Abstract
HIV/AIDS continue to devastate populations worldwide. Recent studies suggest that vaccines that induce beneficial immune responses in the mucosal compartment may improve the efficacy of HIV vaccines. Adenovirus serotype 5 (Ad5)-based vectors remain a promising platform for the development of effective vaccines. In an effort to improve the efficacy of Ad5-based vaccines, even in the presence of preexisting Ad5 immunity, we evaluated the potential for an Ad5-based HIV vaccine to induce antigen-specific immune responses following sublingual (s.l.) administration, a route not previously tested in regard to Ad-based vaccines. s.l. vaccination with an Ad5-based HIV-Gag vaccine resulted in a significant induction of Gag-specific cytotoxic T-lymphocyte (CTL) responses in both the systemic and the mucosal compartment. We also show that s.l. immunization not only avoided preexisting Ad5 immunity but also elicited a broad repertoire of antigen-specific CTL clones. Additionally, we confirm for the first time that oral delivery of a vaccine expressing a potent Toll-like receptor (TLR) agonist can stimulate innate immune responses through induction of cytokines and chemokines and activation of NK cells, NKT cells, and macrophages in vivo. These results positively correlated with improved antigen-specific CTL responses. These results could be achieved both in Ad5-naïve mice and in mice with preexisting immunity to Ad5. The simplicity of the s.l. vaccination regimen coupled with augmentation of TLR-dependent pathways active in the oral cavity makes s.l. delivery a promising method for HIV vaccine development specifically, as well as for many other vaccine applications in general.
As the number of worldwide cases of HIV/AIDS continues to rise, the generation of an effective HIV/AIDS vaccine remains a global priority. A recent clinical study using a canarypox viral vector expressing gp120, Gag, and Pol followed by boosting with a gp120 protein formulation showed some evidence of a protective effect; however, the data reached statistical significance in only one of three analyses, and the study was conducted using a cohort of low-risk volunteers with an annual incidence of infection of only ≈0.3% (25). While these results provide hope, more-sobering failures in other recent HIV vaccine clinical trials (in higher-risk populations) illustrate the continued need for development of more potent HIV/AIDS vaccination strategies that provide protection for individuals at both low and high risk for HIV transmission (7, 14, 23). The mucosal lining of the gastrointestinal and reproductive tissues is a primary area of HIV transmission, with the draining lymph nodes associated with these sites providing a reservoir of CD4+ CCR5+ cells susceptible to HIV infection and viral replication. The replication of HIV in mucosal sites results in the rapid systemic destruction of CD4+ T cells, an early marker of progressive HIV infection (18). The generation of a robust, HIV-specific cytotoxic T-lymphocyte (CTL) response in mucosal-compartment lymph nodes may preserve CD4+ T-cell populations at these sites and may provide important protection against viral replication, viremia, and/or lower viral loads in HIV-infected individuals (2, 6, 19).
Adenovirus serotype 5 (Ad5)-based vaccine vectors continue to be one of the most promising platforms for the development of newer classes of potent vaccines. For example, the induction of antigen-specific T-cell responses elicited by Ad5-based vaccines has been shown to surpass that generated by other virus- and nonvirus-based vaccine platforms, relative to the generation of systemic CTL responses to expressed antigens (including HIV-derived antigens), in both animal models and human clinical trials (8, 12). However, the presence of Ad5 neutralizing antibodies in some of the human populations residing in both developed and underdeveloped countries may be a limitation to the widespread use of Ad5-based vaccine platforms for HIV/AIDS prophylaxis (1, 22). Attempts to address this complication have included pseudotyping the Ad5 capsid with proteins derived from highly divergent (non-cross-reactive) human and nonhuman Ad serotypes, constructing altogether new Ad-based vectors derived from rare human and nonhuman Ad serotypes, and artificially masking the Ad5 capsid with chemical modifications to avoid neutralizing antibodies (these strategies and others are reviewed extensively in reference 26). Although promising, these strategies mitigate the advantages of the Ad5-based platform, such as an extensively proven safety record in numerous human clinical trials, confirmed biodistribution and safety profiles, and production capability compliant with current good manufacturing practices.
Altering the route of vaccination can significantly impact the induction of antigen-specific adaptive responses. For example, it has been shown that intranasal (i.n.), oral gavage (o.g.), and enteric vaccination strategies with Ad vectors can each induce antigen-specific systemic and mucosal responses and, in some instances, can circumvent preexisting Ad5 immunity (9, 17, 32). Another rarely reported route of oral vaccination is the sublingual (s.l.) route. Historically used as a strategy to induce tolerance to allergens, the sublingual mucosa has only recently been evaluated as a site that could be used for vaccine delivery (10, 11, 15, 27, 28). Importantly, it has been shown that s.l. administration of an adjuvanted, nonreplicating antigen vaccine can induce adaptive immune responses in mucosal tissues, such as the female genital tract, thereby providing protection against papillomavirus infection (10). In addition, a recent study reported that s.l. vaccination with an adjuvanted influenza vaccine induced immune responses that were sufficient to protect mice from lethal viral infection (28). Utilization of s.l. administration of Ad-based vectors has not been described previously.
In this study, we investigated the ability of a replication-incompetent [E1-]Ad serotype 5-based HIV-Gag (Ad5-HIV-Gag) vaccine to induce systemic and mucosal immune responses in both naïve and Ad5-immune mice when delivered using the s.l. route of vaccination. In addition, we sought to determine if coadministration of an Ad5-based vector expressing a potent Toll-like receptor (TLR) agonist could improve the induction of antigen-specific, adaptive immune responses. Our results confirm that s.l. delivery of Ad5-HIV-Gag induced antigen-specific CTL responses in the systemic and mucosal compartments of both naïve and Ad5-immune mice. Furthermore, we show that these HIV-specific responses can be improved upon by inclusion of a TLR agonist expressing an Ad5-based vector, validating the importance of TLR-mediated pathogen recognition systems present in the oral cavity.
MATERIALS AND METHODS
Vector construction.
The construction of Ad5-HIV-Gag, Ad5-GFP, and Ad5-GFP/rEA vectors has been described previously (3) (GFP is green fluorescent protein and rEA is recombinant Eimeria tenella antigen). Vectors were purified using CsCl centrifugation per the method described by Ng and Graham (21).
Animal procedures.
