Abstract
Most strains of Neisseria gonorrhoeae carry the 57-kb gonococcal genetic island (GGI), as do a few strains of Neisseria meningitidis. The GGI is inserted into the chromosome at the dif site (difA) and is flanked by a partial repeat of the dif site (difB). Since dif is a sequence recognized by the site-specific recombinases XerC and XerD and the GGI shows evidence of horizontal acquisition, we hypothesized that the GGI may be acquired or lost by XerCD-mediated site-specific recombination. We show that while the GGI flanked by wild-type dif sites, difA and difB, is not readily lost from the gonococcal chromosome, the substitution of difB with another copy of difA allows the frequent excision and loss of the GGI. In mutants carrying two difA sites (difA+ difA+), the GGI can be detected as an extrachromosomal circle that exists transiently. A mutation of xerD diminished GGI excision from the chromosome of a difA+ difA+ strain, while mutations in recA or type IV secretion genes had no effect on the loss of the GGI. These data indicate that the GGI is maintained by the replication of the chromosome and that GGI excision and loss are dependent upon the dif sequence and xerD. The detection of a circular form of the GGI in a wild-type strain suggests that GGI excision may occur naturally and could function to facilitate GGI transfer. These data suggest a model of GGI excision and loss explaining the absence of the GGI from some gonococcal strains and the maintenance of variant GGIs in some gonococcal and meningococcal isolates.
Relative to the core genomic DNA of Neisseria gonorrhoeae (the gonococcus [Gc]), the gonococcal genetic island (GGI) is a genetic element of low G+C content, differing dinucleotide frequencies, and few DNA uptake sequences (15, 18), indicative of horizontal acquisition. Most strains of N. gonorrhoeae carry the 57-kb GGI, as do a few strains of Neisseria meningitidis (18, 38). The GGI encodes numerous homologues of type IV secretion system (T4SS) genes, the encoded products of which are necessary for DNA secretion and facilitate natural transformation (15, 18, 19, 25, 34). Several versions of the GGI exist, and certain versions of the island have been correlated with disseminated gonococcal infection (15, 19). Similar to the characteristic flanking repeats of many mobile elements (17), a duplicated sequence is present at the location of GGI insertion. The GGI of N. gonorrhoeae is inserted at the dif site difA and is flanked by an imperfect direct repeat, a degenerate dif site, difB. The dif site is a 28-bp recognition sequence for the site-specific recombinases XerC and XerD. In Escherichia coli, these tyrosine recombinases act to resolve chromosomal and plasmid dimers during replication (3). In some pathogens, as well as in E. coli, these enzymes can also act on dif in the excision and integration of nonself DNA, such as phages and plasmids (11, 20).
The dif site is present in the majority of sequenced prokaryotic genomes and is highly conserved among them (10). In most bacterial species, including N. gonorrhoeae and E. coli, the dif site consists of an 11-bp XerC binding site, followed by a 6-bp spacer region and an 11-bp XerD binding region (3-5, 10). Although the functions of N. gonorrhoeae XerC and XerD homologues have not yet been described, E. coli XerC and XerD have been characterized in great detail. In E. coli, the heterodimer comprising the site-specific recombinase XerCD recognizes the dif site located near the replication terminus and resolves chromosomal dimers. Such dimers arise from an odd number of recombination events between parent and newly synthesized chromosomes during DNA replication (7, 33). In resolving these dimers, the enzymes XerC and XerD recognize the 28-bp dif site, bind sequentially and cooperatively to the recognition site, and catalyze both cleavage and strand exchange in the resolution reaction (5-7). Although mutations in dif leading to the inability of E. coli to resolve chromosomal dimers are not lethal, chromosomal concatemers inhibit proper septation and give rise to filamentous cells in a portion of the population (2, 26). The advantage of chromosomal monomers for efficient septation likely contributes to the conservation of dif.
In addition to acting on chromosomal DNA, XerCD can also act on plasmids, phages, and other horizontally acquired DNA. In E. coli, XerCD binds and cleaves at dif-like sites to resolve plasmid multimers (3). These plasmid dif-like sites that XerC and XerD recognize in E. coli include cer, ckr, nmr, mwr, and psi (9, 14, 37, 40, 41). Research into several other systems has suggested an additional role for site-specific recombination: the chromosomal integration and excision of horizontally acquired DNA. The XerCD recombinases are responsible for the integration of CTXφ, the bacteriophage encoding cholera toxin, into the dif/attB site of Vibrio cholerae (20).
In gonococci, the presence of a conserved difA site and a degenerate difB site flanking the GGI along with evidence for horizontal acquisition and the absence of the GGI from some strains suggest that the GGI may be a mobile genetic element acquired or excised via XerCD site-specific recombination (15, 18). Because the integration as well as the excision of DNA can occur at the dif site, we have investigated a potential role for the site-specific recombinase XerCD. Previously, it was demonstrated that the GGI can be deleted from the chromosome by transformation and homologous recombination with DNA from a GGI-negative (GGI−) strain, thereby creating an isogenic ΔGGI mutant. Additionally, a nonreplicating plasmid containing dif and an erythromycin (Erm) resistance cassette can integrate into the isogenic ΔGGI mutant strain and can be readily excised from the chromosome. This “Erm island” exists transiently as an extrachromosomal entity and is eventually lost during cell division (18). Here we demonstrate that the excision and loss of the GGI occurs spontaneously, yet infrequently, in wild-type (WT) strains carrying both difA and difB sites. The frequencies of the loss of the GGI in xerD and recA mutants indicate that excision at dif is dependent upon XerCD site-specific recombination and independent of RecA homologous recombination, while the replacement of the degenerate difB with the conserved difA sequence allows the frequent excision and loss of the GGI. Additionally, we show that the type IV secretion system encoded by the GGI does not affect GGI loss and, therefore, that DNA secretion is not the means for excision and loss. Based on the data presented here combined with previously reported data, we conclude that the GGI is not a conjugative replicative plasmid, as suggested previously by others (38). Finally, we propose a model in which the GGI excises at a low frequency and transiently exists extrachromosomally, and without reincorporation into the chromosome, the GGI is lost upon replication and cell division.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial plasmids and strains used in this study are described in Table 1. Insertions for mutagenesis and cloning of xerD were constructed from N. gonorrhoeae MS11 (42). Gonococci were grown on GCB (Difco) agar plates containing Kellogg's supplements (23) in the presence of 5% CO2 or with aeration in GCB liquid (GCBL) medium containing Kellogg's supplements and 0.042% NaHCO3 (29). In the GGI loss assay, gonococci were plated onto GCB-Tris-XP (5-bromo-4-chloro-3-indolylphosphate) agar plates (8) containing 40 μg/ml XP with or without streptomycin. For gonococcal plating, the concentrations of streptomycin, chloramphenicol (Cm), and erythromycin used in agar plates were 100 μg/ml, 10 μg/ml, and 10 μg/ml, respectively. Kanamycin was used at a concentration of 80 μg/ml for the selection of the xerD insertion. Tetracycline was used at a concentration of 2 μg/ml for the selection of the recA insertion. E. coli strains were grown in Luria-Bertani (LB) broth or on LB agar plates. The concentration of erythromycin used for E. coli selection was 500 μg/ml, while kanamycin was used at a concentration of 40 μg/ml.
TABLE 1.
