Abstract
Rickettsia japonica pathogenesis and reservoir potential in dogs were evaluated by both experimental inoculation and epidemiologic survey. In the experimental inoculation study, dogs 1 and 2 were pretreated with an immunosuppressive dose of cyclosporine 14 days before inoculation and became ill after exposure to R. japonica. Dogs exhibited clinical signs, including fever, anorexia, depression, and decreased water consumption, between 36 and 96 h after inoculation, but these signs disappeared spontaneously by 5 days after inoculation. Dogs 3 and 4 were not pretreated with cyclosporine, and no clinical signs were detected in them throughout the 14-day observation period. The control dog was clinically normal and had a normal rectal temperature throughout the study period. We attempted to detect rickettsial DNA from peripheral blood and aspiration samples from kidney and spleen by nested PCR, but all samples examined were negative. The control dog lacked detectable titers to R. japonica antigen on day 14, while positive antibodies to R. japonica were detected in all four experimentally infected dogs, with titers of 1:160 to 1:80. In the epidemiologic survey, 24 (1.8%) of the 1,363 dogs examined throughout Japan had antibodies against R. japonica, with titers of 1:40 or more. However, we observed neither clinical signs at the time of sample collection nor nested PCR results indicative of rickettsial infection in these dogs. In conclusion, dogs in Japan can be exposed to R. japonica, and infected dogs with immunosuppressive conditions can temporarily develop clinical symptoms, including fever, anorexia, depression, and decreased water consumption.
Rickettsiae belong to the order Rickettsiales and are obligate intracellular, Gram-negative bacteria. Several species cause disease in humans and other animals and are distributed worldwide. This genus comprises the spotted fever group (SFG) rickettsiae and the typhus group (TG) rickettsiae (18). In Japan, Rickettsia japonica, classified within the SFG, is the causative agent of Japanese spotted fever (JSF) (15). In 1984, the first JSF patient was reported in Tokushima Prefecture, and since then, most JSF patients have been identified in western Japan (15). Recent epidemiologic studies clarified both the vector and reservoirs of JSF in Japan. Dermacentor taiwanensis and Haemaphysalis flava are confirmed vectors of R. japonica (9). Isolation of R. japonica from wild mice indicated that mice are a mammalian reservoir for the pathogen (22). Dogs have also been thought to be a mammalian reservoir for R. japonica, as antibodies against R. japonica have been detected in canine serum (6, 8). A recent epidemiologic study revealed that antibodies against R. japonica were detected in 20 of 1,207 dogs in Japan (21). Dogs are often exposed to a large number of tick species, depending on the distribution of these arthropod vectors in the environment (19). They most likely have an increased risk of tick bites compared to humans, due at least in part to their activity in woodland and bush areas. However, no previous studies have addressed whether dogs that are naturally infected with R. japonica readily exhibit detectable clinical signs or whether dogs are important sources of infection. The present study evaluated R. japonica pathogenesis and the reservoir potential of dogs through experimental inoculation and an epidemiologic survey.
MATERIALS AND METHODS
Experimental inoculation of R. japonica in dogs.
Five 6-month-old male beagle dogs weighing between 8 and 11 kg were provided by a commercial breeder. They were housed indoors and maintained in a biosafety level P3 animal care facility, as dictated by the Animal Care and Use Committee regulations of Obihiro University of Agriculture and Veterinary Medicine (permission number 20-86). During a 2-week acclimatization period, we monitored clinical signs, food consumption, and rectal temperatures on a daily basis. During that time 2 milliliters of blood was obtained from the cephalic vein of each dog. Complete blood counts were performed using EDTA-anticoagulated blood. All hematological parameters fell within normal ranges (1). Extracted serum was subjected to an indirect immunofluorescence assay (IFA) to confirm the absence of reactive antibodies to R. japonica.
Four dogs were randomly selected for experimental inoculation, and the remaining one was monitored as a control. Two dogs (dogs 1 and 2) in the inoculation group were orally administered cyclosporine (Atopica; Novartis, Basel, Switzerland) with a daily dose of 50 mg/head, beginning at 14 days before inoculation (day −14) and lasting until the final experimental day (day 14), to suppress their immunity. Serum cyclosporine concentrations in dogs 1 and 2 on day 1 were measured by a commercial laboratory and were 35 and 39 ng/ml, respectively. Dogs 3 and 4 of the inoculation group were not treated with any drugs during the experimental period.
