Abstract
Vaccines represent a strategic successful tool used to prevent or contain diseases with high morbidity and/or mortality. However, while vaccines have proven to be effective in combating pathogenic microorganisms, based on the immune recognition of these foreign antigens, vaccines aimed at inducing effective antitumor activity are still unsatisfactory. Nevertheless, the effectiveness of the two licensed cancer-preventive vaccines targeting tumor-associated viral agents (anti-HBV [hepatitis B virus], to prevent HBV-associated hepatocellular carcinoma, and anti-HPV [human papillomavirus], to prevent HPV-associated cervical carcinoma), along with the recent FDA approval of sipuleucel-T (for the therapeutic treatment of prostate cancer), represents a significant advancement in the field of cancer vaccines and a boost for new studies in the field. Specific active immunotherapies based on anticancer vaccines represent, indeed, a field in continuous evolution and expansion. Significant improvements may result from the selection of the appropriate tumor-specific target antigen (to overcome the peripheral immune tolerance) and/or the development of immunization strategies effective at inducing a protective immune response. This review aims to describe the vast spectrum of tumor antigens and strategies to develop cancer vaccines.
CANCER IMMUNOTHERAPY
Cancer immunotherapy may be classified into passive as well as active strategies, with the latter being specific or nonspecific (117). Passive or “adoptive” immunotherapy is based on administration of antitumor antibodies or transfer of tumor-reactive lymphocytes. Active immunotherapy is aimed either at eliciting a specific de novo host immune response against selected tumor antigens (Ags) by employing cancer vaccines or at amplifying the existing antitumor immune response by administering nonspecific proinflammatory molecules or adjuvants. In this context, considering the disappointing results up to now, the quest for specific and selective tumor antigens for developing tumor-specific cancer vaccines, optimal delivery systems (i.e., dendritic cell [DC]-based vaccines), adjuvants, and strategies to overcome immune tolerance and regulatory T (Treg) cell responses is the main goal for several research groups and leading health care companies.
QUEST FOR THE APPROPRIATE TUMOR ANTIGEN
The role of the immune system in tumor containment and/or “rejection” has been studied for decades, showing the possibility of inducing an immune response able to reject an experimentally transplanted tumor. However, the “immunosurveillance of tumors” theory independently postulated by Burnet (19-21) and Thomas (173) has not held the original promise, and much skepticism has been raised by different authors. More recently, the original concept of immunosurveillance has been further elaborated by Schreiber et al. (53, 54) into the “cancer immunoediting” hypothesis, which postulates three main phases: elimination, equilibrium, and escape. In particular, in the elimination phase, cells of the innate and adaptive immune responses may eradicate the developing tumor and protect the host from tumor formation. If the elimination process is not successful, the tumor cells may enter the equilibrium phase and be immunologically shaped by immune “editors” to produce new populations of tumor variants. These variants may eventually evade the immune system and become clinically detectable in the escape phase (53, 54).
The cells playing a key role in this process have been identified in both the innate (e.g., natural killer cells, natural killer T cells, macrophages, and dendritic cells) and the adaptive (e.g., CD4+ Th1 and CD8+ T cells) immune systems, whose final goal is to kill the antigen-bearing tumor cells. More recently, a relevant role for an additional subset of CD4+ T helper cells (named Th17) in the immune response to cancer has been proposed and described by several authors (reviewed in reference 203).
However, different approaches have failed to induce an effective antitumor immune response, suggesting the notion of “nonimmunogenicity” of tumors (69). However, more recently it has been shown that the low tumor immunogenicity is not due to the lack of target “tumor” antigens but to their inability to induce an effective immune response. Among several possible biological reasons, this would be consequent to the growth of tumors in the absence of an inflammation process necessary to establish the tissue microenvironment essential to recruit and induce activation and maturation of the antigen-presenting cells (APCs), which represent the key point of initiating an effective adaptive humoral and cellular immune response.
In this perspective, the search for human tumor antigens as potential targets for cancer immunotherapy has led to the discovery of several molecules expressed mainly or selectively on cancer cells.
Antigens used in cancer vaccines, indeed, should preferably be molecules differently expressed on normal and tumor cells; however, most antigens are derived from mutated or modified self-proteins, which may induce immune tolerance (61). This aspect represents a challenge for the appropriate design of vaccines that have to overcome such tolerance in order to elicit specific antitumor immunity without “undesired” autoimmunity side effects (130).
MULTIPLE “UNDEFINED” ANTIGENS
It is well known that tumors show the accumulation of several genetic modifications in somatic cells (63, 186), which provide cancer cells with the selective growth advantage to initiate clonal expansion (66).
In this context, cancer genes have been originally studied based on their possible relation to cancer (59). More recently, high-throughput technologies have enabled the identification of mutated genes in cancers without any hypothesis-driven bias, whose number has resulted to be surprisingly high, with a functional heterogeneity broader than previously thought (140, 169). These studies have been performed in breast, colorectal, pancreatic, and lung cancers and glioblastoma and, overall, have identified almost 400 candidate cancer genes (CAN-genes) (48, 83, 134, 199). Interestingly, systems-level analyses show that, despite the low degree of overlap in terms of gene identity, cancer signatures converge on specific biological processes, as defined by significant molecular and functional associations between genes and/or proteins (68, 163, 170).
Considering the high number of potential tumor antigens for each individual type of cancer, the concept of immunizing with whole tumor cells to avoid the exclusion of potentially relevant antigens from the vaccine is still valid. A further advantage is that since whole tumor cells express an array of antigens, this vaccine approach circumvents the major histocompatibility complex (MHC) restriction and the need for specific patient-tailored epitope identification. The efficacy of autologous tumor cells as a cancer vaccine has been tested in several clinical trials targeting different tumor types, including colorectal cancer (67, 185) and melanoma (3, 12). Alternatively, to overcome the limitations of patient-tailored vaccines (e.g., standardization of large-scale production, variability in the quality and composition of the vaccines, and lack of reliable comparative analysis of clinical outcome), the use of allogeneic tumor cell lines as cancer vaccines has been tested for prostate cancer (114, 160, 162).
However, the effectiveness of such a vaccine strategy is dramatically hampered by the immune system's inherent tolerance to several tumor antigens, as they may be expressed by normal tissues or presented to T cells in a nonstimulatory context. As a consequence, the breaking of tolerance and the containment of immune suppression need a potent and specific immune stimulus combining antigens and immunological adjuvants (reviewed in references 32 and 192). Whole tumor cell vaccines can be made more immunogenic by modifying tumor cells to express costimulatory molecules and/or cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), which has proven to be more effective than others in inducing recruitment, maturation, and function of dendritic cells (DCs), the most potent type of antigen-presenting cell (APC) (50, 57, 81).
In particular, vaccination with genetically engineered, irradiated melanoma cells, modified to secrete GM-CSF, was shown to improve tumor antigen presentation through increased DC and macrophage recruitment, enabling the generation of effective melanoma-specific CD4+ and CD8+ T cells, CD1-restricted NKT cells, and antibodies (51, 57). Although the advantages of using GM-CSF as an adjuvant for cancer vaccines have been reported, recent observations have suggested the potential of GM-CSF to induce immune suppression, which may negatively impact the management of cancer patients (reviewed in reference 34). Whole tumor cell vaccines expressing high levels of costimulatory molecules are currently being pursued to treat aggressive cancers such as acute myeloid leukemia (AML) (26, 31).
Another approach based on multiple “undefined” antigens takes into account the heat shock proteins (HSPs), which are ubiquitous, intracellular molecular protein chaperons whose expression increases under conditions of elevated temperatures and metabolic stress (105) to enhance the antigen processing and presentation by MHC molecules (167). Consequently, HSPs are associated with a large repository of peptides consisting of self-peptides as well as the entire antigenic peptide repertoire of cancer cells. Therefore, the cross-presentation and cross-priming of tumor antigens mediated by HSPs may result in a valid strategy, especially when the amount of antigen is a limiting factor (reviewed in references 15 and 119). Heat shock protein-based vaccines have been shown to be effective in mice when the HSP was purified from tumor cells matching the implanted tumor (166, 171, 179). Vaccines based on proteins complexed with HSPs, purified from autologous tumors (HSP-protein complex), have been evaluated in clinical trials targeting different cancers. In particular, the immunogenicity and efficacy of HSP-protein complex 96 (HSPPC-96; vitespen) made of tumor peptides associated with the heat shock protein gp96 has been extensively assessed in preclinical and clinical trials for a wide range of cancers, including phase I and II trials in colorectal cancer (110), melanoma (7, 138), and renal cell carcinoma (82) and two phase III studies of melanoma and renal cell carcinoma (165, 172, 197, 198). Furthermore, a full-length human papillomavirus type 16 (HPV16) E7 antigen fused to HSP65 from Mycobacterium bovis BCG (HspE7) (33) has been evaluated in a phase II clinical trial, resulting in lesion regression in women with grade III cervical intraepithelial neoplasia (CIN III) (55, 183).
DEFINED ANTIGENS
Cancer vaccines based on defined specific tumor antigens should elicit a very specific effector and memory cell response with a limited chance of inducing autoimmunity. Such an approach may have opposite biological effects. One effect is the possible undesired selection and expansion of tumor variants which lack the target tumor antigen and are biologically resistant to the vaccine-induced immune response. Such tumor variants, however, may in turn induce a beneficial effect, broadening the immune response against newly expressed antigens not present in the original vaccine in a process defined “epitope spreading” (23, 144).
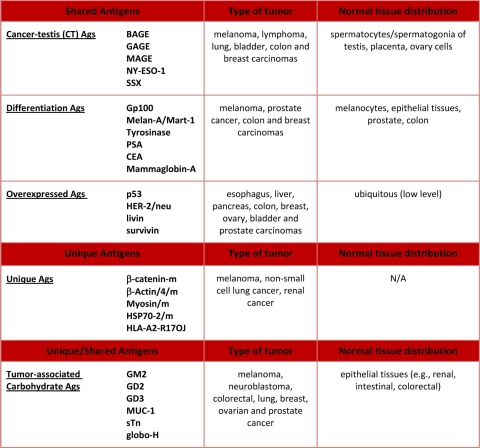
Since the identification of MAGE-1, the first gene reported to encode a human tumor antigen recognized by T cells (182), a large number of tumor antigens have been described (Table 1). Initial classification was based on expression profiles, with tumor-specific antigens (TSAs) being expressed only by cancer cells and tumor-associated antigens (TAAs) representing the mutated counterparts of proteins expressed by normal tissues. The currently accepted classification, however, includes only TAAs, which are divided into shared and unique TAAs and further classified into class I and class II HLA-restricted TAAs, according to the HLA allele restriction (reviewed in reference 125).
