Abstract
The generation of robust T-cell-dependent humoral immune responses requires the formation and expansion of germinal center structures within the follicular regions of the secondary lymphoid tissues. B-cell proliferation in the germinal center drives ongoing antigen-dependent selection and the generation of high-affinity class-switched plasma and memory B cells. However, the mechanisms regulating B-cell proliferation within this microenvironment are largely unknown. Here, we report that cyclin D3 is uniquely required for germinal center progression. Ccnd3−/− mice exhibit a B-cell-intrinsic defect in germinal center maturation and fail to generate an affinity-matured IgG response. We determined that the defect resulted from failed proliferative expansion of GL7+ IgD− PNA+ B cells. Mechanistically, sustained expression of cyclin D3 was found to be regulated at the level of protein stability and controlled by glycogen synthase kinase 3 in a cyclic AMP-protein kinase A-dependent manner. The specific defect in proliferative expansion of GL7+ IgD− PNA+ B cells in Ccnd3−/− mice defines an underappreciated step in germinal center progression and solidifies a role for cyclin D3 in the immune response, and as a potential therapeutic target for germinal center-derived B-cell malignancies.
The T-cell-dependent (TD) adaptive immune response is typified by the generation of high-affinity antigen (Ag)-specific memory B cells and antibody-secreting plasma cells (PCs) that have undergone class switch recombination (CSR) to IgG or IgE isotypes. The generation of these cells has long been known to occur in secondary lymphoid tissues within the unique microenvironment of the germinal center (GC). After Ag binding and activation via the B-cell receptor complex (BCR), B cells migrate to the T-cell zone, where additional costimulation is provided by cognate helper T cells. A cohort of these activated B cells go on to colonize GCs, which upon maturation represent an oligoclonal expansion of Ag-specific B cells (14, 18).
Within the GC, B cells undergo rapid proliferative expansion in response to Ag bound by follicular dendritic cells (FDCs). In humans, histological examination of the GC reveals distinct dark and light zones defined by the presence of proliferating centroblasts and largely nonproliferating centrocytes, respectively. Evidence suggests that centroblasts represent early GC cells that undergo somatic hypermutation (SHM), CSR and proliferative expansion, while centrocytes undergo affinity-based selection and differentiation events, leading to commitment to the memory or PC sublineages and exit from the GC. Although the organization of the GC is less distinct in mice, recent work has shown that chemokine-directed migration, FDC interaction, and proliferation may distinguish early and late GC B cells (1). Despite significant advances in understanding GC B-cell differentiation using multiparameter flow cytometry and intravital imaging, the initial colonization of GC “founder” B cells and their subsequent proliferative expansion is not well understood.
Rapid proliferation is a defining characteristic of GC B cells. Therefore, understanding how proliferation is regulated within the GC will provide insight into associated differentiation and selection events. In eukaryotic cells, cell cycle progression is regulated by four broad categories of proteins: cyclins, cyclin-dependent kinases (CDKs), pocket proteins or retinoblastoma proteins (pRBs), and the WAF/CIP and INK4 families of CDK inhibitors. These proteins regulate the cell cycle at different phases. The enabling checkpoint, known as the restriction point, is at the G1/S boundary and is regulated by the D-type and E-type cyclins. Specifically, D-type cyclins regulate G1 progression and E-type cyclins regulate the G1/S transition. Extracellular mitogenic signals induce transcription of three isoforms of D-type cyclins (D1, D2, and D3). D-type cyclins pair with CDK4/6 to facilitate pRB phosphorylation, leading to its degradation and the release and activation of transcription factors, including E2F. Thus, D-type cyclins are key mediators connecting extracellular signals and G1-phase progression.
Normal B cells induce expression of both cyclin D2 and D3 (but not cyclin D1) upon stimulation by mitogenic and prosurvival factors (28, 35), and studies conducted by multiple groups suggest that cyclin D2 and D3 play nonredundant roles during B-cell development and activation (7, 16, 19, 20, 22, 36). B-cell development in cyclin D2-deficient (Ccnd2−/−) animals is relatively normal, with the exception of B1-cell development, and Ccnd2−/− B cells show reduced proliferation and delayed S-phase entry in response to BCR ligation (7, 16, 36). In addition, cyclin D3 may be overexpressed in a compensatory fashion in Ccnd2−/− B cells (16). In contrast, recent work has shown that cyclin D3 is important for early B-cell proliferation and maturation, a process relying on pre-BCR signaling, whereas cyclin D2 was dispensable (7). In the periphery, histological analyses showed that GCs in human lymphoid tissues express cyclin D3 (38, 39). Together, this body of work suggests that cyclin D2 and D3 exert both overlapping and distinct stage-specific roles in B-cell development.
The findings presented here focus on the role of cyclin D3 in the TD immune response. We provide the first direct evidence that B cells critically require cyclin D3 for GC-dependent high-affinity TD immune responses. This outcome is dictated by a requirement for cyclin D3 in the proliferative expansion of IgD− GL7+ mature GC B cells and the generation of IgG+ cells derived from this subset. We also show that cyclin D2 is regulated at the level of transcription and is repressed in GC B cells, correlating with BCL6 expression, whereas cyclin D3 is regulated at the level of protein stability via the cyclic AMP (cAMP)/protein kinase A (PKA)/glycogen synthase kinase 3 (GSK3) signaling cascade.
MATERIALS AND METHODS
Animals.
Ccnd3−/− mice were provided by Piotr Sicinski. Wild-type (WT) controls were Ccnd3+/+ and were generated along with Ccnd3−/− mice by interbreeding Ccnd3+/− animals. bWT and bCcnd3−/− were generated as follows: μMT animals (Jackson Laboratories) were exposed to a 5-Gy dose of irradiation. Approximately 6 h postirradiation, the animals were injected intravenously with bone marrow from WT or Ccnd3−/− mice. In some experiments, bone marrow cells from a single donor animal were divided among four to eight recipient animals. At 6 weeks after bone marrow transfer, the efficiency of reconstitution was tested by flow cytometric analysis of peripheral blood leukocyte populations. Animals showing full reconstitution of the B-cell population were used for experimentation.
Immunizations.
Immediately prior to injection, citrated sheep blood (Colorado Serum Company) was pelleted by centrifugation and washed twice with phosphate-buffered saline (PBS). Red blood cells were resuspended in PBS to a final concentration of 10% (vol/vol). Animals were injected intraperitoneally (i.p.) with 0.1 or 0.2 ml. NP19-KLH (Biosearch Technologies) was prepared in PBS, and a 4:1 (vol/vol) emulsion of NP19-KLH solution and CFA (Sigma) was prepared containing a final NP19-KLH concentration of 0.05 mg/ml. Animals were i.p. injected with 0.2 ml (i.e., 0.01 mg of NP19-KLH per animal).
Flow cytometry.
