between 24 and 48 h postchallenge (hpc), while eight of the piglets receiving rTbpA or rTbpB vaccines (four from each group) died between 24 and 72 hpc, with survival rates of 33.3 and 20%, respectively, in these two latter groups. In contrast, all of the animals immunized with the NPAPTM, NPAPTCp, or PG vaccines survived challenge with 3 × 108 CFU of H. parasuis Nagasaki strain (Fig. 3). These three later vaccines showed significantly higher survival rates (P < 0.0001) compared to the rTbpA, rTbpB, and nonimmunized groups.
FIG. 3.
Survival rates after challenge with H. parasuis for various experimental groups.
The nonvaccinated control animals showed high temperatures until death (3°C above those at the time of challenge), and significant differences were obtained at 24 and 48 hpc compared to challenge (P < 0.0005) (Table 2). A similar tendency was observed for the groups receiving rTbpA or rTpbB formulations: significantly higher temperatures were also recorded at 24 hpc for rTbpA (P < 0.005) and until 48 hpc for rTbpB (P < 0.005 for both times), but these increases reached only about 1 to 1.5°C compared to challenge. However, no significant differences in temperature were observed at different times after challenge for the NPAPTM, NPAPTCp, and PG groups. When we compared test groups to one another, control piglets had significantly higher temperatures than the rTbpA group (P < 0.05 at 24 hpc and P < 0.005 at 48 hpc), the rTbpB group (P < 0.005 at 24 hpc and P < 0.0005 at 48 hpc), and the NPAPTM, NPAPTCp, and PG groups (P < 0.0005 at 24 and 48 hpc).
TABLE 2.
Rectal temperatures (°C) in surviving piglets at different intervals after challenge with H. parasuis
| Expt group (n)a | Mean temp (°C) ± SD at various intervals after challenge (n)b |
||||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 144 h | |
| Control (4) | 38.1 ± 0.33 (4) | 41.0 ± 0.70A (2) | 41.2 ± 0.49A (2) | — (0) | — (0) |
| rTbpA (6) | 39.0 ± 0.40 (6) | 40.1 ± 0.78BC (6) | 39.7 ± 0.31D (4) | 39.4 ± 0.55 (2) | 39.2 ± 0.17 (2) |
| rTbpB (5) | 38.4 ± 0.27 (5) | 39.6 ± 1.02BD (5) | 39.7 ± 1.07BE (5) | 39.1 (1) | 39.2 (1) |
| NPAPTM (6) | 39.4 ± 0.31 (6) | 39.4 ± 0.31E (6) | 39.0 ± 0.22E (6) | 38.9 ± 0.31 (6) | 39.3 ± 0.17 (6) |
| NPAPTCp (6) | 39.4 ± 0.27 (6) | 39.2 ± 0.15E (6) | 39.2 ± 0.36E (6) | 38.8 ± 0.21 (6) | 39.2 ± 0.10 (6) |
| PG (6) | 38.7 ± 0.62 (6) | 39.2 ± 0.19E (6) | 39.0 ± 0.16E (6) | 39.1 ± 0.53 (6) | 39.4 ± 0.07 (6) |
Experimental groups: Control, no vaccine; rTbpA, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; rTbpB, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTM, native proteins with affinity for porcine-transferrin plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTCp, native proteins with affinity for porcine-transferrin plus neuraminidase from Clostridium perfringens (100 mU/ml); PG, Porcilis Glässer (Intervet). n, Number of animals per group.
The numbers of surviving piglets at each time point (n) are indicated in parentheses. Superscript capital letters indicate significant differences as follows: A, P < 0.0005 from result at 0 h (challenge); B, P < 0.005 from result at 0 h (challenge); C, P < 0.05 compared to control group; D, P < 0.005 compared to control group; and E, P < 0.0005 compared to control group.
Clinical signs suspicious of Glässer's disease, such as limb uncoordination, swollen joints, severe dyspnea, and coughing, together with other more nonspecific signs (apathy, weakness, and anorexia), were seen in all of the nonimmunized control piglets. Similar but considerably milder clinical signs were observed in piglets vaccinated with rTbpA or rTbpB; however, two animals belonging to rTbpA group and one belonging to rTbpB group showed as only signs weakness, loss of appetite, and a mild transient rise in temperature until 3 dpc, resulting in a complete recovery at 7 dpc. No appreciable clinical signs were seen in piglets immunized with the NPAPTM, NPAPTCp, or PG vaccines. On the other hand, no adverse reactions to any of the four formulations developed in the present study (i.e., the rTbpA, rTbpB, NPAPTM, and NPAPTCp vaccines) or to the commercial bacterin were detected.
