Bacteria express a variety of adhesive proteins, termed adhesins, on their cell surface. Adhesive activity is required for many aspects of the bacterial lifestyle, including attachment to both abiotic and biotic surfaces, bacterium-bacterium interactions, and biofilm formation. For pathogenic bacteria, adhesins mediate specific binding to host cells and initiate the infectious process (6, 9). Adhesins are critical virulence factors; they are key determinants of the tissue tropism of a particular pathogen and dictate how and where bacterial colonization may occur. A given pathogen may encode multiple different adhesins to allow binding to different sites within the host, as well as for interactions outside the host in the environment or in a vector. The expression of adhesins must be regulated and coordinated so that the proper adhesin is expressed at the proper time and in the proper environment. In addition, bacteria must be able to modulate adhesive activity to allow detachment from a particular niche and dispersal to new sites. Adhesins, which are by necessity surface-exposed structures, are also prominent targets of the host immune system. Therefore, the expression of adhesins is typically tightly controlled and subject to complex regulatory mechanisms, including phase and antigenic variation (1, 16). Phase variation allows for on/off expression of an adhesin, while antigenic variation changes the amino acid composition of surface-exposed regions of the adhesin to prevent recognition by the host immune system. Repetitive sequence elements have been shown to provide a source of expression and antigenic variation in surface-exposed adhesins in both bacterial and eukaryotic cells (2, 4, 7, 11). In this issue of Journal of Bacteriology, Sheets and St. Geme reveal a new role for repetitive sequence elements in controlling bacterial adhesion (14). The authors demonstrate that variation of a tandem repeated sequence results in the direct and gradual modulation of adhesive activity.
The focus of the study by Sheets and St. Geme is the Cha adhesin, expressed by the Haemophilus cryptic genospecies. The Haemophilus cryptic genospecies is a Gram-negative bacterial pathogen and an important causative agent of maternal genital tract and neonatal respiratory tract infections (10, 17). In an earlier study, Sheets and colleagues identified Cha as a novel adhesin that is conserved among different isolates of the Haemophilus cryptic genospecies but absent from H. influenzae strains (13). Mutagenesis of Cha caused a loss of bacterial adhesion to epithelial cells of the genital and respiratory tract, suggesting that Cha may be the primary adhesin responsible for the apparent tissue tropism of the Haemophilus cryptic genospecies (13). Cha belongs to the autotransporter family of secreted proteins (5, 13). Autotransporters are characterized by the presence of a conserved outer membrane or β-domain in their C terminus and an N-terminal passenger domain that contains the functional activity. An N-terminal signal sequence directs translocation of the autotransporter across the cytoplasmic membrane to the periplasm via the Sec general secretion machinery. Once in the periplasm, the C-terminal domain inserts into the outer membrane, forming a β-barrel structure. The passenger domain is secreted to the cell surface, either directly through the channel provided by the β-domain or with the assistance of the Bam outer membrane machinery (12). Following secretion, the passenger domain may remain anchored to the cell surface by the β-domain or may be proteolytically cleaved and released to the external milieu. Cha is a new member of the trimeric autotransporter family (8, 13), all members of which function as adhesins. Well-studied trimeric autotransporters include the Yersinia YadA and Haemophilus influenzae Hia adhesins (3, 15). For trimeric autotransporters, the C-terminal domains trimerize to form a single outer membrane β-barrel, which displays three intertwined passenger domains on the cell surface. The passenger domains are composed of modular and repetitive structural elements (8). Typically, an N-terminal head domain is connected via a neck region to a trimeric coiled-coil stalk region, which is in turn connected to the β-domain membrane anchor. The trimeric autotransporters form prominent surface structures that can be visualized by electron microscopy as lollipop-like appendages or extended hair-like structures (8). Cha shares these conserved features but contains in addition a unique 28-residue repeated sequence, located in the region corresponding to the stalk domain. Consistent with this, the Cha repeat sequence has a heptad periodicity of small hydrophobic residues, suggesting a coiled-coil architecture (14).
In the Haemophilus cryptic genospecies strain originally analyzed by Sheets and colleagues, Cha contained nine of the 28-residue sequence repeats (13). However, the authors noted size differences among cha-hybridizing genomic fragments from different strains, and they speculated that variation in the number of repeats might be responsible and might result in the generation of antigenic or functional diversity. In their current study, Sheets and St. Geme show that the repeat-containing region of Cha indeed undergoes spontaneous variation (14). Through analysis of a set of Cha repeat variants containing from none to approximately 100 repeats, the authors show an inverse correlation between repeat length and Cha-mediated adhesion; i.e., the greater the number of repeats and the longer the Cha sequence, the lower the adhesion. This correlation holds true both for adhesion to host cells and for bacterium-bacterium interactions (autoaggregation). Moreover, the authors show that the impact of repeat variation on adhesive activity cannot be explained by alterations in the expression, surface localization, or proper folding of Cha. These findings demonstrate that adhesion may be modulated gradually, instead of the on/off regulation generated by phase variation, and that longer structures are not necessarily advantageous in conferring adhesive activity. The latter finding is surprising, because many bacterial adhesins are presented on long, polymeric fibers, such as pili or fimbriae, which are generally thought to enhance adhesion by moving the binding domain away from the bacterial surface.