All animal procedures were approved by the Michigan State University Animal Care and Use Committee. Adult male C57BL/6 mice (7 to 10 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME). Ad-Null (an [E1-,E3-]Ad5 vector not expressing any antigen) (1 × 1010 virus particles [vps]) was injected intramuscularly (i.m.) in the right hind limb in a total of 20 μl phosphate-buffered saline (PBS) to generate animals with preexisting Ad5 immunity (Ad5-preimmune animals). The titers of Ad5-neutralizing antibodies were determined on 293 cells essentially as previously described (22). At 3 weeks postexposure, significant titers (>1:3,200) of total IgG specific for the Ad5 capsid were detected, confirming that these animals were in fact Ad5 immune (data not shown). Neutralizing antibody titers were evaluated and found to have an average of ≥1:200, and no significant difference in neutralizing antibody titers between s.l.- and o.g.-treated animals was detected prior to treatment (data not shown). The following day, mice were randomly separated into two groups and vaccinated by either the s.l. or the o.g. route of administration. Specifically, for s.l. delivery, mice were transiently anesthetized using inhaled isoflurane. Next, 1 × 1010 vps of Ad5-HIV-Gag was either diluted in PBS or mixed with equivalent particles of Ad5-GFP or Ad5-GFP/rEA to a total volume of 8 μl in PBS (pH 7.4) and slowly released under the tongue using a micropipette to minimize swallowing. Oral gavage vaccinations of 1 × 1010 vps of Ad5-HIV-Gag were diluted to a total of 100 μl in PBS (pH 7.4) and administered into the stomach using a 22-gauge, 1.5-in. reusable feeding needle with a 1.25-mm ball at the tip (Roboz Surgical Instrument Co., Gaithersburg, MD) attached to a tuberculin syringe (Becton Dickinson, Franklin Lakes, NJ). Animals were restricted from food for 8 h prior to oral gavage vaccination.
Isolation of SMLNs and splenocytes.
Splenocytes were prepared by physical disruption of spleen tissue and passage of the resulting cell suspension through a 40-μm sieve, followed by red blood cell (RBC) lysis and resuspension in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin-amphotericin B (Fungizone). Submandibular lymph node (SMLN) suspensions were prepared by dissection of the submandibular gland and isolation of mandibular lymph nodes, accessory mandibular lymph nodes, and the superficial parotid lymph node (31). The tissue was incubated for 1 h at 37°C with vortexing at 15-min intervals in collagenase-DNase containing RPMI 1640 supplemented with 10% FBS and penicillin-streptomycin-amphotericin B. Then, the tissue was passed through a 40-μm sieve, followed by RBC lysis and resuspension in complete RPMI medium.
ELISpot assays.
Enzyme-linked immunospot (ELISpot) assays were completed per manufacturer's protocol using a Ready-set Go gamma interferon (IFN-γ) and interleukin-2 (IL-2) mouse ELISpot kit purchased from eBioscience (San Diego, CA). Briefly, cells were plated at a density of 1 × 106 cells/well in a 96-well polyvinylidene difluoride (PVDF) microtiter plate. Gag-specific 15-mer peptides were obtained from the NIH AIDS Reagent and Reference Program (catalog no. 8117, lot no. 9). Complete RPMI 1640 medium containing peptide (2 μg/ml final concentration) was added to each well. Cells were incubated no less than 16 h. Plates were washed and developed per manufacturer's protocol. Resulting spot-forming cells (SFCs) were quantified using an automated ELISpot reader system (Cellular Technology, Cleveland, OH).
Flow cytometry and FACS analysis.
For NK, NKT, B-cell, and T-cell activation studies, splenocytes and SMLNs were initially incubated with CD16/32 FcRII/III antibody to minimize nonspecific binding. Cells were then stained with CD69-phycoerythrin (PE) (3 μg/ml), CD3-allophycocyanin (APC), NK1.1-PE-Cy7, CD8-Pacific blue, CD4-Alexa Fluor 700, and CD19-peridinin chlorophyll protein (PerCP)-Cy5.5. For macrophage and dendritic cell (DC) activation/maturation analyses, SMLN cells and splenocytes were initially incubated with CD16/32 FcRII/III antibody to minimize nonspecific binding, followed by staining with Cd11c-PE-Cy7, Cd11b-APC-Cy7, major histocompatibility complex class II (MHC-II)-Alexa Fluor 700, CD80-APC, and CD86-Pacific blue (all obtained from BD Biosciences, San Diego, CA). All antibodies were diluted in fluorescence-activated cell sorter (FACS) buffer (1% FBS and sodium azide). Cells were incubated on ice with the appropriate antibodies for 30 min, washed, sorted using an LSR II instrument, and analyzed using FlowJo software.
Cytokine and chemokine analysis.
A mouse multiplex-based assay including IL-6, IL-12(p40), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), keratinocyte-derived chemokine (KC), and RANTES was used per the manufacturer's instructions (Bio-Rad, Hercules, CA) to determine the indicated cytokine/chemokine plasma concentrations via Luminex 100 technology (Luminex, Austin, TX) as previously described (3).
RESULTS
Sublingual vaccination with Ad5-HIV-Gag induces Gag-specific cytotoxic T-cell responses in spleen and submandibular lymph nodes of Ad5-naïve mice.