Strains and plasmids
| Plasmid or N. gonorrhoeae strain | Description | Reference |
|---|---|---|
| Plasmids | ||
| pIDN1 | Cloning vector (Ermr) | 19 |
| pIDN2 | Cloning vector (Ermr) | 19 |
| pAIB1 | GGI-flanking DNA near yheS introducing difA (497 bp) into pIDN2 (Ermr) | This work |
| pAIB2 | PCR-amplified GGI-flanking DNA near yheS (497 bp) in pIDN2 (difB Ermr) | This work |
| pARM3 | FA1090 difA and flanking DNA in pIDN2 | 18 |
| pHH18 | PCR-amplified ′traG′ of MS11 cloned into pKC1 | 18 |
| pHH23 | MS11 parA, dif, and flanking DNA cloned into pIDN2 | 18 |
| pHH35 | ΔtraH; 990-bp in-frame deletion generated in pJD1181 | This work |
| pJD1181 | traH′ region in pNH9.9 | 18 |
| pJD1197 | MS11 difB and 460-bp flanking DNA and 459-bp DNA internal to GGI near traD cloned into pIDN2 | This work |
| pJD1199 | PCR substitution of difA for difB and introduction of SmaI site into pJD1197 | This work |
| pKC1 | rpsL cloned into pIDN1 | 13 |
| pKH35 | Complementation vector (Cmr) | 18 |
| pKH93 | PCR-amplified xerD from MS11 cloned into pIDN2 | This work |
| pKH98 | 242-bp deletion of xerD by PCR, restriction digestion, and self-ligation of pKH93 | This work |
| pKH100 | Kanamycin marker inserted into xerD of pKH98 (Ermr Kanr) | This work |
| pNMD24 | xerD from pKH93 cloned into pKH35; xerD complementation | This work |
| pNMD26 | DNA flanking GGI of the parA side from ND500 cloned into pAIB2 (2-bp mutation in dif) | This work |
| pNMD31 | difB introduction and deletion of the GGI sequence by restriction digestion and self-ligation of pJD1199 | This work |
| pNMD34 | difA and flanking DNA from pNMD26 cloned into pNMD31 (difB) | This work |
| pNMD36 | Ligation of digested PCR amplification products from pJD1199, pNMD31, and pHH18; construct for introducing ermC rpsL cassette with difB adjacent to traD | This work |
| pNMD38 | Replacement of dif and flanking DNA from pNMD36 with digested PCR-amplified difA and flanking DNA of pAIB1; construct for introducing ermC rpsL cassette with difA adjacent to traD | This work |
| pNMD44 | 587-bp deletion of parA by PCR, restriction digestion, and self-ligation of pHH23 | This work |
| N. gonorrhoeae strain | ||
| MS11 | Wild-type N. gonorrhoeae | 42 |
| FA1090recA4 | tetM insertion in recA | 36 |
| HH522 | MS11 transformed with pCBB-1 (exp1::mTnCmPhoA) | 19 |
| HH549 | MS11 transformed with pHH35; traH deletion mutant | This work |
| KH570 | ND519 transformed with pKH100 (exp1::mTnCmPhoA xerD) | This work |
| ND500 | MS11 transformed with pARM3 (ΔGGI) | 19 |
| ND515 | MS11 transformed with HH522 (exp1::mTnCmPhoA) | This work |
| ND519 | ND515 transformed with pJD1199 (exp1::mTnCmPhoA difA+difA+) | This work |
| ND525 | ND519 transformed with FA1090recA4 (exp1::mTnCmPhoA difA+difA+recA) | This work |
| ND531 | KH570 transformed with pNMD24 (exp1::mTnCmPhoA difA+difA+xerD complemented) | This work |
| ND532 | ND500 transformed with pNMD26 (Erm island difA+difA A26T and A27C) | This work |
| ND538 | ND532 transformed with pAIB1 (Erm island difA+difA+) | This work |
| ND554 | ND515 transformed with pNMD36 (exp1::mTnCmPhoA difA+difB+) | This work |
| ND555 | ND500 transformed with pNMD34 (Erm island difA+difB+) | This work |
| ND558 | ND554 transformed with FA1090recA4 (exp1::mTnCmPhoA difA+difB+recA) | This work |
| ND560 | KH570 transformed with pNMD36 (exp1::mTnCmPhoA difB xerDrpsL) | This work |
| ND569 | ND519 transformed with pNMD38 (exp1::mTnCmPhoA difA+difA+rpsL) | This work |
| ND570 | ND569 transformed with FA1090 recA4 (exp1::mTnCmPhoAdifA+difA+rpsLrecA4) | This work |
| ND574 | HH549 (ΔtraH) transformed with ND515 (ΔtraHexp1::mTnCmPhoA difA+difA+) | This work |
| ND576 | ND574 transformed with pNMD38 (ΔtraHexp1::mTnCmPhoAdifA+difA+rpsL) | This work |
| ND593 | ND515 transformed with pNMD44 (parAexp1::mTnCmPhoA difA+difB+) | This work |
| ND596 | ND593 transformed with pNMD38 (parAexp1::mTnCmPhoA difA+difA+rpsL) | This work |
Determination of dif alleles.
The dif sequences were identified by using the Basic Local Alignment Search Tool (BLAST) from the NCBI and Broad Institute websites (1) (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi and http://www.broadinstitute.org/annotation/genome/neisseria_gonorrhoeae/MultiHome.html, respectively). Meningococcal dif regions were amplified by using primers 77F (5′-TAACAGCAGACGCTCCATTC-3′) and 138R (5′-GAGAGTGCAGGCATTTATCA-3′), 77F and 82R (5′-ATCGGTGACGAATTCATTCC-3′), and 73F (5′-AGCCATCAGGGAGGCGGATA-3′) and 41606R (5′-TGCCTACCACCACATCAAAG-3′). These amplified products were then sequenced by using 77F or 41606R and assigned a dif allele.
Mutation construction and transformation.
E. coli transformants were screened via a cracking gel using the lysis solution described previously by Kado and Liu (22). Plasmids were purified by using a QIAprep Spin miniprep kit (Qiagen) according to the manufacturer's instructions. All transformations of N. gonorrhoeae were performed by spot transformation according to a method described previously by Gunn and Stein (16). All strains used in the loss assays were in a background of a previously described PhoA+ strain (18). DNA used for PCR screening for transformants was generated by lysing isolated colonies as previously described by Wright et al. (44). Constructs and strains carrying difA or difB were confirmed by DNA sequencing.
The generation of difA (from difB) was accomplished by PCR amplification introducing a 4-bp change (C23T, A24T, T26A, and C27A) (see Fig. 2B). The recA mutation was introduced by transformation with chromosomal DNA of strain FA1090recA4 and selection for tetracycline resistance (36).
The xerD insertion mutation was created by the insertion of a kanamycin cassette into xerD. Transformants were selected for resistance to kanamycin. The complementation of xerD was accomplished by cloning xerD into complementation vector pKH35 (18) and transforming the resulting plasmid into xerD mutant KH570. Transformants were selected for chloramphenicol resistance and screened by PCR for the presence of xerD at the complementation site and the deletion of xerD in the native site.
Constructs for the GGI loss selection assay were created by cloning the streptomycin sensitivity marker rpsL, derived from N. gonorrhoeae strain F62, from pHH18 into constructs containing DNA flanking the left junction of the GGI and either difA or difB, generating pNMD38 and pNMD36, respectively. Specifically, pNMD36 was generated by PCR amplification of DNA flanking the left side of the GGI and dif from pNMD31 to reintroduce difB, and the internal GGI sequence between dif and traD was PCR amplified from pJD1199. These two fragments were digested and ligated together, altering the sequence such that the DNA farther from the GGI was ligated into the inverted GGI sequence. The ligation product was PCR amplified and cloned into the fragment of pHH18 carrying an E. coli origin of replication, the erythromycin marker, and rpsL. The generation of pNMD38 was accomplished by cloning difA and GGI flanking DNA from pAIB1 into pNMD36 and screening for the introduction of an additional MseI restriction site created by the presence of difA. The resulting constructs were transformed into recipient gonococcal strains. Transformants were selected for resistance to erythromycin, and dif sites were confirmed by DNA sequencing.
The traH deletion of pHH35 was generated by the restriction digestion of pJD1181 and the insertion of the 1.4-kb SspI-BsaWI fragment of traH from MS11, generating a 990-bp traH in-frame deletion. The partial deletion of ′parA was constructed by PCR amplification around pHH23, deleting 499 bp of the parA N-terminal coding sequence and an additional 88 bp upstream of parA, and then religation to generate pNMD44. These constructs were used to transform gonococci, and mutations were confirmed by PCR amplification.
GGI loss screen.
Bacterial strains were initially plated onto GCB agar plates and grown overnight. Nonpiliated colonies were restreaked onto GCB agar plates containing chloramphenicol to select for isolates carrying the GGI and grown overnight. Cultures containing 4 ml of GCBL medium with Kellogg's supplements (23) and 0.042% NaHCO3 were inoculated (optical density at 540 nm [OD540] of ∼0.2 to 0.4) with nonpiliated Gc strains by using a Dacron swab. Additionally, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to liquid cultures of the xerD-complemented strain. Strains were grown for 3 to 5 h and diluted to an OD540 of 0.18 to 0.33 in GCBL medium plus supplements and NaHCO3 (23). Cultures were grown for 18 to 20 h, and dilutions were plated onto GCB and GCB-Tris-XP containing 40 μg/ml XP (8). After 2 days of growth on GCB-Tris-XP, isolated colonies were restreaked onto GCB-Tris-XP. White colonies were patched onto GCB agar plates containing chloramphenicol.
PCR screen for the absence or presence of GGI.