Gross and histopathological findings.
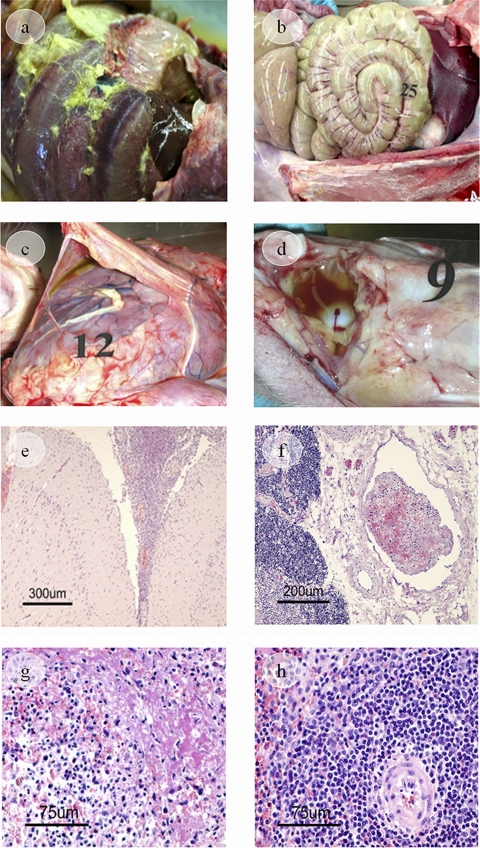
All nonimmunized piglets and most of those receiving rTbpA or rTbpB vaccines showed the characteristic inflammatory changes caused by a H. parasuis infection (Table 3). Severe fibrinous polyserositis was observed in the pericardial, pleural, and peritoneal cavities, characterized by the presence of fibrin strands or layers on serosal surfaces (Fig. 4a and 4c). Moreover, some piglets presented a moderate fibrinous polyarthritis, located mainly in carpal and hock joints (Fig. 4d), and a fibrinous exudate was also identified in the occipito-cervical joint in a piglet immunized with rTbpB. Another frequent inflammatory lesion was a moderate to severe fibrinous-suppurative meningitis identified by histopathological examination (Fig. 4e). Likewise, most organs showed vascular alterations of varying severity such as edema, congestion, hemorrhages, disseminated intravascular coagulation (consumptive coagulopathy), and vascular thrombosis (Table 3 and Fig. 4f). The spleens of these animals seemed normal in gross appearance, but microscopic examination revealed that the spleens in some of the piglets presented a transudation of plasma proteins (fibrin deposits) in the marginal zone of white pulp and in red pulp, as well as a lymphocyte reduction in periarteriolar lymphoid sheaths and follicles due to a lympholysis, with the production of nuclear debris (Table 3 and Fig. 4g). These splenic lesions and the vascular alterations described above were compatible with an acute septicemia, and death by septic shock was suspected.
TABLE 3.
Pathological changes found in each experimental group after challenge with H. parasuis
| Pathology | Treatment group (n)a |
|||||
|---|---|---|---|---|---|---|
| Control (4) | rTbpA (6) | rTbpB (5) | NPAPTM (6) | NPAPTCp (6) | PG (6) | |
| Vascular lesions | ||||||
| Congestion | + | + | + | + | + | + |
| Pulmonary edema | ++ | + | + | ± | − | ± |
| Gallbladder edema | ++ | ++ | ++ | − | − | ± |
| Brain edema | ++ | ± | ± | − | − | − |
| Hemorrhages | ++ | ++ | ++ | ± | ± | ± |
| Disseminated intravascular coagulation | + | + | + | − | − | − |
| Vascular thrombosis | ++ | ++ | ++ | − | − | ± |
| Inflammatory lesions | ||||||
| Meningitis | ++ | + | ++ | − | − | − |
| Fibrinous pleuritis | ++ | ++ | ++ | − | − | − |
| Fibrinous pericarditis | ++ | ++ | ++ | − | − | − |
| Fibrinous peritonitis | ++ | ++ | ++ | + | + | ± |
| Fibrinous polyarthritis | + | + | + | − | − | ± |
| Splenic changes | ||||||
| Lymphoid hyperplasia | − | ± | ± | ++ | ++ | ++ |
| Fibrin deposits | ± | ± | ± | − | − | − |
| Lympholysis | ± | ± | ± | − | − | − |
Experimental groups: control, no vaccine; rTbpA, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; rTbpB, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTM, native proteins with affinity for porcine-transferrin plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTCp, native proteins with affinity for porcine-transferrin plus neuraminidase from Clostridium perfringens (100 mU/ml); PG, Porcilis Glässer vaccine (Intervet). Severity: -, no changes; ±, minimal to mild changes; +, moderate changes; and ++, severe changes. n, Number of animals per group.