After the acclimatization period, four dogs (dogs 1 to 4) were inoculated subcutaneously with 20 ml of L929 cell suspension medium infected with R. japonica strain Aoki (day 0). Each inoculum dose contained approximately 2 × 107 infected cells suspended in minimum essential medium (MEM) with 1% fetal bovine serum (FBS). The control dog was inoculated with the same amount of uninfected L929 cells suspended at the same concentration. Clinical signs and food consumption were monitored, and rectal temperatures were recorded twice a day. Blood from the cephalic vein was collected in EDTA tubes every 2 days from day 0 until day 14. Each blood sample was subjected to hematological analysis and PCR. Peripheral blood smears were examined for neutrophil counts and evidence of platelet aggregation. Sera were collected and IFA was conducted on day 14. Kidney and spleen samples were aspirated for PCR use on day 14.
IFA.
Detection of antibodies against R. japonica was carried out using IFA as described previously (21). Serum samples were screened at a 1:20 dilution in phosphate-buffered saline (pH 7.2) with 0.5% Tween 20 (PBST). Fluorescein isothiocyanate (FITC)-labeled rabbit anti-canine IgG (Fc) conjugates (Rockland Inc., Gilbertsville, PA) or FITC-labeled rabbit anti-feline IgG (Fc) conjugates (Rockland Inc.) were used as secondary antibodies for the IFA. Reactive antibodies were then detected using a fluorescence light microscope. Samples that reacted with the R. japonica antigen at the screening dilution were then titrated to the endpoint. We considered antibody titers 1:40 or above to be positive, as this ratio was used in a previous survey (6).
DNA extraction and PCR.
DNA was extracted from EDTA blood samples using a QIAamp DNA minikit (Qiagen GmbH, Hilden, Germany). DNA samples were stored at −20°C in 200 μl of TE (Tris-EDTA) buffer until further analysis. Nested PCR was performed with genus-specific primers for the rickettsial citrate synthase (gltA) gene used in our previous study (7); the primer pair RpCS.877p and RpCS.1273r was used for the first amplification. The first round of PCR was carried out in a 25-μl reaction mixture (5 μl of DNA template) under the following settings: 35 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s, and extension at 72°C for 90 s. The resulting PCR products were then used as a template for the second amplification with primers RpCS.896f and RpCS.1258n. PCR settings for the second round were the same as those for the first round, except that annealing was carried out at 56°C for 30 s. DNA extracted from the Rickettsia AT-1 strain was used as a positive control, and distilled water was used for the blank control.
Epidemiologic study.
Between January 2006 and June 2008, blood and sera were collected from 1,363 domestic dogs and processed in animal hospitals located within 31 prefectures in Japan (40 dogs in Hokkaido, 40 dogs in Aomori, 60 dogs in Miyagi, 40 dogs in Fukushima, 35 dogs in Tochigi, 50 dogs in Tokyo, 40 dogs in Chiba, 40 dogs in Kanagawa, 40 dogs in Yamanashi, 40 dogs in Shizuoka, 37 dogs in Aichi, 39 dogs in Mie, 40 dogs in Fukui, 40 dogs in Osaka, 40 dogs in Kyoto, 40 dogs in Shiga, 40 dogs in Nara, 40 dogs in Wakayama, 64 dogs in Hyogo, 22 dogs in Tottori, 40 dogs in Shimane, 40 dogs in Hiroshima, 57 dogs in Yamaguchi, 167 dogs in Tokushima, 40 dogs in Kochi, 40 dogs in Fukuoka, 34 dogs in Nagasaki, 40 dogs in Kumamoto, 30 dogs in Oita, 40 dogs in Miyazaki, and 8 dogs in Kagoshima). All animals in the study were routinely active outdoors. Clinical status was determined by the veterinarians treating these animals. Blood samples from each animal were collected in EDTA tubes for DNA extraction, and both blood and sera were stored at −20°C until transfer to the Obihiro University of Agriculture and Veterinary Medicine. DNA was extracted from each blood sample and evaluated for Rickettsia infection using the nested PCR protocol described above. Each serum sample was also examined for the presence of antibodies against R. japonica using the IFA method. The samples that were positive for antibodies against R. japonica were also examined for antibodies against other domestic Rickettsia species, including Rickettsia helvetica strain IP-1, Rickettsia tamurae strain AT-1, and Rickettsia asiatica strain IO-1. The method was exactly the same as that described above.