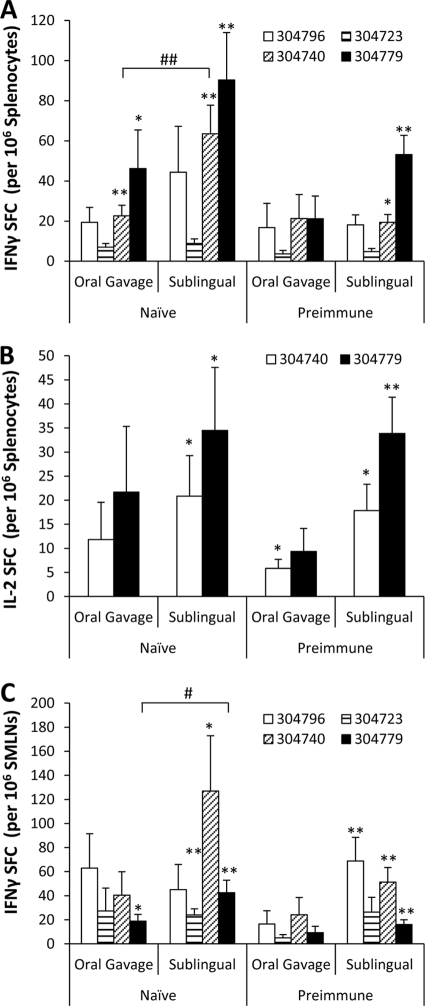
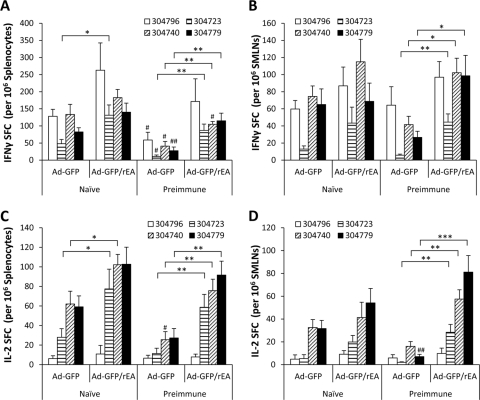
To test whether s.l. vaccination of mice with a conventional [E1-]Ad5-based vector was capable of inducing cell-mediated adaptive immune (CMI) responses, we vaccinated Ad5-naïve C57BL/6 mice s.l. with 1 × 1010 vps of an Ad5 vector expressing the HXB2 clade B HIV-Gag protein. In recent studies, the oral gavage (o.g.) method of vaccine delivery has been proposed as an alternative method to deliver Ad5-based vaccines in order to induce antigen-specific CMI responses in mucosal tissue. Therefore, a second cohort of mice vaccinated using the previously reported o.g. route of administration was also evaluated. At 14 days postvaccination, animals were terminally sacrificed and the number of antigen-specific T cells present in splenocyte and SMLN preparations (SMLN preparations included cells derived from mandibular lymph nodes, accessory mandibular lymph nodes, and the superficial parotid lymph node [31]) was determined using IFN-γ ELISpot analysis (Fig. 1). Cells derived from these tissues were either incubated in medium alone or exposed to immunodominant HIV-Gag-specific peptides representative of different epitopes within the HIV-Gag sequence (Table 1). In the spleens of Ad5-naïve animals, statistically significant inductions of HIV-Gag-specific T-cell responses were observed in both o.g.- and s.l.-vaccinated animals when splenocytes were incubated with peptides 304740 (P < 0.01) and 304779 (P < 0.05) (Fig. 1A). Moreover, we observed a significantly greater number of T cells recalled by peptide 304740 (P < 0.05) present in splenocytes harvested from s.l.-vaccinated mice than in those derived from mice treated by the o.g. route. Similar results were also observed when the numbers of antigen-specific T cells secreting IL-2 in the spleen were quantified (Fig. 1B). Specifically, whereas peptides 304796 and 304723 did not induce levels of IL-2-secreting T cells over that of unstimulated cells in any animal tested (data not shown), a significant induction of HIV-Gag-specific IL-2-secreting T cells was recalled from splenocytes harvested from Ad5-naïve s.l.-vaccinated animals, but not o.g.-treated animals, when incubated with peptides 304740 and 304779 (P < 0.05).
FIG. 1.
HIV-Gag-specific T-cell responses in naïve and Ad5-preimmune C57BL/6 mice following o.g. or s.l. administration of Ad5-HIV-Gag. Naïve or Ad5-preimmune C57BL/6 mice (n = 6 for all treatment groups) were immunized with 1 × 1010 vps of Ad5-HIV-Gag by either the s.l. or the o.g. route of delivery. (A) Fourteen days postvaccination, IFN-γ ELISpot analyses were used to quantify HIV-Gag-specific T-cell responses in splenocyte preparations. Cells were stimulated with 4 different peptides as indicated. Spot-forming cells (SFCs) are reported per 1 × 106 total cells plated per well. The induction of T-cell responses was considered significant if the average number of SFCs recalled with the indicated peptide was statistically higher than the number of SFCs from cells incubated in medium alone (no peptide), as determined using a homoscedastic two-tailed t test (*, P < 0.05; **, P < 0.01). In naïve mice, the average numbers of background SFCs derived from splenocytes incubated in medium alone were 2.8 and 4.3 for o.g. and s.l. preparations, respectively. In Ad5-preimmune mice, the average numbers of background SFCs derived from splenocytes incubated in medium alone were 2.8 and 4.8 for o.g. and s.l. preparations, respectively. Significant differences between o.g. and s.l. groups for one peptide (e.g., 304740 in naïve mice) are indicated (#, P < 0.05; ##, P < 0.01) and were calculated using one-way analysis of variance (ANOVA) followed by a Newman-Keuls post hoc test using data from all groups incubated with that peptide. Bars represent means ± standard errors (SE). (B) IL-2 ELISpot analyses were used to quantify HIV-Gag-specific T-cell responses in splenocyte preparations. The average numbers of background splenocyte SFCs from naïve mice were 0.5 and 1 for o.g.- and s.l.-treated mice, respectively. The average numbers of background splenocyte SFCs from Ad5-preimmune mice were 1.1 and 5 for o.g.- and s.l.-treated mice, respectively. (C) IFN-γ ELISpot analyses were used to quantify HIV-Gag-specific T-cell responses in SMLN preparations. Statistics were completed as described for panel A. In naïve mice, the average numbers of background SFCs derived from SMLN cells incubated in medium alone were 2.3 and 1.5 for o.g.- and s.l.-treated mice, respectively. In Ad5-preimmune mice, the average numbers of background SFCs derived from SMLN cells incubated in medium alone were 2.1 and 3 for o.g.- and s.l.-treated mice, respectively.
TABLE 1.
HIV-1 consensus subtype B Gag (15-mer) peptidesa
| QBI no. | Peptide sequence | Position (aa)b | p55 Gag region | NIH catalog no. | NIH lot no. |
|---|---|---|---|---|---|
| 304723 | EELRSLYNTVATLYC | 73-87 | p17 matrix protein | 7890 | 49859 |
| 304740 | QMVHQAISPRTLNAW | 141-155 | p24 core antigen capsid | 7907 | 49876 |
| 304779 | VDRFYKTLRAEQASQ | 297-311 | p24 core antigen capsid | 7946 | 49915 |
| 304796 | EAMSQVTNSATIMMQ | 365-379 | p2-p7 nucleocapsid | 7963 | 270207P1 |
Gag 15-mer peptides were provided by the NIH AIDS Reagent and Reference Program (catalog no. 8117, lot no. 9).
aa, amino acids.
In addition to the spleen, we measured mucosal T-cell responses in submandibular lymph node (SMLN) preparations derived from Ad5-naïve mice vaccinated by either the s.l. or the o.g. route of administration (Fig. 1C). In s.l.-vaccinated animals, a positive T-cell recall response was detectible when SMLNs were incubated with peptides 304723 (P < 0.01), 304740 (P < 0.05), and 304779 (P < 0.01). In contrast, only peptide 304779 (P < 0.05) was able to recall a significant HIV-Gag-specific IFN-γ T-cell response in SMLNs derived from o.g.-vaccinated mice. In addition, the quantity of T cells recalled by peptide 304779 was significantly higher in s.l.-treated mice than in mice vaccinated using the o.g. route (P < 0.05). No antigen-specific IL-2-secreting T cells were detected in SMLN preparations when stimulated ex vivo with any peptide tested.
Sublingual vaccination with Ad5-HIV-Gag induces Gag-specific cytotoxic T-cell responses in spleen and submandibular lymph nodes even in mice with preexisting immunity to Ad5.