As indicated, animals were sacrificed and single cell suspensions were prepared from spleens by physical dissociation between glass slides. Red blood cells were removed by hypotonic lysis. Cells were blocked with anti-CD16/32 and stained with the indicated antibodies, according to the manufacturers' suggested protocols. All antibodies were from eBioscience or BD Biosciences. Where indicated, cells were first stained with 12.5 μg of biotin-conjugated PNA (Vector Laboratories)/ml, followed by staining with fluorescence-labeled streptavidin (eBioscience). For intracellular staining of BCL6, the cells were stained for surface markers, fixed with Cytofix/Cytoperm (BD Biosciences), washed and permeabilized with permeabilization buffer (eBioscience), and then permeabilized with 0.01% Triton X-100 (Sigma). Cells were fixed again before staining them with rabbit anti-BCL6 (Cell Signaling Technology), followed by fluorescein isothiocyanate-labeled anti-rabbit IgG (Jackson Immunoresearch). The data were acquired on a FACSCanto (BD Biosciences) using FACSDiva software (BD Biosciences). The data were analyzed with FlowJo software (Treestar, Inc.).
Histology.
As indicated, the animals were sacrificed, and the spleens were harvested and immediately frozen, following embedding in Tissue-Tek O.C.T compound (Sakura Finetek). Sections (5-μm thick) were prepared on glass slides, fixed in acetone, blocked with 5% fetal bovine serum (FBS), and stained with allophycocyanin-conjugated anti-B220 (eBioscience), fluorescein-conjugated PNA, and rabbit anti-Ki67 (Vector Laboratories), followed by anti-rabbit Cy3 (Jackson ImmunoResearch). Slides were mounted with Fluoro-Gel (Electron Microscopy Sciences) prior to imaging with a Zeiss Axio ImagerM1, using Slidebook software (Intelligent Imaging innovations).
In vivo proliferation.
Five days postimmunization animals were injected i.p. with 2 mg of bromodeoxyuridine (BrdU; Sigma) in PBS. At 6 to 7 h after BrdU injection, flow cytometric analysis of BrdU incorporation and DNA content was performed as follows: after surface staining, cells were fixed with Cytofix/Cytoperm (BD Biosciences) and washed and permeabilized with permeabilization buffer (eBioscience), followed by permeabilization with 0.01% Triton X-100 (Sigma). The cells were fixed again and treated with 300 μg/ml of DNase I (Sigma). The cells were then stained with fluorescence-labeled anti-BrdU (Invitrogen). Prior to acquisition, the cells were resuspended in buffer containing 7-amino-actinomycin D (7AAD) for staining of total DNA content.
Enzyme-linked immunosorbent assay (ELISA).
Polystyrene plates were coated with NP4-bovine serum albumin (BSA) or NP23-BSA, followed by blocking with BSA. Dilutions of serum collected at the indicated time points were added followed by detection using anti-IgM or anti-IgG coupled to AP. PNPP was added and absorbance was measured at 405 nm.
Germinal center sorting.
At 5 days postimmunization, the animals were sacrificed, and single cell suspensions were prepared from spleens by physical dissociation between glass slides. Cells were pooled and red blood cells were removed by hypotonic lysis. Between 10 and 90% of the total splenocytes were used for non-GC B-cell and GC B-cell sorting, respectively. The cells were labeled with a biotin-conjugated antibody cocktail, including anti-CD43 (BD Bioscience), anti-CD11c (eBioscience), and anti-GL7 (eBioscience) for non-GC or anti-CD43 (BD Bioscience), anti-CD11c (eBioscience), and anti-IgD (eBioscience) for GC. Target cells were purified by depletion of labeled nontarget cells using anti-biotin microbeads and LS columns (Miltenyi Biotech) according to the manufacturer's recommended protocol.
Reverse transcription-PCR (RT-PCR).
Total RNA was purified from ∼106 cells using NucleoSpin RNA II columns (Macherey-Nagel), according to the manufacturer's protocol. cDNA synthesis was performed using an Advantage RT-for-PCR kit (Clontech) with oligo(dT) primer. PCR was carried out with a serial dilution of cDNA using D2 forward (5′-TTC ATT GAG CAC ATC CTT CG-3′) and D2 reverse (5′-ATG CTG CTC TTG ACG GAA CT-3′) or using D3 forward (5′-CGC CCC TGA CTA TTG AGA AG-3′) and D3 reverse (5′-ATC CGC AGA CAT AGA GCA GG-3′) primers (IDT).
Inhibitor studies and Western blot analysis.
A total of 106 freshly isolated cells were cultured in 1 ml of RPMI 1640 containing 10% FBS and 10 μM H-89 dihydrochloride (Calbiochem), 10 μM LY294002 (Calbiochem), 10 mM LiCl (Sigma), or 100 μM N6,2′-o-dibutyryladenosine 3′,5′-cyclic monophosphate (Sigma), as indicated. At the indicated time points, cells were collected, whole-cell lysates were prepared, and equivalent cell numbers were resolved by SDS-PAGE. Where indicated, whole-cell lysates prepared from 24 h lipopolysaccharide (LPS)-stimulated CD43-negative B cells were used as a positive control. Western blotting and immunodetection were conducted with the following antibodies, followed by the appropriate horseradish peroxidase-labeled secondary (Jackson Immunoresearch): cyclin D2 (M-20) (Santa Cruz Biotechnology), cyclin D3 (DCS22), and phospho-GSK-3β (5B3) (Cell Signaling Technology). β-Actin, Erk, or Akt was used as a loading control.
RESULTS
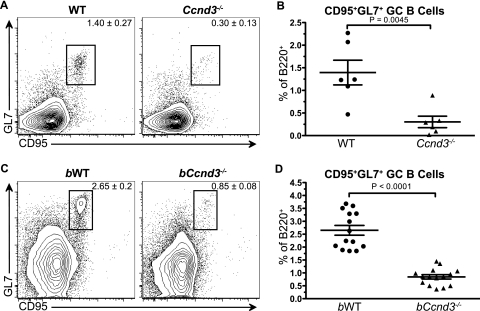
To directly assess the requirement for cyclin D3 during the adaptive immune response, we immunized WT and cyclin D3-deficient (Ccnd3−/−) animals with the TD Ag NP-KLH. At 10 days postimmunization, the presence of GCs was assessed by flow cytometry. Ccnd3−/− animals were found to have an ∼4-fold reduction in CD95+ GL7+ GCs as a proportion of B220+ B cells (Fig. 1A and B), indicating that cyclin D3 is required for the GC response and that cyclin D2 cannot compensate for its loss.
FIG. 1.
B cells require cyclin D3 for TD Ag-induced germinal centers. WT (•) and Ccnd3−/− (▴) animals (A and B) or sublethally irradiated (5 Gy) μMT mice reconstituted 8 weeks earlier with bone marrow from WT (bWT) (•) or Ccnd3−/− (bCcnd3−/−) (▴) mice (C and D) were immunized i.p. with NP19-KLH in CFA. At 10 days postimmunization, GC B-cell generation, indicated by CD95 and GL7 staining, was assessed by flow cytometry. Values indicate the gated population as a percentage of B220+ cells. The data shown represent two independent experiments with two to four animals per group (A and B) or four independent experiments with three to five animals per group (C and D). Graphs show the means and standard errors of the mean (SEM), along with the P value, as determined by using an unpaired t test.
