Abstract
The ability to utilize serum or plasma samples interchangeably is useful for tuberculosis (TB) serology. We demonstrate a strong correlation between antibody titers to several mycobacterial antigens in serum versus plasma from HIV-infected and non-HIV-infected TB and non-TB patients (r = 0.99 to 0.89; P < 0.0001). Plasma and serum can be used interchangeably in the same antibody detection assays.
An estimated 9.4 million new cases of active tuberculosis (TB) occur each year globally, and many are challenging to diagnose, especially those that occur in HIV-infected individuals (18). Much progress has been made in the field of TB serology in the past decade (10, 13, 15; reviewed in reference 16), and recent studies have indicated that serodiagnosis in the form of detection of antibodies (Abs) to immunodominant antigens of Mycobacterium tuberculosis could be a useful adjunct test to diagnose TB earlier (2, 3, 17). Traditionally, serum is used to test for Abs to mycobacterial antigens. Serum differs from plasma in that it does not contain fibrinogen and clotting factors. However, plasma, obtained from samples for routine clinical or research-related tests, is often leftover and sometimes stored, especially from HIV-infected individuals. It would be beneficial if serological studies could use serum and plasma, including stored samples, interchangeably.
Although one would expect similar levels of proteins detected in serum and in plasma, several studies suggest that blood sample preparation and storage conditions could have an influence on concentrations. For example, some proteins, such as beta-2-microglobulin or Plasmodium falciparum histidine-rich protein 2, have been detected in significantly lower concentrations in human plasma than in serum (5, 11), and while very high and significant correlations between plasma and serum levels were obtained for C-reactive protein or insulin (4, 7), no statistically significant correlation was found for cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) (7). It is also conceivable that storage of plasma samples, which can lead to precipitation of some proteins due to polymerization of fibrin, could result in Ab levels different from those found in serum.
Many Ab detection assays are licensed for the use of serum or plasma samples, but to our knowledge, few studies have correlated Ab titers to microbial antigens between simultaneously obtained serum and plasma samples. One study, using a commercial test, showed a very strong and statistically significant correlation between serum and plasma immunoglobulin G (IgG) Ab responses to a herpes simplex virus 2 glycoprotein (6). We are aware of only one study in the field of TB serology that evaluated results of a commercial serodiagnostic test (ICT Tuberculosis test; Amrad Corporation, Melbourne, Australia) in simultaneously obtained serum and plasma samples (9). This card-based test detects IgG Abs to 5 M. tuberculosis antigens in 4 lanes on a test strip which does not allow for the evaluation of Ab levels. Although sensitivities and specificities of the ICT Tuberculosis test for plasma and serum were similar, correlation of Ab titers between the different sample preparations was not possible. To our knowledge, no studies have compared the levels of Abs to mycobacterial antigens between simultaneously obtained serum and plasma. Therefore, the objective of our study was to correlate Ab titers to mycobacterial antigens between concurrently obtained serum and plasma and determine whether these samples could be used interchangeably in serologic assays.
Serum and plasma samples were obtained concurrently from 37 subjects and stored at −70°C until tested. Heparin was used as the anticoagulant to obtain plasma, and all tubes were centrifuged for 10 min at 2,500 rpm to separate cells from serum or plasma. The mean age of subjects was 44 ± 13 years, 26/37 (70%) were male, 10/37 (27%) had microbiologically proven TB, and 11/37 (30%) were known to be HIV infected. One subject was TB/HIV coinfected. Approval for research with human subjects was obtained from the institutional review boards of the New York University School of Medicine and the Albert Einstein College of Medicine, NY. Two recombinant proteins of M. tuberculosis, the 81-kDa malate synthase (MS; Rv1837c) and the 27-kDa protein MPT51 (Rv3803c), were expressed and purified as previously described (3), and the mycobacterial polysaccharide antigen arabinomannan (AM) was isolated and purified from the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine strain as previously described (14). These antigens were selected because of their immunogenicity. The two proteins MS and MPT51 have promising potential for the serodiagnosis of TB (reviewed in reference 16), and studies have indicated that Abs to AM could mediate protection against TB (1, 8). Enzyme-linked immunosorbent assays (ELISAs) were performed basically as previously described (3). Briefly, wells of 96-well microtiter plates (Immulon 2HB; Therma) were coated with either MS or MPT51 at 4 μg/ml or with AM at 50 μg/ml (50 μl/well). Serum and plasma samples, diluted at 1:50, were added in duplicates to the antigen-coated wells, and the bound Abs were detected with either protein A-alkaline phosphatase (1:1,000; Sigma) for detection of IgG or anti-human IgA-alkaline phosphatase (1:1,000; Sigma) followed by p-nitrophenyl phosphate substrate (60 min at 37°C). The optical densities (OD) were measured at 405 nm. Each plate contained corresponding serum and plasma samples that were placed in mirrored positions. Negative controls were processed as described above, except for the addition of serum. Each assay was repeated on two separate days. Prism software, version 5.02 (GraphPad Inc., CA), was used for statistical analysis. Because Ab responses to some antigens were not normally distributed, results for all serum and plasma Ab titers were compared using the nonparametric Spearman rank correlation test.