One of the most notable limitations for Ad serotype 5-based vaccine strategies is that preexisting Ad5 immunity can significantly limit the effectiveness of vaccine vectors engineered using the Ad5 platform (26). It has been reported that o.g. vaccination with a conventional [E1-]Ad5-based vector can potentially avoid the lack of antigen-specific immune responses when Ad vaccines are administered to mice with preexisting Ad5 immunity (9, 32). For this reason, we sought to determine if s.l. vaccination could also induce CMI responses in Ad5-immune mice. To accomplish this, mice with preexisting immunity to Ad5 were vaccinated by either the o.g. or the s.l. route with the HIV-Gag-expressing Ad5 vaccine, and HIV-Gag-specific CMI responses were evaluated at 14 days postvaccination (Fig. 1). Importantly, a significant number of HIV-Gag-specific IFN-γ-expressing T cells was detected, but only in s.l.-vaccinated mice, and once again, these T cells responded only to epitopes 304740 (P < 0.05) and 304779 (P < 0.01) (Fig. 1A). Significant HIV-Gag-specific IFN-γ-expressing T cells were not recalled by any epitope tested in splenocytes derived from o.g.-vaccinated mice. In addition, we observed a significant induction of HIV-Gag-specific T cells secreting IL-2 in splenocyte preparations derived from o.g.- and s.l.-treated mice when incubated with peptide 304740 (P < 0.05) and in splenocytes from s.l.- but not o.g.-treated mice when incubated with peptide 304779 (P < 0.01) (Fig. 1B).
As a measure of CMI responses in mucosal tissue, we also measured HIV-Gag-specific T cells in SMLNs derived from s.l.- or o.g.-vaccinated mice with preexisting immunity to Ad5. Again, we did not detect a significant number of HIV-Gag-specific IFN-γ-secreting T cells in SMLNs derived from mice vaccinated using the o.g. route. In contrast, peptides 304796, 304740, and 304779 (P < 0.01) were able to recall HIV-Gag-specific CMI responses in SMLNs harvested from mice treated s.l. Together, these data indicate that compared to the o.g. route of administration, the s.l. vaccination strategy can induce equivalent, and many times improved, levels of HIV-Gag-specific CMI responses in both splenic and mucosal peripheral lymphoid tissues.
Neither s.l. nor o.g. vaccination of mice with Ad5-HIV-Gag was capable of inducing significant Ad5 capsid-specific CMI responses in peripheral lymphoid tissues.
Another limitation of using adenoviruses as vaccine vectors is the supposed inability to use the same vector as both priming and boosting vaccines, based on the generation of significant neutralizing antibody titers following the primary injection. Therefore, we also investigated whether s.l. and o.g. vaccination routes were capable of inducing Ad5 capsid-specific T cells in peripheral lymphoid tissues. Interestingly, no IFN-γ (Fig. 2 A)- or IL-2 (Fig. 2B)-producing T cells specific to Ad5 capsid proteins were observed in any SMLN preparation, inclusive of those derived from mice with preexisting immunity to Ad5, thus suggesting that a mucosal route of Ad5-based vaccine delivery may be ideal to avoid Ad5-specific T cells at the site of administration. As expected, we did observe a significant population of Ad5 capsid-specific T cells expressing IFN-γ and/or IL-2 in splenocyte preparations derived from animals with preexisting Ad5 immunity (P < 0.05), but not in Ad5-naïve mice. Importantly, we noted that although the induction was significant as a group, the numbers of Ad5-specific T cells in the spleens of individual Ad5-preimmune animals were variable. It is therefore possible that those animals with low levels of Ad5-specific T cells were the same animals with higher levels of HIV-Gag-specific T cells. To take this important observation into consideration, we completed correlation analyses (confidence interval of 95%) to determine if mice with the highest level of Ad5-specific T cells as measured by IFN-γ or IL-2 ELISpot assays correlated with low levels of Gag-specific T-cell responses in the same animals. No significant correlations were found.
FIG. 2.
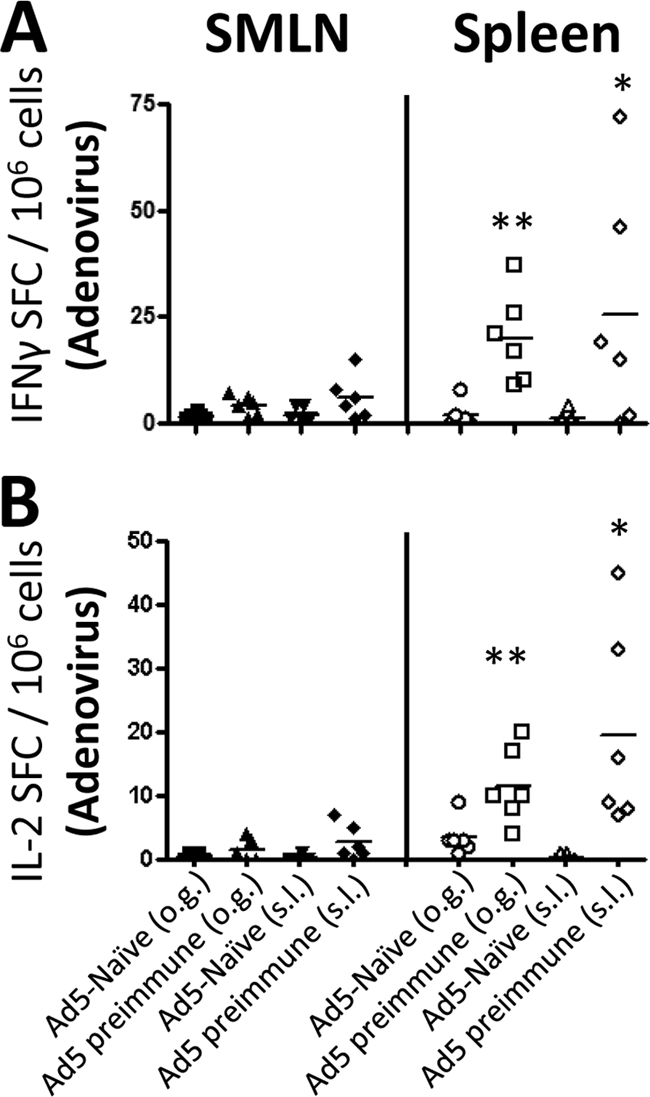
Ad5-specific T-cell responses in SMLNs and spleens of C57BL/6 mice with or without preexisting Ad5 immunity. Naïve or Ad5-preimmune C57BL/6 mice (n = 6 for both s.l. and o.g. treatment groups) were immunized with 1 × 1010 vps of Ad5-HIV-Gag by either the s.l. or the o.g. route of delivery. Fourteen days postvaccination, IFN-γ (A) and IL-2 (B) ELISpot analyses were used to quantify Ad5-specific T-cell responses in both SMLN and splenocyte preparations. Cells were stimulated with heat-inactivated Ad5 particles. Spot-forming cells (SFCs) are reported per 1 × 106 total cells plated per well. Each symbol represents one sample, and the horizontal line indicates the mean of the presented data. The induction of T-cell responses was considered significant if the average number of SFCs recalled with the indicated peptide was statistically higher than the number of SFCs derived from cells incubated in medium alone, as determined using a homoscedastic two-tailed t test (*, P < 0.05; **, P < 0.01). The average numbers of background SFCs are reported in the legend for Fig. 1.
