Abstract
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia in the United States and globally. Despite the availability of pneumococcal capsular polysaccharide (PPS) and protein conjugate-based vaccines, the prevalence of antibiotic-resistant pneumococcal strains, serotype (ST) replacement in nonconjugate vaccine strains, and uncertainty as to whether the PPS vaccine that is used in adults protects against pneumonia emphasize the need for continued efforts to understand the nature of protective PPS antibody responses. In this study, we generated mouse monoclonal antibodies (MAbs) to a conjugate consisting of the PPS of serotype 8 (PPS8) S. pneumoniae and tetanus toxoid. Thirteen MAbs, including four IgMs that bound to PPS8 and phosphorylcholine (PC) and five IgMs and four IgG1s that bound to PPS8 but not PC, were produced, and their nucleotide sequences, epitope and fine specificity, and efficacy against lethal challenge with ST8 S. pneumoniae were determined. MAbs that bound to PPS8 exhibited gene use that was distinct from that exhibited by MAbs that bound to PC. Only PPS8-binding MAbs that did not bind PC were protective in mice. All 13 MAbs used germ line variable-region heavy (VH) and light (VL) chain genes, with no evidence of somatic hypermutation. Our data reveal a relationship between PPS specificity and VH gene use and MAb efficacy in mice. These findings provide insight into the relationship between antibody molecular structure and function and hold promise for the development of novel surrogates for pneumococcal vaccine efficacy.
Streptococcus pneumoniae (pneumococcus) is the most common bacterial cause of meningitis, otitis media, and pneumonia in the United States and globally. Worldwide, pneumococcus is associated with the highest prevalence of all vaccine-preventable diseases (16, 28, 42) and is the cause of approximately 1 million deaths among children under the age of 5 years annually in the developing world (38). Currently available pneumococcal vaccines are composed of either unconjugated or protein-conjugated pneumococcal capsular polysaccharides (PPSs). A 23-valent unconjugated vaccine is used in adults, and a 7-valent pneumococcal conjugate vaccine (PCV7) or a newly introduced 13-valent PCV is used in infants and children. Since 2000, the use of the PCV has led to a dramatic decrease in invasive pneumococcal disease in children (1) and in adults due to herd immunity (28). However, the ongoing problem of pneumococcal antibiotic resistance, uncertainty about the efficacy of the adult vaccine against pneumonia (29, 36), insufficient protection of immunocompromised patients, and the phenomenon of serotype (ST) replacement with use of the pediatric vaccine (17) highlight the need for improved pneumococcal vaccines and surrogates for vaccine efficacy.
PPS-based vaccines elicit antibodies with two types of reactivity: reactivity with PPS and reactivity with phosphorylcholine (PC) (20, 45). PC is a major structural component of the pneumococcal cell wall and is present in all pneumococcal strains (49). PC is covalently linked to the pneumococcal virulence factor C-polysaccharide (23), binds to the platelet-activating factor receptor (PAFr) on host cells, and is required for pneumococcal host invasion (39). PC is also found in purified PPS as a contaminant from the PPS purification process (31, 44). Data on the efficacy of PC antibodies in mouse models of pneumococcal infection suggest that the ability of these antibodies to confer protection is model dependent (3-6, 49). Naturally occurring antibodies to PC that express the T15 idiotype were shown to protect mice against intravenous challenge with ST3, and mouse monoclonal antibodies (MAbs) expressing the same idiotype were protective when administered as passive immunogens to mice before intravenous infection with ST3 (5, 6). Although some protection against other STs has been demonstrated, mouse MAbs to PC appear to protect principally against intravenous infection with ST3, with IgG3 being more protective than IgM (5). To our knowledge, the efficacy of PC-binding MAbs has not been evaluated in pulmonary infection models. The role of PC antibodies in human pneumococcal infection is less well understood, although recent studies link naturally occurring PC-reactive IgM to protection against atherosclerosis (11, 25, 47). The efficacy of type-specific antibody to PPS against pneumococcus is incontrovertible (41).
S. pneumoniae ST8 is a strain that, unlike the serotypes that are included in PCV7, increases in prevalence in individuals over the age of 10 years (22). ST8 expresses a nonhemolytic allele of pneumolysin (30) and is highly virulent in systemic (intraperitoneal [i.p.]) and pulmonary (intranasal [i.n.]) infection models in mice (9, 55). The current 23-valent PPS vaccine includes a PPS serotype 8 (PPS8) moiety, but PPS8 is not included in available PCV7 or PCV13 vaccines. In the study reported herein, we compared the molecular genetic structures of PPS8- and PC-reactive mouse MAbs and their efficacies in mice to further our understanding of the relationship between antibody gene use, specificity, and efficacy.
MATERIALS AND METHODS
Bacteria and PPS conjugates.
The Streptococcus pneumoniae ST8 and purified PPS8 strains used in this report were obtained from the American Type Culture Collection (ATCC; Manassas, VA). S. pneumoniae ST8 (strain 6308; ATCC) was grown in tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) to mid-log phase at 37°C in 5% CO2 as described previously (55). In some experiments, S. pneumoniae ST3 (WU2) (43, 51) was also used. All bacteria used in this study were frozen in 10% glycerol in TSB at −80°C prior to use. A conjugate of purified PPS8 (ATCC 6308) and tetanus toxoid (TT) (PPS8-TT) was produced according to the methods described for another TT conjugate (14). The total protein content of the conjugates was assayed using the bicinchoninic acid and microassay (Pierce, Rockford, IL), using TT as a standard.
Mice.