Since Ccnd3−/− animals lack cyclin D3 in all tissues and it has been shown to play a role in early T-cell development (34), we could not conclude that the GC B-cell defect observed in Ccnd3−/− animals was B cell intrinsic. This issue was addressed by using chimeric animals generated by reconstituting sublethally irradiated B-cell-deficient μMT mice with bone marrow from WT or Ccnd3−/− animals, thereby creating mice with cyclin D3-sufficient (bWT) or -deficient (bCcnd3−/−) B cells. After complete reconstitution, bWT and bCcnd3−/− animals were immunized with NP-KLH. At 10 days postimmunization, the presence of GCs was assessed by flow cytometry. bCcnd3−/− animals were found to have a 3- to 4-fold reduction in CD95+ GL7+ GCs as a proportion of B220+ B cells (Fig. 1C and D); thus recapitulating the defect observed in Ccnd3−/− animals. This finding indicates that B cells specifically require cyclin D3 for the GC response and that the defect observed in Ccnd3−/− animals is B cell autonomous.
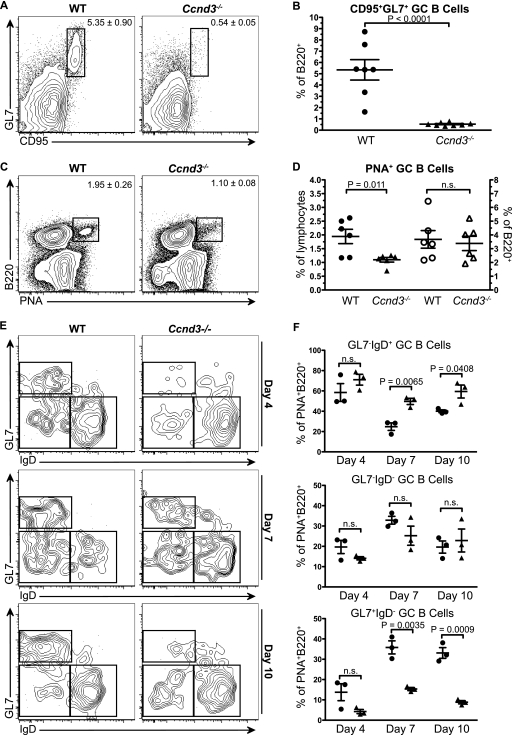
Although this initial observation clearly shows that B cells require cyclin D3 for a robust GC response, the Ccnd3−/− animals did generate a small but measurable population of CD95+ GL7+ GC B cells (Fig. 1). The dramatic reduction of GCs observed at the peak of the response could have resulted from reduced entry of B cells into the GC or from a failure of these early GC founder cells to properly expand and mature. To distinguish between these possibilities and to more clearly elucidate the role of cyclin D3 in the GC, we incorporated sheep red blood cell (SRBC) immunizations. The SRBC-induced GC response has well-defined kinetics and the added benefit of eliciting a more robust TD response than hapten-carrier immunization. The intensity of the SRBC response allows for better resolution of poorly defined subsets of cells within the GC and the ability to distinguish early GC cells from late/mature GC cells. We first confirmed that the GC defect observed in NP-KLH-immunized Ccnd3−/− animals was mirrored using SRBCs. WT and Ccnd3−/− mice were immunized with SRBCs and analyzed at the peak (day 7) of the immune response. Relative to WT controls, Ccnd3−/− animals were found to have a 10-fold reduction in CD95+ GL7+ GCs as a proportion of B220+ B cells (Fig. 2A and B), affirming that cyclin D3 is required for robust GC responses. In addition to formally addressing the contribution of cyclin D2 to GC formation, we also immunized Ccnd2−/− mice, which produced a robust GC response that was indistinguishable from WT mice (data available upon request). Thus, GC maturation is a cyclin D3-dependent process that does not require cyclin D2.
FIG. 2.
Cyclin D3 is required for germinal center maturation but is dispensable for germinal center initiation. (A and B) WT and Ccnd3−/− animals were immunized i.p. with SRBCs, and GC generation, as indicated by the presence of CD95+ GL7+ B cells, was assessed by flow cytometry 7 days postimmunization. (C and D) WT and Ccnd3−/− animals were immunized i.p. with SRBCs, and GC initiation, as indicated by the presence of PNA+ B cells as a percentage of total lymphocytes (filled symbols) or as percentage of B220+ cells (open symbols), was assessed by flow cytometry 4 days postimmunization. (E and F) WT (•) and Ccnd3−/− (▴) animals were immunized i.p. with SRBCs, and GC maturation, as indicated by GL7 and IgD profile of PNA+ B cells, was assessed by flow cytometry 4, 7, and 10 days postimmunization. The data shown represent two independent experiments with three to five animals per group (A to D) or three animals per group (E and F) per time point. Graphs show means and SEM, along with the P value, as determined by using an unpaired t test.
All GC B cells bind PNA beginning at the earliest stages of GC formation (33). We used this parameter as a means to determine whether the observed GC defect was a result of reduced early entry of B cells into the GC response. WT and Ccnd3−/− mice were immunized with SRBCs and examined at the initiation of the GC response (day 4 to 5) by flow cytometry (Fig. 2) and histology (data available upon request). Although Ccnd3−/− animals had a 40 to 50% reduction in PNA+ GC B cells as a percentage of total lymphocytes, the proportion of PNA+ B cells was unaltered in Ccnd3−/− animals (Fig. 2C and D). This observation was not surprising since it has been previously reported that Ccnd3−/− mice have a reduction in peripheral B cells resulting from defects in early B-cell development (7). However, the presence of normal numbers of PNA+ GC B cells as a proportion of total B220+ B cells at this time point (Fig. 2C and D) suggests that cyclin D3 is not required for GC initiation. This interpretation is further supported by the identification of proliferating (Ki67+) PNA+ cells in the GC at this stage (data available upon request).
Since Ccnd3−/− mice have an intact PNA+ GC B-cell compartment early in the response but display a dramatic reduction in CD95+GL7+ B cells at the peak of the response, we sought to better understand the changes that occur during GC expansion to define the role of cyclin D3 in this process. Although GL7 is commonly used as a marker for GC B cells, it has been shown that 30 to 40% of PNA+ GC B cells are GL7− at day 8 of the response (33). Furthermore, fluctuations in the GL7+ and GL7− populations may occur throughout the response, suggesting some heterogeneity in the B-cell subsets identified by this marker.