Interestingly, we also found that both the spleen and the SMLNs of Ad5-naïve mice vaccinated with Ad5-HIV-Gag were devoid of Ad5 capsid-specific T cells 14 days after s.l. or o.g. vaccination (Fig. 2A and B). These data indicate that these routes of delivery do not induce heightened Ad5-specific T-cell responses at a time point that is typically regarded as the peak of these responses using alternate routes of vaccination (i.e., subcutaneous/intramuscular).
Sublingual administration of an Ad5-based vaccine expressing a TLR agonist stimulates innate immune cell populations in spleen and lymph nodes.
In addition to reporting its technical simplicity and repeated consistency, we have provided evidence that the s.l. route of vaccination is, at minimum, as effective as the o.g. route at inducing CMI responses in both naïve mice and mice with Ad5 immunity. For this reason, we focus all subsequent experiments in this study on testing the ability to further improve upon this s.l. delivered HIV-Gag antigen vaccine by using an Ad-based vector expressing a TLR agonist as a vaccine adjuvant.
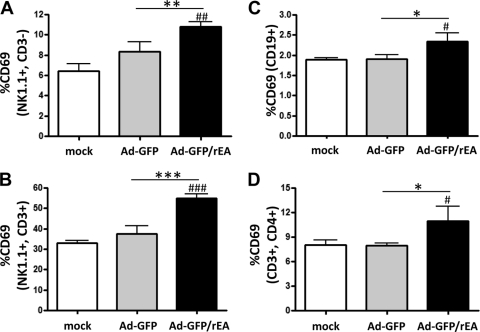
We have recently shown that Ad vaccines interact with the TLR arm of the innate immune system and that these interactions significantly contribute to the ability of Ad5 vaccines to elicit potent, antigen-specific adaptive immune responses (4, 5, 16). Capitalizing upon those findings, we have developed an Ad5-based vaccine platform that expresses a TLR agonist derived from E. tenella (i.e., recombinant Eimeria antigen [Ad5-GFP/rEA]). We have shown that vaccine cocktails containing the rEA-expressing Ad5 can induce heightened activation of innate immune responses compared to that found with controls when administered via the intravascular or intramuscular route (3). These responses include the induction of systemic cytokines and chemokines as well as increased activation/maturation of macrophage, DC, NK cell, and NKT cell populations in both the liver and the spleen. Therefore, we sought to determine if Ad5-GFP/rEA can promote increased induction of innate immune responses when delivered s.l. to the oral cavity. Animals were treated s.l. with 1 × 1010 vps of Ad5-HIV-Gag plus 1 × 1010 vps of either Ad5-GFP or Ad5-GFP/rEA (total inoculum of 2 × 1010 vps), and the activation of innate immune cell populations was determined 14 h posttreatment (Fig. 3). Using the surface expression of CD69 as a marker of activation, we observed a significant activation of NK cells (Fig. 3A; P < 0.01), NKT cells (Fig. 3B; P < 0.001), B cells (Fig. 3C; P < 0.05), and CD4+ T cells (Fig. 3D; P < 0.05) in the spleens of animals treated with Ad5-GFP/rEA compared to that for either mock-treated or Ad5-GFP-treated mice. No significant inductions of these cell types were observed in animals treated with the control Ad5-GFP virus. While NK cells were slightly activated by both Ad5-GFP and Ad5-GFP/rEA in SMLNs, no significant difference between treatment groups was observed (data not shown) (B cells, T cells, and NKT cells did not show significant inductions).
FIG. 3.
Ad5-GFP and Ad5-GFP/rEA induced NK, NKT, B-cell, and CD4+ T-cell activation in the spleen in vivo. C57BL/6 mice (n = 3 for all groups) were either mock treated or treated s.l. with 1 × 1010 vps of either Ad5-GFP or Ad5-GFP/rEA premixed with 1 × 1010 vps of Ad5-HIV-Gag. Lymphocytes from spleen tissue were harvested 14 h after s.l. treatment and stained for expression of surface markers specific to NK cells (A), NKT cells (B), B cells (C), or CD4+ T cells (D) and FACS sorted. Bars represent means ± SE. Data were analyzed using one-way ANOVA followed by a Newman-Keuls post hoc test. Significant differences from mock-injected mice are indicated by number symbols: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Significant differences between the indicated groups are shown by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
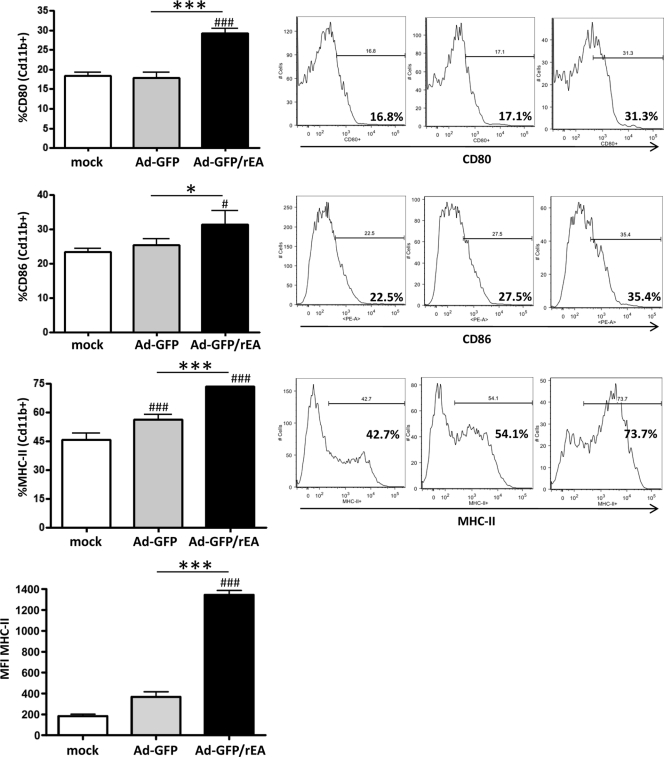
Antigen-presenting cells, such as macrophages and dendritic cells (DCs), are a critical component of the innate immune system and can directly affect the generation of robust adaptive immune responses by presenting antigens to T cells and providing secondary costimulatory signals (24). For this reason, we also evaluated the activation/maturation of these two cell types in the spleens of s.l.-vaccinated mice (Fig. 4). Unlike results for Cd11c+ DCs (data not shown), we found that the activation/maturation of Cd11b+ macrophages were significantly higher in Ad5-GFP/rEA-treated mice than in control animals. Specifically, the percentage of Cd11b+ macrophages expressing CD80 (P < 0.001) or CD86 (P < 0.05) was significantly higher than that for both mock-treated and Ad5-GFP-treated mice (Fig. 4). In addition, the percentage of cells expressing MHC-II was also significantly higher in the Ad5-GFP/rEA group, as was the amount of expression on a per-cell basis (as measured by the mean fluorescence intensity), indicating a potentially enhanced ability to present antigen to T cells.