BALB/c mice (National Cancer Institute, Bethesda, MD) were used for generating hybridomas and for protection experiments. All mice used in this study were maintained by the Institute for Animal Studies at the Albert Einstein College of Medicine (AECOM) in accordance with the rules and regulations of animal welfare at AECOM.
Generation of PPS8-specific mouse MAbs.
Thirteen MAbs were generated from two separate fusions. For each fusion, BALB/c mice were vaccinated subcutaneously at the base of the tail with a total dose of 2.5 μg PPS8-TT in incomplete Freund's adjuvant (Brenntag Biosector, Frederikssund, Denmark). The splenocytes were isolated on day 7 for the first fusion and 3 days after the boost for the second fusion, and hybridomas were generated by fusing isolated splenocytes with the mouse myeloma cell line NSO as described previously (14). Cells were propagated with a cell-cloning system (ClonaCell-HY; Stem Cell Technologies, Inc., Vancouver, British Columbia, Canada) according to the manufacturer's instructions. Hybridoma cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 10% NCTC-109 (Gibco, Grand Island, NY), 1% nonessential amino acids (Mediatech, Herndon, VA), and 1% penicillin-streptomycin (Mediatech, Herndon, VA).
Identification of PPS8-binding MAbs.
The isotype and specificity of secreted antibody from hybridoma cells were evaluated as previously described (14). Supernatants were absorbed with purified pneumococcal cell wall polysaccharide (CWPS) (Statens Seruminstitut, Copenhagen, Denmark) before use. To evaluate PPS8 binding, polystyrene enzyme-linked immunosorbent assay (ELISA) plates (Corning GlassWorks, Corning, NY) were coated with 10 μg/ml of PPS8 (ATCC 6308) and incubated with serially diluted hybridoma supernatants. After the washing step, the plates were incubated at 37°C for 1 h with alkaline phosphatase-conjugated goat anti-human secondary immunoglobulins of different isotypes (Southern Biotechnology, Birmingham, AL). After the plates were washed, bound antibody was detected by developing the plates with ρ-nitrophenyl phosphate substrate (Sigma-Aldrich, St. Louis, MO). A Sunrise absorbance reader (Tecan US, Durham, NC) was used to record all optical densities (ODs) in this study. MAbs were also tested for their binding to double-stranded DNA (dsDNA) (Sigma-Aldrich, St. Louis, MO) and bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO).
Identification of PC-binding MAbs.
To ascertain the reactivity of the MAbs with PC, microtiter plates were coated with 1.0 μg/ml of PC-BSA (Sigma-Aldrich, St. Louis, MO) and binding of the MAbs to PC was determined as described above. The PC-binding mouse IgA s107.3.4.E (24) (provided by M. Scharff, Albert Einstein College of Medicine) was used as a positive control.
Quellung-type and IF studies of binding of MAb to bacteria.
Quellung-type reactions were performed as described previously (21). Immunofluorescence (IF) experiments were performed as follows. A suspension of approximately 108 ST3 (WU2) or ST8 (ATCC 6308) cells was incubated with the PPS8-specific MAb 31B12 IgG1[κ] (this study), the PPS3-specific MAb 1E2 IgG1[κ] (52), or the PC-reactive MAb 5G6 IgM[κ] (this study) in TSB for 1 h at 37°C. After three washes, anti-mouse IgG-TRICH and IgM-FICH (BD-Bioscience, San Jose, CA) were added to the mixtures, followed by incubation overnight at 4°C in TSB. After three washes, cells were resuspended in 20 μl of ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA). The PPS3-specific MAb 1E2 IgG1[κ] (52) was used as a positive control for PPS3 binding.
Inhibition ELISA with different PPSs.
The PPS specificity of PPS8-binding MAbs was determined by inhibition ELISA. Plates were coated with 10 μg/ml of PPS8 (ATCC) and blocked with 1% BSA-phosphate-buffered saline (PBS) as described above. Then, PPS3 (ATCC 17-X), PPS4 (ATCC 18-X), PPS9N (ATCC 21-X), PPS14 (ATCC 13-X), PPS19F (ATCC 24-X), PPS23F (ATCC 25-X), and PPS8 (ATCC 20-X) (as a positive control) were added at different concentrations, beginning with 10 μg/ml, after which the MAbs were added at a concentration that achieved 50% saturation by direct binding to PPS8-coated plates. MAb binding to PPS8 was determined as described above.
Binding assay with PC structural analogues.
To determine the specificity of PC-binding MAbs, PC-reactive MAbs were incubated with serial dilutions of choline, dsDNA, l-α-glycero PC, phosphocholine, and acetylcholine (Sigma-Aldrich, St. Louis, MO), beginning with 500 μg/ml, on 5% BSA-PBS-coated plates for 1 h at 37°C. After incubation, 100 μl of the samples was transferred to plates coated with 1.0 μg of PC-BSA. Binding of the MAbs was determined by ELISA as described above.
Nucleic acid sequence analysis.
MAb nucleic acid sequences were determined as described previously (14, 52). Briefly, mRNA was isolated from approximately 106 hybridoma cells and reverse transcribed to cDNA by Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen, Carlsbad, CA). To identify gene use, V region heavy (VH) and V region light (VL) chains were amplified by primers that anneal to a leader and to constant regions by use of a mouse Ig primer set (Invitrogen, Carlsbad, CA). PCR products were gel purified according to the manufacturer's instructions (Qiagen, Valencia, CA) and sequenced by the Albert Einstein College of Medicine Sequencing facility. The mouse V region sequences that were obtained were compared to the database of mouse immunoglobulin sequences by using VBASE2 (40) and BLAST (National Center for Biotechnology Information, Bethesda, MD). The nucleic acid translation was performed by the Expasy program (Swiss Institute of Bioinformatics, Basel, Switzerland).