TABLE 1.
List of most relevant TAAs recognized by T cellsa
References for antigens listed here are reported in the text. N/A, not applicable.
Among the shared TAAs, the following three main groups can be identified: (i) cancer-testis (CT) antigens, (ii) differentiation antigens, and (iii) widely occurring, overexpressed antigens.
Among shared tumor-specific antigens, cancer-testis (CT) antigens are expressed in histologically different human tumors and, among normal tissues, in spermatocytes/spermatogonia of the testis and, occasionally, in placenta. CT antigens result from the reactivation of genes which are normally silent in adult tissues (46) but are transcriptionally activated in different tumor histotypes (45). Many CT antigens have been identified and used in clinical trials, although little is known about their specific functions, especially with regard to malignant transformation. This group of TAAs includes MAGE-A1 (30, 177), NY-ESO-1 (78), and SSX-2 (1).
Differentiation antigens are shared between tumors and the normal tissue of origin and found mostly in melanomas and normal melanocytes (Gp100, Melan-A/Mart-1, and Tyrosinase) (4, 89-91, 131, 190), although they are also found in epithelial tissues and tumors such as prostate tumors (prostate-specific antigen [PSA]) (37, 38) and breast carcinomas (mammaglobin-A) (79). Moreover, expression of several oncofetal antigens appears to be increased in many adult cancer tissues, including carcinoembryonic antigen (CEA), which is highly expressed in colon cancer (178). TAAs from this group, despite representing self-antigens, have been and still are commonly used in current cancer vaccination trials, often together with CT antigens.
Widely occurring, overexpressed TAAs have been detected in different types of tumors as well as in many normal tissues, and their overexpression in tumor cells can reach the threshold for T cell recognition, breaking the immunological tolerance and triggering an anticancer response. Among the most interesting TAAs of this group are the antiapoptotic proteins (livin and survivin) (154, 155), hTERT (116, 187, 188), and tumor suppressor proteins (e.g., p53) (2, 180).
Unique TAAs, on the other hand, are products of random somatic point mutations induced by physical or chemical carcinogens and therefore expressed uniquely by individual tumors and not by any normal tissue, representing the only true tumor-specific antigens (Ags) (reviewed in reference 133). Such Ags characterize each single neoplasm and were shown to be diverse between tumors induced in the same animal or even in different tissue fragments from the same tumor nodule (61, 141, 200). A relevant feature of unique Ags is their potential resistance to immunoselection if the mutated protein is crucial to the oncogenic process and thus indispensable for maintaining the neoplastic state. As a consequence, unique Ags should elicit an immune response clinically more effective than that of shared Ags. However, identification of unique tumor antigens for solid human tumors requires sequencing of the whole genome of each individual tumor in order to identify mutated genes and select peptides whose motifs are predicted to be presented by the patient's HLA alleles. Moreover, each tumor bears highly heterogeneous sets of defects in dozens of different genes (25, 83, 85, 142, 199) which need to be further verified for their substantial contribution to the tumor development and progression and, consequently, for their relevance as vaccine targets (58).
On the contrary, unlike for the solid tumors, the strategy of identifying unique TAAs is relatively easy and feasible for tumors of hematological origin, such as B cell lymphomas, for which the target antigen is well known, being represented by the immunoglobulin idiotype (Ig Id) included in the B cell receptor (BCR). Therefore, sequencing analysis for the identification of cancer-related mutations can be selectively focused on the Ig Id, which can be used for developing a patient-specific vaccine (8, 168). More recently, however, it has been demonstrated that the BCR repertoire expressed by clonal B cells sustaining hepatitis C virus (HCV)-associated non-Hodgkin's lymphoma (NHL) is not random, with a restricted representation of immunoglobulin idiotypes in different patients (28, 43, 137). As a consequence, it is possible to overcome the limitations of tailor-made individual vaccines by designing shared idiotype vaccines to elicit immunity, targeting the B cell clone sustaining the HCV-associated NHL in a broad spectrum of patients (18, 44).
An additional class of tumor antigens is represented by tumor-associated carbohydrate antigens (TACAs), which are glycans uniquely or excessively expressed on the cancer cell surface (143) and correlate with various stages of cancer development (42, 65). However, TACAs do not elicit T cell responses and are usually poorly immunogenic given their structural similarity to normal antigens (80). Nevertheless, conjugation to a carrier protein increases their immunogenicity, enhancing the presentation of carbohydrate antigens to antigen-presenting cells as well as induction of helper T cell activation (92, 106). In particular, keyhole limpet hemocyanin (KLH) has been shown to be the most effective carrier for TACAs (86), and KLH conjugates of GM2 for melanoma (27) as well as those of sTn for breast cancer (73) have both entered phase III clinical trials. However, the disappointing outcomes of these vaccine clinical trials, in terms of time-to-disease progression and overall survival, have driven the generation of several forms of fully synthetic carbohydrate vaccines, which have been shown to be immunogenic regardless of the use of a protein carrier or external adjuvant (6, 13, 22, 76, 176, 194). Unfortunately, despite all efforts, TACA-based cancer vaccines have failed to induce sufficient T cell-mediated immune responses in cancer patients, and none has been approved for clinical use yet (64).
APPLICATION OF DEFINED ANTIGENS AS CANCER VACCINES
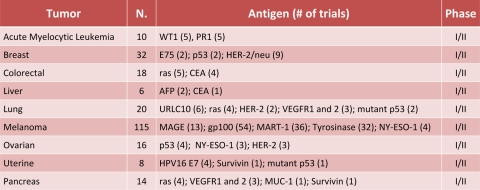
Most cancer vaccine clinical trials have been performed with peptide-based vaccines, employing either cancer-testis antigens or differentiation TAAs, and despite the induction of a high frequency of specific T cells, the clinical outcomes have been disappointingly limited (29, 132, 136, 148, 149) (Table 2). There are many possible reasons for these unsatisfactory results, including immune tolerance induced by shared TAAs (35, 112) and limited cytotoxic T lymphocyte (CTL) expansion due to activation of regulatory T lymphocytes (122). Furthermore, single peptides elicit a CD8+ T cell response with a narrow epitope specificity, which may result in limited immunological efficacy and in the induction of immune escape mechanisms (146).
TABLE 2.
Peptide-based cancer vaccine clinical trials for most representative tumorsa
Further information on current cancer vaccine clinical trials is available at www.clinicaltrials.gov. N. = number of total clinical trials registered for the corresponding tumors.
Several strategies have been adopted to overcome such limitations, including the introduction of inflammatory cytokines in the vaccination protocol, such as alpha interferon (IFN-α) (94, 135) and interleukin-2 (IL-2) (104, 147, 150, 151), with conflicting results. An alternative strategy is to generate peptide variants of TAAs (47, 72, 84), including mimotopes, heteroclitic peptides, altered-peptide ligands, and superagonists, introducing MHC-anchor residue modifications (127, 181), systematic residue substitutions (161), combinatorial peptide libraries (111, 139), and genetically encoded peptide libraries (39, 191). However, considering the overall disappointing results of clinical trials testing such peptide variants, additional strategies have been developed to broaden the repertoire of responding T cells by introducing amino acid substitutions in the peptide-MHC binding surface (16, 74, 102).
Significant improvement in the immunogenicity of single-peptide vaccines has been achieved using long peptides deriving either from the chemical linkage of multiple immunogenic epitopes (71, 159) or from naturally occurring linked CTL and Th epitopes, as shown for human papillomavirus (HPV) E6-E7 proteins (93, 193), the CT antigen NY-ESO-1 (202), and HER-2/neu (49, 95). The enhanced immunological potency of long peptides, which do not bind directly to MHC class I molecules as 8-mer to 10-mer CTL epitopes do, is most likely due to their efficient presentation to CTL precursors through processing by DCs (9, 14, 113, 156), which should dramatically reduce transient CTL responses or tolerance (174, 175). Furthermore, long peptides may persist longer in inflamed lymph nodes in close proximity to the vaccination site, resulting in the clonal expansion of IFN-γ-producing effector T cells and improved antitumor CTL response (14). Finally, the presence of multiple immunogenic epitopes in long peptides would ensure the interaction with different HLA class I and class II alleles, eliciting a broad T cell response against many epitopes, including the immunodominant ones, and reducing the emergence of tumor escape variants.
DENDRITIC CELLS AS AN ANTIGEN DELIVERY SYSTEM
An effective vaccine needs to efficiently hit the innate immune system and, downstream, the adaptive humoral and cellular immunity to elicit an adequate level of effector cells and establish the immunological memory. To this aim, vaccine strategies effective in activating both innate and adaptive immunity are actively pursued by several groups, and among the different possible strategies, dendritic cell (DC)-based vaccines represent one of the most promising strategies (reviewed in references 96 and 126).
Dendritic cells can be generated in vitro from CD34+ progenitor cells derived from the patient's bone marrow or peripheral blood, and maturation can be obtained ex vivo with a cocktail of several cytokines.
The original protocol for DC generation has been designed, including GM-CSF and IL-4 (121, 157), but it has been shown that functionally distinct DC subsets can be generated by using different cytokines. Indeed, activated monocytes induced with IFN-α/β, thymic stroma lymphopoietin (TSLP), tumor necrosis factor (TNF), or IL-15 will differentiate into IFN DCs, TSLP DCs, TNF DCs, or IL-15 DCs, respectively, able to induce different types of immune responses (5). For example, melanoma-peptide-pulsed IL-15 DCs are much more efficient than IL-4 DCs in inducing antigen-specific CTL differentiation in vitro (52), whereas IFN-α DCs show improved activation of T helper cells (128). Similarly, different DC maturation pathways may significantly impact their capacity to elicit T cell immunity (99). Indeed, GM-CSF/IL-4 DCs activated with a cocktail of IFN-α, poly(I:C), IL-1β, TNF, and IFN-γ are much more effective in inducing specific CTLs than “gold standard” DCs matured with a cocktail of macrophage cytokines, including IL-1β/TNF/IL-6/prostaglandin E2 (PGE2) (109). Moreover, PGE2 can skew the differentiation of T helper cells to Th2 cells, blocking the production of IL-12 p70 (87, 88).