GC B cells are widely regarded as being IgD-negative; however, several reports over the past 2 decades have shown that a fraction of GC B cells in both humans and mice express surface IgD (23, 33) and that this subset may represent the initial expansion of the cohort of B cells that seed the GC (17). It has also been shown that the proportion of IgD+ cells in the GC may fluctuate throughout the response, a finding consistent with ongoing CSR (33). Although GL7 and IgD have been useful markers to costain GC B cells, they have not been used in conjunction with the pan-GC marker PNA to resolve subsets of B cells during maturation of the GC response. Therefore, we incorporated PNA, GL7, and IgD staining to elucidate the stage at which cyclin D3 expression becomes necessary to drive GC B-cell proliferation. WT and Ccnd3−/− mice were immunized with SRBCs, and GC maturation was assessed at multiple time points postimmunization using GL7, PNA, and IgD (Fig. 2E and F). At day 4 postimmunization, 60 to 70% of PNA+ B cells were identified as surface IgD+ and GL7− in both WT and Ccnd3−/− animals, suggesting that early GC cells are indeed IgD+ and GL7− and confirming that the initiation of the GC is intact in Ccnd3−/− mice. As the response progressed from days 7 to 10, the proportion of IgD− GL7+ GC B cells increased in WT animals to comprise the majority of GC B cells, accompanied by a concomitant decrease of IgD+ GL7− early GC B cells (Fig. 2E and F). However, Ccnd3−/− animals showed a dramatic 2- to 3-fold reduction in the proportion of IgD− GL7+ GC B cells (Fig. 2E and F). Accordingly, the population of IgD+ GL7− early GC B cells was overrepresented by ∼2-fold at the peak of the response in Ccnd3−/− mice. Thus, Ccnd3−/− mice can initiate a GC response, but the response fails to mature.
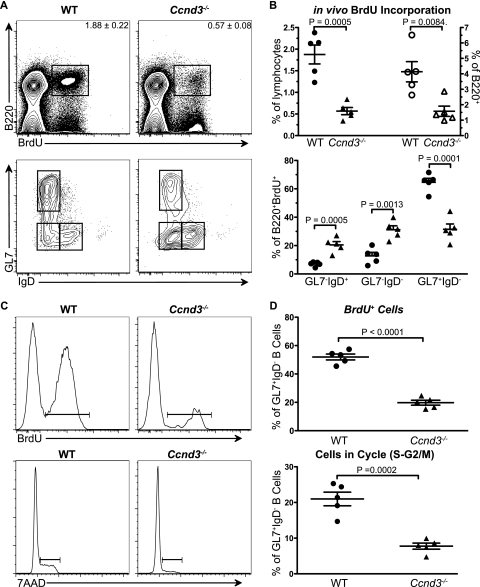
The failure of Ccnd3−/− mice to generate mature GCs may derive from defective proliferative expansion of GC B cells during the course of maturation. To address this possibility, we measured in vivo proliferation within the GC B-cell subsets. WT and Ccnd3−/− mice were immunized with SRBCs and administered BrdU for 6 to 7 h at 5 days postimmunization. Flow cytometric analysis revealed a 2- to 3-fold reduction in the percentage of BrdU+ proliferating B220+ B cells in Ccnd3−/− mice (Fig. 3A and B upper panels). Subsetting of BrdU+ B cells using IgD and GL7 markers showed a similar subset distribution as the PNA+ GC population in untreated mice (Fig. 2 and 3A and B, lower panels). This finding indicates that both early and more mature GC B cells proliferate in WT animals and that proliferation is not uncoupled as GC B cells mature.
FIG. 3.
Cyclin D3 is specifically required for the proliferative expansion of GL7+ IgD− mature germinal center B cells. WT and Ccnd3−/− animals were immunized i.p. with SRBCs. At 5 days postimmunization, the animals were injected i.p. with 2 mg of BrdU. (A and B) At 6 to 7 h postinjection, the in vivo proliferation and cell cycle status of GC B cells was measured by flow cytometry. B-cell proliferation, as indicated by BrdU incorporation (upper panels) as a percentage of total lymphocytes (filled symbols) and as a percentage of B220+ (open symbols) and the GC maturation state (GL7, IgD profile) of proliferating B cells (B220+ BrdU+) (lower panels), was assessed by flow cytometry. (C and D) Proliferation (upper panels) and cell cycle status (lower panels) of total IgD− GL7+ GC B cells from WT and Ccnd3−/− animals were measured. The data shown represent at least two independent experiments with two to three animals per group. Graphs show means and SEM, along with the P value, as determined by using an unpaired t test.
To determine whether cyclin D3 is required for the proliferative expansion of more mature GC B cells, we assessed in vivo proliferation and cell cycle progression in the IgD− GL7+ subset. In Ccnd3−/− mice, 2- to 3-fold fewer mature GC B cells incorporated BrdU during the labeling period (Fig. 3C and D, upper panels). Consistent with this result, we observed, in Ccnd3−/− mice, that 2- to 3-fold fewer mature GC B cells were in cell cycle, as measured by 7AAD staining of DNA content (Fig. 3C and D, lower panels). The percentage of subdiploid (apoptotic/necrotic) IgD− GL7+ cells was not increased in Ccnd3−/− mice 14 days postimmunization relative to controls. These data confirm that cyclin D3 is required specifically in IgD− GL7+ mature GC B cells for cell cycle progression and proliferative expansion, defining a previously unappreciated stage in the expansion and maturation of the GC response.
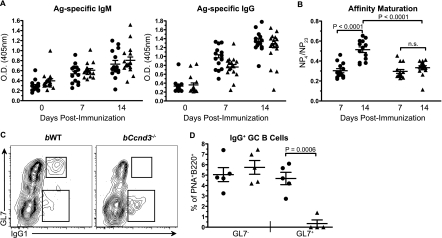
It is known that both the processes of CSR and PC differentiation are intimately linked to proliferation (12, 21). Therefore, we sought to determine the impact of a B-cell-intrinsic GC defect on humoral immunity. bWT and bCcnd3−/− mice were immunized with NP-KLH. Total Ag-specific (NP23) IgM and IgG were measured in sera collected 7 and 14 days postimmunization (Fig. 4A). NP-specific IgM and IgG antibody responses were generated in Ccnd3−/− animals, suggesting that cyclin D3 is dispensable for initial CSR, PC differentiation, and antibody secretion. This finding was unexpected given the recent study demonstrating a role for cyclin D3 in pre-BCR mediated proliferation during early B-cell development (7) but is consistent with our observation that initiation of the GC response is intact in Ccnd3−/− mice. Our data also highlight a functional distinction between pre-BCR signaling and Ag-induced BCR signaling on mature B cells and complements previous reports that implicate cyclin D2 as the critical D-type cyclin downstream of BCR activation (6).
FIG. 4.