FIG. 4.
Gross and histopathological findings. Images in the peritoneal cavity show severe fibrinous peritonitis (nonvaccinated control piglet) (a) and mild fibrinous peritonitis (NPAPTM-vaccinated piglet) (b). (c) Image of the pericardial cavity and heart showing a fibrinous pericarditis (rTbpA piglet). (d) Image of the hock joint showing fibrinous arthritis (inflammatory fluid and fibrin strands, rTbpB piglet). (e) Image of the meninges showing fibrinous-suppurative meningitis. Note a thickening of the meninges covering the brain due to the presence of fibrin and inflammatory cells in the subarachnoid space (H&E) (rTbpA piglet). (f) Image of a blood vessel showing vascular thrombosis (H&E) (rTbpB piglet). (g) An image of spleen tissue shows lympholysis of the follicular center cells, with the production of nuclear debris. Transudation of plasma proteins is also seen (H&E) (nonvaccinated control piglet). (h) No necrotic lymphocytes of the white pulp are seen in spleen tissue (H&E) (NPAPTCp piglet).
In contrast, all piglets immunized with the NPAPTM, NPAPTCp, or PG vaccine survived the challenge with the lethal dose of H. parasuis Nagasaki strain, and a considerable reduction of pathological changes was shown (Table 3). The only lesions observed grossly were a mild (PG vaccine) to moderate (NPAPTM and NPAPTCp vaccines) fibrinous peritonitis in some animals (Fig. 4b), as well as a mild fibrinous polyarthritis in some piglets immunized with the PG commercial vaccine. In addition, microscopically, generalized congestion was observed, and mild edema and hemorrhages were found occasionally in piglets immunized with the NPAPTM or NPAPTCp vaccines. Vascular thrombosis was only seen in a piglet immunized with PG vaccine. A lymphoid hyperplasia of spleen, characterized by multiple prominent nodules of white pulp in its cut surface, was a constant finding in these three test groups, these nodules being made up of expanded periarteriolar lymphoid tissue and follicles due to an increase in lymphocytes. No necrotic lymphocytes in the white pulp were seen (Fig. 4 h).
Bacteriological findings.
No H. parasuis was isolated from the heart blood, kidneys, and pleural cavities from any of the six groups compared. However, pure cultures were recovered from the brains, spleens, and peritonea of all of the control piglets and from the livers, lungs, and pericardial cavities of most of them. H. parasuis was isolated from the eight sites shown in Table 4 in two control piglets. Similar decreased isolation rates were found for rTbpA group, but H. parasuis could be recovered from all of the samples taken in at least one of the dead animals. In rTbpB group, H. parasuis was isolated from the liver parenchyma in three piglets, and from the brains, lungs, pericardial and peritoneal cavities, and carpal and hock joints in two piglets. In the rTbpA and rTbpB treatment groups, H. parasuis was only recovered from the piglets that died (Table 4). All of these positive cultures were confirmed by PCR. In contrast, no H. parasuis recovery was obtained from any of the swabs taken from piglets vaccinated with the NPAPTM, NPAPTCp, or PG formulations.
TABLE 4.
Recovery of H. parasuis from piglets after challenge
| Expt group and piglet category (n)a | No. of piglets positive for H. parasuis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Brain parenchyma | Liver parenchyma | Lung parenchyma | Spleen parenchyma | Pericardial swab | Peritoneal swab | Carpal joint | Hock joint | |
| Control | ||||||||
| Dead piglets (4) | 4 | 3 | 3 | 4 | 3 | 4 | 2 | 2 |
| rTbpA | ||||||||
| Dead piglets (4) | 4 | 3 | 1 | 3 | 2 | 1 | 1 | 1 |
| Surviving piglets (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| rTbpB | ||||||||
| Dead piglets (4) | 2 | 3 | 2 | 0 | 2 | 2 | 2 | 2 |
| Surviving piglets (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NPAPTM | ||||||||
| Surviving piglets (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NPAPTCp | ||||||||
| Surviving piglets (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PG | ||||||||
| Surviving piglets (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Experimental groups: control, no vaccine; rTbpA, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; rTbpB, vaccine containing rTbpA plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTM, native proteins with affinity for porcine-transferrin plus Montanide IMS 2215 VG PR in a 1:4 ratio; NPAPTCp, native proteins with affinity for porcine-transferrin plus neuraminidase from Clostridium perfringens (100 mU/ml); PG, Porcilis Glässer vaccine (Intervet). n, Number of animals per group.