PCR amplification for the presence and absence of the GGI from the chromosome was performed on lysates of isolated blue (PhoA+) and white (PhoA−) colonies by using primers 77F and 86R (5′-CAAGCGCATGGTACATGAAT-3′), 77F and 138R, and 73F and 41606R (melting temperature [Tm] 55°C; 60-s extension time; 30 cycles). For PCR detection of the GGI as an extrachromosomal entity, primers 98F (5′-GCGGCTGCAGACAATATTAC-3′) and dif-R (5′-GAGAGCCATTAGCCAGGAA-3′) were used (Tm 55°C; 30-s extension time; 40 cycles).
DNA isolation and Southern blotting.
Attempts to isolate the circular form of the GGI included DNA purification using a Qiagen DNeasy blood and tissue kit and a Qiagen large-construct kit according to the manufacturer's instructions. Plasmid Safe was added to purified DNA according to the manufacturer's recommendations (Epicentre). DNA was also isolated from cells embedded in 1% agarose plugs according to the manufacturer's recommendations (Bio-Rad). Southern blots to detect the extrachromosomal form of the GGI were performed according to standard procedures (35). Undigested and EcoRV-digested DNA preparations were separated by gel electrophoresis in a 0.8% agarose Tris-borate-EDTA (TBE) gel. The probe for Southern hybridization was the traD coding sequence generated by PCR amplification using primers 131F (5′-GGCGGTATTGATATTGGCAC-3′) and 86R.
Selection for GGI loss.
Piliated N. gonorrhoeae colonies were streaked onto GCB agar plates containing chloramphenicol and grown overnight. Four milliliters of GCBL medium containing Kellogg's supplements (23) and 0.042% NaHCO3 was inoculated with strains, grown for 4 to 6 h, and then diluted to an OD540 of 0.20 to 0.35. After overnight growth in liquid medium, strains were plated onto GCB and GCB-Tris-XP containing streptomycin. After 2 days of growth on GCB-Tris-XP with streptomycin, isolated colonies were restreaked onto GCB-Tris-XP. White colonies were patched onto GCB containing chloramphenicol.
RESULTS
Variations in dif among Neisseria strains.
We are seeking to understand the mechanisms of GGI acquisition and loss. The GGI is present in 80% of N. gonorrhoeae strains and some N. meningitidis strains (15, 38). The T4SS which it carries acts to secrete DNA that is active in natural transformation (15, 18, 19, 25, 34). Also, gonococci expressing the T4SS have an additional mechanism of iron acquisition during intracellular growth, indicating that the T4SS is expressed during infection and may affect infection processes (45). Therefore, knowledge of how the GGI is gained and lost may shed light on virulence differences between Neisseria strains and species and mechanisms of horizontal gene transfer in these highly variable species. Previous studies suggested that the dif sequences and the recombinase XerCD might be involved in GGI acquisition and loss (18).
Because (i) the GGI is flanked by dif as well as a degenerate dif, (ii) the GGI encodes genes for a T4SS with some similarities to the F-plasmid conjugation system (18), and (iii) plasmids with dif-like sites can integrate into the chromosome (26), we speculate that the GGI was once a conjugative plasmid that found residence in a neisserial chromosome via site-specific recombination at dif. Once integrated, mutations in dif may have accumulated, decreasing the frequency of GGI excision and leading to the preservation of the GGI within the chromosome. Previous sequence data for dif sites in both GGI+ and GGI− strains revealed not only that GGI+ strains of Neisseria have a second dif site but also that a largely conserved dif sequence exists among Neisseria strains (18). Because of the presence of two dif sites in GGI-carrying isolates, we have designated difA as the locus conserved among sequenced Neisseria genomes and difB as the degenerate second site that is found only in pathogenic species carrying the GGI (Fig. 1). In N. gonorrhoeae, the degenerate site difB, which flanks the side of the GGI nearest the majority of the T4SS genes, differs from difA by 4 bp in the XerD binding region (19) (Fig. 1And 2A).
FIG. 1.
Conservation of dif. Shown are sequence variations and alleles of dif among Neisseria species. difA is highly conserved with at most one base change, while difB differs from difA alleles by several base changes, all of which are localized to the XerD binding domain. Conserved bases are shown in uppercase boldface type, while nonconserved bases are shown in regular lowercase type. Abbreviations in parentheses indicate the Neisseria species (Gc, N. gonorrhoeae; Mc, N. meningitidis; La, N. lactamica) in which each allele is found. The regions where E. coli XerC and XerD bind dif (3) are indicated.
FIG. 2.
difA and difB sites in WT and mutant strains as well as resolution products. (A) Depiction of dif sites flanking the GGI in WT (difA+ difB+) and difA+ difA+ (2 copies of difA) strains. The region labeled tra contains 27 genes, most of which are required for the transfer of DNA via the gonococcal T4SS. (B) Sequence differences between difA1 and difB1. Arrows indicate base pair substitutions made at positions 23, 24, 26, and 27 in difB to create a second difA site and base pair substitutions made at positions 26 and 27 to create a difB carrying two of the changes found in difB1. (C) Recombination at dif to excise the GGI results in the maintenance of difA in the chromosome and re-creation of difB in the excised GGI, since recombination occurs in the spacer region and difB is identical to difA in the XerC binding domain. The conserved XerC binding domain is depicted as a black box, while the XerD binding region is shown as a white box for difA and a gray box for difB. The black rectangle connecting the XerC and XerD binding regions is the spacer region where recombination occurs.
In Neisseria, difA appears to be conserved, with four alleles of difA observed. The three alternate alleles (difA2 to difA4) differ from difA1 by a single-base-pair change (Fig. 1). Multiple alleles of neisserial difB also exist. However, difB differs from difA by a minimum of 3 bp changes, and the variation between difB alleles is more extensive. Sequence variation in difB is found primarily within the XerD binding site (Fig. 1).
Using DNA sequencing and comparisons of published genome sequences, we determined the dif alleles for 28 gonococcal strains and 12 meningococcal strains (Table 2). These data show a largely conserved difA sequence among Neisseria species. The greater variation and consistency of variation within the XerD binding domain in difB of these isolates reveal a means by which the GGI could become stabilized within the genome, i.e., by diminishing XerD activity at difB. It is interesting that since XerCD-mediated recombination at dif sequences results in the cleavage and recombination of DNA in the dif spacer regions between the XerC- and XerD-bound regions (5), the resolution of the GGI will result in the maintenance of a difA in the chromosome and create a difB in the resolved circle (Fig. 2C). Therefore, increased variation in the XerD binding region of difB may be favored because it increases GGI maintenance but still allows the re-creation of a consensus difA site for bacteria that excise the GGI. However, the occurrence of sequence variation in the XerD binding region of difB instead of the XerC binding region of difA may indicate a more important role for XerD in GGI excision.
TABLE 2.
dif allelic composition of gonococcal and meningococcal strainsa
| Strain | Presence of GGI | difA allele | difB allele |
|---|---|---|---|
| DGI18b,c | − | A1 | |
| FA6140b | − | A1 | |
| 1291b,d | − | A1 | |
| PID24-1b | − | A1 | |
| 35/02b | − | A1 | |
| SK-92-679b,c | − | A1 | |
| FA1090c,d,g | − | A1 | |
| FA19b,c,d | + | A1 | B1 |
| PID332b,c | + | A1 | B1 |
| PID18b,c | + | A1 | B1 |
| SK-93-1035b,c | + | A1 | B1 |
| MS11b,c | + | A1 | B1 |
| NCCP11945g | + | A1 | B1 |
| IN522c,f | + | A1 | B1 |
| DGI14c | + | A1 | B1 |
| DGI20c | + | A1 | B1 |
| PID1b,c | + | A2 | B1 |
| DGI2b,c | + | A2 | B1 |
| PID2075c,d | + | A1 | B1 |
| RUN5290c,d | + | A1 | B1 |
| IN113831c,d | + | A1 | B1 |
| JC1c,d | + | A1 | B1 |
| IN644c,d | + | A1 | B1 |
| RUN5287c,d | + | A1 | B1 |
| LT37971c,d | + | A1 | B1 |
| PID2004c,d | + | A1 | B1 |
| LT38089c,d | + | A1 | B1 |
| LT38093c,d | + | A1 | B1 |
| MC58g (Mc) | − | A1 | |
| FAM18g (Mc) | − | A1 | |
| 053442g (Mc) | − | A1 | |
| Alpha14g (Mc) | − | A1 | |
| 8013g (Mc) | − | A3 | |
| Z2491g (Mc) | − | A3 | |
| A22e,f (Mc) | + | A3 | B2 |
| 98/250521e,f (Mc) | + | A3 | B2 |
| Alpha275g (Mc) | + | A3 | B2 |
| 01/241471e,f (Mc) | + | A3 | B3 |
| 97/252675e,f (Mc) | + | A3 | B3 |
| 00/240868e,f (Mc) | + | A3 | B3 |
All meningococcal strains are indicated (Mc); all others are gonococcal strains.