FIG. 4.
Ad5-GFP and Ad5-GFP/rEA induced macrophage activation in spleens in vivo. C57BL/6 mice (n = 3 for all groups) were either mock treated or treated s.l. with 1 × 1010 vps of either Ad5-GFP or Ad5-GFP/rEA premixed with 1 × 1010 vps of Ad5-HIV-Gag. Lymphocytes from spleen tissue were harvested 14 h after s.l. treatment and stained for expression of surface markers specific to Cd11b+ macrophages and expression of CD80, CD86, and MHC-II. Bars represent means ± SE. Data were analyzed using one-way ANOVA followed by a Newman-Keuls post hoc test. Significant differences from mock-injected mice are indicated by number symbols: #, P < 0.05; ###, P < 0.001. Significant differences between the indicated groups are shown by asterisks: *, P < 0.05; ***, P < 0.001. Representative histograms are found at right. MFI, mean fluorescence intensity.
Sublingual coadministration of Ad5-HIV-Gag and Ad5-GFP/rEA results in heightened innate immune responses and improved antigen-specific T-cell responses in both Ad5-naïve and Ad5-preimmune mice.
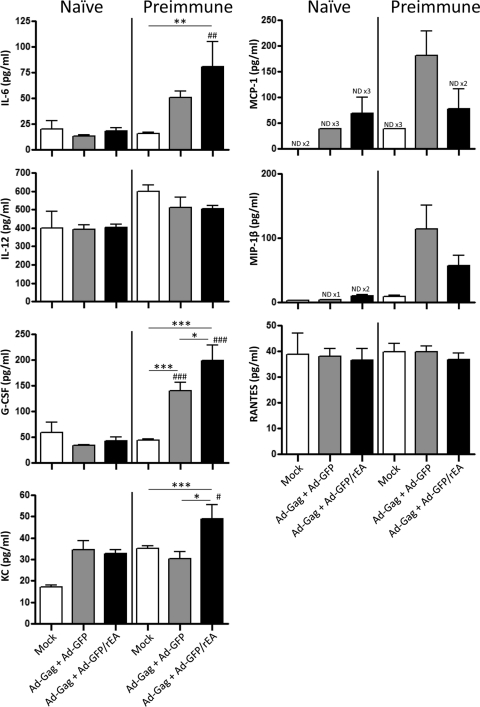
Our recent work has shown that inclusion of a TLR agonist in an Ad vaccine formulation can significantly improve adaptive immune responses toward a coadministered transgene (HIV-Gag) when the formulation is administered i.m. (3). Therefore, we sought to determine if oral TLR systems can also be harnessed to improve antigen-specific responses in naïve or Ad5-immune mice after s.l. vaccination with Ads. To accomplish this, naïve or Ad5-preimmune mice were coadministered Ad5-HIV-Gag premixed with equivalent amounts of either Ad5-GFP/rEA or the Ad5-GFP control virus (total of 2 × 1010 vps). As a primary measure of the effect of rEA expression on innate immune system activation in naïve and Ad5-immune animals, blood was drawn at 6 h after s.l. administration, and the presence of serum cytokines was tested (Fig. 5). No significant inductions in serum IL-6, IL-12(p40), granulocyte colony-stimulating factor (G-CSF), KC, MCP-1, MIP-1β, or RANTES were observed in treated, Ad5-naïve mice. In contrast, significant elevations of IL-6 (P < 0.01) and KC (P < 0.001) were detected in the serum of Ad5-immune mice compared to that of mock-treated mice when the Ad5-GFP/rEA-supplemented vaccine formulation was administered. Interestingly, we observed a significant induction of serum G-CSF levels (P < 0.001) in animals treated with the control Ad5-GFP plus Ad5-HIV-Gag formulation compared to those in identically injected naïve mice or mock-treated Ad5-immune animals. Moreover, significantly higher levels of this chemokine were detected in Ad5-preimmune animals treated with the Ad5-GFP/rEA-supplemented vaccine than in Ad5-preimmune mice treated with the control vaccine formulation (P < 0.05).
FIG. 5.
Heightened Ad5-GFP/rEA induced cytokine responses. Naïve or Ad5-preimmune C57BL/6 mice (n = 6 for all groups) were either mock treated or treated s.l. with 1 × 1010 vps of either Ad5-GFP or Ad5-GFP/rEA premixed with 1 × 1010 vps of Ad5-HIV-Gag. Plasma was harvested, and cytokines and chemokines were measured at 6 h postinfection. Bars represent means ± SE. Data were analyzed using one-way ANOVA followed by a Newman-Keuls post hoc test. Significant differences from identically treated naïve animals are indicated by number symbols: #, P < 0.05; ##, P < 0.01; ###, P < 0.001. Significant differences between the indicated groups are shown by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ND ×3, not detectable in 3 out of 6 samples.
To evaluate the primary, antigen-specific adaptive immune response induced after s.l. vaccination with rEA-expressing Ad vaccines, mice were terminally sacrificed and T-cell responses were measured in cells derived from SMLNs and spleens at 14 days after s.l. treatment (Fig. 6). Significant IFN-γ ELISpot assay responses were again detected in both splenocyte and SMLN preparations in both naïve mice and animals with preexisting Ad5 immunity (Fig. 6A and B). Moreover, in naïve mice, the inclusion of Ad5-GFP/rEA significantly improved HIV-Gag-specific splenic T-cell responses over those of control mice as measured by stimulation with Gag-specific peptide 304723 (P < 0.05) (Fig. 6A). A significant improvement was not observed in SMLN cells harvested from the same naïve animals (Fig. 6B). In contrast, we observed a significant improvement in T-cell responses in both splenocyte (Fig. 6A)- and SMLN (Fig. 6B)-derived T cells in Ad5-immune mice when Ad5-GFP/rEA was included in the vaccine formulation compared to those for mice treated with the control Ad5-GFP virus. This was particularly true for cells stimulated with peptides 304723 (P < 0.01), 304740 (P < 0.05), and 304779 (P < 0.05). A slight, yet significant, reduction in one HIV-Gag-specific T-cell clone within the splenocyte population, specific for the 304740 epitope (P < 0.05), was noted in mice with preexisting Ad5 immunity compared to the level in identically treated naïve mice in the Ad5-GFP/rEA-supplemented vaccine group. Significant decreases in HIV-Gag-specific CMI responses were noted for all epitopes in Ad5-preimmune mice treated with the control Ad5-GFP-supplemented Ad5-HIV-Gag vaccine formulation compared to responses in naïve animals, confirming that inclusion of rEA in Ad5-based vaccines can overcome reductions in antigen-specific CMI responses caused by the presence of preexisting Ad5 immunity (P < 0.05).