RESULTS
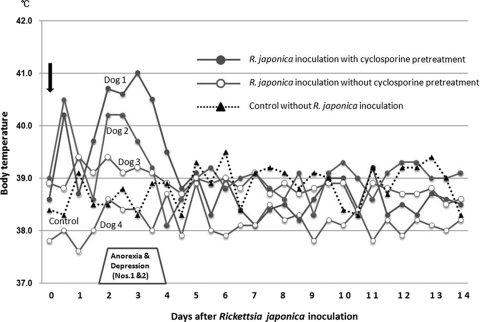
Changes in the body temperatures and conditions of dogs inoculated with R. japonica are shown in Fig. 1. A temperature above 40.0°C was temporally recorded in the afternoon on the day of inoculation in dogs pretreated with cyclosporine (dog 1, 40.2°C; dog 2, 40.5°C). Body temperatures returned to normal by the next morning (day 1), but anorexia and depression appeared in both dogs on the afternoon of day 1. The body temperature of dog 1 increased again to 39.7°C in the afternoon on day 1 and remained higher than 39.5°C until the morning on day 4. Dog 2 also exhibited a temperature of 40.2°C from the morning on day 2 until the morning on day 3. Both dogs exhibited severe anorexia and depression and less water consumption on days 2 and 3; however, their appetites and water consumption improved by the morning of day 4 and had returned to normal by the morning of day 5. Animals that did not receive cyclosporine pretreatment (dogs 3 and 4) exhibited no clinical signs throughout the 14-day observation period. The control dog was also clinically normal and had a normal rectal temperature throughout the study period.
FIG. 1.
Changes in body temperature of dogs inoculated with R. japonica from the day of inoculation (day 0) to day 14. The body temperature of each dog was measured twice a day (at 8 a.m. and 5 p.m.).
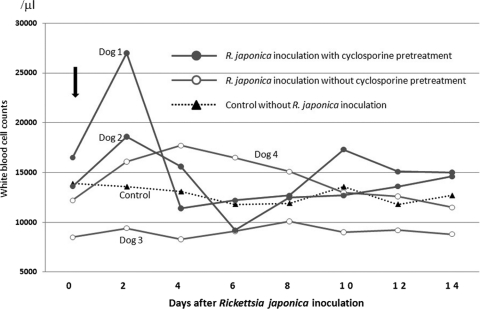
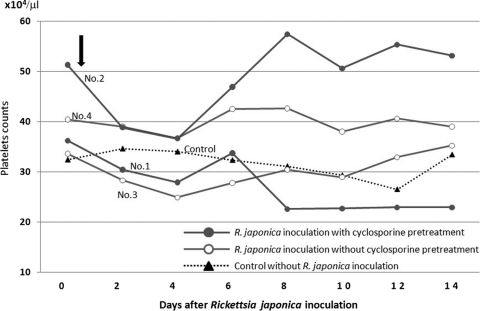
Figure 2 shows changes in white blood cell counts. On day 2, increased white blood cell counts were observed in dogs 1 (27,000/μl) and 2 (18,600/μl). Dog 1 also had an increased white blood cell count (17,300/μl) on day 10. Blood smears revealed that this increased white blood cell count was due to an increase in neutrophils (data not shown). Dog 4 showed a slight increase in white blood cell count on day 4 (18,000/μl). Thrombocytopenia with a platelet count of less than 200,000/μl was not observed in any of the dogs examined (Fig. 3).
FIG. 2.
Changes in white blood cell counts in peripheral blood. Increased counts were observed in both dog 1 (27,000/μl) and dog 2 (18,600/μl) on day 2. Dog 1 also exhibited an increased white blood cell count (17,300/μl) on day 10. Dog 4 showed a slight increase in white blood cell count on day 4 (18,000/μl).
FIG. 3.
Changes in platelet counts in peripheral blood. Thrombocytopenia with a platelet count of less than 200,000/μl was not observed in any of the dogs examined.
We attempted to detect rickettsial DNA from peripheral blood and aspiration samples of kidney and spleen using nested PCR, but all samples were negative.