Presence or absence of GGI and/or dif site alleles determined by genome sequences provided by the Broad Institute (http://www.broadinstitute.org/annotation/genome/neisseria_gonorrhoeae/MultiHome.html).
Presence or absence of the GGI previously reported by Dillard and Seifert (15).
Presence or absence of GGI and/or dif site alleles previously reported by Hamilton et al. (18).
Presence or absence of the GGI previously reported by Snyder et al. (38).
dif sites sequenced for this work.
Presence or absence of GGI and dif sequences determined by genome sequences reported in the GenBank database.
Sequence variation at dif affects the loss of the GGI and the loss of the Erm island.
Previous studies using a 2-kb DNA fragment carrying an erythromycin marker and flanked by two identical difA sites, designated the Erm island, showed that this engineered island can excise from the gonococcal chromosome and is highly unstable (18). The frequent loss of this Erm island and the conservation of dif among Neisseria species implicate difA as being the functional dif site for recombination in gonococci. To determine whether the difB sequence affects the excision of inserted DNA, N. gonorrhoeae strains that carried an Erm island flanked by either two difA sites or difA and difB were constructed. Also, a strain that carried the Erm island with 2 mutations in difB (A26T and A27C) relative to the dif consensus sequence was constructed (Fig. 2B). Screening for the loss of the Erm island showed that the dif sequence had a substantial effect on the loss frequency. After overnight growth, 31.7% (±8.3%) of strains carrying two difA sites lost the Erm island and were erythromycin sensitive. In contrast, mutation of 2 bp (A26T and A27C) in difB relative to difA reduced the Erm island loss to 1.7% (±1.5%), and the strain carrying difA and difB lost the Erm island at a frequency of 0.17% (±0.29%). Loss frequency values for these strains were significantly different from that of the difA+ difA+ strain (P < 0.05). These data indicate that sequence variation at difB affects the excision of this engineered island and that sequence changes found in difB in the gonococcal chromosome stabilize DNA inserts at this location.
To determine whether it is plausible for the entire 57-kb GGI to be excised and lost via recombination at dif, we constructed a strain carrying two identical copies of difA (difA+ difA+) flanking the GGI in the background of a previously described gonococcal strain, a derivative of strain MS11 carrying a GGI-harbored phoA fusion and a chloramphenicol resistance marker (Fig. 2A). Previous efforts to enumerate the frequency of spontaneous loss of the GGI in a strain carrying one difA copy and one difB copy (WT) revealed the GGI loss frequency to be less than 1 × 10−4 (18). All strains were grown overnight in liquid medium and plated onto medium containing XP, the PhoA indicator substrate. In this loss assay, strains that maintain the GGI and the productive PhoA fusion formed blue colonies, while strains that have lost the GGI would appear white. After overnight growth without antibiotic selection, no white colonies were observed on indicator plates inoculated with the WT strain (Table 3), confirming the stability of the GGI in MS11. Numerous white colonies grew on indicator plates inoculated with the difA+ difA+ strain, indicating a high frequency of loss of the GGI. The loss of the GGI, when flanked by duplicate difA sites, occurs at a frequency of 2.33 × 10−3, much more frequently than when flanked by difA and difB yet 100-fold less frequently than the loss of the Erm island. Since the loss of the GGI in the WT background (difA+ difB+) occurs less frequently than does loss in the difA+ difA+ background, these data suggest that the differences in difB1 from difA1 stabilize the GGI within the chromosome.
TABLE 3.
Loss of the GGI is dependent upon xerD and dif but independent of recA
| Strain | Avg loss frequencya | Confidence interval (95%)c |
|---|---|---|
| WT (ND515 difA+difB+) | <2.00 × 10−4b | NA |
| difA+difA+ (ND519) | 2.33 × 10−3 | 8.70 × 10−4, 6.21 × 10−3 |
| difA+difA+recA (ND525) | 8.31 × 10−4 | 5.19 × 10−4, 1.3 3 × 10−3 |
| difA+difA+xerD (KH570) | <2.00 × 10−4b | NA |
| difA+difA+xerDxerD+ (ND531) | 2.17 × 10−3 | 9.21 × 10−4, 5.13 × 10−3 |
Calculated as the geometric mean.
No white colonies were observed. The value is expressed as the limit of detection.
Calculated confidence intervals indicate that the loss of the GGI in the corresponding strain is not statistically significantly different from that of the difA+ difA+ strain. NA, not applicable.
The loss of the GGI is independent of recA but dependent upon xerD and dif sequences.
Since homologous recombination is a plausible mechanism for the excision of the GGI at dif, we next examined the requirement for RecA in the loss of the GGI. From the difA+ difA+ PhoA fusion strain, a recA mutant strain was constructed by the insertion of a tetracycline resistance marker into recA (36), and this strain was tested for the frequency of loss of the GGI. The recA mutant lost the GGI at a frequency comparable to that of its parent difA+ difA+ strain, with a frequency of 8.31 × 10−4, suggesting that the excision of the GGI is not due to homologous recombination between difA sites (Table 3). Since chromosomal dimers would not be expected to form in recA mutants, the loss of the GGI in the recA mutant also indicates that chromosomal dimers are not necessary for the activation of XerCD site-specific recombination, as was observed for E. coli (32, 39).
Since XerC and XerD recombinases act upon dif and dif-like sites in E. coli (3, 14), we examined the role of XerCD in the loss of the GGI. To determine whether or not site-specific recombination could be a means to excise the GGI, we constructed a gonococcal xerD mutant strain by inserting a kanamycin cassette into xerD. Examination of the xerD mutant by Gram staining and light microscopy showed no gross differences in cell morphology. However, the xerD mutant did show slightly more cells in aggregates of 4 or more cells than the wild-type strain, suggesting that in a portion of the population, an odd number of homologous recombination events occurs, and the xerD mutant is unable to resolve them. This situation is similar to filamentation occurring in xerD mutants of E. coli (27). N. gonorrhoeae does not filament, since it divides in alternating planes (43). We tested the xerD strain for the frequency of loss of the GGI. Unlike the high frequency of loss observed for the recA mutant, there was no detectable GGI loss observed for the xerD mutant (Table 3). Complementation of xerD restored the frequency of loss to that of the difA+ difA+ strain. The stabilization of the GGI in the xerD mutant and the instability of the GGI in the complemented strain indicate that xerD is necessary for the excision of the GGI.
To confirm that white (PhoA−) colonies arose from loss of the GGI rather than from inactive PhoA, white colonies were tested for the presence or absence of the GGI within the chromosome by PCR amplification (Fig. 3). DNA from lysates of white colonies was PCR amplified with primers spanning the left junction of the GGI (Fig. 3A). The presence of a product indicates the presence of the GGI in the chromosome, as shown by the lane containing difA+ difA+ chromosomal DNA (Fig. 3B), while no product indicates the absence of the GGI from the chromosome, as shown by the lane containing chromosomal DNA from the previously described GGI deletion strain ND500 (18). The absence of a PCR product (77F and 86R) from the white isolates indicates that they no longer carry the GGI within the chromosome. These white isolates were confirmed to be Cms. Since a chloramphenicol marker is adjacent to the PhoA fusion within the GGI of the parent strain, the Cm sensitivity further indicates that white colonies from the difA+ difA+, recA, and xerD complementation strains are in fact GGI−. Together, the PCR data and the chloramphenicol sensitivity confirm the absence of the GGI in white colonies and validate the GGI loss assay.