DISCUSSION
It has become evident that many bacterial virulence determinants are synthesized in response to specific environmental signals, and those that are expressed in host are especially attractive for studies of pathogenesis and rational vaccine development (31). In this way, the transferrin-binding proteins produced by some Gram-negative organisms play a pivotal role in virulence (11). Indeed, their potential utility as vaccine immunogens has been supported by studies carried out in some members of the Pasteurellaceae family, such as A. pleuropneumoniae (36) or H. influenzae (20), in which TbpB behaved as a protective antigen. Therefore, we have developed in the present study a subunit vaccine from H. parasuis composed of several native Omp proteins with affinity to porcine transferrin, which were adjuvanted with two types of compounds (the commercial mineral oil Montanide IMS 2215 VG PR and a neuraminidase from C. perfringens), and we have compared these two formulations to two other recombinant peptide vaccines (including rTbpA or rTbpB as immunogens) and to a commercially available bacterin against Glässer's disease. We chose an intratracheal inoculation for challenge with a lethal dose of H. parasuis because this is the most commonly used route for inducing a successful infection under experimental conditions (3, 6, 7, 21, 40); in addition, this administration route circumvented the innate immune system located in the upper respiratory tract of hosts.
The groups immunized with recombinant TbpA or TbpB fragments showed a similar behavior against challenge, with low survival rates and clinical signs resembling those of control piglets, although of a milder intensity in some cases. Moreover, as in the nonvaccinated group, rTbpA- and rTbpB-immunized animals had significantly higher temperatures at 24 hpc compared to the challenge group, and for rTbpB-immunized animals this was also the case at 48 hpc. In addition, the gross histopathological and bacteriological findings were also similar to those seen in nonvaccinated animals. Based on these results, a partial-to-minimal protection could be attributed to these two recombinant vaccines designed in our laboratory. Consequently, these two subunit vaccines cannot be recommended for an effective protection against Glässer's disease. Very similar results were previously reported by Martín de la Fuente et al. (21) when testing 200 μg of the same rTbpB described in our study (that is, an antigenic concentration just half of that inoculated by us) but adjuvanted with a different mineral oil that also belonged to the Montanide series.
Myers et al. (25), after generating antibodies against rTbpA from Moraxella catarrhalis, showed that anti-rTbpA antiserum was capable of recognizing epitopes located on the surfaces of entire cells, but it was not bactericidal. Loosmore et al. (20) confirmed that infant rats passively immunized with rabbit anti-H. influenzae rTbpA serum were not protected from challenge with H. influenzae. The observations seen in these two studies in other closely related Gram-negative organisms seem to corroborate the scarce level of protection obtained for H. parasuis rTbpA antigen in the present study. Nevertheless, Potter et al. (31) demonstrated that the combination of the native TbpA and the recombinant TbpB from Mannheimia haemolytica (an organism also belonging to the Pasteurellaceae family) enhanced considerably the protection conferred by these two proteins when administered separately to calves to prevent shipping fever and that TbpA solely might contribute to protection through a cell-mediated immune response. The scarce protection conferred by rTbpA formulation in our study might be explained by the reduced length of the protein fragment that was cloned (only 200 amino acids) and/or by the fact that the selected sequence might not have included the most exposed epitopes in the bacterial surface and, consequently, this antigen could not have been accessible to the immune response.
Retzer et al. (34) cloned and expressed a larger N-terminal portion of TbpB from different pathogens of the Pasteurellaceae and Neisseriaceae families and proved that this portion possesses a high binding affinity for iron-loaded transferrin. Several reports have demonstrated the immunoprotective capacity of the A. pleuropneumoniae (36), H. influenzae (20), and Neisseria meningitidis (35) full-length recombinant TbpBs in pigs, rats, and in vitro tests, respectively. According to these studies in other Gram-negative organisms, the successful protection of this recombinant protein against challenge with H. parasuis might be achieved with the inoculation of either a larger N-terminal portion of H. parasuis rTbpB or the entire rTbpB, thus resulting in a greater antigenic exposure to the porcine immune system than that obtained by us with the first 102 amino acids of N-terminal domain of TbpB.
