Abstract
LDL-related protein 6 (LRP6) is a coreceptor of WNTs and a key regulator of the WNT/β-catenin pathway. Upon activation, LRP6 is phosphorylated within its intracellular PPPS/TP motifs. These phosphorylated motifs are required to recruit axin and to inhibit glycogen synthase kinase 3 (GSK3), two basic components of the β-catenin destruction complex. On the basis of a kinome-wide small interfering RNA (siRNA) screen and confirmative biochemical analysis, we show that several proline-directed mitogen-activated protein kinases (MAPKs), such as p38, ERK1/2, and JNK1 are sufficient and required for the phosphorylation of PPPS/TP motifs of LRP6. External stimuli, which control the activity of MAPKs, such as phorbol esters and fibroblast growth factor 2 (FGF2) control the choice of the LRP6-PPPS/TP kinase and regulate the amplitude of LRP6 phosphorylation and WNT/β-catenin-dependent transcription. Our findings suggest that cells not only recruit one dedicated LRP6 kinase but rather select their LRP6 kinase depending on cell type and the external stimulus. Moreover, direct phosphorylation of LRP6 by MAPKs provides a unique point for convergence between WNT/β-catenin signaling and mitogenic pathways.
The WNT signaling pathway is a highly conserved cascade that plays vital roles in development and cell differentiation and whose aberrant activation has been implicated in many types of oncogenic diseases. The initiation of WNT/β-catenin signaling requires the interaction of the WNT ligand with a seven-span transmembrane receptor called Frizzled (FZD) and low-density lipoprotein receptor-related proteins LRP5 or -6. In the absence of these interactions, cytoplasmic β-catenin is phosphorylated and subsequently degraded by a destruction complex that includes axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3 (GSK3). In the WNT-stimulated cell, β-catenin is not targeted for degradation by this ubiquitin-proteasome pathway. Instead, it accumulates in the nucleus, where it binds TCF/LEF transcription factors and serves as a transcriptional coactivator of WNT target genes, which regulate cell proliferation and cell cycle progression (10).
Recently, remarkable progress has been made in understanding how the aforementioned signal is relayed through LDL-related protein 6 (LRP6) further into the cytoplasm (3, 14, 35, 36). Two groups of residues in the intracellular domain (ICD) have been identified as crucial for the function of LRP6: (i) a PPPS/TP motif that is reiterated five times and is evolutionarily conserved among species; and (ii) serines surrounding these PPPS/TP motifs in the position +2 from serine/threonine in the PPPS/TP motifs. At least 4 intact PPPS/TP motifs are required for efficient signal transduction, and it was hypothesized that PPPS/TP motifs reiterated five times serve as a built-in signaling amplifier and that individual motifs cooperate in downstream signal transduction (22, 33). Additionally, the extracellular domain of LRP6 can exert an inhibitory effect on the ICD by preventing oligomerization (21). One possibility is that WNT binding triggers LRP6 oligomerization in “signalosomes,” which serve to localize adaptor molecules and cytoplasmic kinases that then phosphorylate the ICD. Dishevelled (DVL), another key downstream WNT signaling component, has been shown to be one such required molecule for signalosome formation and LRP6 phosphorylation (3). Phosphorylated LRP6 then interferes with the function of the destruction complex by recruiting the scaffold protein axin (30) and directly inhibiting GSK3 (12, 26, 34), thereby enabling the accumulation of β-catenin and promoting the expression of β-catenin-regulated gene programs.
Several candidate kinases have been identified, which are thought to mediate phosphorylation of LRP6. Zeng et al. introduced GSK3 as the first kinase known to phosphorylate PPPS/TP motifs and thereby implicated GSK3 in both upstream and downstream regulation of WNT signaling events (36). In a different set of studies, Davidson et al. provided convincing evidence that CK1γ is responsible for phosphorylation of serine residues surrounding PPPS/TP sites (mainly T1479) (14). Recent data have implicated two other kinases as well, namely, G protein-coupled receptor kinases GRK5 and -6 and a complex of cyclin Y/PFTK, which is active in the G2/M phase of the cell cycle (9, 13).
These findings suggest that a broad spectrum of kinases may be capable of activating LRP6 and that individual kinases may have redundant or replaceable roles. Several of these kinases, including GSK3 and cyclin Y/PFTK, are members of the “CMGC kinase superfamily,” which contains mitogen-activated protein kinases (MAPKs), CDKs, glycogen synthase kinases (GSKs), and CDK-like kinases (CLKs). With a few notable exceptions, most of the proteins in this group share a preference for proline-enriched substrates (16). We have performed a kinome-wide small interfering RNA (siRNA) screen for regulators of the WNT/β-catenin signaling and examined whether the multiple kinases from the CMGC group that we identified as putative WNT regulators might in fact be targeting the LRP6 PPPSP sites. Here we demonstrate for the first time that PPPS/TP motifs can be specifically phosphorylated by several proline-targeted kinases from the MAPK family and that these kinases are in turn sufficient and required for LRP6-initiated downstream signaling. Our findings raise the possibility that LRP6-kinase specificity is dependent on the extracellular stimulus, cell type, and cell-specific MAPK signaling status. It also provides a novel cross-talk between growth factors and the WNT signaling system.
MATERIALS AND METHODS
Cell culture, treatments, and conditioned medium.
HEK293 and rat chondrosarcoma (RCS) cells were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), and antibiotics. Cells were transfected with Lipofectamine Plus (Invitrogen), Fugene 6 (RCS cells; Roche), or polyethyleneimine.
HEK293 cells were treated in the 24-well format with the following inhibitors: BIRB 796 (10 μM) (Axon Medchem); U0126 (10 μM), SP600125 (1 to 50 μM), AS601245 (10 μM), D4476 (10 μM), IC261 (10 μM), and SU5402 (5 μM) (Calbiochem); and SB203580 (10 μM) (Sigma). One microliter of Lipofectamine (Invitrogen) was added to each well to improve cell permeability of the compounds. The following agonists were added 2 h after inhibitor treatment: phorbol-12,13-dibutyrate (PDBu; 1 μM) (Sigma) and phorbol-12-myristate-13-acetate (TPA; 0.2 μM) (Sigma). Recombinant mouse WNT-3A (30 ng/μl) (R&D Systems) or 0.1% bovine serum albumin (BSA)-phosphate-buffered saline (PBS) (control) was added 5 min later. RCS cells were treated as described previously (19). For immunoblotting, cells were harvested 2 h following stimulation; for the luciferase reporter assay, cells were harvested after an overnight treatment. Plasmids encoding vesicular stomatitis virus G protein (VSVG)-tagged LRP6ΔN (30), DVL2-Myc (20), CK1ɛ (25), S33A-β-catenin (24), V5-LRP6 and V5-LRP6 5A (33), ERK1 and ERK2 (27), and MAPKs-hemagglutinin (HA) have been described previously (11). The LRP6 construct was obtained from Bart Williams (Van Andel Institute, Grand Rapids, MI), and derivative constructs were mutagenized as previously described (33).