Mouse protection experiments. (i) i.p. infection model.
Purified MAbs were diluted in 100 μl sterile PBS and administered to mice by the intraperitoneal (i.p.) route 2 h prior to the injection of bacteria by the i.p. route, as described previously (7, 55). ST8 (6308) and ST3 (WU2) were thawed prior to use and diluted to the desired concentration in 100 μl of TSB. For infection, mice were given 50, 100, or 1,000 CFU of ST8 (6308) or 100 or 200 CFU of ST3 (WU2). This infection model is based on the historical serum potency assay (8) and has been used previously to study the efficacy of MAbs to PPS in vivo (14, 43, 52, 55).
(ii) i.n. infection model.
MAbs were prepared and administered as described for the i.p. infection model. Intranasal (i.n.) infection was induced as described previously (35). The mice were minimally anesthetized using isoflurane (Henry Schein, Melville, NY) with an inhalation anesthesia system (Vetequip, Pleasanton, CA) and inoculated with 5 × 103 or 5 × 104 CFU of ST8 pneumococcal cells in 40 μl of TSB by applying 20 μl to the opening of each naris by a pipette. The PPS3-specific MAb 1E2 IgG1[κ] (52) was used as a negative control for protection against ST8, and the Cryptococcus neoformans glucuronoxylomannan (GXM)-specific mouse MAb 2D10 IgM[κ] (provided by A. Casadevall, Albert Einstein College of Medicine) and PBS were used as a negative isotype (2D10) control and a control for MAb administration, respectively, in both infection models. The 50% lethal dose (LD50) for each mouse strain was determined by Reed and Muench LD50 calculations previously (data not shown).
Statistical analysis.
Mouse survival was evaluated statistically by Kaplan-Meier plotting and the log rank test (14, 55). All statistical analyses were performed using Prism (version 5.02 for Windows; GraphPad Software, San Diego, CA). A P value of <0.05 was considered statistically significant.
RESULTS
Characterization of MAbs produced from PPS8-TT conjugate-vaccinated mice.
Thirteen PPS8-reactive MAbs (nine IgM and four IgG1 MAbs) were produced from PPS8-TT immunized BALB/c mice (Table 1). 2B3B3, 28H11, 13Q2, 22B10, 25A1, 26E4, 28A2, 31B12, and 30H9 bound PPS8 only, and 24A4, 5G6, 30A12, and 20H4 bound both PPS8 and PC. The isotype, PC reactivity, and variable-region heavy (VH) chain and light (VL) chain use of the MAbs were determined (Table 1). None of the MAbs demonstrated measurable reactivity with BSA or TT (data not shown).
TABLE 1.
MAb germ line gene usage and specificity
| Group | Cell line | Isotype | PC bindinga | Gene family |
GenBank accession no.d |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| VHb | DHb | JHb | VKc | JKc | VH | VL | ||||
| 1 | 24A4 | IgM | + | Igh-VS107 | DFL16.1 | JH1 | IgVk-7 | JK5 | FJ147348 | FJ147349 |
| 2 | 5G6 | IgM | + | Igh-VS107 | DFL16.2 | JH1 | IgVk-7 | JK5 | FJ147338 | FJ147339 |
| 3 | 30A12 | IgM | + | Igh-VS107 | DFL16.1 | JH1 | IgVk-4 | JK5 | FJ147356 | FJ147357 |
| 4 | 20H4 | IgM | + | Igh-VS107 | DFL16.1 | JH1 | IgVk-8 | JK1 | FJ147342 | FJ147343 |
| 5 | 2B3B3 | IgM | − | Igh-VX24 | DSP2.11 | JH2 | IgVk-6 | JK2 | FJ147336 | FJ147337 |
| 6 | 28H11 | IgM | − | Igh-VX24 | DSP2.3 | JH1 | Igk-V8 | JK2 | FJ147354 | FJ147355 |
| 7 | 13Q2 | IgM | − | Igh-VQ52 | DFL16.1 | JH1 | IgVk-4 | JK2 | FJ972833 | FJ972834 |
| 8 | 22B10 | IgM | − | Igh-VQ52 | DFL16.1 | JH4 | Igk-V8 | JK1 | FJ147344 | FJ147345 |
| 9 | 25A1 | IgM | − | Igh-VQ52 | DSP2.9 | JH1 | IgVk-2 | JK2 | FJ147350 | FJ147351 |
| 10 | 26E4 | IgG1 | − | Igh-V7183 | DSP2.12 | JH4 | IgVk-1 | JK5 | FJ147352 | FJ147353 |
| 11 | 28A2 | IgG1 | − | Igh-V7183 | DSP2.12 | JH4 | IgVk-3 | JK2 | FJ972831 | FJ972832 |
| 12 | 31B12 | IgG1 | − | Igh-V7183 | DSP2.12 | JH4 | IgVk-2 | JK2 | FJ972829 | FJ972830 |
| 13 | 30H9 | IgG1 | − | Igh-VX24 | DSP2.10 | JH2 | IgVk-4 | JK2 | FJ147358 | FJ147359 |
Antibody binding to (+) or lack of binding to (−) phosphorycholine (PC).
Germ line gene usage of the variable-region heavy chain (VH).
Germ line gene usage of the variable-region light chain (VL).
GenBank accession numbers of MAb nucleotide sequences.
PPS specificity of PPS8-binding MAbs.