On the other hand, of the nine native proteins showing affinity to porcine transferrin described here, the function of three of them (catalase, elongation factor Tu, and Omp2) have never been associated, to our knowledge, with the acquisition, binding, or transport of iron in H. parasuis. In a recent immunoproteomic analysis of N. meningitidis (43), several immunoreactive proteins were found, including an ABC transporter, periplasmic protein, which was also identified in our NPAPT antigen. Similarly, previous studies have reported as immunogenic proteins elongation factor Tu in Bordetella pertussis (1) or elongation factor Tu and Omp2 in Helicobacter pylori (19). In addition, Zhou et al. (45) demonstrated a strong potential for Omp2 of H. parasuis serovar 5 as a vaccine candidate, among other Omps. Catalase has been used in other organisms, such as Haemophilus ducreyi (18) and Gardnerella vaginalis (14), as an iron source. The identification of catalase in NPAPT antigen after purification could be explained by the affinity of this enzyme for the iron that was present in the CNBr-activated Sepharose column.
In complete contrast to the results obtained for the rTbpA or rTbpB formulations, piglets receiving the NPATP antigen were resistant to challenge with H. parasuis serovar 5, regardless of the compound used as potentiator and the administration route. Therefore, both of these vaccines provided strong protection against Glässer's disease, as corroborated by the absence of deaths, hyperthermia (or any other relevant clinical sign), and H. parasuis recovery from any of the locations sampled, as well as by the scarce gross and histopathological changes recorded. That the results obtained with the NPATPM and NPATPCp groups were practically identical suggests that the efficacy of these vaccine formulations was not related to the adjuvant chosen and/or the administration route but rather to the quality of the antigens themselves.
Montanide adjuvant could not be used for the formulation of the vaccine being inoculated intratracheally because the administration of mineral oils is not recommended by this route. Neuraminidase from C. perfringens (type VI) was used as a replacement. This glycoprotein possesses enzymatic action degrading the sialic acid, and desialylation of cell surface glycoconjugates is frequently observed during inflammation and infection episodes (39). In this sense, Kuroiwa et al. (17) reported that neuraminidase increased interleukin-8 (IL-8) production in human lung epithelial cell cultures, while Stamatos et al. (39) showed that the desialylation of glycoconjugates on the surfaces of monocytes by neuraminidase activated increased production of specific cytokines such as IL-6, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β. Based on these findings, we decided to use this glycoprotein as a potentiator in the vaccine administered intratracheally in order to enhance the local immune response, because the secretion of proinflammatory cytokines would attract phagocytes and other antigen-presenting cells, thus improving the presentation of NPAPT antigens.
The protection results exhibited by NPAPTM and NPAPTCp vaccines were similar to those obtained by the commercial PG vaccine, also formulated with an H. parasuis strain belonging to serovar 5. Other commercial inactivated vaccines containing serovar 5 strains demonstrated equally strong protection against experimental infection with the homologous serovar (5, 13).
Some gross and microscopic lesions have already been reported in similar experiments (7, 21, 24, 26, 42). The vascular lesions seen in piglets receiving rTbps, which are more severe in nonvaccinated animals, were previously described by Amano et al. (3) in pigs with acute septicemia after intratracheal inoculation with lower concentrations (105 to 107 CFU) of the H. parasuis Nagasaki strain. These authors associated septicemia in these animals with endotoxin shock caused by the endotoxins released by bacterial lysis, resulting in disseminated intravascular coagulation and death within a short time. Interestingly, the piglets that survived challenge (the NPAPTM, NPAPTCp, and PG groups) showed a reactive hyperplasia in spleen, which was considerably milder in the minimally protected animals (rTbpA and rTbpB groups) and absent in nonvaccinated piglets. This finding could be explained by the invasiveness of this pathogen and by the activation and clonal expansion of memory cells as a response to bacterial antigens.
In a previous trial with conventional piglets (data not shown), the animals were inoculated with the same concentrations of rTbpA, rTbpB, and NPAPT antigens by the same administration routes. No clinical signs, fibrinous peritonitis or polyarthritis, or other macroscopic lesions were seen; consequently, the lesions reported here must be attributable to H. parasuis invasion. On the other hand, no H. parasuis was isolated from piglets that survived until they were euthanized 15 days after challenge (not even from those surviving in rTbpA and rTbpB groups), and this finding seems to indicate that H. parasuis could have been largely cleared by this time.