Conditioned media for the whole-kinome siRNA screens have been obtained using L cells that contain either WNT-3A expression plasmid or an empty vector (ATCC). A 10-cm culture dish of confluent L cells was grown in 10 ml of DMEM with 10% FBS and antibiotics. After 3 days, fresh medium was replaced over the cells for an additional 3 days and the two 10-ml volumes were mixed in a 1:1 mixture. Stocks were made up of 100 ml and kept at −80°C in 10-ml aliquots. Once initially thawed for an experiment, the conditioned medium was kept at −20°C and could be reused up to five freeze-thaw cycles.
Kinome/phosphatome siRNA screen.
HEK293 cells stably transfected with the Super TopFlash reporter plasmid (courtesy of Bart Williams) were split into poly-d-lysine-coated 96-well culture plates. The Super TopFlash reporter contains a promoter with several TCF4-binding repeats whose activation drives firefly luciferase expression. Seeded cells were grown overnight to 60 to 70% confluence at 37°C and 5% CO2 in DMEM, 10% fetal bovine serum (FBS), and antibiotics and were then transfected with a mixture of Lipofectamine Plus reagent, serum-free DMEM, and DNA for 3 h at 37°C. We transfected two separate wells with different siRNA constructs (Qiagen) at concentrations of 50 nM for each of the 518 human kinases. After 3 days of growth in culture, the cells were stimulated with WNT-3a-conditioned medium for 6 h, and reporter activity was analyzed in a luminometer. Kinases were scored as hits if transfection of their siRNA was able to influence luciferase activity compared to that of a reference siRNA. For the follow-up siRNA experiments, HEK293 cells were transfected with siRNA as previously described (7). siRNAs were used targeting the following kinases: MAPK1 (Qiagen catalogue no. SI00300755), MAPK3 (Qiagen catalogue no. SI00605997), MAPK8 (Qiagen catalogue no. SI02757209), and MAPK11 (Qiagen catalogue no. SI00606053). Control siRNA (Santa Cruz Biotechnology) was used as a negative control.
Purification of GST fusion protein substrates.
E. coli strain BL21 cells containing pGEX-4T3 plasmid were grown for 12 to 18 h in 500 ml of LB broth. Following 60 min of isopropyl-β-d-thiogalactopyranoside (IPTG) stimulation, cells were spun at 3,000 rpm for 30 min. The pellet was resuspended in a lysis buffer and affinity purified with washed glutathione-agarose beads. The purified glutathione S-transferase (GST) fusion proteins were washed three times in buffer and suspension in a glycerol solution at −80°C.
In vitro kinase assay.
Ten-centimeter plates of HEK293 cells at 50% confluence were transfected with 3 μg of an HA-tagged kinase and either (i) 1 μg of its respective upstream activating kinase (e.g., p38 and Mek3) or (ii) a green fluorescent protein (GFP) control. After 2 days, cells were starved for >2 h and then lysed in 0.5 ml 20 mM HEPES buffer (other additives were 10 mM EGTA [pH 8], 40 mM β-glycerophosphate, 1% NP-40, 2.5 mM MgCl2, 2 mM orthovanadate, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin-leupeptin). Lysates were centrifuged at 15,000 × g for 20 min at 4°C, and supernatants were incubated with 2 μl of HA antibody for 1 h at 4°C. We then added equal volumes of washed G-Sepharose beads solution to each experimental condition and incubated at 4°C for 1 h. Immunoprecipitates were washed 3 to 5 times in phosphate-buffered saline (PBS) with 1% NP-40 and 2 mM sodium vanadate. An additional wash was performed with 100 mM Tris (pH 7.5) and 0.5 M LiCl. The washed precipitates were placed in a kinase reaction buffer (12.5 mM MOPS [morpholinepropanesulfonic acid], pH 7.5, 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM sodium fluoride, 0.5 mM vanadate, 1 μCi [γ-32P]ATP, 20 μM cold ATP, 3.3 μM DTT) to a final volume of 30 μl. Reactions continued for 30 min at 30°C and were terminated with 10 μl 6× Laemmli buffer. The samples were heated at 95°C for 5 min and analyzed by sodium dodecyl sulfate (SDS)-gel electrophoresis on 12% polyacrylamide gels.
Cell-free MAPK assays were carried out with 300 ng of active ERK1, p38α, or JNK1 kinases (Cell Signaling Technology, Beverly, MA), and 300 ng of recombinant LRP6-ICD was used as a substrate. Kinase reactions were performed in a kinase buffer containing 25 mM Tris (pH 7.5), 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4, 10 mM MgCl2 for 30 min at 30°C in the presence of 200 μM ATP. MAPK-mediated phosphorylation of LRP6 was detected by Western blotting.
Western blotting.
Immunoblotting and sample preparations were performed as previously described (6). The antibodies were purchased from Cell Signaling Technology (phospho-LRP6 [Ser1490] 2568; LRP6 2560; phospho-p38 MAPK [Thr180/Tyr182] 9212; phospho-p44/42 MAPK [Thr202/Tyr204] 4376; p44/42 MAPK 9102; DVL3 3218), Santa Cruz Biotechnology (p38, sc-7972; JNK1, sc-571; actin, sc-1615; α-tubulin, sc-8035), Invitrogen (V5, R960-25), Covance (HA.11, MMS-101R), Sigma (VSVG, V4888), and BD Biosciences (β-catenin, BD610153).
Immunocytochemistry and axin cluster assay.
HEK293 and RCS cells were seeded on collagen-coated coverslips in 24-well plates at approximately 2 × 105 cells/well. Twenty-four hours after seeding, cells were cotransfected using calcium transfection with 0.4 μg GFP-tagged axin construct and either 0.4 μg V5-tagged LRP6 wild-type (WT) construct or 0.4 μg kinase construct (p38, JNK, ERK1, ERK2). Twenty-four hours posttransfection, cells were treated with 100 ng/μl WNT-3A (R&D) for 2 h, fixed in fresh 4% paraformaldehyde, permeabilized in 0.25% Triton X-100, and incubated overnight with PBTA (3% BSA, 0.25% Triton, 0.01% NaN3). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole; 1:5,000). Cells were visualized using a Zeiss LSM 710 confocal microscope. Two hundred GFP-positive cells per coverslip were scored for the pattern of GFP-axin localization into one of the following categories: even, punctate, and membranous. If a cell had a uniform background with only a few prominent dots, it was classified as even. If a uniform background was lacking and cells contained several prominent clusters, it was classified as punctate. If a continuous portion of the membrane contained axin, it was classified as membranous localization. The graph in Fig. 4H shows percentages of the pattern with standard deviations from three independent experiments.
Reporter assays.