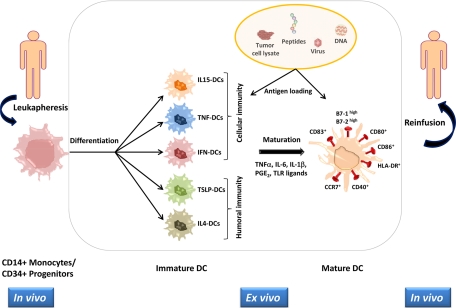
Matured DCs are then loaded with tumor antigens as peptides (24), tumor lysates (121), or apoptotic debris (129), which are processed and presented on the DC surface in the context of MHC class I and II molecules. The matured and antigen-loaded DCs are then transferred back to the patients for the generation of an antitumor immune response (reviewed in references 5 and 157) (Fig. 1).
FIG. 1.
Schematic representation of a DC-based vaccine preparation. CD14+ monocytes or CD34+ hematopoietic progenitors are derived from patients. Different DC subsets are generated in vitro, with distinct specialization in driving adaptive immunity to the Th1 or Th2 response. Mature DCs are loaded with one of the indicated sources of tumor antigens and reinfused in the patient. The most relevant cell markers characterizing the different activation stages of DCs are indicated.
Concerning the strategy of using tumor lysates or apoptotic debris as source of tumor antigens, the original and standard procedure is to use autologous DCs loaded with autologous tumor cells, both derived from the treated patient. However, the preparation of sufficient amounts of autologous tumor cells might be a significant limiting factor, and to overcome this hurdle, the use of allogeneic tumor cell lines as sources of tumor antigens has been proposed. Tumor cell lines, indeed, share many TAAs with the patients' autologous tumor cells and can be efficiently expanded in vitro. Cellular fusions generated by autologous DCs and allogeneic tumor cell lines have been shown to induce antigen-specific polyclonal CTLs, with cytotoxic activity against autologous tumor cells (10, 97, 98). A further possible alternative is to use allogeneic DCs from healthy donors as a fusion partner, given that unprimed T cells from an individual react against the foreign MHC antigens of another individual. It has been demonstrated, indeed, that fusions of both autologous and allogeneic DCs are effective in inducing antitumor immunity in humans (62), although this approach can be applied only in selected situations (reviewed in reference 96).
The effectiveness of DC-based vaccines has been demonstrated in specific tumor stages, and very recently, the first autologous cellular immunotherapy has received FDA approval for the treatment of asymptomatic or minimally symptomatic metastatic hormone-refractory prostate cancer (HRPC). Sipuleucel-T consists of autologous PBMCs loaded with recombinant human prostatic acid phosphatase (PAP) linked to granulocyte-macrophage colony-stimulating factor (PAP-GM-CSF), which has proven to be effective in phase III clinical trials (70).
However, results from multiple clinical trials with DC-based cancer vaccines have been contradictory, and only fractions of the enrolled patients show potent antitumor immune responses (reviewed in references 96 and 103). Several reasons may account for this modest clinical outcome, including the reproducible efficiency of DC generation and the possible induction of adaptive CD4+ CD25+ Foxp3+ regulatory T (Treg) cells in the presence of transforming growth factor β (TGF-β) or IL-10 derived from the tumor microenvironment (189).
HARNESSING IMMUNE TOLERANCE AND Treg CELLS
Naturally occurring regulatory T (Treg) cells account for 5% to 10% of peripheral CD4+ T cells (60, 152), whose key role is to inhibit self-reactive effector T cells, inducing peripheral T cell tolerance (152). Moreover, Treg cells have been found to be increased in peripheral blood and tumors in a variety of human cancers (75, 107, 196), resulting in poorer prognosis and reduced survival (153, 158, 195, 201). The presence of an increased percentage of circulating Treg cells may, indeed, represent a major obstacle to the success of cancer vaccines, and partial depletion of Treg cells has been shown to enhance DC vaccine-induced immune responses in cancer patients (41, 123). In this perspective, cancer vaccines may be more effective when combined with therapeutic interventions aimed at eliminating and/or controlling naturally occurring CD4+ CD25+ regulatory T cells. Studies done in the 1980s showed that pretreatment with cytostatic drugs (i.e., cyclophosphamide) was significantly enhancing the efficacy of adoptive cancer immunotherapy in preclinical (124) as well as clinical (11) settings. Several clinical trials are currently ongoing to assess the efficacy of cyclophosphamide to control Treg cells and improve the immune response to cancer vaccines in humans (56). Alternative strategies to eliminate and/or control naturally occurring Treg cells are represented by the use of a recombinant IL-2 diphtheria toxin conjugate (Ontak), which has been shown to enhance tumor-specific T cell responses to vaccines (41, 118) as well as to improve immune responses in patients with metastatic melanoma (108).
Furthermore, depletion of CD4+ CD25+ Treg cells may also be achieved using an anti-CD25 monoclonal antibody (MAb), as shown for melanoma or breast cancer vaccine (77, 115, 145).
The role of Toll-like receptor (TLR) agonists as inhibitors of Treg cell function (36, 184) is still controversial. It has been reported, indeed, that different TLR agonists can be effective in limiting tumor progression when used as adjuvants to coadministered a cancer vaccine (120, 164) or as stand-alone immunotherapeutics, eliciting an immune response to tumor self-antigens (17, 100, 101). Nevertheless, such effects are not univocal, given that TLR agonists may induce differentiation, proliferation, or activation of Treg cells. Human CD4+ CD25+ Treg cells stimulated with the TLR5 agonist flagellin, indeed, show enhanced expression of Foxp3 and increased suppressive function (40).
CONCLUSIONS AND FUTURE DIRECTIONS
The cancer vaccine field is constantly growing and generating a considerable amount of information in terms of antigen target identification and delivery as well as immune modulation, which will represent the knowledge platform to accomplish the ultimate aim of developing an effective cancer vaccine. However, large-scale clinical trials of current strategies and protocols have not yet proved to be as efficacious as needed for complete tumor regression.
Several reasons may account for these disappointing results, including (i) tumor evasion from immune recognition, (ii) inefficient induction of high-affinity adaptive immunity, and (iii) tumor-induced immunosuppression. Each of these aspects needs to be addressed and possibly solved in order to increase the chances of success.
In this discovery process, the systems biology approach can have a great impact not only on the comprehension of multiple pathways involved in tumor development and progression but also on the dissection of molecular mechanisms involved in the efficient induction of effective innate and adaptive immunity. Such an approach, ultimately, will have a significant impact on cancer vaccine development for the identification of both novel potential target antigens and molecular prediction markers of immunogenicity.
This would represent the real switch from the “empirical” to the “knowledge-based” age of cancer vaccinology, enabling the development of strategies with enhanced therapeutic efficacy to significantly improve the quality of life of cancer patients.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Ayyoub, M., S. Stevanovic, U. Sahin, P. Guillaume, C. Servis, D. Rimoldi, D. Valmori, P. Romero, J. C. Cerottini, H. G. Rammensee, M. Pfreundschuh, D. Speiser, and F. Levy. 2002. Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma. J. Immunol. 168:1717-1722. [DOI] [PubMed] [Google Scholar]
- 2.Azuma, K., S. Shichijo, Y. Maeda, T. Nakatsura, Y. Nonaka, T. Fujii, K. Koike, and K. Itoh. 2003. Mutated p53 gene encodes a nonmutated epitope recognized by HLA-B*4601-restricted and tumor cell-reactive CTLs at tumor site. Cancer Res. 63:854-858. [PubMed] [Google Scholar]
- 3.Baars, A., A. M. Claessen, A. J. van den Eertwegh, H. E. Gall, A. G. Stam, S. Meijer, G. Giaccone, C. J. Meijer, R. J. Scheper, J. Wagstaff, J. B. Vermorken, and H. M. Pinedo. 2000. Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann. Oncol. 11:965-970. [DOI] [PubMed] [Google Scholar]
- 4.Bakker, A. B., M. W. Schreurs, A. J. de Boer, Y. Kawakami, S. A. Rosenberg, G. J. Adema, and C. G. Figdor. 1994. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 179:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau, J., and A. K. Palucka. 2005. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5:296-306. [DOI] [PubMed] [Google Scholar]
- 6.Bay, S., S. Fort, L. Birikaki, C. Ganneau, E. Samain, Y. M. Coic, F. Bonhomme, E. Deriaud, C. Leclerc, and R. Lo-Man. 2009. Induction of a melanoma-specific antibody response by a monovalent, but not a divalent, synthetic GM2 neoglycopeptide. ChemMedChem 4:582-587. [DOI] [PubMed] [Google Scholar]