In conclusion, we developed a strategy to obtain Omps from H. parasuis grown under iron-restricted conditions, and the pool of nine native proteins with affinity to porcine transferrin reported in the present study provides effective subunit vaccines to control Glässer's disease caused by H. parasuis serovar 5, Nagasaki strain. Further studies are required to demonstrate whether the two vaccine formulations described here might be also effective in cross-protection experiments with other serovars.
Acknowledgments
We thank Concepción Gil García (Proteomic Service, Complutense University of Madrid-Scientific Park of Madrid, Spain, ProteoRed Network) for help in the identification of H. parasuis native proteins with affinity for porcine transferrin.
Research in the laboratory of R.F. and S.M. was supported by long-predoctoral fellowships from the Spanish Ministry of Science and Innovation, as well as by grant AGL2008-00110/GAN (Spanish Ministry of Science and Innovation).
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Altindis, E., B. E. Tefon, V. Yildirim, E. Ozcengiz, D. Becher, M. Hecker, and G. Ozcengiz. 2009. Immunoproteomic analysis of Bordetella pertussis and identification of new immunogenic proteins. Vaccine 27:542-548. [DOI] [PubMed] [Google Scholar]
- 2.Amano, H., M. Shibata, N. Kajio, and T. Morozumi. 1994. Pathologic observations of pigs intranasally inoculated with serovars 1, 4, and 5 of Haemophilus parasuis using immunoperoxidase method. J. Vet. Med. Sci. 56:639-644. [DOI] [PubMed] [Google Scholar]
- 3.Amano, H., M. Shibata, K. Takahashi, and Y. Sasaki, Y. 1997. Effects on endotoxin pathogenicity in pigs with acute septicemia of Haemophilus parasuis infection. J. Vet. Med. Sci. 59:451-455. [DOI] [PubMed] [Google Scholar]
- 4.Angen, O., S. Oliveira, P. Ahrens, B. Svensmark, and T. D. Leser. 2007. Development of an improved species specific PCR test for detection of Haemophilus parasuis. Vet. Microbiol. 119:266-276. [DOI] [PubMed] [Google Scholar]
- 5.Bak, H., and H. J. Riising. 2002. Protection of vaccinated pigs against experimental infections with homologous and heterologous Haemophilus parasuis. Vet. Rec. 151:502-505. [DOI] [PubMed] [Google Scholar]
- 6.Blanco, I., A. Canals, G. Evans, M. A. Mellencamp, C. Cia, N. Deeb, L. Wang, and L. Galina-Pantoja. 2008. Differences in susceptibility to Haemophilus parasuis infection in pigs. Can. J. Vet. Res. 72:228-235. [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco, I., L. Galina-Pantoja, S. Oliveira, C. Pijoan, C. Sánchez, and A. Canals. 2004. Comparison between Haemophilus parasuis infection in colostrums deprived and sow-reared piglets. Vet. Microbiol. 103:21-27. [DOI] [PubMed] [Google Scholar]
- 8.del Río, M. L., C. B. Gutiérrez, and E. F. Rodríguez Ferri. 2003. Value of indirect hemagglutination and coagglutination tests for serotyping Haemophilus parasuis. J. Clin. Microbiol. 41:880-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Río, M. L., C. B. Gutiérrez-Martín, J. I. Rodríguez Barbosa, J. Navas, and E. F. Rodríguez Ferri. 2005. Identification and characterization of the TonB region and its role in transferrin-mediated iron acquisition in Haemophilus parasuis. FEMS Immunol. Med. Microbiol. 45:75-86. [DOI] [PubMed] [Google Scholar]
- 10.Goethe, R., O. Flores Gonzáles, T. Lindner, and G. F. Gerlach. 2001. A novel strategy for protective Actinobacillus pleuropneumoniae subunit vaccines: detergent extraction of cultures induced by iron restriction. Vaccine 19:966-975. [DOI] [PubMed] [Google Scholar]
- 11.Gray-Owen, S. D., and A. B. Schryvers. 1996. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 4:185-191. [DOI] [PubMed] [Google Scholar]