FIG. 6.
Increased HIV-Gag-specific CMI responses in naïve and Ad5-immune mice coadministered Ad5-HIV-Gag and Ad5-GFP/rEA. Naïve or Ad5-preimmune C57BL/6 mice (n = 6 for all groups) were either mock treated or treated s.l. with 1 × 1010 vps of either Ad5-GFP or Ad5-GFP/rEA premixed with 1 × 1010 vps of Ad5-HIV-Gag. Lymphocytes from spleen (A and C) and SMLN (B and D) tissue were harvested 14 days after s.l. treatment. IFN-γ and IL-2 ELISpot analyses were used to quantify HIV-Gag-specific T-cell responses in splenocyte and SMLN preparations. Cells were stimulated with 4 different peptides as indicated. SFCs in each well were background subtracted from the number in the appropriate unstimulated wells. SFCs are reported per 1 × 106 total cells plated per well. Bars represent means ± SE. Asterisks indicate a significant difference between Ad5-GFP- and Ad5-GFP/rEA-treated mice (*, P < 0.05; **, P < 0.01; ***, P < 0.001), as determined using a homoscedastic two-tailed t test. Number symbols indicate a significant reduction in levels for Ad5-preimmune mice compared to those for identically treated naïve mice, as determined by one-way ANOVA followed by a Newman-Keuls post hoc test, inclusive of all data obtained with stimulation by the indicated peptide (#, P < 0.05; ##, P < 0.01).
Additional experiments were also completed to evaluate HIV-Gag-specific IL-2-secreting antigen-specific T-cell responses in both the spleen (Fig. 6C) and the SMLNs (Fig. 6D) of the experimental and control mice. Similarly to the results described above, supplementing the Ad5-HIV-Gag vaccine with Ad5-GFP/rEA significantly improved the antigen-specific IL-2-secreting T-cell responses in both SMLNs and spleens compared to those for control animals. Specifically, the numbers of splenic T cells that recognize HIV-Gag peptides 304723 (P < 0.05) and 304740 (P < 0.05) were significantly increased in naïve mice treated with Ad5-HIV-Gag and Ad5-GFP/rEA relative to those for animals given control vaccinations. The same T-cell clones, with the addition of peptide 304779-specific T cells, were also found to be present in higher numbers in Ad5-GFP/rEA-treated Ad5-preimmune mice than in control vaccine-treated mice. A significantly increased expansion of these same HIV-Gag-specific T cells was also observed in SMLN preparations derived from Ad5-preimmune animals vaccinated with the Ad5-HIV-Gag- and Ad5-GFP/rEA-expressing vaccine cocktail. However, no improvement in CMI responses was observed in the SMLNs of naïve mice treated with the Ad5-GFP/rEA-supplemented vaccine formulation relative to those for identically treated control mice.
DISCUSSION
The vast majority of HIV infections are due to sexual transmission followed by viral penetration of the mucosal epithelium, either by disruption or trauma to the mucosal tissue or by a mechanism of transcytosis that is not completely understood (20). The reservoir of CD4+ CCR5+ T cells contained within mucosa-associated lymph nodes is especially susceptible to virus replication, facilitating dissemination and ultimately resulting in a rapid and systemic destruction of the CD4+ T-cell populations in lymphoid and nonlymphoid tissues (6). Therefore, an HIV vaccine capable of generating HIV antigen-specific CTL responses in mucosal sites could theoretically have a very good chance of minimizing the viremia and CD4+ T-cell destruction in HIV-infected individuals.
Mucosal vaccination strategies may improve the ability to generate adaptive immune responses in the mucosal compartment (6, 19). The oral cavity has been a site of application for vaccines in the past, and this route of immunization can facilitate the induction of immune responses in the mucosal compartments of the body. Wild-type Ads have been administered enterically to military recruits for over 30 years to facilitate the induction of respiratory mucosa immunity (13, 29, 30). Similarly, enteric administrations of Ad vaccines (by the oral gavage route [9, 32], introduction into the intestinal tract by use of enteric capsules [29, 30], or direct administration [17]) can facilitate the induction of mucosal adaptive immune responses to targeted antigens. Furthermore, oral gavage administration of Ads can allow for repeated vaccination, a benefit that limits the efficacy of Ad vaccines when administered via other routes, such as the intramuscular route (9).
The s.l. route of vaccination is another method to trigger mucosal immunity. For example, s.l. administration of a peptide and adjuvant combination has been shown to induce adaptive immune responses in the female genital tract (10). However, the utilization of s.l. administration of Ad-based vaccines has not been described previously. In this study, we evaluated the ability of an Ad5-based vaccine to stimulate innate and adaptive immune responses via an s.l. vaccination strategy to generate antigen-specific splenic and mucosal CTL responses. These outcomes were compared to responses generated in animals treated with the oral gavage method of vaccine delivery both in Ad5-naïve mice and in mice with preexisting Ad5 immunity.
The results of our work indicate that s.l. vaccination of mice with an Ad5-HIV-Gag vaccine elicits both systemic and mucosal CTL responses in Ad5-naïve mice and in mice with preexisting Ad5 immunity, inclusive of responses detected in T cells derived from both SMLNs and the spleen. Furthermore, antigen-specific CMI responses following s.l. administrations were consistently equivalent to, and in some cases more robust than, those measured in animals treated using the o.g. method. We also confirmed that these responses were directed against multiple epitopes within the Gag protein, indicating that the s.l. vaccination strategy promoted and expanded the breadth of HIV-Gag-responsive T-cell clones that could express IFN-γ and/or IL-2 in these tissues.
We also evaluated the anti-Ad5 T-cell responses in Ad5-naïve mice 14 days after s.l. treatment with Ad5-HIV-Gag. Interestingly, Ad5-specific T-cell responses were not detected in either SMLNs or spleen tissues at the 14-day time point. Therefore, using s.l. vaccination as a priming vaccination may foster the efficacy of boosting vaccinations using an Ad5-based vaccine platform.