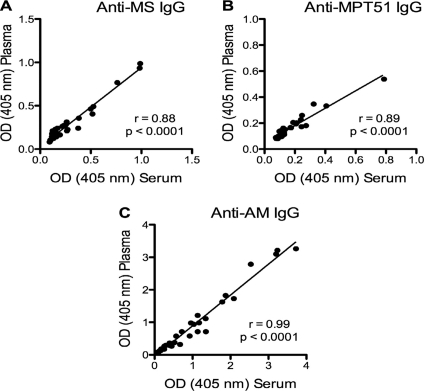
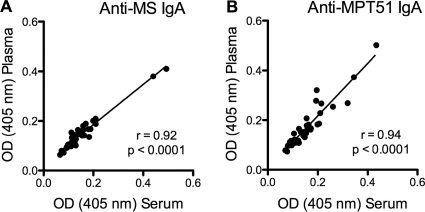
We found a very strong and highly statistically significant correlation between serum and plasma IgG Ab responses to both mycobacterial proteins (r = 0.88 and P < 0.0001 for MS; r = 0.89 and P < 0.0001 for MPT51) as well as to the mycobacterial polysaccharide AM (r = 0.99; P < 0.0001) (Fig. 1). Similarly, we found a very strong and highly statistically significant correlation between serum and plasma IgA Ab responses to both mycobacterial proteins (r = 0.92 and P < 0.0001 for MS; r = 0.94 and P < 0.0001 for MPT51) (Fig. 2). In subgroups categorized by HIV status, correlations between serum and plasma Ab responses were equally strong and statistically significant for both IgG and IgA regardless of the presence or absence of HIV infection (Table 1). Due to the limited amounts of AM available to us, we were not able to test for IgA Ab responses to this polysaccharide antigen.
FIG. 1.
Correlation graphs for the assays comparing serum and plasma IgG Ab titers to the three mycobacterial antigens malate synthase (MS), MPT51, and arabinomannan (AM). The Spearman rank correlation was used to test for statistical significance.
FIG. 2.
Correlation graphs for the assays comparing serum and plasma IgA Ab titers to malate synthase (MS) and MPT51. The Spearman rank correlation was used to test for statistical significance.
TABLE 1.
Correlation between serum and plasma IgG and IgA antibody (Ab) titers to mycobacterial antigens in subgroups stratified by HIV status
| Antigen and Ab isotype |
r value (P value) for correlationa between serum and plasma Ab titers |
|
|---|---|---|
| HIV+ (n = 11) | HIV− (n = 26) | |
| Anti-MS IgG | 0.88 (<0.001) | 0.87 (<0.0001) |
| Anti-MPT51 IgG | 0.89 (<0.001) | 0.89 (<0.0001) |
| Anti-AM IgG | 0.99 (<0.0001) | 0.97 (<0.0001) |
| Anti-MS IgA | 0.89 (<0.001) | 0.94 (<0.0001) |
| Anti-MPT51 IgA | 0.95 (<0.0001) | 0.94 (<0.0001) |
Spearman rank correlation.
This study demonstrates a very strong and highly significant correlation between serum and plasma Ab titers to different mycobacterial antigens in samples obtained from a variety of patients, including those with active TB and HIV infection. The very strong correlation was observed regardless of Ab isotype studied, protein or polysaccharide antigen tested, or HIV status of subjects. Thus, the polyclonal B cell response commonly seen in HIV-infected individuals (12) appeared to have no impact on the degree of correlation between serum and plasma Ab titers. Frozen storage seems to have negligible or no influence on the correlation between serum and plasma Ab titers. Also, the strong correlations did not appear to be different between lower and higher Ab levels. This is an important observation because prior studies concluded that the significant differences observed between serum and plasma concentrations for certain proteins, such as some cytokines or a plasmodial protein, could have been due in part to the overall low concentrations of these proteins (7, 11). Another study that reported significant differences in the detection of microglobulin levels between serum and plasma samples suggested that the differences observed could have been due to a nonspecific effect of heparin or citrate on the microglobulin because they were not observed between serum and EDTA plasma (5). Our study was limited by the lack of comparison between serum and EDTA or citrate plasma. However, most leftover plasma in clinical practice or research contains heparin, which appeared to have no impact on the strong correlation between serum and plasma Ab titers.
In conclusion, our results indicate that serum and plasma samples from both HIV-infected and non-HIV-infected subjects can be used interchangeably to test for Ab responses to mycobacterial antigens, even in the same assay. These findings can facilitate research in the field of TB serology.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NIH/NIAID) (AI-067665 to J.M.A. and AI-033774, AI-052733, HL-059842, and AI-033146 to A.C.), the Center for AIDS Research (AI-51519), the Macromolecular Therapeutic Development Facility at the Albert Einstein College of Medicine, the Clinical Translational Science Institute (NCRR 1UL1RR029893) at the New York University School of Medicine, the Howard Hughes Medical Institute, and the Aeras Global TB Vaccine Foundation. M.S. was supported by the Roth Scholarship of the Summer Undergraduate Research Program (SURP) of the Graduate Division of Biomedical Sciences, Albert Einstein College of Medicine.