None of the dogs used in this experiment showed any detectable antibody (titer < 1:20) to R. japonica antigen on day 0, just before the inoculation. On the final day of the study (day 14), positive antibodies against R. japonica were detected in all four inoculated dogs, with titers being 1:160 (dogs 1, 2, and 3) and 1:80 (dog 4), while the control dog lacked detectable antibody to R. japonica.
Among the 1,363 dogs examined, 24 (1.8%) exhibited antibodies against R. japonica with titers of 1:40 or more. The profiles of the positive animals are shown in Table 1. Among these 24 positive dogs, 5 (20.8%) lived in prefectures in eastern Japan (1 dog, Miyagi; 3 dogs, Tochigi; and 1 dog, Kanagawa Prefecture) and the other 19 (79.2%) were in prefectures in western Japan (3 dogs, Wakayama; 1 dog, Osaka; 1 dog, Hyogo; 6 dogs, Hiroshima; 1 dog, Yamaguchi; 1 dog, Tokushima; 1 dog, Kochi; 1 dog, Nagasaki; 1 dog, Kumamoto; and 3 dogs, Miyazaki). The ages of the positive dogs ranged from 2 to 12 years. The endpoint titers against R. japonica of the positive animals ranged from 1:40 to 1:320. Among the 24 dogs seropositive for antibodies against R. japonica, 12 dogs also had antibodies against one or more of the other Rickettsia antigens. A total of 15 dogs showed the highest titers against R. japonica, 1 dog (Tochigi-7) showed the highest titer against R. helvetica, and 8 other dogs showed the same titers against two or more antigens. These dogs did not show any clinical signs at the time of sample collection. Nested PCR did not reveal rickettsial infection for any of the 1,363 dogs examined.
TABLE 1.
Profiles of dogs that reacted with R. japonica antigens with titers of 1:40 or morea
| Location and dog identifier | IFA titer |
Prefecture | Age (yr) | Breed | Sex | Present illness | |||
|---|---|---|---|---|---|---|---|---|---|
| R. japonica | R. helvetica | R. asiatica | Rickettsia tamurae | ||||||
| Eastern Japan | |||||||||
| D1 | 40 | <40 | <40 | <40 | Miyagi | 10 | Mix | ♀ | None |
| D2 | 40 | 40 | <40 | 40 | Tochigi | 4 | G. retriever | ♂ | None |
| D3 | 40 | 80 | <40 | <40 | Tochigi | NR | Mix | ♀ | None |
| D4 | 80 | 80 | 80 | 80 | Tochigi | 4 | G. retriever | ♂ | None |
| D5 | 40 | <40 | <40 | <40 | Kanagawa | 10 | Shiba | ♂ | None |
| Western Japan | |||||||||
| D6 | 40 | <40 | 80 | <40 | Wakayama | 7 | G. retriever | ♀ | Babesiasis |
| D7 | 40 | <40 | <40 | <40 | Wakayama | 2 | Mix | ♂ | None |
| D8 | 40 | <40 | <40 | <40 | Wakayama | 10 | Mix | ♀ | None |
| D9 | 40 | <40 | <40 | <40 | Osaka | 10 | L. retriever | ♀ | None |
| D10 | 40 | <40 | <40 | <40 | Hyogo | 2 | Mix | ♂ | None |
| D11 | 160 | 80 | 40 | 40 | Hiroshima | 12 | G. retriever | ♂ | None |
| D12 | 40 | <40 | 40 | <40 | Hiroshima | 10 | L. retriever | ♂ | None |
| D13 | 40 | <40 | 40 | <40 | Hiroshima | 8 | Akita | ♀ | None |
| D14 | 80 | <40 | 40 | 40 | Hiroshima | 3 | Mix | ♀ | None |
| D15 | 320 | 80 | 160 | 80 | Hiroshima | 7 | Mix | ♂ | None |
| D16 | 160 | <40 | 80 | <40 | Hiroshima | 4 | Plot hound | ♂ | None |
| D17 | 40 | <40 | <40 | <40 | Yamaguchi | 2 | Min. pincher | ♀ | Kennel cough |
| D18 | 40 | <40 | <40 | <40 | Tokushima | 8 | G. retriever | ♀ | Otitis externa |
| D19 | 80 | 40 | <40 | <40 | Kochi | 5 | Min. dach. | ♀ | None |
| D20 | 40 | <40 | <40 | <40 | Nagasaki | NR | NR | NR | NR |
| D21 | 40 | <40 | <40 | <40 | Kumamoto | 6 | Mix | ♀ | None |
| D22 | 80 | <40 | <40 | <40 | Miyazaki | NR | NR | NR | NR |
| D23 | 40 | 40 | <40 | <40 | Miyazaki | NR | NR | NR | NR |
| D24 | 40 | <40 | <40 | <40 | Miyazaki | NR | NR | NR | NR |
G. retriever, golden retriever; L. retriever, Labrador retriever; Min. pincher, miniature pincher; Min. dach., miniature dachshund; NR, not recorded. Boldface numbers indicate titers of 40 or greater.