HEK293 cells were transiently transfected with 0.1 μg Super 8X TopFlash construct and 0.1 μg Renilla luciferase (for HEK-Super TopFlash cells only with Renilla luciferase), with the indicated amount of V5-tagged LRP6 constructs or VSVG-tagged LRP6ΔN construct, and with 0.4 μg of plasmid encoding the indicated kinase in a 24-well plate. Luciferase assays were performed 24 h later with a Dual-Glo luciferase kit (Promega) and a Victor 3V luminometer (PerkinElmer) or MLX luminometer (Dynex Technologies). The firefly luciferase value in each well was normalized to the corresponding Renilla readout in order to control for transfection efficiency and general cell viability. Results of reporter assays are shown as means with a standard deviation of at least three independent experiments.
RESULTS
MAPKs promote WNT/β-catenin signaling.
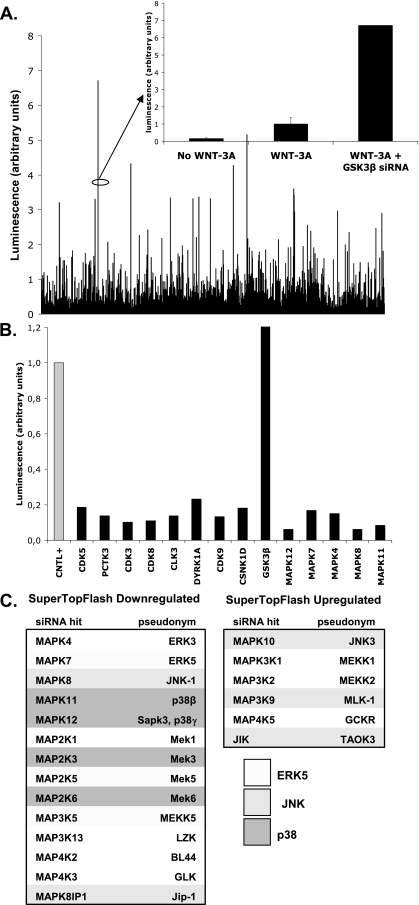
In order to identify candidate kinases involved in WNT signaling, we performed a whole-kinome siRNA screen. We transfected a siRNA library targeting all 518 human kinases (Qiagen) into HEK293 cells and used the Super TopFlash reporter as a luminescent readout for the downstream WNT signal. As proof of principle, we noted that transfection of siRNA sequences specific for GSK3β, a negative regulator of WNT signaling, had the anticipated stimulatory effect on the reporter activity. We compared all transfection conditions against a nonsense siRNA transfection and compiled a list of significant kinases regulating WNT/β-catenin signaling.
During the screen we identified several MAPKs and components of the MAPK pathway, which acted as putative positive regulators of WNT/β-catenin signaling in HEK293 (Fig. 1). It has been well established that kinases from the MAPK family generally have high substrate affinity for serines/threonines in proline-rich regions (8, 28). Based on these predictions, we hypothesized that MAPKs might influence WNT signaling by acting on LRP5 and/or LRP6 and their PPPS/TP motifs.
FIG. 1.
A whole-kinome siRNA screen identifies MAPKs as regulators of WNT signaling. (A) A whole-kinome siRNA screen using a luminescent readout for WNT activity revealed many potential regulators of WNT signaling. GSK3β, a known pathway inhibitor, served as an internal control. (B) A secondary screen using some of the strongest hits from the original library was performed for confirmation. (C) List of components of the MAPK cascade identified in the screen. Shades of gray indicate the association with the specific MAPK cascade (ERK5, JNK1, and p38).
MAPKs phosphorylate PPPS/TP motifs in LRP6 in vitro.
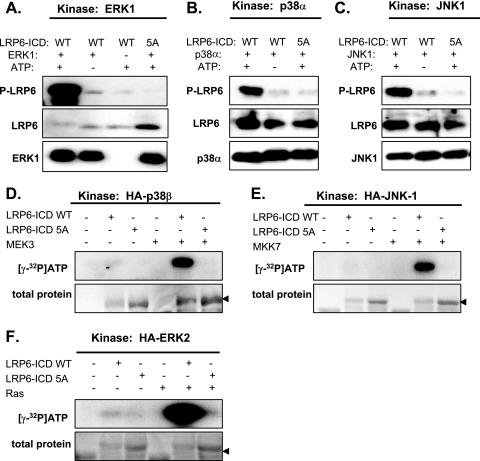
Phosphorylated PPPS/TP clusters are capable of directly inhibiting GSK3 and promoting axin recruitment to the membrane, which together result in decreased phosphorylation and degradation of β-catenin (12, 26, 30, 34). To test whether MAPKs can phosphorylate LRP6-ICD, we performed in vitro kinase reactions using three bacterially produced recombinant MAPKs: ERK1, p38α, and JNK1. As we show in Fig. 2A to C, all three tested kinases efficiently phosphorylated a bacterially purified, GST-fused LRP6-ICD substrate, as detected by a phosphospecific antibody recognizing serine/threonine phosphorylation within the first PPPSP cluster (anti-Ser1490-LRP6 antibody). Importantly, phosphorylation of an ICD with all PPPS/TP motifs mutated to alanines (LRP6-ICD-5A) by all three MAPKs tested was negligible, which suggests that MAPKs very specifically phosphorylate only serines and threonines within PPPS/TPs. In the next step, we tested phosphorylation of ICDs by MAPKs in a more physiological context. Selected MAPKs (ERK1, p38β, JNK1, ERK5) were transfected into HEK293 cells with or without upstream activators (MAPKKs or RAS), and the MAPKs were immunoprecipitated. The isolated kinases were then incubated with ICD substrates, and the phosphorylation was assessed by the incorporation of 32P into LRP6-ICD. We observed that physiologically activated forms of ERK1, p38β, and JNK1 all phosphorylated WT ICD substrates, but not ICD-5A. We also examined ERK5 (data not shown), because in our kinase screen it had emerged as a putative WNT-pathway kinase; however, it did not appear capable of phosphorylating LRP6-ICD in vitro.
FIG. 2.
MAPKs efficiently and specifically phosphorylate PPPS/TP motifs of LRP6 in vitro. Recombinant ERK1 (A), p38α (B), and JNK1 (C) were incubated with the wild-type (WT) or mutated (5A; all serines/threonines within PPPS/TP motifs mutated to alanines) recombinant intracellular domain of LRP6 (LRP6-ICD) in the presence or absence of ATP. Level of phosphorylation was determined by Western blotting with phospho-LRP6 (phospho-Ser1490) antibody. Antibodies against LRP6 and the kinase were used to confirm equal loading. (D to F) Indicated HA-tagged kinases were overexpressed in HEK293 cells with or without an upstream activator (MEK3 for p38β, MKK7 for JNK1, and RAS for ERK2) and purified by immunoprecipitation. Using GST-tagged LRP6-ICD substrates, a series of in vitro kinase reactions were performed with different MAP kinases with or without activation. If the reaction was successful, [γ-32P]ATP was incorporated into ICD substrate and detected on film. To identify whether the reaction involved the PPPS/TP motifs, the same reactions were performed using mutated LRP-ICD lacking functional motifs (LRP6-5A). Coomassie-stained gels demonstrate an equal amount of substrate in individual conditions (total protein).