Cyclin D3 is required for affinity maturation but is dispensable for early Ag-specific antibody production. bWT (•) and bCcnd3−/− (▴) animals were immunized i.p. with NP-KLH in CFA, and serum was collected at the indicated time points. Total Ag-specific IgM and IgG (A), and affinity maturation (B), determined by the ratio of high-affinity IgG to Ag-specific IgG, was measured by ELISA. The data shown is from four independent experiments with three to five mice per group. (C and D) The generation of IgG class switched B cells from GL7+ and GL7− GC B cells (B220+, PNA+) 14 days postimmunization was measured by flow cytometry. IgG1+ cells are represented as a percentage of PNA+ B cells. The data from five animals are shown. Graphs show means and SEM, along with the P value, as determined by using an unpaired t test.
Proliferation within the GC is known to be required for affinity-matured humoral immunity (40). However, resolution of the proliferative compartment of the GC response has not been achieved. Given the dramatic defect in GC maturation observed in Ccnd3−/− mice, we hypothesized that affinity maturation may be altered in these animals. High-affinity (NP4) and total (NP23) Ag-specific antibodies were compared as a measure of affinity maturation. Strikingly, affinity maturation was ablated in Ccnd3−/− mice (Fig. 4B). To characterize this defect at the cellular level, we quantified the generation of IgG class switched cells from GL7− and GL7+ PNA+ GC B cells in bWT and bCcnd3−/− mice by flow cytometry. Although there was no alteration in the proportions of GL7− IgG1+ GC B cells in bCcnd3−/− mice, a dramatic 10-fold reduction in GL7+ IgG1+ cells was observed (Fig. 4C and D). These data not only indicate that B cells require cyclin D3 for the process of affinity maturation but, taken together with the observed defects in GC B-cell subset, suggest that high-affinity B-cell clones are derived from the IgD− GL7+ pool.
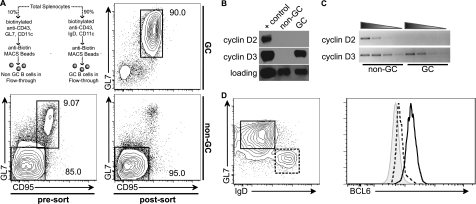
Given previous work indicating that both cyclin D2 and cyclin D3 are induced in B cells upon stimulation with diverse mitogenic stimuli, it is surprising that cyclin D3 would have such a profound role in the GC. This requirement could be a result of functional differences between cyclin D2 and cyclin D3, or a result of distinct expression patterns. To discriminate between these possibilities, we designed a novel magnetic bead separation scheme that would allow for the purification of “untouched” mature GC and non-GC B cells from the spleens of immunized mice. Using this scheme, we attained GC and non-GC (predominantly follicular) B-cell purities of ≥90% (Fig. 5A). Cyclin D2 protein was not detected in GC or non-GC B cells; however, cyclin D3 was only detected in GC B cells (Fig. 5B). To determine whether the lack of cyclin D2 protein in GC B cells was a result of transcriptional regulation, we performed semiquantitative RT-PCR on GC and non-GC B cells (Fig. 5C). Both cyclin D2 and cyclin D3 transcripts were readily detected in non-GC cells. However, only cyclin D3 could be detected in GC B cells. These data suggest that in follicular B cells both cyclin D2 and cyclin D3 are translationally or posttranslationally regulated, whereas in GC B cells cyclin D2 is transcriptionally repressed and cyclin D3 is regulated posttranslationally.
FIG. 5.
Cyclin D3 but not cyclin D2 is expressed in germinal center B cells. (A) GC and non-GC B cells were sorted from spleens pooled from 5 to 10 WT animals at 5 days after immunization with SRBCs, as indicated. Values indicate the gated population as a percentage of total cells and represent at least three experiments. Total protein or RNA was prepared from the sorted cells and subjected to Western blot (B) or semiquantitative RT-PCR (C) analysis for cyclin D2 or cyclin D3, as indicated. BCL-6 levels in GL7+ IgD− (solid line) and GL7− IgD+ (dashed line) PNA+ GC B cells were assessed by intracellular staining and flow cytometric analysis (D). The shaded histogram indicates the PNA− non-GC B-cell gate. The data shown are an average derived from at least three mice.
The presence of both cyclin D2 and cyclin D3 transcripts in follicular B cells and the absence of cyclin D2 transcripts in GC B cells suggests that GC B cells express a transcriptional repressor that is specific for cyclin D2. BCL6 is the most widely studied transcriptional repressor expressed in and required for GC formation. Recent studies have identified Ccnd2 as a bona fide target of BCL6 repression (3). Therefore, we hypothesized that BCL6 expression should mirror the requirement for cyclin D3 in the GC. We found that, whereas early GC cells slightly upregulated BCL6, full expression was only observed in mature GCs (Fig. 5D). These data correlate with the lack of cyclin D2 expression in mature GCs and the requirement for cyclin D3 specifically at this stage.
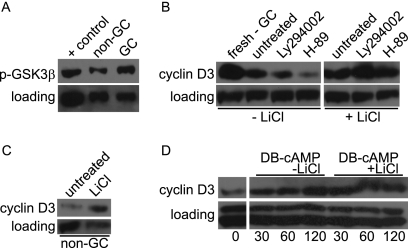
Based on our observation that cyclin D3 transcripts were observed in both follicular and GC B cells, whereas cyclin D3 protein was only detected in GC cells, and previous reports showing that cyclin D3 was regulated by pre-BCR mediated inhibition of proteosomal degradation (7), we hypothesized that GC-specific signaling events promote cyclin D3 protein stability. The proteosomal degradation of D-type cyclins upon phosphorylation of a conserved threonine residue by GSK3α/β has been previously reported (10). In addition, phosphorylation of GSK3α/β on serine 21/9 residues leads to reduced kinase activity (27). We hypothesized that GSK3α/β is phosphorylated and inactive in GC B cells, allowing for cyclin D3 protein accumulation. To test this hypothesis, we performed Western blot analysis on freshly isolated non-GC and GC B cells. More serine phosphorylated (S9) GSK3β was detected in GC B cells compared to non-GC B cells (Fig. 6A), suggesting that GSK3β is less active in GCs. To further test the hypothesis that GSK3β is regulating cyclin D3 in B cells, we utilized the GSK3-specific inhibitor LiCl. LiCl treatment of cultured GC B cells, which lose cyclin D3 when cultured in medium alone, prevented the loss of cyclin D3 (Fig. 6B). Similarly, treatment of non-GC B cells with LiCl resulted in enhanced cyclin D3 protein levels (Fig. 6C). Therefore, we conclude that GSK3α/β is active in follicular B cells, leading to cyclin D3 degradation and that GSK3β is phosphorylated and thereby inactivated in GC B cells, which is sufficient for cyclin D3 protein accumulation.