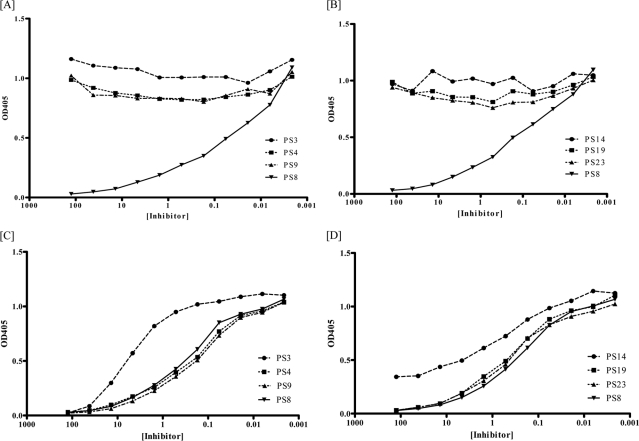
The specificity of the 13 MAbs was evaluated by a PPS inhibition assay. The PPS8 binding of nine MAbs, 2B3B3, 28H11, 13Q2, 22B10, 25A1, 26E4, 28A2, 31B12, and 30H9, was inhibited by soluble PPS8 but not by any of the other PPSs used (Fig. 1A and B). The PPS binding of 28H11 IgM[κ] shown in the figure is representative of all of the PPS8-only-binding MAbs (data from the other MAbs are not shown).
FIG. 1.
PPS8 specificity of PPS8- and PC-binding MAbs. PPS binding of the PPS8-binding MAb 28H11 (A, B) and the PC-binding MAb 24A4 (C, D) as determined by inhibition ELISA. The soluble PPSs shown in the legend were used to inhibit the binding of the indicated MAbs to PPS8-coated plates. Similar binding patterns were obtained for the other PPS8- and PC-binding MAbs (not shown).
PC specificity of PC-binding MAbs.
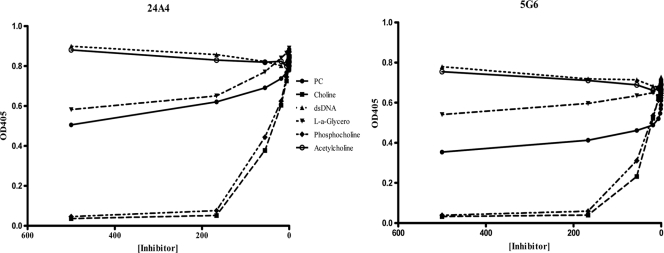
The binding of the four PC-binding MAbs, 24A4, 5G6, 30A12, and 20H4, to PPS8 was inhibited by soluble PPS3, PPS4, PPS8, PPS9, PPS14, PPS19, and PPS23. The degrees of inhibition by PPS3 and PPS14 were less than that observed with PPS8 or other PPSs (Fig. 1C and D). The PPS binding of 24A4 IgM[κ] shown in Fig. 1 is representative of the other PC-binding MAbs (data for the other MAbs are not shown). The specificity of the PC-binding MAbs was further analyzed with assays for direct binding to PC analogues, as described previously (46). The binding of all four PC-binding MAbs to PC-BSA was inhibited by PC-BSA (control) and choline, as shown for MAbs 24A4 and 5G6 but not by any of the other PC analogs (Fig. 2).
FIG. 2.
Specificity of PC-binding MAbs. The binding of PC-binding MAbs to the analogs of phosphorylcholine indicated in the legend was determined by inhibition ELISA for 24A4 (left) and 5G6 (right), which differs from 24A4 by 1 amino acid (Y to D) in the D region.
VH and VL gene use of PC-reactive and PPS8-specific MAbs.
The VH and VL gene use of the MAbs produced in this study and their GenBank accession numbers are shown in Table 1. The murine VH gene family s107 was used by the four PC-binding MAbs, and three families, Vx24, VQ52, and V7183, were used by the nine PPS8-binding MAbs (Table 1). Three murine VL gene families, Vκ4, Vκ7, and Vκ8, were used by the PC-binding MAbs, and six murine VL gene families, Vκ1, Vκ2, Vκ3, Vκ4, Vκ6, and Vκ8, were used by the PPS8-binding MAbs (Table 1). No PC- or PPS8-binding MAb had evidence of somatic hypermutation.
CDR3 analysis of PC-reactive and PPS8-specific MAbs.
Table 2 shows the CDR3 regions of the PC- and PPS8-binding MAbs. Each PC-binding MAb was 12 amino acids in length, with identical sequences, except MAb 5G6, which has aspartic acid (D) instead of tyrosine (Y) in the D region (Table 2). The lengths of the CDR3 regions of the PPS8-specific MAbs ranged from 8 to 16 amino acids (Table 2). The mean CDR3 length (± standard deviation [SD]) for the four PC-binding MAbs was 12.0 ± 0.0 amino acids, with values of 10.1 ± 2.8 amino acids for the nine PPS8-binding MAbs, 10.2 ± 0.9 amino acids for the nine (both PC- and PPS-binding) IgM MAbs, and 11.8 ± 3.5 amino acids for four IgG MAbs (Table 2).
TABLE 2.
Amino acid sequences of CDR3 regions of PC- and PPS8-binding MAbs
| MAb | MAb specificity | CDR3 amino acid sequence | No. of amino acids in CDR3 |
|---|---|---|---|
| S107 | 4 | ||
| 20H4 | PC | D Y Y G S S Y W Y F D V | 12 |
| 24A4 | PC | D Y Y G S S Y W Y F D V | 12 |
| 5G6 | PC | D Y Y G S S D W Y F D V | 12 |
| 30A12 | PC | D Y Y G S S Y W Y F D V | 12 |
| Vx24 | |||
| 30H9 | PPS8 | Q G Y R Y F D Y | 8 |
| 2B3B3 | PPS8 | P Y Y R Y F D Y | 8 |
| 28H11 | PPS8 | P W F R Y F D V | 8 |
| VQ52 | |||
| 13Q2 | PPS8 | N R G S P C W Y F D V | 11 |
| 22B10 | PPS8 | Q L G H Y A M D Y | 9 |
| 25A1 | PPS8 | D G G W Y F D V | 8 |
| V7183 | |||
| 26E4 | PPS8 | L L G S R N L S H R L L S | 13 |
| 28A2 | PPS8 | S H R L L S Q N D T P I R X S L | 16 |
| 31B12 | PPS8 | L L G S R N L S H R | 10 |
Based on DNAPLOT Query (40), the non-germ line nucleotide sequences of the MAbs were derived from nucleotide addition and P deletion.