- 7.Belli, F., A. Testori, L. Rivoltini, M. Maio, G. Andreola, M. R. Sertoli, G. Gallino, A. Piris, A. Cattelan, I. Lazzari, M. Carrabba, G. Scita, C. Santantonio, L. Pilla, G. Tragni, C. Lombardo, F. Arienti, A. Marchiano, P. Queirolo, F. Bertolini, A. Cova, E. Lamaj, L. Ascani, R. Camerini, M. Corsi, N. Cascinelli, J. J. Lewis, P. Srivastava, and G. Parmiani. 2002. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J. Clin. Oncol. 20:4169-4180. [DOI] [PubMed] [Google Scholar]
- 8.Bendandi, M. 2009. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat. Rev. Cancer 9:675-681. [DOI] [PubMed] [Google Scholar]
- 9.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 10.Berard, F., P. Blanco, J. Davoust, E. M. Neidhart-Berard, M. Nouri-Shirazi, N. Taquet, D. Rimoldi, J. C. Cerottini, J. Banchereau, and A. K. Palucka. 2000. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 192:1535-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berd, D., and M. J. Mastrangelo. 1988. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 48:1671-1675. [PubMed] [Google Scholar]
- 12.Berd, D., T. Sato, H. C. Maguire, Jr., J. Kairys, and M. J. Mastrangelo. 2004. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J. Clin. Oncol. 22:403-415. [DOI] [PubMed] [Google Scholar]
- 13.Bettahi, I., G. Dasgupta, O. Renaudet, A. A. Chentoufi, X. Zhang, D. Carpenter, S. Yoon, P. Dumy, and L. BenMohamed. 2009. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol. Immunother. 58:187-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bijker, M. S., S. J. van den Eeden, K. L. Franken, C. J. Melief, S. H. van der Burg, and R. Offringa. 2008. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur. J. Immunol. 38:1033-1042. [DOI] [PubMed] [Google Scholar]
- 15.Bolhassani, A., and S. Rafati. 2008. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev. Vaccines 7:1185-1199. [DOI] [PubMed] [Google Scholar]
- 16.Borbulevych, O. Y., T. K. Baxter, Z. Yu, N. P. Restifo, and B. M. Baker. 2005. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: implications for vaccine design. J. Immunol. 174:4812-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandau, S., and H. Suttmann. 2007. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed. Pharmacother. 61:299-305. [DOI] [PubMed] [Google Scholar]
- 18.Buonaguro, L., A. Petrizzo, M. Tornesello, M. Napolitano, D. Martorelli, G. Castello, G. Beneduce, R. A. De, O. Perrella, L. Romagnoli, V. Sousa, R. De, V. R. Dolcetti, and F. M. Buonaguro. 2010. Immune signatures in human PBMCs of idiotypic vaccine for HCV-related lymphoproliferative disorders. J. Transl. Med. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnet, F. M. 1970. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13:1-27. [DOI] [PubMed] [Google Scholar]
- 20.Burnet, M. 1957. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1:841-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnet, M. 1957. Cancer: a biological approach. I. The processes of control. Br. Med. J. 1:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buskas, T., S. Ingale, and G. J. Boons. 2005. Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated tn antigen. Angew. Chem. Int. Ed. Engl. 44:5985-5988. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield, L. H., A. Ribas, V. B. Dissette, S. N. Amarnani, H. T. Vu, D. Oseguera, H. J. Wang, R. M. Elashoff, W. H. McBride, B. Mukherji, A. J. Cochran, J. A. Glaspy, and J. S. Economou. 2003. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 9:998-1008. [PubMed] [Google Scholar]
- 24.Celluzzi, C. M., J. I. Mayordomo, W. J. Storkus, M. T. Lotze, and L. D. Falo, Jr. 1996. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 183:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerami, E., E. Demir, N. Schultz, B. S. Taylor, and C. Sander. 2010. Automated network analysis identifies core pathways in glioblastoma. PLoS One 5:e8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan, L., N. Hardwick, D. Darling, J. Galea-Lauri, J. Gaken, S. Devereux, M. Kemeny, G. Mufti, and F. Farzaneh. 2005. IL-2/B7.1 (CD80) fusagene transduction of AML blasts by a self-inactivating lentiviral vector stimulates T cell responses in vitro: a strategy to generate whole cell vaccines for AML. Mol. Ther. 11:120-131. [DOI] [PubMed] [Google Scholar]
- 27.Chapman, P. B., D. M. Morrissey, K. S. Panageas, W. B. Hamilton, C. Zhan, A. N. Destro, L. Williams, R. J. Israel, and P. O. Livingston. 2000. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-keyhole limpet hemocyanin + QS21 vaccine: a dose-response study. Clin. Cancer Res. 6:874-879. [PubMed] [Google Scholar]
- 28.Charles, E. D., R. M. Green, S. Marukian, A. H. Talal, G. V. Lake-Bakaar, I. M. Jacobson, C. M. Rice, and L. B. Dustin. 2008. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood 111:1344-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhuri, D., R. Suriano, A. Mittelman, and R. K. Tiwari. 2009. Targeting the immune system in cancer. Curr. Pharm. Biotechnol. 10:166-184. [DOI] [PubMed] [Google Scholar]
- 30.Chaux, P., R. Luiten, N. Demotte, V. Vantomme, V. Stroobant, C. Traversari, V. Russo, E. Schultz, G. R. Cornelis, T. Boon, and B. P. van der. 1999. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1. J. Immunol. 163:2928-2936. [PubMed] [Google Scholar]
- 31.Cheuk, A. T., J. W. Wells, L. Chan, N. B. Westwood, S. A. Berger, H. Yagita, K. Okumura, F. Farzaneh, G. J. Mufti, and B. A. Guinn. 2009. Anti-tumor immunity in a model of acute myeloid leukemia. Leuk. Lymphoma 50:447-454. [DOI] [PubMed] [Google Scholar]
- 32.Chiang, C. L., F. Benencia, and G. Coukos. 2010. Whole tumor antigen vaccines. Semin. Immunol. [DOI] [PMC free article] [PubMed]
- 33.Chu, N. R., H. B. Wu, T. Wu, L. J. Boux, M. I. Siegel, and L. A. Mizzen. 2000. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin. Exp. Immunol. 121:216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clive, K. S., J. A. Tyler, G. T. Clifton, J. P. Holmes, E. A. Mittendorf, S. Ponniah, and G. E. Peoples. 2010. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev. Vaccines 9:519-525. [DOI] [PubMed] [Google Scholar]
- 35.Colella, T. A., T. N. Bullock, L. B. Russell, D. W. Mullins, W. W. Overwijk, C. J. Luckey, R. A. Pierce, N. P. Restifo, and V. H. Engelhard. 2000. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J. Exp. Med. 191:1221-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conroy, H., N. A. Marshall, and K. H. Mills. 2008. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene 27:168-180. [DOI] [PubMed] [Google Scholar]
- 37.Corman, J. M., E. E. Sercarz, and N. K. Nanda. 1998. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin. Exp. Immunol. 114:166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correale, P., K. Walmsley, C. Nieroda, S. Zaremba, M. Zhu, J. Schlom, and K. Y. Tsang. 1997. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J. Natl. Cancer Inst. 89:293-300. [DOI] [PubMed] [Google Scholar]
- 39.Crawford, F., E. Huseby, J. White, P. Marrack, and J. W. Kappler. 2004. Mimotopes for alloreactive and conventional T cells in a peptide-MHC display library. PLoS Biol. 2:E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crellin, N. K., R. V. Garcia, O. Hadisfar, S. E. Allan, T. S. Steiner, and M. K. Levings. 2005. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J. Immunol. 175:8051-8059. [DOI] [PubMed] [Google Scholar]
- 41.Dannull, J., Z. Su, D. Rizzieri, B. K. Yang, D. Coleman, D. Yancey, A. Zhang, P. Dahm, N. Chao, E. Gilboa, and J. Vieweg. 2005. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115:3623-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis, J. W., M. Granovsky, and C. E. Warren. 1999. Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta 1473:21-34. [DOI] [PubMed] [Google Scholar]
- 43.De Re, V., S. De Vita, A. Marzotto, M. Rupolo, A. Gloghini, B. Pivetta, D. Gasparotto, A. Carbone, and M. Boiocchi. 2000. Sequence analysis of the immunoglobulin antigen receptor of hepatitis C virus-associated non-Hodgkin lymphomas suggests that the malignant cells are derived from the rheumatoid factor-producing cells that occur mainly in type II cryoglobulinemia. Blood 96:3578-3584. [PubMed] [Google Scholar]
- 44.de Re, V., M. P. Simula, A. Pavan, M. Garziera, D. Marin, R. Dolcetti, V. S. De, D. Sansonno, S. Geremia, and G. Toffoli. 2009. Characterization of antibodies directed against the immunoglobulin light kappa chain variable chain region (VK) of hepatitis C virus-related type-II mixed cryoglobulinemia and B-cell proliferations. Ann. N. Y. Acad. Sci. 1173:152-160. [DOI] [PubMed] [Google Scholar]