DISCUSSION
JSF is the most important spotted fever in Japan. JSF onset in humans occurs 2 to 10 days after a person has worked in the fields and is abrupt. Common symptoms include headache, high fever, and shaking chills. Other major objective signs of JSF in humans include skin eruptions and tick bite eschar (13). Although some domestic and wild animals are suspected to be involved in JSF epidemiology, little is known about JSF reservoir animals. The dog is one potential reservoir animal (14), but the epidemiologic role of dogs with regard to JSF is unknown, and the pathogenesis of the agent in a canine host has never been examined. Therefore, the present study examined the pathogenesis of R. japonica in dogs and the reservoir potential of dogs for R. japonica through experimental inoculation and by conducting an epidemiologic survey.
In our inoculation experiment, the two dogs pretreated daily with an immunosuppressive dose of cyclosporine 14 days before inoculation (dogs 1 and 2) became ill after exposure to R. japonica. These two dogs exhibited increased body temperatures in the afternoon on the day of inoculation. This could have been a reaction to pathogen inoculation, but it was observed only in dogs 1 and 2. The exact reason for the temporary increase in body temperatures is unknown. Clinical signs in dogs 1 and 2 included fever, anorexia, depression, and decreased water consumption, all of which were observed between 36 and 96 h after inoculation. Increased white blood cell counts, likely as a reaction against the infection, were also recorded on day 2 in dogs 1 and 2. Clinical signs disappeared from these dogs without any treatment. These clinical signs were similar to those observed in canine cases of Rocky Mountain spotted fever (RMSF); however, R. rickettsii infection causes more severe disease in dogs. When dogs infected with R. rickettsii develop clinical illness, a fever usually develops 2 to 3 days postinfection, while cutaneous lesions consisting of vesicles and/or petechial and ecchymotic hemorrhage develops 4 to 6 days postinfection (10, 12). Other clinical signs of RMSF in affected dogs not receiving any pretreatment include depression, listlessness, anorexia, ocular and nasal discharge, scleral injection, and increased bronchovesicular lung sounds (2, 4, 5, 17). Skin and vascular lesions were not observed in the present study.
No clinical signs were observed in the Rickettsia-inoculated dogs without pretreatment with cyclosporine (dogs 3 and 4). A recent study reported that cyclosporine inhibits lymphocyte activation and expression of cytokine mRNA in dogs (11). These immunosuppressive effects of cyclosporine might affect the susceptibility of dogs to R. japonica. Cyclosporine has been widely used to suppress transplant rejection and control pruritus in allergic dermatitis in both humans and dogs. An oral dose of 5 mg/kg of body weight once a day is the most popular dosage administered to atopic dogs (20). For the present experiment, dogs were administered a similar cyclosporine dose of 50 mg per 8 to 11 kg body weight. Thus, a similar situation could occur in dogs with allergic dermatitis that are treated with cyclosporine in areas where R. japonica is endemic. Veterinarians should note that R. japonica can cause clinical disease in immunosuppressed domestic dogs. It is possible that aged dogs might be more susceptible to the infection of R. japonica because of the age-related loss of immune capacity.