These results demonstrated that several kinases from the MAPK family are able to specifically phosphorylate PPPS/TP motifs of LRP6. This phosphorylation in vitro does not require any priming by other kinases, thus suggesting that MAPKs can serve as important positive regulators of WNT/β-catenin signaling.
Active p38 and JNK1 are required for LRP6 phosphorylation and WNT/β-catenin pathway activation in HEK293 cells.
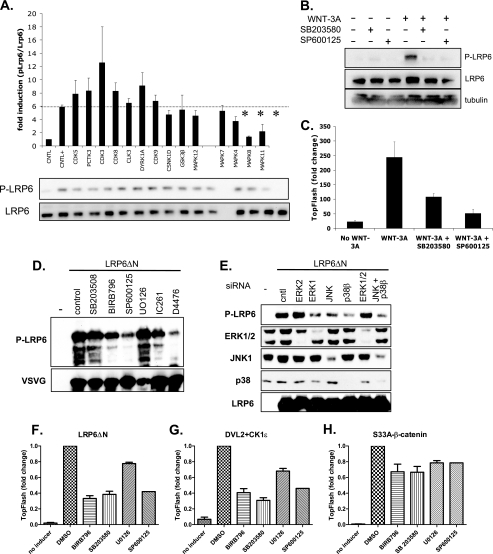
It was shown previously that several different kinases contribute to the WNT-induced phosphorylation of LRP6 (9, 13, 14, 36). Thus, we next analyzed the level of WNT-3A-induced phosphorylation of Ser1490-LRP6 in HEK293 cells transfected with siRNAs against several kinases identified by the siRNA screen (Fig. 1). As we show in Fig. 3A, the WNT-3A-induced phosphorylation of endogenous LRP6 was significantly attenuated by knockdown of JNK1 (MAPK8) and p38β (MAPK11). These results were confirmed by pharmacological inhibition of p38 by SB203580 and that of JNK by SP600125 (Fig. 3B). Changes in the phosphorylation of LRP6 were paralleled by changes in TCF/LEF-dependent transcription analyzed by the TopFlash reporter system (Fig. 3C). Phosphorylation of LRP6 is under normal conditions under tight control. However, it was shown earlier that deletion of the extracellular domain of LRP6 (LRP6ΔN) results in the constitutive phosphorylation of PPPS/TP clusters, likely via its oligomerization, and subsequent activation of downstream signaling (21). In Fig. 3D, we show that inhibition of p38 and JNK, but not of MEK/ERK, downregulates the phosphorylation of LRP6ΔN. Inhibitors of CK1, which were previously shown to phosphorylate LRP6 (14, 36), were used as a positive control. Knockdown of JNK1 and of p38β, but not of ERK1/2, showed similar effects toward constitutively active LRP6ΔN in line with the pharmacological inhibition of the kinases (Fig. 3E). These findings demonstrate that JNK and p38 contribute to the phosphorylation of endogenous LRP6 in response to WNT and of constitutively active LRP6.
FIG. 3.
JNK1 and p38β are required for LRP6 phosphorylation and WNT/β-catenin pathway activation in HEK293 cells. (A) Using siRNAs specific for the previously identified hits from the kinome screen, we tested whether these kinases affect the phosphorylation of LRP6. Results from Western blotting were quantified via densitometry. Knockdown of MAPK8 (p38) and MAPK11 (JNK) appeared to most strongly correlate with reduced LRP6 phosphorylation. HEK293 cells were then treated with specific chemical inhibitors for JNK (SP10025; 1 μM) and p38 (SB203580; 10 μM) and assayed for pLRP6 (B) or TopFlash luminescence (C). (D) HEK cells were transfected with constitutively active LRP6 (LRP6ΔN) and were treated with the indicated inhibitors of MAPKs (SB203508 and BIRB769 for p38, SP600125 for JNK1, UO126 for the MEK/ERK1/2 pathway, and IC261 and D4476 for CK1). The contribution of individual kinases to the phosphorylation of PPPS/TP motifs of LRP6 was determined by Western blotting using pSer1490-LRP6 antibody. Probing against VSVG visualized LRP6ΔN. (E) Indicated MAPKs were downregulated in HEK293 cells using siRNA targeted against the indicated MAPK. Phosphorylation of LRP6ΔN was determined 2 days later by Western blotting of phospho-Ser1490-LRP6. Efficiency of the knockdown was confirmed by probing against ERK1/2, JNK1, p38, and LRP6. (F to H) TCF/LEF-dependent transcription in HEK293 cells was induced by transfection of LRP6ΔN (F), the combination of DVL2 and CK1ɛ (G), and by constitutively active S33A-β-catenin (H). Cells were treated with 10 μM BIRB796 and 10 μM SB203580 to inhibit p38 kinases, with 50 μM SP600125 to inhibit JNK kinases and with 10 μM UO126 to inhibit activation of ERK1/2. TCF/LEF-dependent transcription was measured using the TopFlash luciferase reporter.
To further identify the level at which MAPK inhibition blocks TCF/LEF-dependent transcription, we performed epistatic experiments. TCF/LEF-mediated transcription was induced by overexpression of constitutively active LRP6 (LRP6ΔN), by overexpression of DVL2 with its activating kinase CK1ɛ, and by overexpression of the activated form of β-catenin (S33A-β-catenin) (Fig. 3F to H). The inhibition of p38 by BIRB796 and SB203580, and by JNK inhibitor SP600125, efficiently blocked TopFlash reporter activation induced by LRP6ΔN and DVL2/CK1ɛ by approximately 60 to 70%, whereas they reduced TopFlash transcription induced by S33A-β-catenin only by 20 to 25% (Fig. 3F to H). Inhibition of the MEK/ERK1/2 pathway by UO126 led to a reduction only by approximately 20% under all conditions (Fig. 3F to H), which suggests that ERK1/2 also interferes with the WNT/β-catenin pathway, but not upstream of β-catenin. In summary, this panel shows that in HEK293 cells, p38 and JNK, but not ERK1/2, are required for both WNT-induced and endogenous phosphorylation of LRP6 and ultimately for the activation of the WNT/β-catenin pathway.
p38β, JNK, and ERK1/2 increase LRP6-mediated activation of the WNT/β-catenin pathway and promote membrane localization of axin clusters.