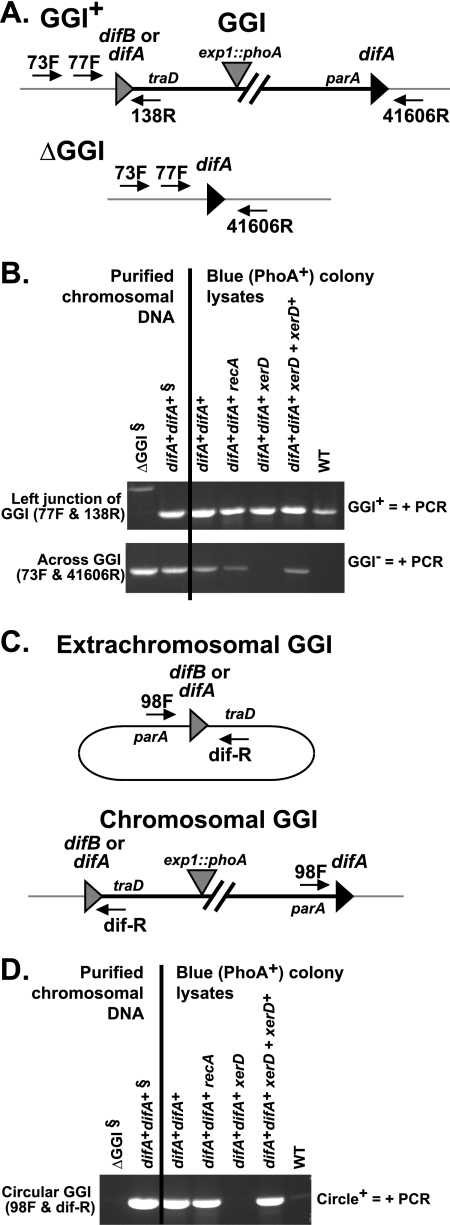
FIG. 3.
Confirmation of GGI loss. (A) Maps of dif loci in strains with or without the GGI showing the locations where oligonucleotide primers 73F, 77F, 86R, and 41606R bind. For all strains shown here, the difB1 site normally present adjacent to traD has been changed to a difA1 site. (B) PCR amplification products confirming the loss of the GGI. ΔGGI chromosomal DNA is the control for the absence of the GGI, while difA+ difA+ is the control for the presence of the GGI. § indicates that chromosomal DNA purified from a population of bacteria was used as a template for the amplification.
These data taken together illustrate that the loss of the GGI at dif is dependent upon XerD activity; however, the loss is neither mediated by RecA nor dependent upon RecA action for XerCD-mediated recombination. These data also indicate that the mechanism by which the GGI can be excised and lost is via site-specific recombination rather than homologous recombination. Furthermore, we can also conclude that the GGI in WT MS11 is stabilized by difB1.
GGI excision is detectable in difA+ difA+ but not in WT or xerD mutant strains.
Because difA+ difA+ strains readily lose the GGI, we hypothesized that excision should be detectable prior to the loss of the GGI. To detect GGI excision from the chromosome, we attempted PCR across the junction of the GGI in the difA+ difA+ strain using primers that hybridize to DNA flanking the GGI, i.e., 73F and 41606R (Fig. 3A). The presence of a product from the PCR amplification across dif indicates excision, as demonstrated by bands in the difA+ difA+ strains (Fig. 3B). Because the chromosomal DNA was prepared from Cmr difA+ difA+ colonies, the GGI should be present in all cells from which the DNA was extracted. The presence of a band in the amplification with 77F and 86R confirms the presence of the GGI in the chromosome of the Cmr difA+ difA+ strain. Since amplification from the Cmr difA+ difA+ strain yields a product for each reaction, it can be concluded that the GGI in the difA+ difA+ strain is excising from the chromosome at a frequency detectable by PCR. This result further supports the high frequency of loss in difA+ difA+ strains observed with the loss assay.
As we were able to detect excision from the difA+ difA+ Cmr strain by PCR, we investigated whether or not we could detect the excision of the GGI from the chromosome in a strain with wild-type dif sites (difA+ difB+) and other strains tested in the loss assay. DNA from lysates of blue (PhoA+) colonies was PCR amplified by using primers that would specifically amplify DNA from the left junction of the GGI near traD to nearby GGI-flanking DNA (77F and 138R) as well as across dif from one flanking side to the other (73F and 41606R) (Fig. 4A). As predicted, all blue colonies yielded a PCR product of the expected size when the junction was amplified (77F and 138R), indicating that the GGI is indeed located within the chromosome (Fig. 4B). However, difA+ difA+ strains carrying a functional copy of xerD also generated a product when amplified with primers located outside the GGI, indicating the presence of a difA locus with the GGI deleted (Fig. 4B). Since excision from the chromosome leads to a loss of the GGI and a white colony, a likely mechanism for a colony that is PCR positive for the excision of the GGI to remain blue (PhoA+) and Cmr is by carrying the GGI extrachromosomally. These data taken together suggest that within the population, some difA+ difA+ cells carry the GGI within the chromosome, while other cells have excised the GGI from the chromosome and carry the GGI extrachromosomally.
FIG. 4.
Identification of GGI location in blue (PhoA+) colonies of XP assay strains. (A) Maps of dif loci in strains with or without the GGI showing the locations where oligonucleotide primers 73F, 77F, 138R, and 41606R bind. For most strains shown here, the difB1 site normally present adjacent to traD has been changed to a difA1 site. The strain designated the wild-type strain (WT) is ND515, and it carries the exp1::mTnCmPhoA fusion but is wild type for dif loci; i.e., it has difB1 adjacent to traD. (B) PCR amplification products for examining the presence or absence of the GGI in the chromosome. PCR amplification across the left junction confirms the chromosomal presence of GGI, while GGI excision from the chromosome is detected by PCR amplification across dif. (C) Maps of dif loci in an extrachromosomal GGI and in the chromosomal GGI showing the locations where oligonucleotide primers 98F and dif-R bind. (D) Detection of GGI as an extrachromosomal circular entity. The GGI appears to exist transiently as an extrachromosomal circle in difA+ difA+ strains unless they carry a mutation in xerD. § indicates that chromosomal DNA purified from a population of bacteria was used as a template for the amplification.
Unlike other difA+ difA+ strains, the difA+ difA+ xerD mutant and a strain carrying wild-type dif sites (both a difA and a difB) maintain the GGI within the chromosome (Fig. 4B). The population of wild-type cells appears to be homogeneous in that the GGI is not readily excised. These data suggest that within the population of difA+ difA+ cells, the GGI can exist both intrachromosomally and extrachromosomally, while in difA+ difA+ xerD or wild-type MS11 derivatives, the GGI does not readily excise and exists predominantly within the chromosome. The lack of detectable excision of the GGI from the wild-type strain carrying the difB site in conjunction with the undetectable frequency of GGI loss suggest that excision is a necessary step in the loss of the GGI.
Existence of the GGI as a transient extrachromosomal circular entity.
Three pieces of evidence led us to hypothesize that the GGI might sometimes exist and/or be maintained as an extrachromosomal circle. The first piece of evidence is the discovery that the GGI appears to exist both within the chromosome and outside the chromosome in blue (PhoA+) colonies of difA+ difA+ xerD+ strains (Fig. 4B). The second is an extension of the hypothesis that excision leads to the loss of the GGI and arises from the observation that a few colonies from difA+ difA+ xerD+ strains sector into blue and white sections (Fig. 5A). For strains where the loss of the GGI is not detectable by the loss assay, as in xerD mutants or in the wild-type strain, sectoring was not observed. Based on this observation, if the GGI were to exist as an extrachromosomal entity, it could do so only in a transient capacity; otherwise, the GGI would be maintained in the population, resulting in an unsectored blue colony. The third observation supporting circular GGI formation is that the Erm island can excise from the chromosome and exist transiently as an extrachromosomal circle (18).
FIG. 5.
GGI excision and loss. The loss of the GGI is shown for our N. gonorrhoeae strains engineered to carry a productive PhoA translational fusion such that bacteria carrying the GGI are blue on XP indicator plates and bacteria that have lost the GGI are white on the XP indicator plates. (A) Loss of the GGI occurs in CFU prior to plating or upon plating, leading to the expansion of ΔGGI and PhoA− cells, as shown by the appearance of white (PhoA−) colonies or sectoring of blue and white colonies. The arrows indicate white colonies. Blue (PhoA+) colonies have a darker center. (B) Model of GGI excision and loss. A GGI+ cell (blue background) can excise the GGI by XerCD-mediated site-specific recombination. If the GGI is not integrated back into the chromosome, upon chromosome replication and cell division, a cell lacking the GGI will be created (white background). As the population expands, more ΔGGI cells may be created. Since the GGI carries the oriT for the T4SS, the excised GGI may be secreted. Gonococcal DNA present in the milieu can be taken up by other Neisseria cells through natural transformation and could transform a GGI− cell to a GGI+ cell.