Binding of MAb to S. pneumoniae as determined by imaging.
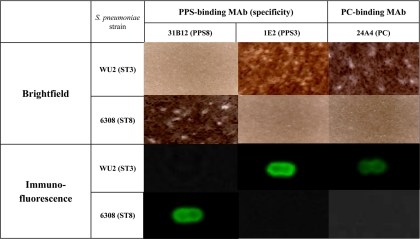
The binding of the PC-binding MAbs to ST3 and ST8 cells was determined by Quellung reaction and immunofluorescence imaging. The PC-binding MAb 24A4 bound to ST3 but not to ST8 (Fig. 3). The binding of 24A4 IgM[κ] is representative of the other PC-binding MAbs (data not shown). The PPS3-binding MAb 1E2 bound to ST3 but not to ST8, and the PPS8-binding MAb 31B12 bound to ST8 but not to ST3 (Fig. 3).
FIG. 3.
Quellung and immunofluorescence (IF) imaging of PPS- and PC-binding MAbs. Binding of the PC-binding MAb 24A4 to ST3 (WU2) and ST8 (6308) was determined by Quellung (bright-field) and IF microscopy. The PC-binding MAb binds both ST3 and ST8. PPS8-binding MAb 31B12 binds ST8 cells but not ST3 cells, and the PPS3-binding MAb 1E2 binds ST3 but not ST8.
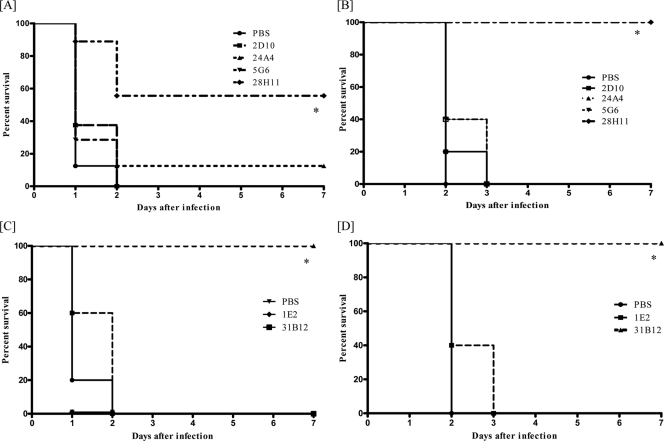
PPS8-binding but not PC-binding MAbs mediate protection against ST8.
The efficacy of PC- and PPS8-binding MAbs was evaluated in vivo in mouse models of i.p. and i.n. infection (Fig. 4). Ten micrograms of the PC-binding MAbs 24A4 and 5G6 did not protect mice from death from ST8 after i.p. (Fig. 4A) or i.n. (Fig. 4B) infection. These MAbs were also not protective when used at a dosage of 100 μg in the same infection models (data not shown). In contrast, 10 μg of the PPS8-binding MAbs 28H11 (IgM) and 31B12 (IgG1) prolonged survival in both infection models (Fig. 4C and D). These PPS8-binding MAbs were also protective against higher inocula (103 CFU for the i.p. route and 5 × 104 CFU for the i.n. route) (see Fig. S1 in the supplemental material). MAb protection experiments with 50 CFU of ST8 could not be performed, because ST8 was not lethal at this inoculum.
FIG. 4.
Effect of MAb administration on lethal ST8 infection in mice. The survival of BALB/c mice treated with 10 μg IgM (A and B) and IgG1 (C and D) MAbs as indicated in each panel after ST8 infection is shown for i.p. (A and C) and i.n. (B and D) infection. The IgM MAbs used in this study were 28H11 (PPS8), 24A4 and 5G6 (PC), and 2D10 (IgM isotype control). The IgG MAbs used in this study were 31B12 (PPS8) and 1E2 (IgG1 isotype control). *, P < 0.01 (Kaplan Meier log rank survival test comparing PPS-binding MAb to isotype control; n = ∼8 to 10 mice per group).
PC-binding MAbs do not mediate protection against ST3 challenge.
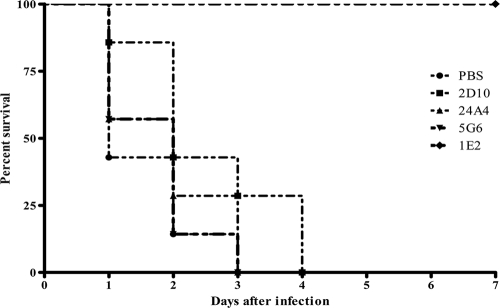
We also tested the efficacy of the PC-binding MAbs 24A4 and 5G6 against ST3 (WU2) in the i.p. model. Ten micrograms of the MAbs did not prolong survival against 100 (Fig. 5) or 200 (data not shown) CFU of WU2.
FIG. 5.