Our work also confirms that TLR systems are active in the oral cavity and that these systems can be manipulated to foster improved induction of antigen-specific adaptive immune responses subsequent to s.l. vaccination. This was confirmed by our use of an Ad5 vaccine platform that expresses a potent TLR agonist (3). Specifically, we have recently shown that expression of the TLR agonist rEA from an Ad5-based vaccine can produce significantly heightened innate immune responses, which were TLR dependent. These responses were positively correlated with an improved induction of antigen-specific adaptive immune responses when Ad5-rEA vectors were coadministered with an Ad5-HIV-Gag vaccine administered i.m. (3). In the present study, we have now confirmed that incorporation of Ad5-rEA could also improve induction of innate and antigen-specific adaptive immune responses in the s.l. model of vaccination in both Ad5-naïve and Ad5-preimmune animals.
Several mechanisms may underlie how s.l. vaccination with Ads in general, and with the rEA-expressing Ad vaccine in particular, imparts improved efficacy. A recent report indicates that dendritic cells (DCs) are at least partially responsible for the effectiveness of a cholera toxin-adjuvanted sublingual vaccine (27). Therefore, Ad5 vaccine direct transduction and/or induced activation of antigen-presenting immune cells (such as DCs and macrophages) present in the sublingual mucosa may result in improved expression of MHC or costimulatory molecules in lymph nodes and/or spleens of s.l.-treated mice. Our results confirmed that NK cells, NKT cells, B cells, and T cells were all stimulated to significantly higher numbers in mice treated s.l. with the Ad5-rEA-expressing vaccine. More importantly, we also observed significantly higher numbers of Cd11b+ macrophages expressing the costimulatory surface proteins CD80 and CD86, as well as an increased fraction of macrophages that express higher levels of MHC-II, in the spleens of Ad5-rEA-treated mice. These data indicate that improvements in antigen presentation and costimulatory signaling may play a significant role in the observed improvements in antigen-specific T-cell responses when Ad5-rEA was included in the vaccine formulation. Interestingly, whereas plasma cytokines and chemokines were not detectably induced 6 h after s.l. treatment in either control or Ad5-GFP/rEA-treated naïve mice, we did detect significant inductions of IL-6, KC, and G-CSF in Ad5-GFP/rEA-treated mice with preexisting immunity to Ad5. These results may indicate that these cytokines and chemokines are particularly important for the improved responses afforded by Ad5-rEA in Ad5-immune animals. Of these factors, G-CSF may be especially important in the Ad5-specific memory response in mucosal tissues, as it was induced both by control Ad vaccines and by Ad5-rEA-expressing vaccines. In all, these results highlight the ability for s.l.-delivered Ad5-based vectors expressing rEA to induce heightened innate immune responses in both Ad5-naïve and Ad5-immune animals that positively correlate with significantly improved antigen-specific adaptive immune responses. These adaptive responses include the increased induction of antigen-specific T cells that secrete IFN-γ and IL-2 in both systemic and mucosal compartments.
In summary, we illustrate for the first time that a recombinant [E1-]Ad5-based HIV vaccine vector expressing HIV-Gag can induce antigen-specific CTL responses in both the systemic and the mucosal compartment following s.l. vaccination that are, at minimum, equivalent to responses induced following o.g. vaccinations. Furthermore, this study shows that the s.l. route of vaccination can elicit these responses both in Ad5-naïve animals and in animals with preexisting immunity to Ad5. Moreover, our results illustrate the ability of Ad vectors expressing a TLR agonist to not only induce heightened innate immune responses but also circumvent preexisting Ad5 immunity and improve the antigen-specific T-cell response in a sublingual model of vaccine delivery.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Abbink, P., et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlers, J. D., and I. M. Belyakov. 2010. Lessons learned from natural infection: focusing on the design of protective T cell vaccines for HIV/AIDS. Trends Immunol. 31:120-130. [DOI] [PubMed] [Google Scholar]
- 3.Appledorn, D. M., et al. 2010. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PloS One 5:e9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appledorn, D. M., S. Patial, S. Godbehere, N. Parameswaran, and A. Amalfitano. 2009. TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses. J. Innate Immun. 1:376-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appledorn, D. M., et al. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134-2144. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., and J. D. Ahlers. 2008. Functional CD8+ CTLs in mucosal sites and HIV infection: moving forward toward a mucosal AIDS vaccine. Trends Immunol. 29:574-585. [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder, S. P., et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casimiro, D. R., et al. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croyle, M. A., et al. 2008. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PloS One 3:e3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuburu, N., et al. 2009. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 183:7851-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuburu, N., et al. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598-8610. [DOI] [PubMed] [Google Scholar]
- 12.Draper, S. J., and J. L. Heeney. 2010. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 8:62-73. [DOI] [PubMed] [Google Scholar]
- 13.Dudding, B. A., et al. 1972. Enteric immunization with live adenovirus type 21 vaccine. I. Tests for safety, infectivity, immunogenicity, and potency in volunteers. Infect. Immun. 5:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn, N. M., et al. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 15.Frew, A. J. 2008. Sublingual immunotherapy. New Engl. J. Med. 358:2259-2264. [DOI] [PubMed] [Google Scholar]
- 16.Hartman, Z. C., et al. 2007. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 81:1796-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemiale, F., et al. 2007. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine 25:2074-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattapallil, J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 19.Mattapallil, J. J., and M. Roederer. 2008. Mucosa and vaccine-induced immune protection in nonhuman primates. Curr. Opin. HIV AIDS 3:387-392. [DOI] [PubMed] [Google Scholar]
- 20.McMichael, A. J., P. Borrow, G. D. Tomaras, N. Goonetilleke, and B. F. Haynes. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. 10:11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng, P., and F. L. Graham. 2002. Construction of first-generation adenoviral vectors. Methods Mol. Med. 69:389-414. [DOI] [PubMed] [Google Scholar]
- 22.Nwanegbo, E., et al. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitisuttithum, P., et al. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 25.Rerks-Ngarm, S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 26.Seregin, S. S., and A. Amalfitano. 2009. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin. Biol. Ther. 9:1521-1531. [DOI] [PubMed] [Google Scholar]
- 27.Song, J. H., et al. 2009. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J. Immunol. 182:6851-6860. [DOI] [PubMed] [Google Scholar]
- 28.Song, J. H., et al. 2008. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Top, F. H., Jr., B. A. Dudding, P. K. Russell, and E. L. Buescher. 1971. Control of respiratory disease in recruits with types 4 and 7 adenovirus vaccines. Am. J. Epidemiol. 94:142-146. [DOI] [PubMed] [Google Scholar]
- 30.Top, F. H., Jr., et al. 1971. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J. Infect. Dis. 124:148-154. [DOI] [PubMed] [Google Scholar]
- 31.Van den Broeck, W., A. Derore, and P. Simoens. 2006. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods 312:12-19. [DOI] [PubMed] [Google Scholar]
- 32.Xiang, Z. Q., et al. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77:10780-10789. [DOI] [PMC free article] [PubMed] [Google Scholar]