- 45.De Smet, C., C. Lurquin, B. Lethe, V. Martelange, and T. Boon. 1999. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol. Cell. Biol. 19:7327-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Smet, C., C. Lurquin, B. P. van der, P. E. De, F. Brasseur, and T. Boon. 1994. Sequence and expression pattern of the human MAGE2 gene. Immunogenetics 39:121-129. [DOI] [PubMed] [Google Scholar]
- 47.de Visser, K. E., T. A. Cordaro, H. W. Kessels, F. H. Tirion, T. N. Schumacher, and A. M. Kruisbeek. 2001. Low-avidity self-specific T cells display a pronounced expansion defect that can be overcome by altered peptide ligands. J. Immunol. 167:3818-3828. [DOI] [PubMed] [Google Scholar]
- 48.Ding, L., G. Getz, D. A. Wheeler, E. R. Mardis, M. D. McLellan, K. Cibulskis, C. Sougnez, H. Greulich, D. M. Muzny, M. B. Morgan, L. Fulton, R. S. Fulton, Q. Zhang, M. C. Wendl, M. S. Lawrence, D. E. Larson, K. Chen, D. J. Dooling, A. Sabo, A. C. Hawes, H. Shen, S. N. Jhangiani, L. R. Lewis, O. Hall, Y. Zhu, T. Mathew, Y. Ren, J. Yao, S. E. Scherer, K. Clerc, G. A. Metcalf, B. Ng, A. Milosavljevic, M. L. Gonzalez-Garay, J. R. Osborne, R. Meyer, X. Shi, Y. Tang, D. C. Koboldt, L. Lin, R. Abbott, T. L. Miner, C. Pohl, G. Fewell, C. Haipek, H. Schmidt, B. H. Dunford-Shore, A. Kraja, S. D. Crosby, C. S. Sawyer, T. Vickery, S. Sander, J. Robinson, W. Winckler, J. Baldwin, L. R. Chirieac, A. Dutt, T. Fennell, M. Hanna, B. E. Johnson, R. C. Onofrio, R. K. Thomas, G. Tonon, B. A. Weir, X. Zhao, L. Ziaugra, M. C. Zody, T. Giordano, M. B. Orringer, J. A. Roth, M. R. Spitz, I. I. Wistuba, B. Ozenberger, P. J. Good, A. C. Chang, D. G. Beer, M. A. Watson, M. Ladanyi, S. Broderick, A. Yoshizawa, W. D. Travis, W. Pao, M. A. Province, G. M. Weinstock, H. E. Varmus, S. B. Gabriel, E. S. Lander, R. A. Gibbs, M. Meyerson, and R. K. Wilson. 2008. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455:1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Disis, M. L., T. A. Gooley, K. Rinn, D. Davis, M. Piepkorn, M. A. Cheever, K. L. Knutson, and K. Schiffman. 2002. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J. Clin. Oncol. 20:2624-2632. [DOI] [PubMed] [Google Scholar]
- 50.Dranoff, G. 2002. GM-CSF-based cancer vaccines. Immunol. Rev. 188:147-154. [DOI] [PubMed] [Google Scholar]
- 51.Dranoff, G., E. Jaffee, A. Lazenby, P. Golumbek, H. Levitsky, K. Brose, V. Jackson, H. Hamada, D. Pardoll, and R. C. Mulligan. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. U. S. A. 90:3539-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubsky, P., H. Saito, M. Leogier, C. Dantin, J. E. Connolly, J. Banchereau, and A. K. Palucka. 2007. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 37:1678-1690. [DOI] [PubMed] [Google Scholar]
- 53.Dunn, G. P., A. T. Bruce, H. Ikeda, L. J. Old, and R. D. Schreiber. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991-998. [DOI] [PubMed] [Google Scholar]
- 54.Dunn, G. P., L. J. Old, and R. D. Schreiber. 2004. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22:329-360. [DOI] [PubMed] [Google Scholar]
- 55.Einstein, M. H., A. S. Kadish, R. D. Burk, M. Y. Kim, S. Wadler, H. Streicher, G. L. Goldberg, and C. D. Runowicz. 2007. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol. Oncol. 106:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emadi, A., R. J. Jones, and R. A. Brodsky. 2009. Cyclophosphamide and cancer: golden anniversary. Nat. Rev. Clin. Oncol. 6:638-647. [DOI] [PubMed] [Google Scholar]
- 57.Emens, L. A. 2009. GM-CSF-secreting vaccines for solid tumors. Curr. Opin. Invest. Drugs. 10:1315-1324. [PubMed] [Google Scholar]
- 58.Fox, E. J., J. J. Salk, and L. A. Loeb. 2009. Cancer genome sequencing—an interim analysis. Cancer Res. 69:4948-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Futreal, P. A., L. Coin, M. Marshall, T. Down, T. Hubbard, R. Wooster, N. Rahman, and M. R. Stratton. 2004. A census of human cancer genes. Nat. Rev. Cancer 4:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavin, M., and A. Rudensky. 2003. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr. Opin. Immunol. 15:690-696. [DOI] [PubMed] [Google Scholar]
- 61.Gilboa, E. 1999. The makings of a tumor rejection antigen. Immunity 11:263-270. [DOI] [PubMed] [Google Scholar]
- 62.Gong, J., N. Nikrui, D. Chen, S. Koido, Z. Wu, Y. Tanaka, S. Cannistra, D. Avigan, and D. Kufe. 2000. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J. Immunol. 165:1705-1711. [DOI] [PubMed] [Google Scholar]
- 63.Greenman, C., P. Stephens, R. Smith, G. L. Dalgliesh, C. Hunter, G. Bignell, H. Davies, J. Teague, A. Butler, C. Stevens, S. Edkins, S. O'Meara, I. Vastrik, E. E. Schmidt, T. Avis, S. Barthorpe, G. Bhamra, G. Buck, B. Choudhury, J. Clements, J. Cole, E. Dicks, S. Forbes, K. Gray, K. Halliday, R. Harrison, K. Hills, J. Hinton, A. Jenkinson, D. Jones, A. Menzies, T. Mironenko, J. Perry, K. Raine, D. Richardson, R. Shepherd, A. Small, C. Tofts, J. Varian, T. Webb, S. West, S. Widaa, A. Yates, D. P. Cahill, D. N. Louis, P. Goldstraw, A. G. Nicholson, F. Brasseur, L. Looijenga, B. L. Weber, Y. E. Chiew, A. DeFazio, M. F. Greaves, A. R. Green, P. Campbell, E. Birney, D. F. Easton, G. Chenevix-Trench, M. H. Tan, S. K. Khoo, B. T. Teh, S. T. Yuen, S. Y. Leung, R. Wooster, P. A. Futreal, and M. R. Stratton. 2007. Patterns of somatic mutation in human cancer genomes. Nature 446:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo, Z., and Q. Wang. 2009. Recent development in carbohydrate-based cancer vaccines. Curr. Opin. Chem. Biol. 13:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hakomori, S. 2001. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 491:369-402. [DOI] [PubMed] [Google Scholar]
- 66.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 67.Harris, J. E., L. Ryan, H. C. Hoover, Jr., R. K. Stuart, M. M. Oken, A. B. Benson III, E. Mansour, D. G. Haller, J. Manola, and M. G. Hanna, Jr. 2000. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J. Clin. Oncol. 18:148-157. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez, P., J. Huerta-Cepas, D. Montaner, F. Al-Shahrour, J. Valls, L. Gomez, G. Capella, J. Dopazo, and M. A. Pujana. 2007. Evidence for systems-level molecular mechanisms of tumorigenesis. BMC Genomics 8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hewitt, H. B., E. R. Blake, and A. S. Walder. 1976. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br. J. Cancer 33:241-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higano, C. S., P. F. Schellhammer, E. J. Small, P. A. Burch, J. Nemunaitis, L. Yuh, N. Provost, and M. W. Frohlich. 2009. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115:3670-3679. [DOI] [PubMed] [Google Scholar]
- 71.Hiranuma, K., S. Tamaki, Y. Nishimura, S. Kusuki, M. Isogawa, G. Kim, M. Kaito, K. Kuribayashi, Y. Adachi, and Y. Yasutomi. 1999. Helper T cell determinant peptide contributes to induction of cellular immune responses by peptide vaccines against hepatitis C virus. J. Gen. Virol. 80(Pt. 1):187-193. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann, T. K., D. J. Loftus, K. Nakano, M. J. Maeurer, K. Chikamatsu, E. Appella, T. L. Whiteside, and A. B. DeLeo. 2002. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264-272) epitope. J. Immunol. 168:1338-1347. [DOI] [PubMed] [Google Scholar]
- 73.Holmberg, L. A., and B. M. Sandmaier. 2004. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 3:655-663. [DOI] [PubMed] [Google Scholar]
- 74.Hou, Y., B. Kavanagh, and L. Fong. 2008. Distinct CD8+ T cell repertoires primed with agonist and native peptides derived from a tumor-associated antigen. J. Immunol. 180:1526-1534. [DOI] [PubMed] [Google Scholar]
- 75.Ichihara, F., K. Kono, A. Takahashi, H. Kawaida, H. Sugai, and H. Fujii. 2003. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 9:4404-4408. [PubMed] [Google Scholar]
- 76.Ingale, S., M. A. Wolfert, T. Buskas, and G. J. Boons. 2009. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting Toll-like receptors. Chembiochem 10:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobs, J. F., C. J. Punt, W. J. Lesterhuis, R. P. Sutmuller, H. M. Brouwer, N. M. Scharenborg, I. S. Klasen, L. B. Hilbrands, C. G. Figdor, I. J. de Vries, and G. J. Adema. 2010. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16:5067-5078. [DOI] [PubMed] [Google Scholar]
- 78.Jager, E., Y. T. Chen, J. W. Drijfhout, J. Karbach, M. Ringhoffer, D. Jager, M. Arand, H. Wada, Y. Noguchi, E. Stockert, L. J. Old, and A. Knuth. 1998. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J. Exp. Med. 187:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaramillo, A., K. Majumder, P. P. Manna, T. P. Fleming, G. Doherty, J. F. Dipersio, and T. Mohanakumar. 2002. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int. J. Cancer 102:499-506. [DOI] [PubMed] [Google Scholar]
- 80.Jennings, H. J., and R. K. Sood. 1994. Synthetic glycoconjugates as human vaccines, p. 325-371. In Y. C. Lee and R. T. Lee (ed.), Neoglycoconjugates: preparation and applications. Academic Press, San Diego, CA.