We have no conflict of interest.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Achkar, J. M., A. Casadevall, and A. Glatman-Freedman. 2007. Immunological options for the treatment of tuberculosis: evaluation of novel therapeutic approaches. Expert Rev. Anti Infect. Ther. 5:461-474. [DOI] [PubMed] [Google Scholar]
- 2.Achkar, J. M., Y. Dong, R. S. Holzman, J. Belisle, I. S. Kourbeti, T. Sherpa, R. Condos, W. N. Rom, and S. Laal. 2006. Mycobacterium tuberculosis malate synthase- and MPT51-based serodiagnostic assay as an adjunct to rapid identification of pulmonary tuberculosis. Clin. Vaccine Immunol. 13:1291-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achkar, J. M., E. Jenny-Avital, X. Yu, S. Burger, E. Leibert, P. W. Bilder, S. C. Almo, A. Casadevall, and S. Laal. 2010. Antibodies against immunodominant antigens of Mycobacterium tuberculosis in subjects with suspected tuberculosis in the United States compared by HIV status. Clin. Vaccine Immunol. 17:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, L., B. Dinesen, P. N. Jorgensen, F. Poulsen, and M. E. Roder. 1993. Enzyme immunoassay for intact human insulin in serum or plasma. Clin. Chem. 39:578-582. [PubMed] [Google Scholar]
- 5.Bjerrum, O. W., and H. S. Birgens. 1986. Measurement of beta-2-microglobulin in serum and plasma by an enzyme-linked immunosorbent assay (ELISA). Clin. Chim. Acta 155:69-76. [DOI] [PubMed] [Google Scholar]
- 6.Cherpes, T. L., L. A. Meyn, and S. L. Hillier. 2003. Plasma versus serum for detection of herpes simplex virus type 2-specific immunoglobulin G antibodies with a glycoprotein G2-based enzyme immunoassay. J. Clin. Microbiol. 41:2758-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dossus, L., S. Becker, D. Achaintre, R. Kaaks, and S. Rinaldi. 2009. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J. Immunol. Methods 350:125-132. [DOI] [PubMed] [Google Scholar]
- 8.Glatman-Freedman, A., A. Casadevall, Z. Dai, W. R. Jacobs, Jr., A. Li, S. L. Morris, J. A. Navoa, S. Piperdi, J. B. Robbins, R. Schneerson, J. R. Schwebach, and M. Shapiro. 2004. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J. Clin. Microbiol. 42:3225-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gounder, C., F. C. Q. Mello, M. B. Conde, W. R. Bishai, A. L. Kritski, R. E. Chaisson, and S. E. Dorman. 2002. Field evaluation of a rapid immunochromatographic test for tuberculosis. J. Clin. Microbiol. 40:1989-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan, I. H., R. Ravindran, J. A. Yee, M. Ziman, D. M. Lewinsohn, M. L. Gennaro, J. L. A. Flynn, C. W. Goulding, K. DeRiemer, N. W. Lerche, and P. A. Luciw. 2008. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin. Vaccine Immunol. 15:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kifude, C. M., H. G. Rajasekariah, D. J. Sullivan, Jr., V. A. Stewart, E. Angov, S. K. Martin, C. L. Diggs, and J. N. Waitumbi. 2008. Enzyme-linked immunosorbent assay for detection of Plasmodium falciparum histidine-rich protein 2 in blood, plasma, and serum. Clin. Vaccine Immunol. 15:1012-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkrands, I., C. Aagaard, K. Weldingh, I. Brock, M. H. Dziegiel, M. Singh, S. Hoff, P. Ravn, and P. Andersen. 2008. Identification of Rv0222 from RD4 as a novel serodiagnostic target for tuberculosis. Tuberculosis (Edinb.) 88:335-343. [DOI] [PubMed] [Google Scholar]
- 14.Schwebach, J. R., A. Casadevall, R. Schneerson, Z. Dai, X. Wang, J. B. Robbins, and A. Glatman-Freedman. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 69:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh, K. K., N. Sharma, D. Vargas, Z. Liu, J. T. Belisle, V. Potharaju, A. Wanchu, D. Behera, and S. Laal. 2009. Peptides of a novel Mycobacterium tuberculosis-specific cell wall protein for immunodiagnosis of tuberculosis. J. Infect. Dis. 200:571-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steingart, K. R., N. Dendukuri, M. Henry, I. Schiller, P. Nahid, P. C. Hopewell, A. Ramsay, M. Pai, and S. Laal. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16:260-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanchu, A., Y. Dong, S. Sethi, V. P. Myneedu, A. Nadas, Z. Liu, J. Belisle, and S. Laal. 2008. Biomarkers for clinical and incipient tuberculosis: performance in a TB-endemic country. PLoS One 3:e2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. 2009. Global tuberculosis control: a short update to the 2009 report. World Health Organization, Geneva, Switzerland.