FIG. 6.
cAMP-PKA-GSK3 signaling regulates cyclin D3 stability. GC and non-GC B cells were sorted from spleens pooled from 5 to 10 WT animals at 5 days postimmunization with SRBCs. (A) Phosphorylation of GSK3 was measured by Western blot analysis. GC B cells were cultured for 120 min in LY294002, H-89, and/or LiCl, as indicated. (B) Cyclin D3 levels were measured by Western blot analysis. (C) Non-GC B cells were cultured for 30 min with or without LiCl, and the relative levels of cyclin D3 were measured by Western blot analysis. (D) Non-GC B cells were cultured in DB-cAMP with or without LiCl for the indicated time points, and the relative cyclin D3 levels were measured by Western blot analysis. Equal loading of the protein samples was confirmed by observation of the levels of β-actin, Erk, or Akt. The data are representative of at least three independent experiments.
Given our observation that GSK3β is phosphorylated in GC B cells, we sought to identify the kinases that act on GSK3α/β to promote cyclin D3 stability. Several kinases, including but not limited to PKA, AKT, PKC, p90Rsk, and p70S6K, have been shown to be capable of phosphorylating GSK3α/β in various cell types (27). Since phosphatidylinositol 3-kinase (PI3K) signaling regulates pre-BCR-induced cyclin D3 stability (7) and is thought to be required for GC B-cell proliferation and maturation (40), we examined the effect of PI3K inhibition on cyclin D3 stability in GC B cells. Surprisingly, treatment with the PI3K chemical inhibitor LY294002 did not enhance degradation of cyclin D3 (Fig. 6B). In contrast, inhibition of PKA, which is active in GCs and augments AID activity (25), with the chemical inhibitor H-89 resulted in a dramatic reduction in cyclin D3 protein (Fig. 6B). This effect was blocked with LiCl treatment (Fig. 6B), further supporting the hypothesis that PKA activity and inhibition of GSK3 results in cyclin D3 accumulation. To determine whether PKA activation is sufficient for preventing cyclin D3 degradation, we treated non-GC B cells with the cell-permeable cAMP analog N6,2′-o-dibutyryladenosine 3′,5′-cyclic monophosphate (DB-cAMP), which is known to activate PKA, in the presence or absence of LiCl. As we predicted, DB-cAMP treated non-GC B cells showed increased cyclin D3 levels relative to untreated samples (Fig. 6D), suggesting that activation of PKA by cAMP is sufficient for preventing cyclin D3 degradation. Taken together, these data support the hypothesis that factors in the GC microenvironment induce cAMP-PKA mediated phosphorylation and inactivation of GSK3, allowing for the sustained presence of cyclin D3 protein.
DISCUSSION
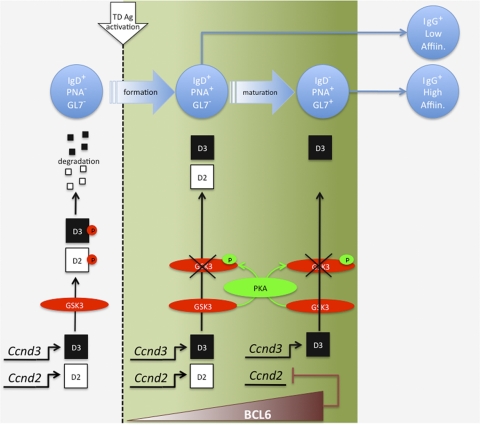
B cells responding to protein antigens recruit T-cell help to enable GC formation and the production of high-affinity memory B cells and antibody-producing cells. However, the subsequent stages of B-cell expansion, differentiation and selection that occur in the GC after initial T-cell encounter are poorly understood. In this report we identify cyclin D3 as the critical D-type cyclin driving B-cell proliferation in the germinal center, while cyclin D2 is dispensable for this process. The nature of this block in GC B-cell differentiation is outlined in Fig. 7. In Ccnd3−/− mice, some PNA+ (and CD95+) B cells are generated and undergo initial activation and expansion, indicating that cyclin D2 and cyclin D3 have redundant roles at this stage. Interestingly, these B cells are competent to undergo class switch recombination and produce PCs secreting low-affinity antigen-specific IgG. However, cyclin D3 expression becomes critical at a stage that is coincident with GL7 upregulation and IgD downregulation (Fig. 7). Hence, very few PNA+ GL7+ IgD− mature GC B cells are generated, and those that are present fail to incorporate BrdU or show evidence of cell cycle progression. These defects culminate in a greatly impaired ability to generate an affinity-matured IgG response. Recent studies using two-photon imaging techniques have shown that GC B cells traffic back and forth between the light (FDC-rich) and dark (non-FDC) zones of the germinal center and that proliferation occurs during this process (2, 13, 31). Although further analysis is required to substantiate GL7 as a marker for B cells undergoing affinity-based selection, GL7 could prove to be a useful marker to further our understanding of the specific processes of migration, proliferation, and affinity-based selection in vivo.
FIG. 7.
Overview of cyclin D2/D3 function and regulation in the germinal center response.
While the manuscript was under review, Peled et al. also reported a GC-specific defect in intact Ccnd3−/− mice (26). Consistent with our findings, the authors found that primary IgG1 responses and affinity maturation were impaired in these mice; however, they also found that generation of high-affinity antibody normalized with repeated immunization. Based on our findings that the affinity-matured IgG response 14 days postimmunization is dramatically reduced in Ccnd3−/− animals and that recruitment of cells into the GC is intact, while proliferative expansion of mature GC cells is reduced, it is possible that cyclin D3 is less crucial for the expansion of memory B cells and that repeated immunization draws more Ag-specific cells into the GC response.
Previous studies of Ccnd2−/− mice revealed a requirement for cyclin D2 in B1 cells and proliferative responses to BCR cross-linking (16, 36). Interestingly, responses to CD40L or LPS were unimpaired, suggesting a redundancy with cyclin D3 (16, 36). More recent studies have focused on the roles of cyclin D2/D3 in early B-cell proliferation. In this case, a predominant role for cyclin D3 was demonstrated for cytokine and pre-BCR-driven pro/pre-B cell expansion (7). However, loss of cyclin D3, but not cyclin D2, resulted in only a partial block at the pre-B cell stage, while the combined loss of cyclin D2 and cyclin D3 resulted in a complete block (7), again indicating some degree of redundancy. Unlike early B-cell differentiation, we demonstrated a completely nonredundant role for cyclin D3 in GC B-cell expansion. Although we readily detected both Ccnd2 and Ccnd3 transcripts in non-GC B cells, Ccnd3 expression could only be detected in GC B cells. The most likely explanation for this finding is the documented direct repression of Ccnd2, but not Ccnd3, expression by BCL6 (32). Accordingly, we observed an increase in BCL6 expression in GC B cells, coinciding with a decrease in Ccnd2 transcripts. These findings and interpretations are consistent with the recent report by Peled et al., who showed that forced expression of BCL6 cannot rescue GC formation in Ccnd3−/− mice (26). Thus, cyclin D2 and cyclin D3 contribute in a redundant fashion to promote early GC B-cell expansion. However, upon full upregulation of BCL-6 expression, cyclin D2 expression is abrogated, leaving cyclin D3 to sustain B-cell proliferation and enable the associated molecular events of class switch recombination and affinity maturation. Since BCL6 is not expressed in memory B cells (5, 15), both cyclin D2 and D3 likely contribute to the initial expansion of memory B cells upon Ag re-encounter.