So what accounts for the authors' observation that the longer the Cha sequence, the lower the adhesion? The authors present several pieces of data suggesting a possible mechanism. First, the authors show that the binding activity of Cha resides in the N-terminal, head region of the passenger domain and that this region is responsible both for binding to host cells and for autoaggregation. Second, autoaggregation appears to be mediated by intermolecular Cha-Cha interactions rather than by the binding of Cha to some other bacterial surface moiety. Finally, the authors show by electron microscopy that expansion of the Cha repeat region results in the appearance of Cha surface structures with elongated stalk regions. Importantly, the electron microscopy images suggest that an increasing length of the stalk region allows the Cha head regions to aggregate together. These results suggest a mechanism whereby the increased stalk length caused by expansion of the repeat region provides sufficient flexibility to allow the N-terminal head regions to bind to each other (Fig. 1). Binding of the head domains to each other would then prevent Cha from binding to host cells or other bacteria. In contrast, the stalk regions of Cha proteins with fewer repeats are presumably too short to allow the neighboring head regions to interact. Other adhesins that are presented on extended structures, such as pili, likely avoid this fate due to a lack of self-binding activity. In addition, many pilus fibers are rigid structures, one function of which might be to decrease interactions between adhesins expressed on the same bacterial surface. How might the repeat-mediated modulation of Cha adhesion benefit the bacterium? Sheets and St. Geme speculate that the alteration of adhesive activity may provide an adaptive advantage within the host, creating variation within the bacterial population to allow binding to specific host sites and facilitating dispersal to new sites. The extension and contraction of the Cha sequence may also provide a source of antigenic variation to prevent detection by the host immune system. Thus, this mechanism of repeat-mediated variation may provide multiple advantages, and it will be interesting to determine its occurrence among other bacterial adhesins.
FIG. 1.
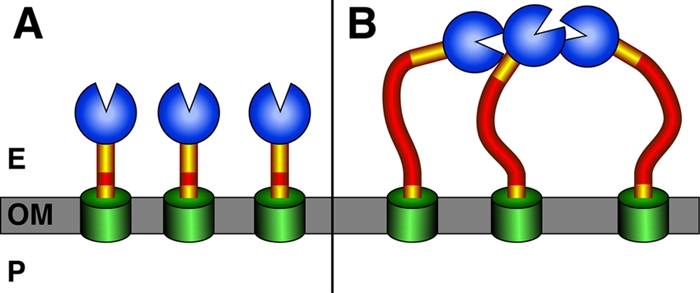
Model for repeat-mediated variation in Cha adhesive activity. The Cha protein is cartooned with the β-domain membrane anchor in green and the head domain, which contains the adhesin activity, in blue. The stalk region of Cha is shown in yellow and red, with red corresponding to the region encoded by the repeated sequence. The outer membrane, OM, periplasm, P, and extracellular environment, E, are indicated. (A) Cha variants containing a small number of repeats have a short stalk region which does not allow head-head interactions. (B) Cha variants containing a large number of repeats have stalks of sufficient length and flexibility to allow the head regions to bind each other.
Acknowledgments
Work in the D.G.T. laboratory is supported by NIH grants GM62987 and AI055621.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 19 November 2010.
REFERENCES
- 1.Blomfield, I. C. 2001. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv. Microb. Physiol. 45:1-49. [DOI] [PubMed] [Google Scholar]
- 2.Dawid, S., S. J. Barenkamp, and J. W. St. Geme III. 1999. Variation in expression of the Haemophilus influenzae HMW adhesins: a prokaryotic system reminiscent of eukaryotes. Proc. Natl. Acad. Sci. U. S. A. 96:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 4.Gravekamp, C., D. S. Horensky, J. L. Michel, and L. C. Madoff. 1996. Variation in repeat number within the alpha C protein of group B streptococci alters antigenicity and protective epitopes. Infect. Immun. 64:3576-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultgren, S. J., et al. 1993. Pilus and non-pilus bacterial adhesins: assembly and function in cell recognition. Cell 73:887-901. [DOI] [PubMed] [Google Scholar]
- 7.Jordan, P., L. A. Snyder, and N. J. Saunders. 2003. Diversity in coding tandem repeats in related Neisseria spp. BMC Microbiol. 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linke, D., T. Riess, I. B. Autenrieth, A. Lupas, and V. A. Kempf. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264-270. [DOI] [PubMed] [Google Scholar]
- 9.Pizarro-Cerda, J., and P. Cossart. 2006. Bacterial adhesion and entry into host cells. Cell 124:715-727. [DOI] [PubMed] [Google Scholar]
- 10.Quentin, R., C. Martin, J. M. Musser, N. Pasquier-Picard, and A. Goudeau. 1993. Genetic characterization of a cryptic genospecies of Haemophilus causing urogenital and neonatal infections. J. Clin. Microbiol. 31:1111-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauceo, J. M., et al. 2006. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot. Cell 5:1664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauri, A., et al. 2009. The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiology 155:3982-3991. [DOI] [PubMed] [Google Scholar]
- 13.Sheets, A. J., S. A. Grass, S. E. Miller, and J. W. St. Geme III. 2008. Identification of a novel trimeric autotransporter adhesin in the cryptic genospecies of Haemophilus. J. Bacteriol. 190:4313-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheets, A. J., and J. W. St. Geme III. 2010. Adhesive activity of the Haemophilus cryptic genospecies Cha autotransporter is modulated by variation in tandem peptide repeats. J. Bacteriol. 193:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St. Geme, J. W., III, and D. Cutter. 2000. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J. Bacteriol. 182:6005-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Woude, M. W., and A. J. Baumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace, R. J., Jr., et al. 1983. Nontypable Haemophilus influenzae (biotype 4) as a neonatal, maternal, and genital pathogen. Rev. Infect. Dis. 5:123-136. [DOI] [PubMed] [Google Scholar]