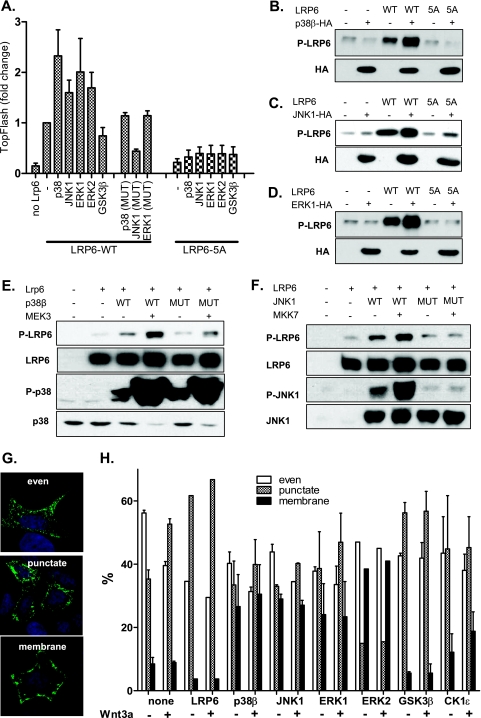
Our results presented in Fig. 2 demonstrate that p38α, p38β, ERK1, ERK2, and JNK1 efficiently phosphorylate LRP6 PPPS/TP motifs in vitro. We have thus tested whether individual MAPKs differ in their abilities to promote LRP6-induced TopFlash reporter activity. As we show in Fig. 4A, all tested MAPKs—p38β, ERK1, ERK2, and JNK1—but not kinase mutants with lower or no kinase activity (p38β-T106M, ERK1-K72R, JNK1-T183A/Y185F), efficiently synergize with LRP6 in the induction of TCF/LEF-driven transcription. Importantly, this effect is dependent on the presence of PPPS/TP motifs. MAPKs fail to promote TopFlash reporter activity when coexpressed with LRP6-5A, which has all serines/threonines within PPPS/TP motifs mutated to alanines (Fig. 4A). The MAPK-mediated increase in the TCF/LEF-driven transcription is accompanied by increased phosphorylation of overexpressed LRP6 but not LRP6-5A at Ser1490. We demonstrate this phenomenon for p38β (Fig. 4B), JNK1 (Fig. 4C), and ERK1 (Fig. 4D). Activation of MAPKs by upstream kinases further potentiates their effects on LRP6 phosphorylation. We show this for p38β and MEK3 (Fig. 4E) as well as JNK1 and MKK7 (Fig. 4F). Kinase variants, which either have decreased kinase activity (such as p38β-T106M) or cannot be activated because of the mutations of key residues required for activation by MAPKK (such as JNK1-T183A/Y185F), show very low or no effect in these assays (Fig. 4E and F).
FIG. 4.
MAPKs promote LRP6 phosphorylation and WNT/β-catenin signaling in cells. (A) HEK293 cells were transfected with plasmids encoding full-length LRP6 (LRP6WT) or LRP6 mutants with all PPPS/TP sites mutated to PPPAP (LRP6-5A) in combination with indicated MAPKs. Synergistic effects of MAPKs (but not their mutated variants, p38β-T106M, ERK1-K72R, and JNK1-T183A/Y185F) and LRP6WT, but not MAPKs and LRP6-5A on TCF/LEF-dependent transcription were found using the TopFlash reporter system. (B and C) Cells were transfected as described for panel A with p38β (B), JNK1 (C), and ERK1 (D), and the phosphorylation of LRP6 was determined by Western blotting. Presence of overexpressed kinases is indicated by probing against the HA tag. (E and F) HEK293 cells were transfected as indicated (mutated MAPKs were identical to those described for panel A), and the phosphorylation of LRP6 at Ser1490 was determined by Western blotting. The activities of MAPKKs (MEK3 and MKK7) were confirmed by probing against activated forms of p38 (phospho-Thr180/Tyr182) and JNK1 (phospho-Thr183/Tyr185). (G) HEK293 cells were transfected with AU-axin1 together with the plasmids encoding proteins as indicated. The cells were either stimulated or not with rmWNT-3A. Cells were fixed and stained for axin, and based on the axin distribution, they were sorted into the following three categories: even, punctate, and membranous. Axin distribution under each condition was determined by counting of at least 200 cells in three independent replicates and is shown as mean ± SEM (H). Blue, DAPI; green, axin-GFP.
Phosphorylation of LRP6 results in the recruitment of axin, visible either as axin membrane localization or as recruitment of axin to so-called signalosomes (3). The functional importance of these two localization patterns of axin is so far unclear, but it is believed that both reflect activation of LRP6, whereas homogenously distributed axin represents axin in the destruction complexes. Overexpressed axin is localized in a mixed pattern, with even distribution in approximately 55% of cells, axin in clusters in 35% of cells, and axin localized to the membrane in 10% of cells (Fig. 4G). The punctate axin pattern was increased at the expense of evenly distributed axin by WNT-3A stimulation or by overexpression of LRP6 (even, 30 to 40%; punctate, 55 to 65%) (Fig. 4H), which correlates with previously described observations that activation of LRP6 promotes formation of axin-containing signalosomes (3). Punctate localization of axin was also promoted by overexpression of GSK3 or CK1ɛ, two kinases already linked to the phosphorylation of LRP6 (36). In line with our previous observations, overexpression of MAPKs—p38β, JNK1, ERK1, and ERK2—led to a more heterogenous axin distribution and promoted cluster formation. These clusters have been predominantly associated with the plasma membrane (10% of membrane localization in control versus 25 to 30% in MAPK-overexpressing cells) (Fig. 4H). These results suggest that MAPKs are capable of promoting WNT/β-catenin signaling by phosphorylation of LRP6, which is accompanied by typical changes in axin localization, and with LRP6 activation.
Stimulus-dependent activation of LRP6 by MAPKs.