- 81.Jinushi, M., F. S. Hodi, and G. Dranoff. 2008. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol. Rev. 222:287-298. [DOI] [PubMed] [Google Scholar]
- 82.Jonasch, E., C. Wood, P. Tamboli, L. C. Pagliaro, S. M. Tu, J. Kim, P. Srivastava, C. Perez, L. Isakov, and N. Tannir. 2008. Vaccination of metastatic renal cell carcinoma patients with autologous tumour-derived vitespen vaccine: clinical findings. Br. J. Cancer. 98:1336-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones, S., X. Zhang, D. W. Parsons, J. C. Lin, R. J. Leary, P. Angenendt, P. Mankoo, H. Carter, H. Kamiyama, A. Jimeno, S. M. Hong, B. Fu, M. T. Lin, E. S. Calhoun, M. Kamiyama, K. Walter, T. Nikolskaya, Y. Nikolsky, J. Hartigan, D. R. Smith, M. Hidalgo, S. D. Leach, A. P. Klein, E. M. Jaffee, M. Goggins, A. Maitra, C. Iacobuzio-Donahue, J. R. Eshleman, S. E. Kern, R. H. Hruban, R. Karchin, N. Papadopoulos, G. Parmigiani, B. Vogelstein, V. E. Velculescu, and K. W. Kinzler. 2008. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jordan, K. R., R. H. McMahan, C. B. Kemmler, J. W. Kappler, and J. E. Slansky. 2010. Peptide vaccines prevent tumor growth by activating T cells that respond to native tumor antigens. Proc. Natl. Acad. Sci. U. S. A. 107:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Junnila, S., A. Kokkola, M. L. Karjalainen-Lindsberg, P. Puolakkainen, and O. Monni. 2010. Genome-wide gene copy number and expression analysis of primary gastric tumors and gastric cancer cell lines. BMC Cancer 10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kagan, E., G. Ragupathi, S. S. Yi, C. A. Reis, J. Gildersleeve, D. Kahne, H. Clausen, S. J. Danishefsky, and P. O. Livingston. 2005. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol. Immunother. 54:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159:28-35. [PubMed] [Google Scholar]
- 88.Kalinski, P., P. L. Vieira, J. H. Schuitemaker, E. C. de Jong, and M. L. Kapsenberg. 2001. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97:3466-3469. [DOI] [PubMed] [Google Scholar]
- 89.Kawakami, Y., S. Eliyahu, C. Jennings, K. Sakaguchi, X. Kang, S. Southwood, P. F. Robbins, A. Sette, E. Appella, and S. A. Rosenberg. 1995. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 154:3961-3968. [PubMed] [Google Scholar]
- 90.Kawakami, Y., S. Eliyahu, K. Sakaguchi, P. F. Robbins, L. Rivoltini, J. R. Yannelli, E. Appella, and S. A. Rosenberg. 1994. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 180:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawakami, Y., P. F. Robbins, X. Wang, J. P. Tupesis, M. R. Parkhurst, X. Kang, K. Sakaguchi, E. Appella, and S. A. Rosenberg. 1998. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J. Immunol. 161:6985-6992. [PubMed] [Google Scholar]
- 92.Kelly, D. F., E. R. Moxon, and A. J. Pollard. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kenter, G. G., M. J. Welters, A. R. Valentijn, M. J. Lowik, D. M. Berends-van der Meer, A. P. Vloon, J. W. Drijfhout, A. R. Wafelman, J. Oostendorp, G. J. Fleuren, R. Offringa, S. H. van der Burg, and C. J. Melief. 2008. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 14:169-177. [DOI] [PubMed] [Google Scholar]
- 94.Kirkwood, J. M., M. H. Strawderman, M. S. Ernstoff, T. J. Smith, E. C. Borden, and R. H. Blum. 1996. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 14:7-17. [DOI] [PubMed] [Google Scholar]
- 95.Knutson, K. L., K. Schiffman, and M. L. Disis. 2001. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J. Clin. Invest. 107:477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koido, S., E. Hara, S. Homma, Y. Namiki, T. Ohkusa, J. Gong, and H. Tajiri. 2009. Cancer vaccine by fusions of dendritic and cancer cells. Clin. Dev. Immunol. 2009:657369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koido, S., E. Hara, S. Homma, A. Torii, Y. Toyama, H. Kawahara, M. Watanabe, K. Yanaga, K. Fujise, H. Tajiri, J. Gong, and G. Toda. 2005. Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clin. Cancer Res. 11:7891-7900. [DOI] [PubMed] [Google Scholar]
- 98.Koido, S., Y. Tanaka, H. Tajiri, and J. Gong. 2007. Generation and functional assessment of antigen-specific T cells stimulated by fusions of dendritic cells and allogeneic breast cancer cells. Vaccine 25:2610-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koski, G. K., P. A. Cohen, R. E. Roses, S. Xu, and B. J. Czerniecki. 2008. Reengineering dendritic cell-based anti-cancer vaccines. Immunol. Rev. 222:256-276. [DOI] [PubMed] [Google Scholar]
- 100.Krieg, A. M. 2006. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5:471-484. [DOI] [PubMed] [Google Scholar]
- 101.Krieg, A. M. 2008. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene 27:161-167. [DOI] [PubMed] [Google Scholar]
- 102.Le Gal, F. A., M. Ayyoub, V. Dutoit, V. Widmer, E. Jager, J. C. Cerottini, P. Y. Dietrich, and D. Valmori. 2005. Distinct structural TCR repertoires in naturally occurring versus vaccine-induced CD8+ T-cell responses to the tumor-specific antigen NY-ESO-1. J. Immunother. 28:252-257. [DOI] [PubMed] [Google Scholar]
- 103.Lesterhuis, W. J., I. J. de Vries, G. J. Adema, and C. J. Punt. 2004. Dendritic cell-based vaccines in cancer immunotherapy: an update on clinical and immunological results. Ann. Oncol. 15(Suppl. 4):iv145-iv151. [DOI] [PubMed] [Google Scholar]
- 104.Li, C. Y., Q. Huang, and H. F. Kung. 2005. Cytokine and immuno-gene therapy for solid tumors. Cell. Mol. Immunol. 2:81-91. [PubMed] [Google Scholar]
- 105.Lindquist, S., and E. A. Craig. 1988. The heat-shock proteins. Annu. Rev. Genet. 22:631-677. [DOI] [PubMed] [Google Scholar]
- 106.Livingston, P. O. 1995. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol. Rev. 145:147-166. [DOI] [PubMed] [Google Scholar]
- 107.Liyanage, U. K., T. T. Moore, H. G. Joo, Y. Tanaka, V. Herrmann, G. Doherty, J. A. Drebin, S. M. Strasberg, T. J. Eberlein, P. S. Goedegebuure, and D. C. Linehan. 2002. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 169:2756-2761. [DOI] [PubMed] [Google Scholar]
- 108.Mahnke, K., K. Schonfeld, S. Fondel, S. Ring, S. Karakhanova, K. Wiedemeyer, T. Bedke, T. S. Johnson, V. Storn, S. Schallenberg, and A. H. Enk. 2007. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int. J. Cancer 120:2723-2733. [DOI] [PubMed] [Google Scholar]
- 109.Mailliard, R. B., A. Wankowicz-Kalinska, Q. Cai, A. Wesa, C. M. Hilkens, M. L. Kapsenberg, J. M. Kirkwood, W. J. Storkus, and P. Kalinski. 2004. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 64:5934-5937. [DOI] [PubMed] [Google Scholar]
- 110.Mazzaferro, V., J. Coppa, M. G. Carrabba, L. Rivoltini, M. Schiavo, E. Regalia, L. Mariani, T. Camerini, A. Marchiano, S. Andreola, R. Camerini, M. Corsi, J. J. Lewis, P. K. Srivastava, and G. Parmiani. 2003. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 9:3235-3245. [PubMed] [Google Scholar]
- 111.McMahan, R. H., J. A. McWilliams, K. R. Jordan, S. W. Dow, D. B. Wilson, and J. E. Slansky. 2006. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J. Clin. Invest. 116:2543-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McWilliams, J. A., R. T. Sullivan, K. R. Jordan, R. H. McMahan, C. B. Kemmler, M. McDuffie, and J. E. Slansky. 2008. Age-dependent tolerance to an endogenous tumor-associated antigen. Vaccine 26:1863-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Melief, C. J., and S. H. van der Burg. 2008. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat. Rev. Cancer 8:351-360. [DOI] [PubMed] [Google Scholar]
- 114.Michael, A., G. Ball, N. Quatan, F. Wushishi, N. Russell, J. Whelan, P. Chakraborty, D. Leader, M. Whelan, and H. Pandha. 2005. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin. Cancer Res. 11:4469-4478. [DOI] [PubMed] [Google Scholar]
- 115.Mills, K. H. 2009. Designer adjuvants for enhancing the efficacy of infectious disease and cancer vaccines based on suppression of regulatory T cell induction. Immunol. Lett. 122:108-111. [DOI] [PubMed] [Google Scholar]
- 116.Minev, B., J. Hipp, H. Firat, J. D. Schmidt, P. Langlade-Demoyen, and M. Zanetti. 2000. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc. Natl. Acad. Sci. U. S. A. 97:4796-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Minev, B. R., F. L. Chavez, and M. S. Mitchell. 1999. Cancer vaccines: novel approaches and new promise. Pharmacol. Ther. 81:121-139. [DOI] [PubMed] [Google Scholar]
- 118.Morse, M. A., A. C. Hobeika, T. Osada, D. Serra, D. Niedzwiecki, H. K. Lyerly, and T. M. Clay. 2008. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 112:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murshid, A., J. Gong, and S. K. Calderwood. 2008. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev. Vaccines 7:1019-1030. [DOI] [PubMed] [Google Scholar]
- 120.Nava-Parada, P., G. Forni, K. L. Knutson, L. R. Pease, and E. Celis. 2007. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 67:1326-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nestle, F. O., S. Alijagic, M. Gilliet, Y. Sun, S. Grabbe, R. Dummer, G. Burg, and D. Schadendorf. 1998. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 4:328-332. [DOI] [PubMed] [Google Scholar]
- 122.Nishikawa, H., E. Jager, G. Ritter, L. J. Old, and S. Gnjatic. 2005. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 106:1008-1011. [DOI] [PubMed] [Google Scholar]
- 123.Nizar, S., J. Copier, B. Meyer, M. Bodman-Smith, C. Galustian, D. Kumar, and A. Dalgleish. 2009. T-regulatory cell modulation: the future of cancer immunotherapy? Br. J. Cancer 100:1697-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.North, R. J. 1984. The murine antitumor immune response and its therapeutic manipulation. Adv. Immunol. 35:89-155. [DOI] [PubMed] [Google Scholar]
- 125.Novellino, L., C. Castelli, and G. Parmiani. 2005. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol. Immunother. 54:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osada, T., T. M. Clay, C. Y. Woo, M. A. Morse, and H. K. Lyerly. 2006. Dendritic cell-based immunotherapy. Int. Rev. Immunol. 25:377-413. [DOI] [PubMed] [Google Scholar]
- 127.Overwijk, W. W., A. Tsung, K. R. Irvine, M. R. Parkhurst, T. J. Goletz, K. Tsung, M. W. Carroll, C. Liu, B. Moss, S. A. Rosenberg, and N. P. Restifo. 1998. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 188:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pace, L., S. Vitale, B. Dettori, C. Palombi, S. La, V. F. Belardelli, E. Proietti, and G. Doria. 2010. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. J. Immunol. 184:5969-5979. [DOI] [PubMed] [Google Scholar]
- 129.Palucka, A. K., H. Ueno, J. Connolly, F. Kerneis-Norvell, J. P. Blanck, D. A. Johnston, J. Fay, and J. Banchereau. 2006. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J. Immunother. 29:545-557. [DOI] [PubMed] [Google Scholar]