Although cyclin D2 expression appears to be regulated at the level of transcription in GC B cells, our results indicate that cyclin D3 expression is regulated posttranslationally. The proteosomal degradation of D-type cyclins upon phosphorylation of a conserved threonine residue by GSK3α/β has been previously reported (10). In addition, phosphorylation of GSK3α/β on serine 21/9 (S21/9) residues is known to reduce kinase activity (27). Consistent with this report, we observed increased S9 phosphorylated GSK3β in GC B cells relative to non-GC B cells, suggesting that GSK3β activity is reduced in GC B cells. In addition, treatment of unstimulated GC B cells with the GSK3 inhibitor LiCl prevented the loss of cyclin D3 protein. Importantly, LiCl treatment of non-GC B cells led to the accumulation of cyclin D3 protein. Therefore, we conclude that GSK3α/β is active in follicular B cells, leading to cyclin D3 degradation, and that GSK3β is phosphorylated and thereby inactivated in GC B cells, allowing the accumulation of cyclin D3 (Fig. 7).
PKA, among several other kinases, has been shown to phosphorylate GSK3 in a cAMP-dependent manner (11). In addition, PKA has recently been shown to be active in GC B cells, where it was found to act on AID during class switch recombination (4). We observed that inhibition of PKA with the chemical inhibitor H-89 resulted in a dramatic reduction in cyclin D3 protein in GC B cells. Significantly, this effect was blocked by LiCl-mediated inhibition of GSK3. Conversely, treatment of non-GC B cells with the cell permeable cAMP analog DB-cAMP, which is known to activate PKA, increased the amount of cyclin D3. In contrast, we found that inhibition of PI3K with the chemical inhibitor LY294002 did not enhance degradation of cyclin D3. This finding may be viewed as surprising since Akt phosphorylates GSK3 on the same residues as PKA. However, a recent report by Rolf et al. revealed that the GC defect observed in PI3K p110δ−/− mice is T-cell intrinsic (29). Coupled with our and others findings that PI3K negatively regulates AID-dependent class switch recombination and somatic hypermutation (9, 24, 37), one may speculate that PI3K signaling is attenuated during GC B-cell differentiation. Taken together, our data support the hypothesis that factors in the GC microenvironment induce cAMP-PKA-mediated phosphorylation and inactivation of GSK3, allowing for the sustained presence of cyclin D3 protein.
In summary, we have identified cyclin D3 as being uniquely required for proliferative expansion of mature GC B cells, demonstrating that the accumulation of cyclin D3 in GCs results from cAMP-PKA-mediated phosphorylation of GSK3α/β and that inactivation of GSK3α/β is required for these processes. In addition, we have shown that cyclin D2 is not expressed in GCs, a finding consistent with it being a target of repression by BCL6. The findings reported here are likely a representation of cyclin D2/D3 regulation and function in the differentiation of human GC B cells (30, 39), which also express cyclin D3 exclusively. Cyclin D3 is also selectively upregulated in GC-derived Burkitt's lymphoma, follicular lymphoma, and some types of diffuse large B-cell lymphoma (30, 39). This pattern of expression is contrasted by studies of B-cell chronic lymphocytic leukemia and lymphoplasmacytic lymphoma, which are more indolent tumors and have been reported to express high amounts of cyclin D2 but do not express cyclin D3 (8).
Acknowledgments
We thank Piotr Sicinski for kindly providing the Ccnd3−/− mice, Alfredo Chavez and Buddy Charbono for animal care, and members of the Rickert lab for many helpful discussions and critical feedback on the manuscript.
This study was supported by the National Institutes of Health (AI041649 to R.C.R.).
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Allen, C. D., T. Okada, and J. G. Cyster. 2007. Germinal-center organization and cellular dynamics. Immunity 27:190-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, C. D., T. Okada, H. L. Tang, and J. G. Cyster. 2007. Imaging of germinal center selection events during affinity maturation. Science 315:528-531. [DOI] [PubMed] [Google Scholar]
- 3.Basso, K., M. Saito, P. Sumazin, A. A. Margolin, K. Wang, W. K. Lim, Y. Kitagawa, C. Schneider, M. J. Alvarez, A. Califano, and R. Dalla-Favera. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood 115:975-984. [DOI] [PMC free article] [PubMed]
- 4.Basu, U., J. Chaudhuri, C. Alpert, S. Dutt, S. Ranganath, G. Li, J. P. Schrum, J. P. Manis, and F. W. Alt. 2005. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature 438:508-511. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya, D., M. T. Cheah, C. B. Franco, N. Hosen, C. L. Pin, W. C. Sha, and I. L. Weissman. 2007. Transcriptional profiling of antigen-dependent murine B-cell differentiation and memory formation. J. Immunol. 179:6808-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiles, T. C. 2004. Regulation and function of cyclin D2 in B lymphocyte subsets. J. Immunol. 173:2901-2907. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, A. B., C. M. Sawai, E. Sicinska, S. E. Powers, P. Sicinski, M. R. Clark, and I. Aifantis. 2006. A unique function for cyclin D3 in early B-cell development. Nat. Immunol. 7:489-497. [DOI] [PubMed] [Google Scholar]
- 8.Delmer, A., F. Ajchenbaum-Cymbalista, R. Tang, S. Ramond, A. M. Faussat, J. P. Marie, and R. Zittoun. 1995. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood 85:2870-2876. [PubMed] [Google Scholar]
- 9.Dengler, H. S., G. V. Baracho, S. A. Omori, S. Bruckner, K. C. Arden, D. H. Castrillon, R. A. DePinho, and R. C. Rickert. 2008. Distinct functions for the transcription factor Foxo1 at various stages of B-cell differentiation. Nat. Immunol. 9:1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang, X., S. X. Yu, Y. Lu, R. C. Bast, J. R. Woodgett, and G. B. Mills. 2000. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. U. S. A. 97:11960-11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forni, L., M. Bjorklund, and A. Coutinho. 1988. Membrane expression of IgG but not maturation to secretion requires DNA replication. J. Mol. Cell. Immunol. 4:59-70. [PubMed] [Google Scholar]
- 13.Hauser, A. E., T. Junt, T. R. Mempel, M. W. Sneddon, S. H. Kleinstein, S. E. Henrickson, U. H. von Andrian, M. J. Shlomchik, and A. M. Haberman. 2007. Definition of germinal-center B-cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity 26:655-667. [DOI] [PubMed] [Google Scholar]