Effect of MAb administration on lethal infection with ST3 in mice. The survival of BALB/c mice treated with 10 μg of the MAbs indicated in the figure after i.p. infection with 100 CFU of ST3 (WU2) is shown. The PPS3-binding MAb 1E2 was used as a positive control. The GXM-binding MAb 2D10 was used as a negative control. The survival curves were not statistically significant with comparison of 24A4 and 5G6 to 2D10 (Kaplan Meier log rank survival test; n = ∼8 to 10 mice per group).
DISCUSSION
This study provides a molecular analysis of PPS8-TT-elicited antibodies to PC and PPS8 and demonstrates a relationship between antibody specificity, gene use, and MAb efficacy in vivo. PC- and PPS8-binding MAbs were derived from distinct VH gene elements; however, only PPS8-binding MAbs were protective against lethal i.p. or i.n. infection with ST8 in mice. The lack of efficacy of PC-binding MAbs against ST3 in our model is interesting, given that naturally occurring PC-binding antibodies were found to enhance resistance to ST3 in naïve mice (6, 27). The biological activities of naturally occurring and vaccine-elicited PC-binding antibodies could differ, with the caveat that the function of endogenously produced antibodies could differ based on the biological niche in which they are produced. On the other hand, the finding that the PC-binding MAbs did not protect against ST8 is not surprising, given that these MAbs did not bind ST8 cells in vitro.
Our data show that the PC-binding IgM MAbs bound to PPS8 and that this binding was inhibited predominantly by PPS8, PPS4, PPS9, PPS19, and PPS2. The most likely explanation for this finding is that the MAbs bound to PC that was contaminating these PPSs. However, this phenomenon was not uniform, as the PPS8 binding of PC-binding MAbs was only weakly inhibited by PPS3 and PPS14. There may be less PC contamination of purified PPS3 than of purified PPS14, given that the ST3 capsule is noncovalently linked to the bacterial cell wall (54). A factor that could limit the PPS14 binding of the PC-binding MAbs is that PPS14 has a neutral charge, which could reduce binding attributable to interactions between positively charged antibody determinants and negatively charged capsular polysaccharide (48). The other PPSs used in this study are negatively charged (53). IgM MAbs with phosphatidylcholine (Ptc) and phosphocholine specificity were previously found to differ in their abilities to protect against bacterial sepsis in mice (2). The PC-binding MAbs in this study exhibited PC and choline specificity but were not protective in our infection models.
The PC- and PPS8-binding MAbs produced in this study use distinct gene elements. All four of the PC-binding MAbs use the VH s107 gene, as reported by other groups (13, 15, 24). These MAbs had identical germ line VH gene sequences, except MAb 5G6, which had a single-amino-acid difference from the other sequences in CDR3. This change was due to the use of a different D gene and resulted in a change in the total charge of the CDR3 (tyrosine to aspartic acid) from −2 (24A4 and others) to −3 (5G6). Previously, single-amino-acid changes in CDR1 and the V-D-joining region of PC antibody were found to alter the specificity (24) and efficacy (26) of PC-binding MAbs. However, the amino acid change in 5G6 did not alter the specificity or efficacy of this MAb.
In contrast to that of the PC-binding MAbs, the VH gene use of the PPS8-binding MAbs was more diverse. These MAbs used three different VH gene families, VQ52, VX24, and V7183, two of which are included in VH clan III, as designated by Kirkham et al. in their categorization schema of mammalian VH genes based on conserved sequences in VH framework regions (32). Of the PC- and PPS8-binding MAbs reported herein, 78.6% (11/14) used clan III gene segments, namely, S107, Vx24, and V718, whereas 21.4% (2/14) used Q52, a clan II gene segment. The use of clan III genes in PPS8-binding MAbs parallels the use of clan III genes in human and mouse MAbs to other capsular polysaccharides, including PPS (12, 14, 34, 55). Hence, our data extend the association between clan III gene use and capsular polysaccharide reactivity, lending credence to the hypothesis that the included group of VH genes could possess structural characteristics that imbue these genes with a propensity to produce antibodies that bind polysaccharide determinants.
Each of the MAbs described herein used germ line VH genes. Hence, the structural characteristics and/or combinatorial diversity of certain VH genes is sufficient to generate antibodies that protect against ST8. Human PPS3-binding MAbs generated from human immunoglobulin transgenic mice also used germ line VH genes (14). The CDR3 lengths of the PC-binding MAbs were identical, 12 amino acids, as reported for other PC MAbs (26). In contrast to the restricted CDR3 lengths that have been reported for antibodies to other polysaccharide antigens (14, 33, 37), the PPS8-binding MAbs had a range of CDR3 lengths. Further studies are required to determine the influence of CDR3 length on MAb specificity and/or function. None of the MAbs reported in this study was somatically mutated. PPS3-binding MAbs generated from PPS3-TT-immunized mice had somatic hypermutation (52), but PC-binding MAbs have a low mutation rate, regardless of their host derivation (18, 19).
There is ample evidence that PPS-binding MAbs can prolong the survival of mice that are infected with the homologous ST (9, 10, 14, 43, 52). The PC-binding IgM MAbs in this study did not mediate protection against lethal ST8 or ST3 infection in either i.n. or i.p. infection models. The efficacy of PC-reactive MAbs in systemic (i.p. or intravenous) infection models has predominantly been demonstrated for ST3, with the caveats that IgM was more protective than IgG and that protection was more robust against intravenous challenge than against i.p. challenge (4). Although the mechanism by which PC antibodies mediate protection is not known, given that PC is a cell wall determinant and that PC-binding MAbs did not bind ST8 cells, the lack of efficacy of these MAbs could reflect an inability to bind to ST8 cells in vivo and/or to engage the appropriate effector cells. Although the PC-binding IgM MAbs were able to bind ST3 cells in vitro, these MAbs did not protect against ST3. PC antibodies are thought to mediate protection by promoting intravascular clearance (4), and they are most effective against intravenous challenge (3). Given that mice die of disseminated disease and bacteremia irrespective of the original route of infection and that the strains that we used were highly lethal, the bacterial burden in our models might have been more than could be cleared by the amount of MAb administered. Recently, i.n. immunization with a PC-keyhole limpet hemocyanin (KLH) conjugate was shown to enhance salivary IgA-mediated clearance of pneumococcus from the nasal tract of BALB/c mice (50). Hence, the efficacy of PC-binding antibody could depend on the ST, the isotype, and/or the infection model.