- 12.Hill, C. E., D. S. Metcalf, and J. I. MacInnes. 2003. A search for virulence genes of Haemophilus parasuis using differential display RT-PCR. Vet. Microbiol. 96:189-202. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann, C. R., and G. Bilkei. 2002. The effect of a homologous bacterin given to sows prefarrowing on the development of Glässer's disease in postweaning pigs after i.v. challenge with Haemophilus parasuis serotype 5. Deut. Tierärztl. Woch. 109:271-276. [PubMed] [Google Scholar]
- 14.Jarosik, G. P. 2000. Binding of catalase by Gardnerella vaginalis. FEMS Microbiol. Lett. 15:191-194. [DOI] [PubMed] [Google Scholar]
- 15.Kielstein, P., and V. J. Rapp-Gabrielson. 1992. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 30:862-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkwood, R. N., S. A. Rawluk, A. C. Cegielski, and A. J. Otto. 2001. Effect of pig age and autogenous sow vaccination on nasal mucosal colonization of pigs by Haemophilus parasuis. J. Swine Health Prod. 9:77-79. [Google Scholar]
- 17.Kuroiwa, A., A. Hisatsune, Y. Isohama, and H. Katsuki, H. 2009. Bacterial neuraminidase increases IL-8 production in lung epithelial cells via NF-κB-dependent pathway. Biochem. Biophys. Res. Commun. 379:754-759. [DOI] [PubMed] [Google Scholar]
- 18.Lee, B. C. 1991. Iron sources for Haemophilus ducreyi. J. Med. Microbiol. 34:317-322. [DOI] [PubMed] [Google Scholar]
- 19.Lin, Y. F., M. S. Wu, C. C. Chang, S. W. Lin, J. T. Lin, Y. J. Sun, D. S. Chen, and L. P. Chow. 2006. Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol. Cell Proteomics. 5:1484-1496. [DOI] [PubMed] [Google Scholar]
- 20.Loosmore, S. M., Y. Yang, D. C. Coleman, J. M. Shortreed, D. M. England, R. E. Harkness, P. S. C. Chong, and M. H. Klein. 1996. Cloning and expression of the Haemophilus influenzae transferrin receptor gene. Mol. Microbiol. 19:575-586. [DOI] [PubMed] [Google Scholar]
- 21.Martín de la Fuente, A. J., C. B. Gutiérrez Martín, C. Pérez Martínez, M. J. García Iglesias, F. Tejerina, and E. F. Rodríguez Ferri. 2009. Effect of different vaccine formulations on the development of Glässer's disease induced in pigs by experimental Haemophilus parasuis infection. J. Comp. Pathol. 140:169-176. [DOI] [PubMed] [Google Scholar]
- 22.Martínez, S., R. Frandoloso, E. F. Rodríguez-Ferri, B. González-Zörn, and C. B. Gutiérrez-Martín. 2010. Characterization of a recombinant transferrin-binding protein A (TbpA) fragment from Haemophilus parasuis serovar 5. FEMS Microbiol. Lett. 307:142-150. [DOI] [PubMed] [Google Scholar]
- 23.Martínez López, R., C. Nombela, R. Díez-Orejas, L. Monteoliva, and C. Gil. 2008. Immunoproteomics analysis of the protective response obtained from vaccination with Candida albicans ecm33 cell wall mutant in mice. Proteomics 8:2651-2664. [DOI] [PubMed] [Google Scholar]
- 24.Miniats, O. P., N. L. Smart, and S. Rosendal. 1991. Vaccination of gnotobiotic primary specific pathogen-free pigs against Haemophilus parasuis. Can. J. Vet. Res. 55:33-36. [PMC free article] [PubMed] [Google Scholar]
- 25.Myers, L. E., Y. P. Yang, R. P. Du, Q. Wang, R. E. Harkness, A. B. Schryvers, M. H. Klein, and S. M. Loosmore. 1998. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect. Immun. 66:4183-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira, S., L. Galina, I. Blanco, A. Canals, and C. Pijoan. 2003. Naturally farrowed, artificially reared pigs as an alternative model for experimental infection by Haemophilus parasuis. Can. J. Vet. Res. 67:146-150. [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira, S., and C. Pijoan. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet. Microbiol. 99:1-12. [DOI] [PubMed] [Google Scholar]
- 28.Palzer, A., M. Ritzmann, and K. Hienritzi. 2007. A field trial for early vaccination against Glässer's disease using Porcilis Glässer. Schweiz. Arch. Tierheilkder. 149:389-394. [DOI] [PubMed] [Google Scholar]
- 29.Peet, R. L., J. Fry, J. Lloyd, J. Henderson, J. Curran, and D. Moir. 1983. Haemophilus parasuis septicemia in pigs. Aust. J. Vet. Res. 60:187. [DOI] [PubMed] [Google Scholar]