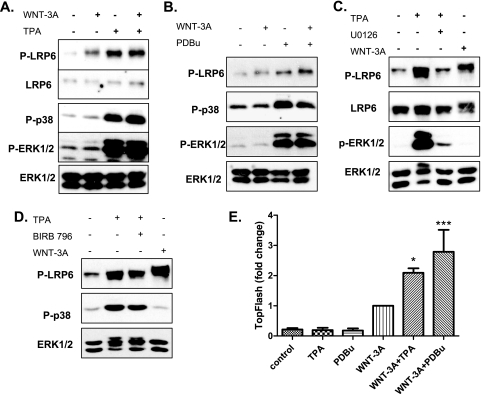
Our results demonstrate that p38, JNK, and ERK1/2 are each able to activate phosphorylation of LRP6, both in cell-free assays and in cells, and similarly promote LRP6 downstream signaling. However, loss-of-function experiments showed that only p38β and JNK1 are required for LRP6 phosphorylation in response to WNT in unstimulated HEK293 cells (Fig. 2). Based on these findings, we decided to test if extracellular stimuli leading to the activation of ERK1/2 may converge with the WNT-induced pathway to promote the phosphorylation and activation of LRP6 in an ERK1/2-dependent manner. Initially, we tested phorbol-12-myristate-13-acetate (TPA) and phorbol-12,13-dibutyrate (PDBu) for their abilities to promote phosphorylation of endogenous LRP6 at Ser1490. Treatment with phorbol esters TPA and PDBu, which activate protein kinase C and subsequently p38 and ERK1/2, increased not only phosphorylation of p38 and ERK1/2 but also that of LRP6 at Ser1490 (Fig. 5A and B). This increase was robust and comparable in intensity to the effects of WNT-3A. However, it is worth noting that although TPA induces Ser1490 LRP6 phosphorylation it does not promote the phosphorylation-dependent shift of LRP6 (compare treatment with TPA and WNT-3A). Importantly, TPA-induced phosphorylation of LRP6 was dependent on the activation of ERK1/2 but not on the activity of p38 (Fig. 5C and D), which suggests context-specific induction of LRP6 kinases, namely, that activation of ERK1/2 kinases by TPA was sufficient to shift the predominant LRP6 kinase from p38/JNK to ERK1/2 kinases (compare Fig. 5C and 3B). Of interest, whereas TPA/PDBu stimulation alone was insufficient to trigger the TopFlash reporter, the effects of TPA/PDBu were synergistic with WNT-3A both at the level of LRP6 phosphorylation and at the level of TCF/LEF-dependent transcription (Fig. 5E).
FIG. 5.
External MAPK activators promote LRP6 phosphorylation and WNT/β-catenin-dependent transcription. (A and B) HEK293 cells were treated with TPA (A) or PDBu (B) and rmWNT-3A as indicated. Activation of LRP6 was monitored using pSer1490 antibody, and activation of MAPKs was monitoral using P-p38 and P-ERK1/2 antibodies. Antibodies against total LRP6 and total ERK1/2 were used as a loading control. (C and D) TPA-induced phosphorylation of LRP6 is dependent on the activation of ERK1/2, as demonstrated by the sensitivity to UO126 treatment (C) and no sensitivity to p38-specific inhibitor BIRB796 treatment (D). (E) TPA- or PDBu-induced phosphorylation of LRP6 promotes WNT-3A-induced activation of the TopFlash reporter. Results represent mean ± SD of three independent replicates. *, P < 0.05; ***, P < 0.001 (one-way analysis of variance [ANOVA]; Tukey's posttest).
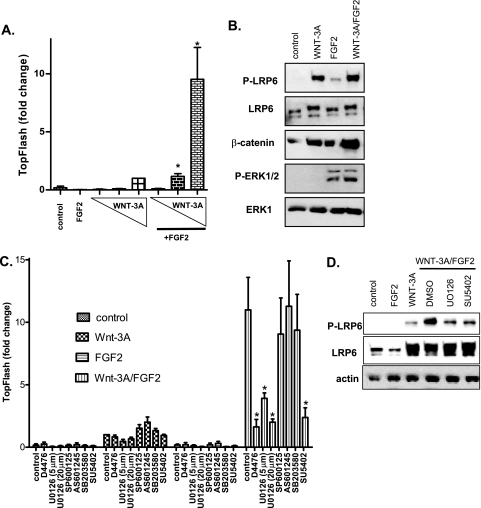
To explore the relevance of our observations regarding the role of MAPKs in WNT signaling in HEK293 cells, we used rat chondrosarcoma (RCS) cells, which have extremely low endogenous activity for MAPKs but retain good responsiveness to external stimuli at the level of MAPK activation and WNT-induced transcriptional response. In RCS cells, fibroblast growth factor 2 (FGF2) induces specific activation of ERK1/2 via the FGFR3 receptor (19). As we show in Fig. 6A, WNT-3A is capable of inducing TCF/LEF-dependent transcription in RCS cells in a dose-dependent manner, as demonstrated by the TopFlash reporter system. Treatment with noncanonical WNT-5a did not show any effect (not shown). Treatment with FGF2 dramatically enhanced WNT-3A-induced TopFlash reporter activity almost 10-fold (Fig. 6A). FGF2 alone promotes (or synergizes with WNT-3A, in the case of cotreatment) the phosphorylation of LRP6 and accumulation of β-catenin (Fig. 6B). To confirm that the effects of FGF2 are mediated by ERK1/2 kinases, we tested a panel of inhibitors directed against FGFR receptors (SU5402), MEK1 (ERK1/2-activating kinases, UO126), JNK (SP600125, AS601245), and p38 (SB203580) for their abilities to block WNT-3A-induced and WNT-3A/FGF2-induced TCF/LEF-dependent transcription. As we show in Fig. 6C, inhibitors of JNK and p38 were unable to block TopFlash reporter induced by WNT-3A/FGF2, whereas inhibition of FGFR3 and ERK1/2-activation almost completely abolished the synergistic effects of FGF2 on WNT-3A-induced transcription. UO126 and SU5402 effects on WNT-driven transcription were mirrored at the level of phosphorylation of LRP6, which was promoted by FGF2 and was sensitive to FGFR3/MEK inhibition (Fig. 6D). In summary, these results demonstrate that FGF2 promotes phosphorylation of LRP6 in an ERK1/2-dependent manner, thereby enhancing WNT-induced signaling, and in a more general view, the results document that LRP6 kinases can differ depending on the cell type and upstream stimulation of mitogenic pathways.
FIG. 6.
FGF2 promotes Lrp6 phosphorylation and TCF/LEF-dependent transcription in an FGFR3/MEK1-dependent manner in RCS cells. (A) RCS cells were transfected with the Super TopFlash reporter and stimulated as indicated in Materials and Methods. Increasing doses of rmWNT-3A (10, 50, and 100 ng/ml) promote TCF/LEF-dependent transcription, which is further potentiated almost 10-fold by the addition of FGF2 (25 ng/ml). (B) Stimulation of RCS cells with FGF2 (25 ng/ml) results in the activation of ERK1/2, increased phosphorylation of LRP6, and increased levels of β-catenin as determined by Western blotting. ERK1 and DVL3 were used as loading controls. (C) FGF2-dependent increase in WNT-induced TopFlash reporter activity is fully dependent on the active FGFR receptor (SU5402 treatment) and activation of the MEK1/ERK1/2 kinases (UO126 treatment) but is not affected by the inhibition of JNK or p38 kinases (SP601245, AS601245, and SB203580 treatments). CK1 inhibitor D4476 was used as a positive control. (D) Similarly, FGF-induced increase in LRP6 phosphorylation is reduced by UO126 and SU5402 treatment, as demonstrated by Western blotting. Actin serves as a loading control. All Western blot data are representative of at least three independent replicates. Graphs show means ± SD from normalized samples (n > 3). Asterisks indicate statistically significant difference (P < 0.001; one-way ANOVA; Tukey's posttest) compared to controls (samples stimulated only with WNT-3A [panel A]; WNT-3A/FGF2/DMSO-treated samples [panel C]).