- 130.Pardoll, D. M. 1999. Inducing autoimmune disease to treat cancer. Proc. Natl. Acad. Sci. U. S. A. 96:5340-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parkhurst, M. R., E. B. Fitzgerald, S. Southwood, A. Sette, S. A. Rosenberg, and Y. Kawakami. 1998. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2). Cancer Res. 58:4895-4901. [PubMed] [Google Scholar]
- 132.Parmiani, G., C. Castelli, P. Dalerba, R. Mortarini, L. Rivoltini, F. M. Marincola, and A. Anichini. 2002. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J. Natl. Cancer Inst. 94:805-818. [DOI] [PubMed] [Google Scholar]
- 133.Parmiani, G., F. A. De, L. Novellino, and C. Castelli. 2007. Unique human tumor antigens: immunobiology and use in clinical trials. J. Immunol. 178:1975-1979. [DOI] [PubMed] [Google Scholar]
- 134.Parsons, D. W., S. Jones, X. Zhang, J. C. Lin, R. J. Leary, P. Angenendt, P. Mankoo, H. Carter, I. M. Siu, G. L. Gallia, A. Olivi, R. McLendon, B. A. Rasheed, S. Keir, T. Nikolskaya, Y. Nikolsky, D. A. Busam, H. Tekleab, L. A. Diaz, Jr., J. Hartigan, D. R. Smith, R. L. Strausberg, S. K. Marie, S. M. Shinjo, H. Yan, G. J. Riggins, D. D. Bigner, R. Karchin, N. Papadopoulos, G. Parmigiani, B. Vogelstein, V. E. Velculescu, and K. W. Kinzler. 2008. An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pehamberger, H., H. P. Soyer, A. Steiner, R. Kofler, M. Binder, P. Mischer, W. Pachinger, J. Aubock, P. Fritsch, H. Kerl, and K. Wolff. 1998. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. Austrian Malignant Melanoma Cooperative Group. J. Clin. Oncol. 16:1425-1429. [DOI] [PubMed] [Google Scholar]
- 136.Perez, S. A., E. von Hofe, N. L. Kallinteris, A. D. Gritzapis, G. E. Peoples, M. Papamichail, and C. N. Baxevanis. 2010. A new era in anticancer peptide vaccines. Cancer 116:2071-2080. [DOI] [PubMed] [Google Scholar]
- 137.Perotti, M., N. Ghidoli, R. Altara, R. A. Diotti, N. Clementi, M. D. De, M. Sassi, M. Clementi, R. Burioni, and N. Mancini. 2008. Hepatitis C virus (HCV)-driven stimulation of subfamily-restricted natural IgM antibodies in mixed cryoglobulinemia. Autoimmun. Rev. 7:468-472. [DOI] [PubMed] [Google Scholar]
- 138.Pilla, L., R. Patuzzo, L. Rivoltini, M. Maio, E. Pennacchioli, E. Lamaj, A. Maurichi, S. Massarut, A. Marchiano, C. Santantonio, D. Tosi, F. Arienti, A. Cova, G. Sovena, A. Piris, D. Nonaka, I. Bersani, F. A. Di, M. Luigi, P. K. Srivastava, A. Hoos, M. Santinami, and G. Parmiani. 2006. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients. Cancer Immunol. Immunother. 55:958-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pinilla, C., V. Rubio-Godoy, V. Dutoit, P. Guillaume, R. Simon, Y. Zhao, R. A. Houghten, J. C. Cerottini, P. Romero, and D. Valmori. 2001. Combinatorial peptide libraries as an alternative approach to the identification of ligands for tumor-reactive cytolytic T lymphocytes. Cancer Res. 61:5153-5160. [PubMed] [Google Scholar]
- 140.Pleasance, E. D., R. K. Cheetham, P. J. Stephens, D. J. McBride, S. J. Humphray, C. D. Greenman, I. Varela, M. L. Lin, G. R. Ordonez, G. R. Bignell, K. Ye, J. Alipaz, M. J. Bauer, D. Beare, A. Butler, R. J. Carter, L. Chen, A. J. Cox, S. Edkins, P. I. Kokko-Gonzales, N. A. Gormley, R. J. Grocock, C. D. Haudenschild, M. M. Hims, T. James, M. Jia, Z. Kingsbury, C. Leroy, J. Marshall, A. Menzies, L. J. Mudie, Z. Ning, T. Royce, O. B. Schulz-Trieglaff, A. Spiridou, L. A. Stebbings, L. Szajkowski, J. Teague, D. Williamson, L. Chin, M. T. Ross, P. J. Campbell, D. R. Bentley, P. A. Futreal, and M. R. Stratton. 2010. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prehn, R. T. 1970. Analysis of antigenic heterogeneity within individual 3-methylcholanthrene-induced mouse sarcomas. J. Natl. Cancer Inst. 45:1039-1045. [PubMed] [Google Scholar]
- 142.Radtke, I., C. G. Mullighan, M. Ishii, X. Su, J. Cheng, J. Ma, R. Ganti, Z. Cai, S. Goorha, S. B. Pounds, X. Cao, C. Obert, J. Armstrong, J. Zhang, G. Song, R. C. Ribeiro, J. E. Rubnitz, S. C. Raimondi, S. A. Shurtleff, and J. R. Downing. 2009. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc. Natl. Acad. Sci. U. S. A. 106:12944-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ragupathi, G. 1996. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol. Immunother. 43:152-157. [DOI] [PubMed] [Google Scholar]
- 144.Ranieri, E., L. S. Kierstead, H. Zarour, J. M. Kirkwood, M. T. Lotze, T. Whiteside, and W. J. Storkus. 2000. Dendritic cell/peptide cancer vaccines: clinical responsiveness and epitope spreading. Immunol. Invest. 29:121-125. [DOI] [PubMed] [Google Scholar]
- 145.Rech, A. J., and R. H. Vonderheide. 2009. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. N. Y. Acad. Sci. 1174:99-106. [DOI] [PubMed] [Google Scholar]
- 146.Rivoltini, L., P. Canese, V. Huber, M. Iero, L. Pilla, R. Valenti, S. Fais, F. Lozupone, C. Casati, C. Castelli, and G. Parmiani. 2005. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control? Expert Opin. Biol. Ther. 5:463-476. [DOI] [PubMed] [Google Scholar]
- 147.Rosenberg, S. A. 2001. Progress in human tumour immunology and immunotherapy. Nature 411:380-384. [DOI] [PubMed] [Google Scholar]
- 148.Rosenberg, S. A., R. M. Sherry, K. E. Morton, W. J. Scharfman, J. C. Yang, S. L. Topalian, R. E. Royal, U. Kammula, N. P. Restifo, M. S. Hughes, D. Schwartzentruber, D. M. Berman, S. L. Schwarz, L. T. Ngo, S. A. Mavroukakis, D. E. White, and S. M. Steinberg. 2005. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 175:6169-6176. [DOI] [PubMed] [Google Scholar]
- 149.Rosenberg, S. A., J. C. Yang, and N. P. Restifo. 2004. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rosenberg, S. A., J. C. Yang, S. L. Topalian, D. J. Schwartzentruber, J. S. Weber, D. R. Parkinson, C. A. Seipp, J. H. Einhorn, and D. E. White. 1994. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271:907-913. [PubMed] [Google Scholar]
- 151.Rosenberg, S. A., J. R. Yannelli, J. C. Yang, S. L. Topalian, D. J. Schwartzentruber, J. S. Weber, D. R. Parkinson, C. A. Seipp, J. H. Einhorn, and D. E. White. 1994. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 86:1159-1166. [DOI] [PubMed] [Google Scholar]
- 152.Sakaguchi, S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345-352. [DOI] [PubMed] [Google Scholar]
- 153.Sasada, T., M. Kimura, Y. Yoshida, M. Kanai, and A. Takabayashi. 2003. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98:1089-1099. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt, S. M., K. Schag, M. R. Muller, M. M. Weck, S. Appel, L. Kanz, F. Grunebach, and P. Brossart. 2003. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood 102:571-576. [DOI] [PubMed] [Google Scholar]
- 155.Schmollinger, J. C., R. H. Vonderheide, K. M. Hoar, B. Maecker, J. L. Schultze, F. S. Hodi, R. J. Soiffer, K. Jung, M. J. Kuroda, N. L. Letvin, E. A. Greenfield, M. Mihm, J. L. Kutok, and G. Dranoff. 2003. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc. Natl. Acad. Sci. U. S. A. 100:3398-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 157.Schuler, G., B. Schuler-Thurner, and R. M. Steinman. 2003. The use of dendritic cells in cancer immunotherapy. Curr. Opin. Immunol. 15:138-147. [DOI] [PubMed] [Google Scholar]
- 158.Shen, L. S., J. Wang, D. F. Shen, X. L. Yuan, P. Dong, M. X. Li, J. Xue, F. M. Zhang, H. L. Ge, and D. Xu. 2009. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin. Immunol. 131:109-118. [DOI] [PubMed] [Google Scholar]
- 159.Shirai, M., C. D. Pendleton, J. Ahlers, T. Takeshita, M. Newman, and J. A. Berzofsky. 1994. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 152:549-556. [PubMed] [Google Scholar]
- 160.Simons, J. W., M. A. Carducci, B. Mikhak, M. Lim, B. Biedrzycki, F. Borellini, S. M. Clift, K. M. Hege, D. G. Ando, S. Piantadosi, R. Mulligan, and W. G. Nelson. 2006. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin. Cancer Res. 12:3394-3401. [DOI] [PubMed] [Google Scholar]
- 161.Slansky, J. E., F. M. Rattis, L. F. Boyd, T. Fahmy, E. M. Jaffee, J. P. Schneck, D. H. Margulies, and D. M. Pardoll. 2000. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity 13:529-538. [DOI] [PubMed] [Google Scholar]
- 162.Small, E. J., N. Sacks, J. Nemunaitis, W. J. Urba, E. Dula, A. S. Centeno, W. G. Nelson, D. Ando, C. Howard, F. Borellini, M. Nguyen, K. Hege, and J. W. Simons. 2007. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 13:3883-3891. [DOI] [PubMed] [Google Scholar]
- 163.Sole, X., N. Bonifaci, N. Lopez-Bigas, A. Berenguer, P. Hernandez, O. Reina, C. A. Maxwell, H. Aguilar, A. Urruticoechea, S. de Sanjose, F. Comellas, G. Capella, V. Moreno, and M. A. Pujana. 2009. Biological convergence of cancer signatures. PLoS One 4:e4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Speiser, D. E., D. Lienard, N. Rufer, V. Rubio-Godoy, D. Rimoldi, F. Lejeune, A. M. Krieg, J. C. Cerottini, and P. Romero. 2005. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 115:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Srivastava, P. K., M. K. Callahan, and M. M. Mauri. 2009. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert Opin. Biol. Ther. 9:179-186. [DOI] [PubMed] [Google Scholar]
- 166.Srivastava, P. K., A. B. DeLeo, and L. J. Old. 1986. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc. Natl. Acad. Sci. U. S. A. 83:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Srivastava, P. K., H. Udono, N. E. Blachere, and Z. Li. 1994. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 39:93-98. [DOI] [PubMed] [Google Scholar]
- 168.Stevenson, G. T., E. V. Elliott, and F. K. Stevenson. 1977. Idiotypic determinants on the surface immunoglobulin of neoplastic lymphocytes: a therapeutic target. Fed. Proc. 36:2268-2271. [PubMed] [Google Scholar]
- 169.Stratton, M. R., P. J. Campbell, and P. A. Futreal. 2009. The cancer genome. Nature 458:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Syed, A. S., M. D'Antonio, and F. D. Ciccarelli. 2010. Network of Cancer Genes: a web resource to analyze duplicability, orthology and network properties of cancer genes. Nucleic Acids Res. 38:D670-D675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tamura, Y., P. Peng, K. Liu, M. Daou, and P. K. Srivastava. 1997. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 278:117-120. [DOI] [PubMed] [Google Scholar]
- 172.Testori, A., J. Richards, E. Whitman, G. B. Mann, J. Lutzky, L. Camacho, G. Parmiani, G. Tosti, J. M. Kirkwood, A. Hoos, L. Yuh, R. Gupta, and P. K. Srivastava. 2008. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J. Clin. Oncol. 26:955-962. [DOI] [PubMed] [Google Scholar]
- 173.Thomas, L. 1959. Reactions to homologous tissue antigens in relation to hypersensitivity, p. 529-532. In H. S. Lawrence (ed.), Cellular and humoral aspects of the hypersensitive states. Hoeber-Harper, New York, NY.