Although fever, anorexia, and depression were observed in R. japonica-inoculated dogs pretreated with cyclosporine, these symptoms disappeared spontaneously by 5 days after inoculation, and no clinical signs were detected in dogs without cyclosporine pretreatment. These results suggest that R. japonica pathogenesis in dogs is much weaker than that of R. rickettsii reported previously (3, 17). Notably, the inoculum amount and the infection route differed between the studies; we subcutaneously inoculated each dog with approximately 2 × 107 infected L929 cells suspended in MEM, which was a much greater inoculum than that administered in the previous study of R. rickettsii. The latter study intraperitoneally inoculated dogs without any pretreatment with only 3,000 Vero cells infected by R. rickettsii suspended in 1 ml of sucrose-phosphate-glutamate buffer, and this caused clinical signs in the dogs (17). Our dogs were inoculated with greater numbers of infected cells, because there are no data available which indicate an optimal amount of pathogen. A quantitative study of experimental inoculation and inoculation route would be useful to this end. It is also possible that inoculation of cell culture-derived rickettsia might differ from that by the tick transmission route with respect to the capacity to cause rickettsemia or disease. The role of tick infestation for rickettsia transmission should be examined in the future study.
A previous study of RMSF observed rickettsemia lasting from 3 to 7 days when dogs were infected by R. rickettsii via intraperitoneal injection with a yolk sac suspension or via parasitism of infected Dermacentor andersoni ticks (16). This indicates that the dog can serve as a reservoir animal for RMSF. However, as the present study found no rickettsial DNA in peripheral blood or from the aspiration samples from kidney and spleen, dogs may not be suitable reservoir animals for R. japonica.
The present study took an epidemiologic approach to evaluate the pathogenesis of R. japonica in dogs as well as the reservoir potential of dogs for R. japonica. Of the 1,363 dogs examined, 24 (1.8%) had antibodies against R. japonica with titers of 1:40 or more. We obtained similar results in a previous study, in that we detected antibodies in 20 of 1,207 dogs examined (21). These results suggest that dogs in Japan can be exposed to R. japonica and produce antibodies against the pathogen under normal conditions. Despite this, none of these dogs from either study had rickettsial DNA in their peripheral blood. As such, we conclude that dogs in Japan have been exposed to this pathogen in the field but do not serve as efficient reservoir animals for R. japonica. Moreover, infected dogs, especially those with immunosuppressive conditions, can temporarily develop clinical signs, including fever, anorexia, and depression.
Acknowledgments
We thank Masaru Okuda (Yamaguchi University), Yasuyuki Endo (Kagoshima University), Aya Matsuu (Tottori University), Naoki Ide (Robins Animal Hospital, Tokushima), Hajime Sugimura (Sugimura Animal Clinic, Hyogo), and other local veterinarians for collecting and transporting canine blood samples.
This study was supported in part by grants H18-Shinkou-Ippan-014 and H21-Shinkou-Ippan-014 for Research on Emerging and Re-Emerging Infectious Diseases from the Japanese Ministry of Health, Labor, and Welfare.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Aeillo, S. E., A. Mays, H. E. Amstutz, D. P. Anderson, J. Armour, L. B. Jeffcott, F. M. Loew, and A. M. Wolf. 1998. Reference guides, p. 2190-2192. In S. E. Aeillo and A. Mays (ed.), The Merck veterinary manual, 8th ed. Merck & Co., Rahway, NJ.
- 2.Breitshwerdt, E. B., D. J. Meuten, D. H. Walker, M. Levy, K. Kennedy, M. King, and B. Curtis. 1985. Canine Rocky Mountain spotted fever: a kennel epizootic. Am. J. Vet. Res. 46:2124-2128. [PubMed] [Google Scholar]