- 30.Pina, S., A. Olvera, A. Barceló, and A. Bensaid. 2009. Trimeric autotransporters of Haemophilus parasuis: generation of an extensive passenger domain repertoire specific for pathogenic strains. J. Bacteriol. 191:576-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potter, A. A., A. B. Schryvers, J. A. Ogunnariwo, W. A. Hutchins, R. T. C. Lo, and T. Watt. 1999. Protective capacity of the Pasteurella haemolytica transferrin-binding proteins TbpA and TbpB in cattle. Microb. Pathog. 27:197-206. [DOI] [PubMed] [Google Scholar]
- 32.Raffie, M., and P. J. Blackall. 2000. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust. Vet. J. 78:172-174. [DOI] [PubMed] [Google Scholar]
- 33.Rapp-Gabrielson, V. J., G. J. Kocur, J. T. Clark, and S. K. Muir. 1997. Haemophilus parasuis: immunity in swine after vaccination. Vet. Med. 92:83-90. [Google Scholar]
- 34.Retzer, M. D., R. Yu, Y. Zhang, G. C. González, and A. B. Schryvers. 1998. Discrimination between apo and iron-loaded forms of transferrin by transferrin binding protein B and its N-terminal subfragment. Microb. Pathog. 25:175-180. [DOI] [PubMed] [Google Scholar]
- 35.Rokbi, B., M. Mignon, G. Maitre-Wilmotte, L. Lissolo, B. Danve, D. A. Caugant, and M. J. Quentin-Millet. 1997. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup b strains. Infect. Immun. 65:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi-Campos, A., C. Anderson, G. F. Gerlach, S. Klashinsky, A. Potter, and P. J. Willson. 1992. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine 10:512-518. [DOI] [PubMed] [Google Scholar]
- 37.Sechi, S., and B. T. Chait. 1998. Modification of cysteine residues by alkylation: a tool in peptide mapping and protein identification. Anal. Chem. 70:5150-5158. [DOI] [PubMed] [Google Scholar]
- 38.Smart, N. L., and O. P. Miniats. 1989. Preliminary assessment of a Haemophilus parasuis bacterin for use in specific pathogen free swine. Can. J. Vet. Res. 53:390-393. [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatos, N. M., S. Curreli, D. Zella, and A. S. Cross. 2003. Desialylation of glycoconjugates on the surface of monocytes activates the extracellular signal-related kinases ERK1/2 and results in enhanced production of specific cytokines. J. Leukoc. Biol. 75:307-313. [DOI] [PubMed] [Google Scholar]
- 40.Takahaschi, L., S. Nagai, T. Yagihashi, T. Idehata, Y. Nakano, K. Senna, T. Maruyama, and J. Murofushi. 2001. A cross protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterins, and evaluation of a bivalent vaccine under laboratory and field conditions. J. Vet. Med. Sci. 63:487-491. [DOI] [PubMed] [Google Scholar]
- 41.Turni, C., and P. J. Blackall. 2005. Comparison of the indirect haemagglutination and gel diffusion test for serotyping Haemophilus parasuis. Vet. Microbiol. 106:145-151. [DOI] [PubMed] [Google Scholar]
- 42.Vahle, J. L., J. S. Haynes, and J. J. Andrews. 1995. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriologic, and morphologic findings. J. Vet. Diagn. Invest. 7:476-480. [DOI] [PubMed] [Google Scholar]
- 43.Williams, J. N., P. J. Skipp, C. D. O'Connor, M. Christodoulides, and J. E. Heckels. 2009. Immunoproteomic analysis of the development of natural immunity in subjects colonized by Neisseria meningitidis reveals potential vaccine candidates. Infect. Immun. 77:5080-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, B., C. Tang, F. Yang, and H. Yue. 2009. Molecular cloning, sequencing and expression of the outer membrane protein A gene from Haemophilus parasuis. Vet. Microbiol. 136:408-410. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, M., Y. Guo, J. Zhao, Q. Hu, Y. Hu, A. Zhang, H. Chen, and M. Jin. 2009. Identification and characterization of novel immunogenic outer membrane proteins of Haemophilus parasuis serovar 5. Vaccine 27:5271-5277. [DOI] [PubMed] [Google Scholar]