- 14.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo, T. C., A. L. Shaffer, J. Haddad, Y. S. Choi, L. M. Staudt, and K. Calame. 2007. Repression of BCL-6 is required for the formation of human memory B cells in vitro. J. Exp. Med. 204:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam, E. W., J. Glassford, L. Banerji, N. S. Thomas, P. Sicinski, and G. G. Klaus. 2000. Cyclin D3 compensates for loss of cyclin D2 in mouse B lymphocytes activated via the antigen receptor and CD40. J. Biol. Chem. 275:3479-3484. [DOI] [PubMed] [Google Scholar]
- 17.Lebecque, S., O. de Bouteiller, C. Arpin, J. Banchereau, and Y. J. Liu. 1997. Germinal center founder cells display propensity for apoptosis before onset of somatic mutation. J. Exp. Med. 185:563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, Y. J., J. Zhang, P. J. L. Lane, E. Y. T. Chan, and I. C. M. Maclennan. 1991. Sites of specific B-cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21:2951-2962. [DOI] [PubMed] [Google Scholar]
- 19.Mandal, M., S. E. Powers, K. Ochiai, K. Georgopoulos, B. L. Kee, H. Singh, and M. R. Clark. 2009. Ras orchestrates exit from the cell cycle and light-chain recombination during early B-cell development. Nat. Immunol. 10:1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mataraza, J. M., J. R. Tumang, M. R. Gumina, S. M. Gurdak, T. L. Rothstein, and T. C. Chiles. 2006. Disruption of cyclin D3 blocks proliferation of normal B-1a cells, but loss of cyclin D3 is compensated by cyclin D2 in cyclin D3-deficient mice. J. Immunol. 177:787-795. [DOI] [PubMed] [Google Scholar]
- 21.McCall, M. N., and P. D. Hodgkin. 1999. Switch recombination and germ-line transcription are division-regulated events in B lymphocytes. Biochim. Biophys. Acta 1447:43-50. [DOI] [PubMed] [Google Scholar]
- 22.Mohamedali, A., I. Soeiro, N. C. Lea, J. Glassford, L. Banerji, G. J. Mufti, E. W. Lam, and N. S. Thomas. 2003. Cyclin D2 controls B-cell progenitor numbers. J. Leukoc. Biol. 74:1139-1143. [DOI] [PubMed] [Google Scholar]
- 23.Nahm, M. H., P. A. Takes, M. B. Bowen, and K. A. Macke. 1989. Subpopulations of B lymphocytes in germinal centers, II. A germinal center B-cell subpopulation expresses sIgD and CD23. Immunol. Lett. 21:201-208. [DOI] [PubMed] [Google Scholar]
- 24.Omori, S. A., M. H. Cato, A. Anzelon-Mills, K. D. Puri, M. Shapiro-Shelef, K. Calame, and R. C. Rickert. 2006. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity 25:545-557. [DOI] [PubMed] [Google Scholar]
- 25.Pasqualucci, L., Y. Kitaura, H. Gu, and R. Dalla-Favera. 2006. PKA-mediated phosphorylation regulates the function of activation-induced deaminase (AID) in B cells. Proc. Natl. Acad. Sci. U. S. A. 103:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peled, J. U., J. J. Yu, J. Venkatesh, E. Bi, B. B. Ding, M. Krupski-Downs, R. Shaknovich, P. Sicinski, B. Diamond, M. D. Scharff, and B. H. Ye. 2010. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 20:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayasam, G. V., V. K. Tulasi, R. Sodhi, J. A. Davis, and A. Ray. 2009. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 156:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid, S., and E. C. Snow. 1996. The regulated expression of cell cycle-related proteins as B-lymphocytes enter and progress through the G1 cell cycle stage following delivery of complete versus partial activation stimuli. Mol. Immunol. 33:1139-1151. [DOI] [PubMed] [Google Scholar]
- 29.Rolf, J., S. E. Bell, D. Kovesdi, M. L. Janas, D. R. Soond, L. M. C. Webb, S. Santinelli, T. Saunders, B. Hebeis, N. Killeen, K. Okkenhaug, and M. Turner. 2010. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 185:4042-4052. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Beato, M., F. I. Camacho, J. C. Martinez-Montero, A. I. Saez, R. Villuendas, L. Sanchez-Verde, J. F. Garcia, and M. A. Piris. 1999. Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 94:765-772. [PubMed] [Google Scholar]
- 31.Schwickert, T. A., R. L. Lindquist, G. Shakhar, G. Livshits, D. Skokos, M. H. Kosco-Vilbois, M. L. Dustin, and M. C. Nussenzweig. 2007. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446:83-87. [DOI] [PubMed] [Google Scholar]
- 32.Shaffer, A. L., X. Yu, Y. He, J. Boldrick, E. P. Chan, and L. M. Staudt. 2000. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13:199-212. [DOI] [PubMed] [Google Scholar]
- 33.Shinall, S. M., M. Gonzalez-Fernandez, R. J. Noelle, and T. J. Waldschmidt. 2000. Identification of murine germinal center B-cell subsets defined by the expression of surface isotypes and differentiation antigens. J. Immunol. 164:5729-5738. [DOI] [PubMed] [Google Scholar]
- 34.Sicinska, E., I. Aifantis, L. Le Cam, W. Swat, C. Borowski, Q. Yu, A. A. Ferrando, S. D. Levin, Y. Geng, H. von Boehmer, and P. Sicinski. 2003. Requirement for cyclin D3 in lymphocyte development and T-cell leukemias. Cancer Cell 4:451-461. [DOI] [PubMed] [Google Scholar]
- 35.Solvason, N., W. W. Wu, N. Kabra, X. Wu, E. Lees, and M. C. Howard. 1996. Induction of cell cycle regulatory proteins in anti-immunoglobulin-stimulated mature B lymphocytes. J. Exp. Med. 184:407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solvason, N., W. W. Wu, D. Parry, D. Mahony, E. W. Lam, J. Glassford, G. G. Klaus, P. Sicinski, R. Weinberg, Y. J. Liu, M. Howard, and E. Lees. 2000. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B-cell development. Int. Immunol. 12:631-638. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, A., T. Kaisho, M. Ohishi, M. Tsukio-Yamaguchi, T. Tsubata, P. A. Koni, T. Sasaki, T. W. Mak, and T. Nakano. 2003. Critical roles of Pten in B-cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 197:657-667.12615906 [Google Scholar]
- 38.Suzuki, R., H. Kuroda, H. Komatsu, Y. Hosokawa, Y. Kagami, M. Ogura, S. Nakamura, Y. Kodera, Y. Morishima, R. Ueda, and M. Seto. 1999. Selective usage of D-type cyclins in lymphoid malignancies. Leukemia 13:1335-1342. [DOI] [PubMed] [Google Scholar]
- 39.Teramoto, N., K. Pokrovskaja, L. Szekely, A. Polack, T. Yoshino, T. Akagi, and G. Klein. 1999. Expression of cyclin D2 and D3 in lymphoid lesions. Int. J. Cancer 81:543-550. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Y., and R. H. Carter. 2005. CD19 regulates B-cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity 22:749-761. [DOI] [PubMed] [Google Scholar]