Similar to the model Erm island, the GGI flanked by two copies of difA (difA+ difA+) is unstable and readily lost from the population. Unlike the Erm island, the GGI of wild-type MS11 is considerably larger and does not carry two direct repeats of difA but rather carries one difA and one difB flanking the GGI. Since the GGI is readily lost from difA+ difA+ strains and color sectoring in colonies has been observed, it is not likely that the excised GGI is a self-replicating plasmid. However, this does not exclude the possibility that the GGI can transiently exist outside the chromosome as an extrachromosomal entity. In E. coli, plasmids containing dif-like sites and no known origin of replication can be maintained by integrating into the chromosome at dif during replication (11). Therefore, it is still plausible that the GGI could exist outside the chromosome and integrate into the chromosome to be maintained. To examine this possibility, lysates of blue colonies from the assay were PCR amplified by using primers located in parA and near traD designed to extend toward dif (Fig. 4C). Since the primers should produce a product only if the GGI exists in a circular form, strains that carry an extrachromosomal circular GGI can be identified. The results of this PCR amplification show that difA+ difA+ xerD+ strains do indeed carry a circular form of the GGI (Fig. 4D); however, 40 amplification cycles were required to generate a detectable product. These data resolve the conflicting previous PCR data indicating both the presence and the absence of the GGI in the chromosome (Fig. 4B) by illustrating the presence of the GGI as a transient extrachromosomal entity. A faint band in the WT lane also indicates that perhaps a few WT cells also excised the GGI and that it was detected as an extrachromosomal circle. This detection of the intra- and extrachromosomal GGI in difA+ difA+ xerD+ strains is consistent with the model Erm island Southern data showing the presence of a circular extrachromosomal entity after the initial integration of the marker and an additional difA site in the chromosome (18). Since excision is occurring in difA+ difA+ xerD+ strains and perhaps to a very small degree in the WT, it is plausible that the GGI could be mobilized by site-specific recombination at dif followed by transfer to a recipient cell.
Because the extrachromosomal circle is detectable via PCR amplification in strains that lose the GGI more frequently, and a faint band was present in the WT strain (Fig. 4D), we attempted to purify the GGI in its circular form. When DNA was purified by standard methods or agarose plugs and then subjected to pulsed-field gel electrophoresis (PFGE) and Southern analysis, we were unable to detect the circular form of the GGI. We also attempted to isolate the circular extrachromosomal entity by using a cosmid purification method and also added Plasmid Safe nuclease to all DNA preparations to digest linearized DNA fragments and isolate the GGI. However, none of these strategies produced detectable amounts of the GGI as a circular extrachromosomal entity in the WT or any difA+ difA+ strains when subjected to Southern analysis. Since the amount of circular DNA in either the WT or difA+ difA+ strains is undetectable by Southern analysis, the circular GGI is likely present in only a very small number of cells. These data suggest that the GGI does not exist predominantly as a circular extrachromosomal entity and support its transient existence outside the chromosome.
Spontaneous loss of the GGI occurs in wild-type gonococci.
As we were unable to detect a loss in a wild-type background using the GGI loss assay, we developed a selection assay for loss. Using the difA+ difA+ PhoA fusion background, we introduced an allele of rpsL that confers streptomycin sensitivity to the GGI between dif and traD. Although MS11 carries an allele of rpsL with a single point mutation conferring streptomycin resistance, the allele conferring sensitivity is dominant to the MS11 rpsL allele that confers resistance (13, 21). With the introduction of the second rpsL allele into the MS11 difA+ difA+ PhoA fusion background, the strains were rendered sensitive to streptomycin as long as the GGI remained within the cell. If the GGI was lost, the cell regained the resistance phenotype. As in the above-described loss assay, gonococci were grown up in liquid medium and then plated onto XP indicator plates, which, in the selection assay, also contained streptomycin. Only cells that have lost the GGI should grow and produce white colonies on the plates. Using the difA+ difA+ strain, we tested the validity of the assay. As the data indicate, the frequency of loss in the selection assay was comparable to that of the previous screen assay (Table 4). However, we did observe numerous blue (PhoA+) colonies. The growth of blue colonies indicates that either a mutation occurred in the allele conferring sensitivity or a recombination event between the two copies of rpsL occurred, resulting in the reproduction of a second rpsL (Strr) copy, as was observed previously by Kohler et al. (24). Both of these scenarios would render a cell streptomycin resistant. To prevent the isolation of these false-positive (GGI+ Strr) isolates, we inserted a tetracycline marker into recA to impair homologous recombination between the two rpsL alleles. This strain was also tested for the loss of the GGI, and as described above, the recA mutation had no effect on the loss frequency (Table 4). The difB allele was then introduced into a Strs Cmr PhoA+ background at the traD end of the GGI, and the recA mutation was incorporated. This strain with WT dif sites flanking the GGI was tested for a loss of the GGI. Since this selection assay allowed greater numbers of gonococci to be plated, we were able to detect less-frequent events (Table 4). The loss of the GGI was confirmed by chloramphenicol sensitivity and PCR amplification (data not shown). Although the GGI in a wild-type background excises considerably less frequently, at 1.15 × 10−6, than the difA+ difA+ strain, these data show that the excision and loss of the GGI can still occur in WT difA+ difB+ isolates (Table 4). The loss of the GGI in a strain carrying wild-type dif sites suggests that XerCD-mediated site-specific recombination excises and circularizes the GGI at an appreciable frequency. This excision and circularization might facilitate the spread of the GGI to other bacteria.
TABLE 4.
Loss of the GGI is affected by the dif sequence but not by recA or type IV secretion mutations
| Strain | Avg loss frequencya | Confidence interval (95%) |
|---|---|---|
| difA+difB+recA (ND558)b | 1.15 × 10−6 | 1.06 × 10−7, 1.24 × 10−5c |
| difA+difA+ (ND569) | 1.22 × 10−3 | 7.66 × 10−4, 1.95 × 10−3 |
| difA+difA+recA (ND570) | 1.84 × 10−3 | 1.30 × 10−3, 2.61 × 10−3 |
| ΔtraH difA+difA+ (ND576) | 1.05 × 10−3 | 7.52 × 10−5, 1.45 × 10−2 |
| ′parA difA+difA+ (ND596) | 2.14 × 10−3 | 1.48 × 10−3, 3.10 × 10−3 |
Calculated as the geometric mean.
Wild-type dif sites.
Statistically significantly different from the difA+ difA+ recA strain.
Loss of the GGI occurs independently of type IV secretion.
Since the GGI encodes a functional T4SS and carries a putative origin of transfer for the secretion of DNA (34), we tested the possibility that the loss of the GGI is dependent upon type IV secretion. We examined GGI loss in strains carrying a mutation in traH or parA, T4SS genes that were previously shown to be required for DNA secretion (18, 19). Neither of the T4SS mutants lost the GGI at a frequency statistically different from that of the parent difA+ difA+ strain (Table 4), indicating that the loss of the GGI occurs independently of type IV secretion.
DISCUSSION
In this study we have shown that GGI recombination occurs at dif and occurs by site-specific recombination. Although several different forms of the GGI exist in N. gonorrhoeae and N. meningitidis, they all carry difB sites that differ from the consensus dif sequence in the XerD binding domain. Each difB has at least 3 changes in the XerD binding region. Reconstitution of the dif consensus sequence, generating a second difA site in the difA+ difA+ strain, facilitated the efficient excision and loss of the GGI (Fig. 3And Tables 3And 4). Mutation of xerD in these difA+ difA+ strains renders the excision and loss of the GGI undetectable (Fig. 4And Table 3). Because homologous recombination between relatively short sequences has been observed for gonococci (19) and the dif sites could be acted upon by RecA, we tested the frequency of loss of the GGI in a recA mutant. The frequency of loss of the GGI in the recA mutant was slightly reduced although not statistically significantly different from that of the wild type (Table 3). Thus, recA is not required for GGI excision, and if it has any effect, it is much less than the effect of a xerD mutation. Together, these data suggest that the GGI can be excised by the action of XerCD at the dif sites. Furthermore, the difB sequences allow the GGI to be maintained in the chromosome.