- 174.Toes, R. E., R. J. Blom, R. Offringa, W. M. Kast, and C. J. Melief. 1996. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J. Immunol. 156:3911-3918. [PubMed] [Google Scholar]
- 175.Toes, R. E., R. Offringa, R. J. Blom, C. J. Melief, and W. M. Kast. 1996. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc. Natl. Acad. Sci. U. S. A. 93:7855-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Toyokuni, T., B. Dean, S. Cai, D. Boivin, S. Hakomori, and A. K. Singhal. 1994. Synthetic vaccines: synthesis of a dimeric Tn antigen-lipopeptide conjugate that elicits immune responses against Tn-expressing glycoproteins. J. Am. Chem. Soc. 116:395-396. [Google Scholar]
- 177.Traversari, C., P. van der Bruggen, I. F. Luescher, C. Lurquin, P. Chomez, A. Van Pel, E. De Plaen, A. Amar-Costesec, and T. Boon. 1992. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 176:1453-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tsang, K. Y., S. Zaremba, C. A. Nieroda, M. Z. Zhu, J. M. Hamilton, and J. Schlom. 1995. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J. Natl. Cancer Inst. 87:982-990. [DOI] [PubMed] [Google Scholar]
- 179.Udono, H., and P. K. Srivastava. 1994. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J. Immunol. 152:5398-5403. [PubMed] [Google Scholar]
- 180.Umano, Y., T. Tsunoda, H. Tanaka, K. Matsuda, H. Yamaue, and H. Tanimura. 2001. Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br. J. Cancer 84:1052-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Valmori, D., J. F. Fonteneau, S. Valitutti, N. Gervois, R. Dunbar, D. Lienard, D. Rimoldi, V. Cerundolo, F. Jotereau, J. C. Cerottini, D. E. Speiser, and P. Romero. 1999. Optimal activation of tumor-reactive T cells by selected antigenic peptide analogues. Int. Immunol. 11:1971-1980. [DOI] [PubMed] [Google Scholar]
- 182.van der Bruggen, P., C. Traversari, P. Chomez, C. Lurquin, E. De Plaen, B. Van den Eynde, A. Knuth, and T. Boon. 1991. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254:1643-1647. [DOI] [PubMed] [Google Scholar]
- 183.Van Doorslaer, K., L. L. Reimers, Y. Y. Studentsov, M. H. Einstein, and R. D. Burk. 2010. Serological response to an HPV16 E7 based therapeutic vaccine in women with high-grade cervical dysplasia. Gynecol. Oncol. 116:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Maren, W. W., J. F. Jacobs, I. J. de Vries, S. Nierkens, and G. J. Adema. 2008. Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology 124:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vermorken, J. B., A. M. Claessen, H. van Tinteren, H. E. Gall, R. Ezinga, S. Meijer, R. J. Scheper, C. J. Meijer, E. Bloemena, J. H. Ransom, M. G. Hanna, Jr., and H. M. Pinedo. 1999. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 353:345-350. [DOI] [PubMed] [Google Scholar]
- 186.Vogelstein, B., and K. W. Kinzler. 2004. Cancer genes and the pathways they control. Nat. Med. 10:789-799. [DOI] [PubMed] [Google Scholar]
- 187.Vonderheide, R. H., K. S. Anderson, W. C. Hahn, M. O. Butler, J. L. Schultze, and L. M. Nadler. 2001. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin. Cancer Res. 7:3343-3348. [PubMed] [Google Scholar]
- 188.Vonderheide, R. H., W. C. Hahn, J. L. Schultze, and L. M. Nadler. 1999. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 10:673-679. [DOI] [PubMed] [Google Scholar]
- 189.Wang, L., K. Pino-Lagos, V. de Vries, I. Guleria, M. H. Sayegh, and R. J. Noelle. 2008. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 105:9331-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang, R. F., E. Appella, Y. Kawakami, X. Kang, and S. A. Rosenberg. 1996. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J. Exp. Med. 184:2207-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang, Y., A. Rubtsov, R. Heiser, J. White, F. Crawford, P. Marrack, and J. W. Kappler. 2005. Using a baculovirus display library to identify MHC class I mimotopes. Proc. Natl. Acad. Sci. U. S. A. 102:2476-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ward, S., D. Casey, M. C. Labarthe, M. Whelan, A. Dalgleish, H. Pandha, and S. Todryk. 2002. Immunotherapeutic potential of whole tumour cells. Cancer Immunol. Immunother. 51:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Welters, M. J., G. G. Kenter, S. J. Piersma, A. P. Vloon, M. J. Lowik, D. M. Berends-van der Meer, J. W. Drijfhout, A. R. Valentijn, A. R. Wafelman, J. Oostendorp, G. J. Fleuren, R. Offringa, C. J. Melief, and S. H. van der Burg. 2008. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 14:178-187. [DOI] [PubMed] [Google Scholar]
- 194.Westerlind, U., A. Hobel, N. Gaidzik, E. Schmitt, and H. Kunz. 2008. Synthetic vaccines consisting of tumor-associated MUC1 glycopeptide antigens and a T-cell epitope for the induction of a highly specific humoral immune response. Angew. Chem. Int. Ed. Engl. 47:7551-7556. [DOI] [PubMed] [Google Scholar]
- 195.Wolf, A. M., D. Wolf, M. Steurer, G. Gastl, E. Gunsilius, and B. Grubeck-Loebenstein. 2003. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res. 9:606-612. [PubMed] [Google Scholar]
- 196.Woo, E. Y., C. S. Chu, T. J. Goletz, K. Schlienger, H. Yeh, G. Coukos, S. C. Rubin, L. R. Kaiser, and C. H. June. 2001. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 61:4766-4772. [PubMed] [Google Scholar]
- 197.Wood, C., P. Srivastava, R. Bukowski, L. Lacombe, A. I. Gorelov, S. Gorelov, P. Mulders, H. Zielinski, A. Hoos, F. Teofilovici, L. Isakov, R. Flanigan, R. Figlin, R. Gupta, and B. Escudier. 2008. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 372:145-154. [DOI] [PubMed] [Google Scholar]
- 198.Wood, C. G., and P. Mulders. 2009. Vitespen: a preclinical and clinical review. Future Oncol. 5:763-774. [DOI] [PubMed] [Google Scholar]
- 199.Wood, L. D., D. W. Parsons, S. Jones, J. Lin, T. Sjoblom, R. J. Leary, D. Shen, S. M. Boca, T. Barber, J. Ptak, N. Silliman, S. Szabo, Z. Dezso, V. Ustyanksky, T. Nikolskaya, Y. Nikolsky, R. Karchin, P. A. Wilson, J. S. Kaminker, Z. Zhang, R. Croshaw, J. Willis, D. Dawson, M. Shipitsin, J. K. Willson, S. Sukumar, K. Polyak, B. H. Park, C. L. Pethiyagoda, P. V. Pant, D. G. Ballinger, A. B. Sparks, J. Hartigan, D. R. Smith, E. Suh, N. Papadopoulos, P. Buckhaults, S. D. Markowitz, G. Parmigiani, K. W. Kinzler, V. E. Velculescu, and B. Vogelstein. 2007. The genomic landscapes of human breast and colorectal cancers. Science 318:1108-1113. [DOI] [PubMed] [Google Scholar]
- 200.Wortzel, R. D., C. Philipps, and H. Schreiber. 1983. Multiple tumour-specific antigens expressed on a single tumour cell. Nature 304:165-167. [DOI] [PubMed] [Google Scholar]
- 201.Yuan, X. L., L. Chen, M. X. Li, P. Dong, J. Xue, J. Wang, T. T. Zhang, X. A. Wang, F. M. Zhang, H. L. Ge, L. S. Shen, and D. Xu. 2010. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin. Immunol. 134:277-288. [DOI] [PubMed] [Google Scholar]
- 202.Zeng, G., Y. Li, M. El-Gamil, J. Sidney, A. Sette, R. F. Wang, S. A. Rosenberg, and P. F. Robbins. 2002. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res. 62:3630-3635. [PMC free article] [PubMed] [Google Scholar]
- 203.Zou, W., and N. P. Restifo. 2010. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]