DISCUSSION
LRP6 is a key receptor required for the induction of WNT/β-catenin signaling. LRP6 is phosphorylated following WNT binding. Several kinases have been previously described to phosphorylate PPPS/TP motifs of LRP6, mainly GSK3 (36), GRK5/6 (9), and the cell cycle-regulated complex of cyclin Y and PFTK (13). Phosphorylation within PPPS/TP motifs cooperates with the WNT-induced phosphorylation of neighboring serines by CK1γ (14). Efficient phosphorylation of both motifs is required for signalosome formation (3), axin recruitment (29), and direct inhibition of GSK3 by the phosphorylated intracellular domain of LRP6 (12, 26, 34). In the present study we demonstrate for the first time that PPPS/TP motifs can be phosphorylated by distinct members of the MAPK family. Furthermore, we provide evidence that the identity of PPPS/TP kinases differs among individual cell types and that the intensity of PPPS/TP phosphorylation depends on the nature of the mitogenic stimulation.
MAPKs, namely, p38 and JNK, have been previously implicated as positive regulators of WNT/β-catenin signaling (1, 2, 32). However, our study identifies a novel mechanism by which MAPKs promote WNT/β-catenin and identifies ERK1/2, p38, and JNK1 as kinases, which contribute to the phosphorylation of PPPS/TP clusters of endogenous LRP6. It is unlikely that all these kinases contribute to LRP6 phosphorylation simultaneously. PPPS/TP modules are multiplied five times in the LRP6 intracellular domain and have been shown to act in a combinatorial fashion (22, 33). One explanation is that each kinase shows a bias toward a certain PPPS/TP motif and that distinct kinases cooperate to achieve full phosphorylation of a single LRP6 molecule. This possibility is supported by the fact that both JNK and p38 are required for LRP6 phosphorylation in HEK293 cells. Alternatively, the choice of a distinct LRP6 kinase can be determined by the cell state, e.g., activity status and compartmentation of putative PPPS/TP kinases. This will depend on many external factors (mitogens) and internal factors (cell fate, which will control expression of upstream mediators of MAPK activation such as FGFRs and EGFRs; cell cycle stage, etc.). Experimental support for this alternative comes from our experiments in HEK cells. WNT-induced LRP6 phosphorylation at Ser1490 is under normal conditions controlled by p38β and JNK1 (as identified by the siRNA screen). However, upon activation of ERK1/2 by acute TPA or PDBu treatment, LRP6 becomes phosphorylated in a U0126-sensitive manner. This suggests that ERK1/2 gets involved in Ser1490 LRP6 phosphorylation upon stimulation with these phorbol esters and that stimulation with TPA was sufficient for a switch in the predominant LRP6 kinase in the same cell type.
Our data do not support the possibility that MAPKs can promote downstream signaling and increase TCF/LEF-dependent transcription without additional WNT stimulation. Neither TPA/PDBu in HEK293 cells nor FGF2 in RCS cells is able to significantly increase TopFlash reporter activity on its own to mimic the effects of WNT-3A, despite the fact that they increase phosphorylation of LRP6 at Ser1490. However, upon WNT-3A stimulation they significantly increased the intensity of WNT/β-catenin signaling (2 to 3 times for TPA/PDBu in HEK293; 10 times for FGF2 in RCS cells). It is thus likely that the key events in the WNT signal transduction—possibly signalosome formation, DVL activation, or CK1γ phosphorylation at LRP6 (visible as a phosphorylation-dependent shift)—are under tight WNT control and that MAPK-mediated phosphorylation controls the amplitude of the signal. These observations may have broad implications, as the cooperation between mitogens and WNT/β-catenin signaling has been extensively documented in many different biological systems during development and tumor formation (18, 23, 31). Different mitogens activate kinase cascades, which result in the activation of MAPKs. Our findings, which demonstrate the involvement of MAPKs in the LRP6 phosphorylation, provide an unexpected and direct molecular link between mitogenic pathways and WNT signaling. Mitogen stimulation can positively interact with the WNT/β-catenin pathway also at other levels. For example, receptor tyrosine kinases can phosphorylate β-catenin at the C terminus (5, 15). Such phosphorylation promotes release of β-catenin from adherens junctions by interference with α-catenin interaction, increases β-catenin binding to BCL9-2, and increases the pool of β-catenin available for TCF/LEF-driven transcription (4, 5). Another described mechanism shows that EGFR-induced ERK1/2 can promote CK2-mediated phosphorylation of α-catenin, which disrupts α-catenin/β-catenin complexes and promotes β-catenin transactivation (17). On the other hand, mitogenic stimulation can also indirectly enhance WNT/β-catenin signaling by increasing the proportion of cells in the G2/M phase when LRP6 is increasingly phosphorylated by cyclin Y/PFTK (13). However, this phenomenon can be excluded from our experiments with RCS cells, which respond to FGF2 by slowing down the cell cycle and accumulation in the G1 cell cycle phase (19).
In summary, our study provides direct evidence that distinct MAPKs, including p38, ERK, and JNK groups, are sufficient and required for the phosphorylation of PPPS/TP motifs of the key WNT receptor LRP6. Direct phosphorylation of LRP6 by MAPKs provides a unique point for cross-talk between the WNT/β-catenin signaling and other major signaling pathways, such as those initiated by polypeptide growth factors, including FGF. Moreover, our findings raise the possibility that rather than the existence of a single LRP6 kinase dedicated to the phosphorylation of PPPS/TP sites in response to WNT, multiple kinases can act as LRP6 kinases, including MAPKs, and their selection within a repertoire of candidate kinases might depend on the cell type, the nature of the external stimuli, and the cell state.
Acknowledgments
This study was supported by grants from the Czech Science Foundation (204/09/0498, 204/09/J030, 204/09/H058, 301/09/0587; V.B. and P.K.), the EMBO Installation Grant (V.B.), and the Ministry of Education Youth and Sports of the Czech Republic (MSM 0021622430; V.B.); by the Academy of Sciences of the Czech Republic (AVOZ50040507, AVOZ50040702); and by grants from the Karolinska Institutet, the Swedish Medical Research Council (K2008-68P-20810-01-4, K2008-68X-20805-01-4), the Knut and Alice Wallenberg Foundation, the Swedish Cancer Foundation, the Swedish Foundation for the International Cooperation in Research and Higher Education (STINT) (G.S.), the National Institutes of Health (NIH 5P01HD022657-21A; W.R.W.), the Intramural Program, the National Institute of Dental and Craniofacial Research (J.W. and J.S.G.), and the Howard Hughes Medical Institute Research Scholars Program (J.W.).