These results have implications for our understanding of how the GGI was acquired by Neisseria and how it may be transferred between bacteria. Since the XerCD reaction at dif can lead to either the excision of DNA or incorporation of DNA into the chromosome, our results examining excision also suggest important roles for dif and XerCD in GGI acquisition. Since XerCD can act on dif-like sequences in plasmids or phage genomes, we speculate that long ago, the GGI was acquired by XerCD acting to integrate either phage-derived DNA or a conjugative plasmid, introduced from another bacterial species. Since the GGI has similarities to the E. coli F plasmid (18), the latter speculation is a more likely explanation. The introduced dif sequence may have had differences from the dif consensus when it was integrated, or it may have acquired mutations after the sequence integrated. The dif alterations exist in the XerD binding region; however, the specific changes are not the same in the different difB sequences. Thus, the greater variability among difB sites may have arisen from mutations within the site after integration, resulting in the stabilization of the GGI in the chromosome. Mutations in this site may also be indicative of multiple independent integration events in an ancestral neisserial species that has given rise to the current pathogenic neisserial strains. Interestingly, two of the difB base changes exist in two different difB alleles. Cytosines are present at positions 23 and 27 in both difB1 (in Gc) and difB3 (in the meningococcus [Mc]). If we assume that the GGI was originally flanked by two difA sequences, this result may mean that those changes occurred in a common ancestor of those species. Alternatively, those changes may be particularly good at stabilizing the GGI in the chromosome and are thus found in strains because the GGI was not excised and lost. Perhaps the GGI, once integrated, confers an advantage such that mutations in the second dif site would act to maintain the GGI and thus the advantageous phenotype.
Although the excision and loss of the GGI occurred at an easily detectable frequency in our strains engineered to carry two difA sequences, the loss of the GGI was not readily detectable in the wild-type strain using the GGI loss screen. Therefore, we developed a selection method for loss of the GGI and were able to measure the loss of the GGI in the wild-type strain at a frequency of 1 × 10−6 GGI− colonies/total colonies after overnight growth. Although this result shows that the excision of the GGI is rare in the wild-type strain, it also shows that the excision and loss of the GGI can occur in wild-type strains even though these strains carry 4 mismatches to the dif consensus sequence. This result may explain the absence of the GGI from some wild-type strains. Dillard and Seifert previously screened a panel of 115 low-passage clinical isolates of N. gonorrhoeae and found that 91 carried the GGI (15). However, the other 24 did not, and for some of these, it was subsequently demonstrated that they did not contain any DNA inserted at dif (18). Since we have found that the GGI can be lost from the chromosome of wild-type strains, it is possible that the GGI− isolates once had the GGI and have since excised and lost it.
Extensive differences in the difB sequence may explain why some N. meningitidis strains maintain mutated forms of the GGI. Some N. meningitidis strains carry the difB2 allele, which has 8 mismatches compared to the difA consensus (Table 2And Fig. 1). Each of these strains has one or more deletions in the T4SS genes, and it appears that some of these islands may be acquiring mutations and degrading over time. For example, sequenced strain Alpha275 carries a deletion of yaa and part of traD and carries an insertion sequence in traK (GenBank accession no. AM889138). It is possible that strains such as this one would have lost the GGI if it were not for the mutations in difB.
The excision of the GGI may allow it to be transferred horizontally. The GGI encodes a T4SS and includes an origin of transfer for the secretion of DNA (34). Chromosomal DNA is secreted from the cell and can be taken up by other gonococci in the population via natural transformation (15, 19, 34). Since it appears that only one origin of transfer exists in the chromosome, we speculate that DNA secretion starts from this spot and proceeds around the chromosome in a unidirectional manner, similarly to how Hfr transfer occurs in E. coli conjugation. If this model were true for Neisseria, then the transfer of the entire chromosomally integrated GGI would be difficult. Because the origin of transfer is internal in the GGI, part of the GGI would be transferred first and the rest of the GGI would not be transferred until the rest of the chromosome, in its entirety, had been secreted. However, if the GGI were to excise, then the secretion of the whole GGI would occur much quicker, and the GGI would have a greater likelihood of being passed to another bacterium.
Nonreplicative circular extrachromosomal intermediates of genomic islands have been detected in a number of other organisms that utilize site-specific recombination for genomic island excision (28, 30, 31). However, the transient existence of excised extrachromosomal genomic islands specifically implicating the XerCD recombinase has not been previously described. Here we demonstrated that a circular extrachromosomal entity exists transiently in difA+ difA+ strains. Although we were able to detect the circular form of the GGI, it did require 40 amplification cycles and loading of twice the normal volume of PCR mixture to observe the PCR product (Fig. 4D). In wild-type strain MS11, the consistent detection of an extrachromosomal GGI was difficult. The inability to directly detect the GGI also verifies that the GGI does not exist predominantly as an extrachromosomal entity. Just as plasmids without an origin of replication but carrying a dif-like site are maintained by integrating into the chromosome during replication (11, 12), the GGI appears to require chromosomal integration to be maintained. Data from the loss assay (Tables 3And 4), coupled with the PCR data (Fig. 3And 4) and the undetectable amount of circularized GGI, suggest that the GGI exists only transiently as an extrachromosomal entity and that in order to be replicated and maintained, it must reside within the chromosome.
These data are not consistent with the previous suggestion of Snyder et al. (38) that the GGI exists both integrated into the chromosome and as a replicating, conjugative plasmid. Our results indicate that the GGI is not a replicating plasmid. In the engineered difA+ difA+ strain, we could increase the level of GGI excision and detect the GGI as an extrachromosomal circle (Fig. 4B and D); however, the GGI was readily lost from these strains, suggesting that it could not autonomously replicate (Tables 3And 4). When we examined the wild-type strain, we could not detect the chromosomal locus that would result from GGI excision (Fig. 4B), and we could only inconsistently detect the circular GGI when we increased the number of PCR amplification cycles (Fig. 4D). These data, together with our measurements of GGI loss frequency, indicate that the GGI rarely excises from the chromosome and is not maintained in a free circular form. It is unlikely that the GGI ever exists as a conjugative plasmid, and there is no evidence that it acts in conjugation. The results of several studies reported previously (15, 19, 34) demonstrated that the GGI-encoded T4SS acts to secrete DNA into the medium, and not directly from cell to cell, and that gene transfer by the T4SS is prevented if DNase I or another nuclease capable of digesting single-stranded DNA is present.
Model of GGI loss.
Based on the data presented here, we propose a model describing the excision and loss of the GGI from Neisseria and the roles of dif and XerCD in this process (Fig. 5). In the model, GGI+ cells are drawn with a blue background, reflecting the PhoA+ phenotype used to track the presence of the GGI in our assays, and GGI− cells are depicted with a white background (Fig. 5B). Although phoA is not present in wild-type gonococci, the presence of the GGI is associated with certain other phenotypes: the ability to secrete DNA and the ability to acquire iron during intracellular growth in a TonB-independent manner (18, 34, 45). This model incorporates two successive steps in the loss of the GGI. The first step is the excision of the GGI from the chromosome, and the second step is the replication of the chromosome without the GGI integrated into the chromosome. In order for GGI loss to occur, the excision of the GGI must occur during or prior to its replication within the chromosome. This model takes into account the observation of colonies sectoring into blue (GGI+) and white (GGI−) sections in our assays using phoA-marked strains (Fig. 5A) as well as the PCR data accounting for the presence of the GGI in the chromosome as well existing as an extrachromosomal entity within a population. This loss model also provides a plausible mechanism for the generation of GGI− strains from WT strains.
A GGI+ bacterium carrying difA and difB will excise the GGI at a low frequency. Since the differences between difA and difB are internal, i.e., in the XerD binding region of difB, the excised GGI will carry difB, and the chromosome will maintain difA (Fig. 2C). It is clear that excision occurs through the action of XerD at dif, since a xerD mutation greatly diminishes loss, and sequence changes in difB rendering the second site more similar to difA lead to increased excision (Tables 3And 4And Fig. 4). It is likely that XerD acts with XerC in this reaction, as in E. coli, but we did not test that idea since we were unsuccessful in obtaining xerC mutants. Since dif-containing molecules can be integrated as well as excised by XerCD, it is likely that an excised GGI can be integrated back into the chromosome. We would expect that such a reaction would be infrequent for excised GGIs, due to the differences in difB compared to the dif consensus sequence.
Once the GGI has excised, GGI− cells can be created as the bacteria replicate their chromosomes and divide. If the GGI could autonomously replicate efficiently, we would expect to see the GGI maintained even after excision. However, in cells that carried two difA sites flanking the GGI and thus increased GGI excision, the GGI was lost from the population at a frequency of around 2 × 10−3 (Table 3). Even in strains carrying difB (the wild-type situation), GGI− colonies arose and could be detected at a frequency of approximately 1 × 10−6 (Table 4). Thus, it is likely that the GGI does not autonomously replicate. An alternative explanation is that the GGI can replicate on its own but does so poorly. In either case, the GGI would be diminished in the population as the cells replicate and divide, diluting out the GGI+ cells. This process may explain how 20% of gonococcal isolates came to be GGI−.