Our data reinforce the concept that acquired immunity to pneumococcus is mediated by serotype-specific antibody to PPS (41) and suggest that VH gene use could predict antibody specificity and function. The PC response to the PPS8 conjugate that we used in this study raises the concern that the apparent specificity or biological function of PPS-binding serum antibodies could be confounded by the presence of PC-binding antibodies, even after CWPS absorption. The PC-binding MAbs that were generated in this study were detected because of their ability to bind PPS8 despite CWPS absorption. Given the discrete gene use of the PPS- and PC-specific MAbs reported herein and the observation that only PPS-specific MAbs were protective, analysis of the gene use of PPS-binding B cells could have the potential to reveal PPS specificity and possibly to predict function. Although certain PPS determinants might elicit antibodies that are less protective than others, the molecular signatures of such antibodies could also be discrete, as described for PPS3-binding MAbs (14, 52). Hence, antibody structure-function relationships could hold promise as biomarkers and/or as a component of a new generation of surrogates for pneumococcal vaccine efficacy.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI45459 and AI44374 to L.-A.P.
Footnotes
Published ahead of print on 10 November 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Anonymous. 2008. Progress in introduction of pneumococcal conjugate vaccine—worldwide, 2000-2008. MMWR Morb. Mortal. Wkly. Rep. 57:1148-1151. [PubMed] [Google Scholar]
- 2.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles, D. E., J. L. Claflin, K. Schroer, and C. Forman. 1981. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature 294:88-90. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles, D. E., C. Forman, S. Hudak, and J. L. Claflin. 1982. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 156:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., et al. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchwald, U. K., A. Lees, M. Steinitz, and L. A. Pirofski. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 9.Burns, T., M. Abadi, and L. A. Pirofski. 2005. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin m monoclonal antibody to serotype 8 capsular polysaccharide. Infect. Immun. 73:4530-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns, T., Z. Zhong, M. Steinitz, and L. A. Pirofski. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrero, J. J., et al. 2009. Low levels of IgM antibodies against phosphorylcholine-A increase mortality risk in patients undergoing haemodialysis. Nephrol. Dial. Transplant. 24:3454-3460. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall, A., et al. 1994. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerny, J., J. S. Smith, C. Webb, and P. W. Tucker. 1988. Properties of anti-idiotypic T cell lines propagated with syngeneic B lymphocytes. I. T cells bind intact idiotypes and discriminate between the somatic idiotypic variants in a manner similar to the anti-idiotopic antibodies. J. Immunol. 141:3718-3725. [PubMed] [Google Scholar]
- 14.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke, S. H., J. J. Kenny, D. G. Sieckmann, and S. Rudikoff. 1984. Amino acid sequence of a phosphocholine-binding antibody from an immune defective CBA/N mouse employing the T15 VH region associated with unusual DH, JH, and V kappa segments. J. Immunol. 132:1544-1549. [PubMed] [Google Scholar]
- 16.Cripps, A. W., D. C. Otczyk, and J. M. Kyd. 2005. Bacterial otitis media: a vaccine preventable disease? Vaccine 23:2304-2310. [DOI] [PubMed] [Google Scholar]
- 17.Dagan, R. 2009. Serotype replacement in perspective. Vaccine 27(Suppl. 3):C22-C24. [DOI] [PubMed] [Google Scholar]
- 18.Desaymard, C., A. M. Giusti, and M. D. Scharff. 1984. Rat anti-T15 monoclonal antibodies with specificity for VH- and VH-VL epitopes. Mol. Immunol. 21:961-967. [DOI] [PubMed] [Google Scholar]
- 19.Diamond, B., and M. D. Scharff. 1984. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc. Natl. Acad. Sci. U. S. A. 81:5841-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekdahl, K., J. H. Braconier, and C. Svanborg. 1997. Impaired antibody response to pneumococcal capsular polysaccharides and phosphorylcholine in adult patients with a history of bacteremic pneumococcal infection. Clin. Infect. Dis. 25:654-660. [DOI] [PubMed] [Google Scholar]
- 21.Fabrizio, K., C. Manix, H. Tian, N. van Rooijen, and L. A. Pirofski. 2010. The efficacy of pneumococcal capsular polysaccharide-specific antibodies to serotype 3 Streptococcus pneumoniae requires macrophages. Vaccine 28:7542-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster, D., et al. 2008. Invasive pneumococcal disease: epidemiology in children and adults prior to implementation of the conjugate vaccine in the Oxfordshire region, England. J. Med. Microbiol. 57:480-487. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie, S. H., et al. 1993. Species of alpha-hemolytic streptococci possessing a C-polysaccharide phosphorylcholine-containing antigen. Infect. Immun. 61:3076-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giusti, A. M., N. C. Chien, D. J. Zack, S. U. Shin, and M. D. Scharff. 1987. Somatic diversification of S107 from an antiphosphocholine to an anti-DNA autoantibody is due to a single base change in its heavy chain variable region. Proc. Natl. Acad. Sci. U. S. A. 84:2926-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronlund, H., et al. 2009. Low levels of IgM antibodies against phosphorylcholine predict development of acute myocardial infarction in a population-based cohort from northern Sweden. Eur. J. Cardiovasc. Prev. Rehabil. 16:382-386. [DOI] [PubMed] [Google Scholar]