We thank S. Yanagawa (Kyoto University, Kyoto, Japan), Mikhail Semenov and Xe He (Harvard Medical School, Boston, MA), Randy Moon (University of Washington, Seattle, WA), J. M. Graff (University of Texas, Dallas, TX), S. Byers (Georgetown University, Washington, DC), and Bart Williams (Van Andel Institute, Grand Rapids, MI) for providing plasmids.
We declare no competing interests.
Footnotes
Published ahead of print on 25 October 2010.
REFERENCES
- 1.Bikkavilli, R. K., M. E. Feigin, and C. C. Malbon. 2008. G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J. Cell Sci. 121:234-245. [DOI] [PubMed] [Google Scholar]
- 2.Bikkavilli, R. K., M. E. Feigin, and C. C. Malbon. 2008. p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J. Cell Sci. 121:3598-3607. [DOI] [PubMed] [Google Scholar]
- 3.Bilic, J., Y. L. Huang, G. Davidson, T. Zimmermann, C. M. Cruciat, M. Bienz, and C. Niehrs. 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316:1619-1622. [DOI] [PubMed] [Google Scholar]
- 4.Brembeck, F. H., M. Rosario, and W. Birchmeier. 2006. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 16:51-59. [DOI] [PubMed] [Google Scholar]
- 5.Brembeck, F. H., T. Schwarz-Romond, J. Bakkers, S. Wilhelm, M. Hammerschmidt, and W. Birchmeier. 2004. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 18:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryja, V., J. Pachernik, K. Soucek, V. Horvath, P. Dvorak, and A. Hampl. 2004. Increased apoptosis in differentiating p27-deficient mouse embryonic stem cells. Cell Mol. Life Sci. 61:1384-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryja, V., G. Schulte, and E. Arenas. 2007. Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate β-catenin. Cell. Signal. 19:610-616. [DOI] [PubMed] [Google Scholar]
- 8.Catling, A. D., H. J. Schaeffer, C. W. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, M., M. Philipp, J. Wang, R. T. Premont, T. R. Garrison, M. G. Caron, R. J. Lefkowitz, and W. Chen. 2009. G protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J. Biol. Chem. 284:35040-35048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell 127:469-480. [DOI] [PubMed] [Google Scholar]
- 11.Coso, O. A., M. Chiariello, J. C. Yu, H. Teramoto, P. Crespo, N. Xu, T. Miki, and J. S. Gutkind. 1995. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81:1137-1146. [DOI] [PubMed] [Google Scholar]
- 12.Cselenyi, C. S., K. K. Jernigan, E. Tahinci, C. A. Thorne, L. A. Lee, and E. Lee. 2008. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc. Natl. Acad. Sci. U. S. A. 105:8032-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson, G., J. Shen, Y. L. Huang, Y. Su, E. Karaulanov, K. Bartscherer, C. Hassler, P. Stannek, M. Boutros, and C. Niehrs. 2009. Cell cycle control of wnt receptor activation. Dev. Cell 17:788-799. [DOI] [PubMed] [Google Scholar]
- 14.Davidson, G., W. Wu, J. Shen, J. Bilic, U. Fenger, P. Stannek, A. Glinka, and C. Niehrs. 2005. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438:867-872. [DOI] [PubMed] [Google Scholar]
- 15.El-Hariry, I., M. Pignatelli, and N. R. Lemoine. 2001. FGF-1 and FGF-2 modulate the E-cadherin/catenin system in pancreatic adenocarcinoma cell lines. Br. J. Cancer 84:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 17.Ji, H., J. Wang, H. Nika, D. Hawke, S. Keezer, Q. Ge, B. Fang, X. Fang, D. Fang, D. W. Litchfield, K. Aldape, and Z. Lu. 2009. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol. Cell 36:547-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katoh, M. 2006. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 5:1059-1064. [DOI] [PubMed] [Google Scholar]
- 19.Krejci, P., V. Bryja, J. Pachernik, A. Hampl, R. Pogue, P. Mekikian, and W. R. Wilcox. 2004. FGF2 inhibits proliferation and alters the cartilage-like phenotype of RCS cells. Exp. Cell Res. 297:152-164. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. S., A. Ishimoto, and S. Yanagawa. 1999. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J. Biol. Chem. 274:21464-21470. [DOI] [PubMed] [Google Scholar]
- 21.Liu, G., A. Bafico, V. K. Harris, and S. A. Aaronson. 2003. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol. 23:5825-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald, B. T., C. Yokota, K. Tamai, X. Zeng, and X. He. 2008. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J. Biol. Chem. 283:16115-16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrew, L. L., S. Hoppler, and R. T. Moon. 1997. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech. Dev. 69:105-114. [DOI] [PubMed] [Google Scholar]
- 24.Orford, K., C. Crockett, J. P. Jensen, A. M. Weissman, and S. W. Byers. 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 272:24735-24738. [DOI] [PubMed] [Google Scholar]
- 25.Peters, J. M., R. M. McKay, J. P. McKay, and J. M. Graff. 1999. Casein kinase I transduces Wnt signals. Nature 401:345-350. [DOI] [PubMed] [Google Scholar]
- 26.Piao, S., S. H. Lee, H. Kim, S. Yum, J. L. Stamos, Y. Xu, S. J. Lee, J. Lee, S. Oh, J. K. Han, B. J. Park, W. I. Weis, and N. C. Ha. 2008. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One 3:e4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 28.Stokoe, D., B. Caudwell, P. T. Cohen, and P. Cohen. 1993. The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2. Biochem. J. 296:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 30.Tamai, K., X. Zeng, C. Liu, X. Zhang, Y. Harada, Z. Chang, and X. He. 2004. A mechanism for Wnt coreceptor activation. Mol. Cell 13:149-156. [DOI] [PubMed] [Google Scholar]
- 31.ten Berge, D., S. A. Brugmann, J. A. Helms, and R. Nusse. 2008. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135:3247-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton, T. M., G. Pedraza-Alva, B. Deng, C. D. Wood, A. Aronshtam, J. L. Clements, G. Sabio, R. J. Davis, D. E. Matthews, B. Doble, and M. Rincon. 2008. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 320:667-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf, J., T. R. Palmby, J. Gavard, B. O. Williams, and J. S. Gutkind. 2008. Multiple PPPS/TP motifs act in a combinatorial fashion to transduce Wnt signaling through LRP6. FEBS Lett. 582:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, G., H. Huang, J. Garcia Abreu, and X. He. 2009. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One 4:e4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng, X., H. Huang, K. Tamai, X. Zhang, Y. Harada, C. Yokota, K. Almeida, J. Wang, B. Doble, J. Woodgett, A. Wynshaw-Boris, J. C. Hsieh, and X. He. 2008. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng, X., K. Tamai, B. Doble, S. Li, H. Huang, R. Habas, H. Okamura, J. Woodgett, and X. He. 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438:873-877. [DOI] [PMC free article] [PubMed] [Google Scholar]