From this model, we can extend GGI excision to possible transfer. If the GGI, which carries an origin of transfer (34), can excise from the chromosome, it could perhaps be secreted as a single fragment of DNA. Other Neisseria cells in the population could then take up the secreted DNA via natural transformation. Since XerCD-mediated site-specific recombination in E. coli has been shown to undergo both intra- and intermolecular recombination, it is plausible that the GGI could be incorporated into the recipient's genome by site-specific recombination. In this manner, the GGI could be or may have been a mobile genetic element.
In conclusion, we have shown that the excision and loss of the GGI require xerD and are affected by the flanking dif sequences. The GGI can excise at low frequencies and can exist as an extrachromosomal entity prior to its loss. Previous studies demonstrated roles for the GGI in natural transformation and infection (15, 18, 19, 45). The present studies have demonstrated how the GGI can be maintained, lost, or possibly transferred to other bacteria.
Acknowledgments
We thank Zachary Benet for DNA sequencing, Holly Hamilton for construction of the traH deletion strain, and Abby Boster for plasmid constructs. We thank Gary Roberts for a critical reading of the manuscript.
We thank the Biotechnology Training Program and the Advanced Opportunity Fellowship for financial support of N.M.D. This work was supported by NIH grants AI047958 and AI072605 to J.P.D. and traineeship on NIH 5 T32 GM008349 for N.M.D.
Footnotes
Published ahead of print on 12 November 2010.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schafer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakely, G., S. Colloms, G. May, M. Burke, and D. Sherratt. 1991. Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol. 3:789-798. [PubMed] [Google Scholar]
- 3.Blakely, G., G. May, R. McCulloch, L. K. Arciszewska, M. Burke, S. T. Lovett, and D. J. Sherratt. 1993. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell 75:351-361. [DOI] [PubMed] [Google Scholar]
- 4.Blakely, G., and D. Sherratt. 1996. Determinants of selectivity in Xer site-specific recombination. Genes Dev. 10:762-773. [DOI] [PubMed] [Google Scholar]
- 5.Blakely, G. W., A. O. Davidson, and D. J. Sherratt. 1997. Binding and cleavage of nicked substrates by site-specific recombinases XerC and XerD. J. Mol. Biol. 265:30-39. [DOI] [PubMed] [Google Scholar]
- 6.Blakely, G. W., A. O. Davidson, and D. J. Sherratt. 2000. Sequential strand exchange by XerC and XerD during site-specific recombination at dif. J. Biol. Chem. 275:9930-9936. [DOI] [PubMed] [Google Scholar]
- 7.Blakely, G. W., and D. J. Sherratt. 1994. Interactions of the site-specific recombinases XerC and XerD with the recombination site dif. Nucleic Acids Res. 22:5613-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle-Vavra, S., and H. S. Seifert. 1995. Shuttle mutagenesis: a mini-transposon for producing PhoA fusions with exported proteins in Neisseria gonorrhoeae. Gene 155:101-106. [DOI] [PubMed] [Google Scholar]
- 9.Bui, D., J. Ramiscal, S. Trigueros, J. S. Newmark, A. Do, D. J. Sherratt, and M. E. Tolmasky. 2006. Differences in resolution of mwr-containing plasmid dimers mediated by the Klebsiella pneumoniae and Escherichia coli XerC recombinases: potential implications in dissemination of antibiotic resistance genes. J. Bacteriol. 188:2812-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnoy, C., and C. A. Roten. 2009. The dif/Xer recombination systems in proteobacteria. PLoS One 4:e6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerget, M. 1984. A 140 base-pair DNA segment from the kanamycin resistance region of plasmid R1 acts as an origin of replication and promotes site-specific recombination. J. Mol. Biol. 178:35-46. [DOI] [PubMed] [Google Scholar]
- 12.Clerget, M. 1991. Site-specific recombination promoted by a short DNA segment of plasmid R1 and by a homologous segment in the terminus region of the Escherichia coli chromosome. New Biol. 3:780-788. [PubMed] [Google Scholar]
- 13.Cloud, K. A., and J. P. Dillard. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 70:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornet, F., I. Mortier, J. Patte, and J. M. Louarn. 1994. Plasmid pSC101 harbors a recombination site, psi, which is able to resolve plasmid multimers and to substitute for the analogous chromosomal Escherichia coli site dif. J. Bacteriol. 176:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 16.Gunn, J. S., and D. C. Stein. 1996. Use of a nonselective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 17.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton, H. L., N. M. Domínguez, K. J. Schwartz, K. T. Hackett, and J. P. Dillard. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 55:1704-1721. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber, K. E., and M. K. Waldor. 2002. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 417:656-659. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 22.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. L. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler, P. L., K. A. Cloud, K. T. Hackett, E. T. Beck, and J. P. Dillard. 2005. Characterization of the role of LtgB, a putative lytic transglycosylase in Neisseria gonorrhoeae. Microbiology 151:3081-3088. [DOI] [PubMed] [Google Scholar]
- 25.Kohler, P. L., H. L. Hamilton, K. Cloud-Hansen, and J. P. Dillard. 2007. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 189:5421-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuempel, P. L., J. M. Henson, L. Dircks, M. Tecklenburg, and D. F. Lim. 1991. dif, a recA-independent recombination site in the terminus region of the chromosome of Escherichia coli. New Biol. 3:799-811. [PubMed] [Google Scholar]
- 27.Leslie, N. R., and D. J. Sherratt. 1995. Site-specific recombination in the replication terminus region of Escherichia coli: functional replacement of dif. EMBO J. 14:1561-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middendorf, B., B. Hochhut, K. Leipold, U. Dobrindt, G. Blum-Oehler, and J. Hacker. 2004. Instability of pathogenicity islands in uropathogenic Escherichia coli 536. J. Bacteriol. 186:3086-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse, S. A., and L. Bartenstein. 1974. Factors affecting autolysis of Neisseria gonorrhoeae. Proc. Soc. Exp. Biol. Med. 145:1418-1421. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, R. A., and E. F. Boyd. 2008. Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J. Bacteriol. 190:636-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajanna, C., J. Wang, D. Zhang, Z. Xu, A. Ali, Y. M. Hou, and D. K. Karaolis. 2003. The Vibrio pathogenicity island of epidemic Vibrio cholerae forms precise extrachromosomal circular excision products. J. Bacteriol. 185:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recchia, G. D., M. Aroyo, D. Wolf, G. Blakely, and D. J. Sherratt. 1999. FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J. 18:5724-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothfield, L. I. 1994. Bacterial chromosome segregation. Cell 77:963-966. [DOI] [PubMed] [Google Scholar]
- 34.Salgado-Pabón, W., S. Jain, N. Turner, C. van der Does, and J. P. Dillard. 2007. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol. Microbiol. 66:930-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Seifert, H. S. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215-220. [DOI] [PubMed] [Google Scholar]
- 37.Sirois, S., and G. Szatmari. 1995. Detection of XerC and XerD recombinases in Gram-negative bacteria of the family Enterobacteriaceae. J. Bacteriol. 177:4183-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder, L. A., S. A. Jarvis, and N. J. Saunders. 2005. Complete and variant forms of the ‘gonococcal genetic island’ in Neisseria meningitidis. Microbiology 151:4005-4013. [DOI] [PubMed] [Google Scholar]
- 39.Steiner, W. W., and P. L. Kuempel. 1998. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J. Bacteriol. 180:6269-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Summers, D. K., and D. J. Sherratt. 1984. Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell 36:1097-1103. [DOI] [PubMed] [Google Scholar]
- 41.Summers, D. K., and D. J. Sherratt. 1988. Resolution of ColE1 dimers requires a DNA sequence implicated in the three-dimensional organization of the cer site. EMBO J. 7:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson, J. 1972. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonococci. J. Exp. Med. 136:1258-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westling-Häggström, B., T. Elmros, S. Normark, and B. Winblad. 1977. Growth pattern and cell division in Neisseria gonorrhoeae. J. Bacteriol. 29:333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright, C. J., A. E. Jerse, M. S. Cohen, J. G. Cannon, and H. S. Seifert. 1994. Nonrepresentative PCR amplification of variable gene sequences in clinical specimens containing dilute, complex mixtures of microorganisms. J. Clin. Microbiol. 32:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zola, T. A., H. R. Strange, N. M. Dominguez, J. P. Dillard, and C. N. Cornelissen. 2010. Type IV secretion machinery promotes Ton-independent intracellular survival of Neisseria gonorrhoeae within cervical epithelial cells. Infect. Immun. 78:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]