- 3.Breitshwerdt, E. B., D. H. Walker, M. G. Levy, W. Burgdorfer, W. T. Corbett, S. A. Hurlbert, M. E. Stebbins, B. C. Curtis, and D. A. Allen. 1988. Clinical, hematological and immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am. J. Vet. Res. 49:70-76. [PubMed] [Google Scholar]
- 4.Comer, K. M. 1991. Rocky Mountain spotted fever. Vet. Clin. North Am. Small Anim. Pract. 21:27-44. [DOI] [PubMed] [Google Scholar]
- 5.Greene, C. E. 1987. Rocky Mountain spotted fever. J. Am. Vet. Med. Assoc. 191:666-671. [PubMed] [Google Scholar]
- 6.Hoshina, K., H. Itogawa, A. Itagaki, M. Gomyoda, and T. Uchida. 1995. Serosurvey for spotted fever group rickettsial infection in vertebrates in Shimane Prefecture. Kansenshogaku Zasshi 69:524-531. [DOI] [PubMed] [Google Scholar]
- 7.Inokuma, H., N. Seino, M. Suzuki, K. Kaji, H. Takahashi, H. Igota, and S. Innoue. 2008. Detection of Rickettsia helvetica DNA from peripheral blood of Sika deer (Cervus nippon yesoensis) in Japan. J. Wildl. Dis. 44:164-167. [DOI] [PubMed] [Google Scholar]
- 8.Inokuma, H., S. Yamamoto, and C. Morita. 1998. Survey of tick-borne diseases in dogs infested with Rhipicephalus sanguineus at a kennel in Okayama Prefecture, Japan. J. Vet. Med. Sci. 60:761-763. [DOI] [PubMed] [Google Scholar]
- 9.Ishikura, M., H. Fujita, S. Ando, K. Matsuura, and M. Watanabe. 2002. Phylogenetic analysis of spotted fever group rickettsiae isolated from ticks in Japan. Microbiol. Immunol. 46:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Kennan, K. P., W. C. Buhles, Jr., D. L. Huxsoll, R. G. Williams, and P. K. Hildebrandt. 1977. Studies on the pathogenesis of Rickettsia rickettsii in the dog: clinical and clinicopathologic changes of experimental infection. Am. J. Vet. Res. 38:851-856. [PubMed] [Google Scholar]
- 11.Kobayashi, T., Y. Momoi, and T. Iwasaki. 2007. Cyclosporine A inhibits the mRNA expression of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J. Vet. Med. Sci. 69:887-892. [DOI] [PubMed] [Google Scholar]
- 12.Lissman, B. A., and J. L. Benach. 1980. Rocky Mountain spotted fever in dogs. J. Am. Vet. Med. Assoc. 176:994-995. [PubMed] [Google Scholar]
- 13.Mahara, F. 2006. Rickettsioses in Japan and the Far East. Ann. N. Y. Acad. Sci. 1078:60-73. [DOI] [PubMed] [Google Scholar]
- 14.Mahara, F. 2007. Clinical aspects and epidemiology of Japanese spotted fever. J. Vet. Med. 60:365-368. (In Japanese.) [Google Scholar]
- 15.Mahara, F., K. Koga, S. Sawaga, T. Taniguchi, F. Shigemi, T. Suto, Y. Tsuboi, A. Ooya, H. Koyama, T. Uchiyama, and T. Uchida. 1985. The first report of the rickettsial infection of spotted fever group in Japan: three clinical cases. Jpn. J. Assoc. Infect. Dis. 59:1165-1172. [DOI] [PubMed] [Google Scholar]
- 16.Norment, B. R., and W. Burgdorfer. 1984. Susceptibility and reservoir potential of the dogs to spotted fever group rickettsia. Am. J. Vet. Res. 45:1706-1710. [PubMed] [Google Scholar]
- 17.Piranda, E. M., J. L. H. Faccini, A. Pinter, T. B. Saito, R. C. Pacheco, M. K. Hagiwara, and M. B. Labrnna. 2008. Experimental infection of dogs with Brazilian strains of Rickettsia rickettsii: clinical and laboratory findings. Mem. Inst. Oswaldo Cruz 103:696-701. [DOI] [PubMed] [Google Scholar]
- 18.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada, Y., T. Beppu, H. Inokuma, M. Okuda, and T. Onishi. 2003. Ixodid tick species recovered from domestic dogs in Japan. Med. Vet. Entomol. 17:38-45. [DOI] [PubMed] [Google Scholar]
- 20.Steffan, J., C. Favrot, and R. Mueller. 2006. A systematic review and meta-analysis of the efficiency and safety of cyclosporine for the treatment of atopic dermatitis in dogs. Vet. Dermatol. 17:3-16. [DOI] [PubMed] [Google Scholar]
- 21.Tabuchi, M., Jilintai, Y. Sakata, N. Miyazaki, and H. Inokuma. 2007. Serological survey of Rickettsia japonica infection in dogs and cats in Japan. Clin. Vaccine Immunol. 14:1526-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto, S., C. Morita, and K. Tsuchiya. 1992. Isolation of spotted fever group Rickettsia from Apodemus speciosus in an endemic area in Japan. Jpn. J. Med. Sci. Biol. 45:81-86. [DOI] [PubMed] [Google Scholar]