- 26.Guo, W. X., et al. 1997. Sequence changes at the V-D junction of the VH1 heavy chain of anti-phosphocholine antibodies alter binding to and protection against Streptococcus pneumoniae. Int. Immunol. 9:665-677. [DOI] [PubMed] [Google Scholar]
- 27.Haas, K. M., J. C. Poe, D. A. Steeber, and T. F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7-18. [DOI] [PubMed] [Google Scholar]
- 28.Hicks, L. A., et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346-1354. [DOI] [PubMed] [Google Scholar]
- 29.Jackson, L. A., et al. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 348:1747-1755. [DOI] [PubMed] [Google Scholar]
- 30.Jefferies, J. M., et al. 2007. Presence of nonhemolytic pneumolysin in serotypes of Streptococcus pneumoniae associated with disease outbreaks. J. Infect. Dis. 196:936-944. [DOI] [PubMed] [Google Scholar]
- 31.Jones, H. E., P. R. Taylor, E. McGreal, S. Zamze, and S. Y. Wong. 2009. The contribution of naturally occurring IgM antibodies, IgM cross-reactivity and complement dependency in murine humoral responses to pneumococcal capsular polysaccharides. Vaccine 27:5806-5815. [DOI] [PubMed] [Google Scholar]
- 32.Kirkham, P. M., F. Mortari, J. A. Newton, and H. W. Schroeder, Jr. 1992. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maitta, R. W., et al. 2004. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect. Immun. 72:4810-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maitta, R. W., K. Datta, A. Lees, S. S. Belouski, and L. A. Pirofski. 2004. Immunogenicity and efficacy of Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan peptide mimotope-protein conjugates in human immunoglobulin transgenic mice. Infect. Immun. 72:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks, M., et al. 2007. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect. Immun. 75:1586-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mykietiuk, A., et al. 2006. Effect of prior pneumococcal vaccination on clinical outcome of hospitalized adults with community-acquired pneumococcal pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 25:457-462. [DOI] [PubMed] [Google Scholar]
- 37.Nakouzi, A., and A. Casadevall. 2003. The function of conserved amino acids in or near the complementarity determining regions for related antibodies to Cryptococcus neoformans glucuronoxylomannan. Mol. Immunol. 40:351-361. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien, K. L., et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893-902. [DOI] [PubMed] [Google Scholar]
- 39.Rajam, G., et al. 2008. A functional epitope of the pneumococcal surface adhesin A activates nasopharyngeal cells and increases bacterial internalization. Microb. Pathog. 44:186-196. [DOI] [PubMed] [Google Scholar]
- 40.Retter, I., H. H. Althaus, R. Munch, and W. Muller. 2005. VBASE2, an integrative V gene database. Nucleic Acids Res. 33:D671-D674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 42.Roush, S. W., and T. V. Murphy. 2007. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298:2155-2163. [DOI] [PubMed] [Google Scholar]
- 43.Russell, N. D., J. R. Corvalan, M. L. Gallo, C. G. Davis, and L. Pirofski. 2000. Production of protective human antipneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect. Immun. 68:1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skovsted, I. C., et al. 2007. Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for adsorption in pneumococcal enzyme-linked immunosorbent assays. Vaccine 25:6490-6500. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen, U. B., R. Agger, J. Bennedsen, and J. Henrichsen. 1984. Phosphorylcholine determinants in six pneumococcal capsular polysaccharides detected by monoclonal antibody. Infect. Immun. 43:876-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spira, G., H. L. Aguila, and M. D. Scharff. 1988. T15 PC-binding monoclonal antibodies retain specificity when they switch from IgM to IgG. J. Immunol. 140:2675-2680. [PubMed] [Google Scholar]
- 47.Su, J., et al. 2006. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 188:160-166. [DOI] [PubMed] [Google Scholar]
- 48.Suresh, M. V., S. K. Singh, D. A. Ferguson, Jr., and A. Agrawal. 2007. Human C-reactive protein protects mice from Streptococcus pneumoniae infection without binding to pneumococcal C-polysaccharide. J. Immunol. 178:1158-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szu, S. C., S. Clarke, and J. B. Robbins. 1983. Protection against pneumococcal infection in mice conferred by phosphocholine-binding antibodies: specificity of the phosphocholine binding and relation to several types. Infect. Immun. 39:993-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka, N., et al. 2007. Intranasal immunization with phosphorylcholine induces antigen specific mucosal and systemic immune responses in mice. Vaccine 25:2680-2687. [DOI] [PubMed] [Google Scholar]
- 51.Tian, H., A. Groner, M. Boes, and L. A. Pirofski. 2007. Pneumococcal capsular polysaccharide vaccine-mediated protection against serotype 3 Streptococcus pneumoniae in immunodeficient mice. Infect. Immun. 75:1643-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian, H., S. Weber, P. Thorkildson, T. R. Kozel, and L. A. Pirofski. 2009. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 77:1502-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Dam, J. E., A. Fleer, and H. Snippe. 1990. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Van Leeuwenhoek 58:1-47. [DOI] [PubMed] [Google Scholar]
- 54.Vollmer, W., and A. Tomasz. 2001. Identification of the teichoic acid phosphorylcholine esterase in Streptococcus pneumoniae. Mol. Microbiol. 39:1610-1622. [DOI] [PubMed] [Google Scholar]
- 55.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.