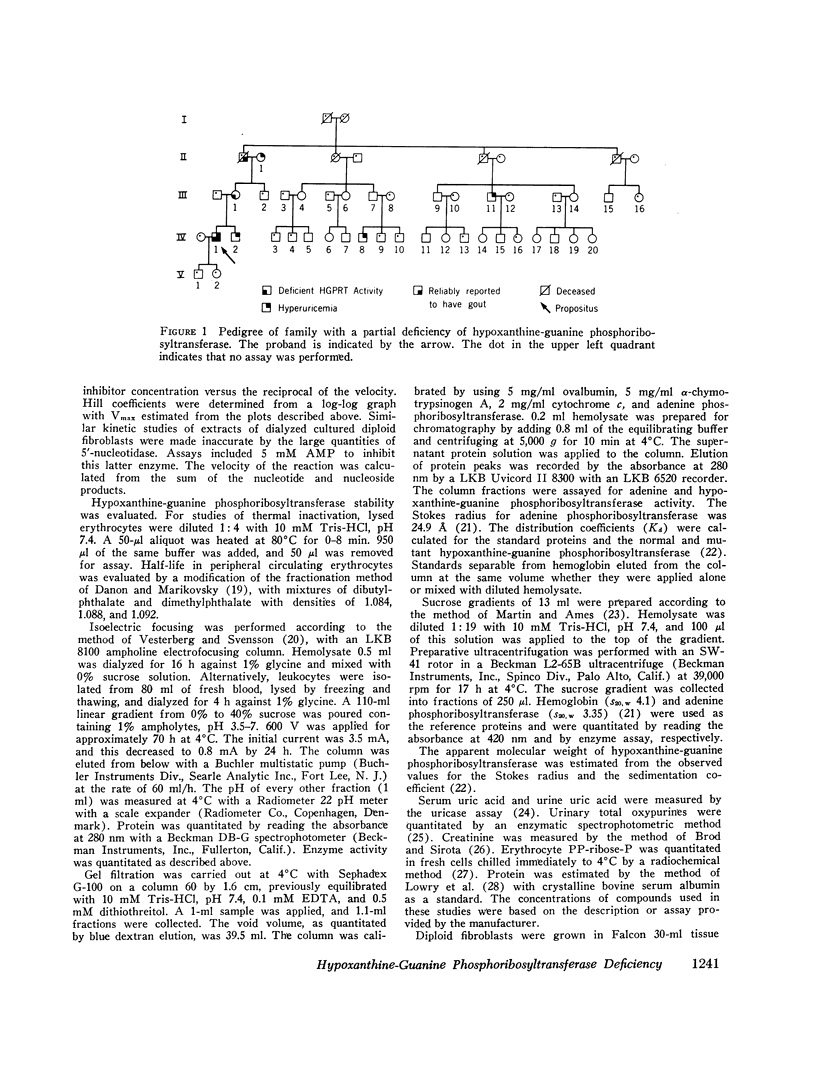
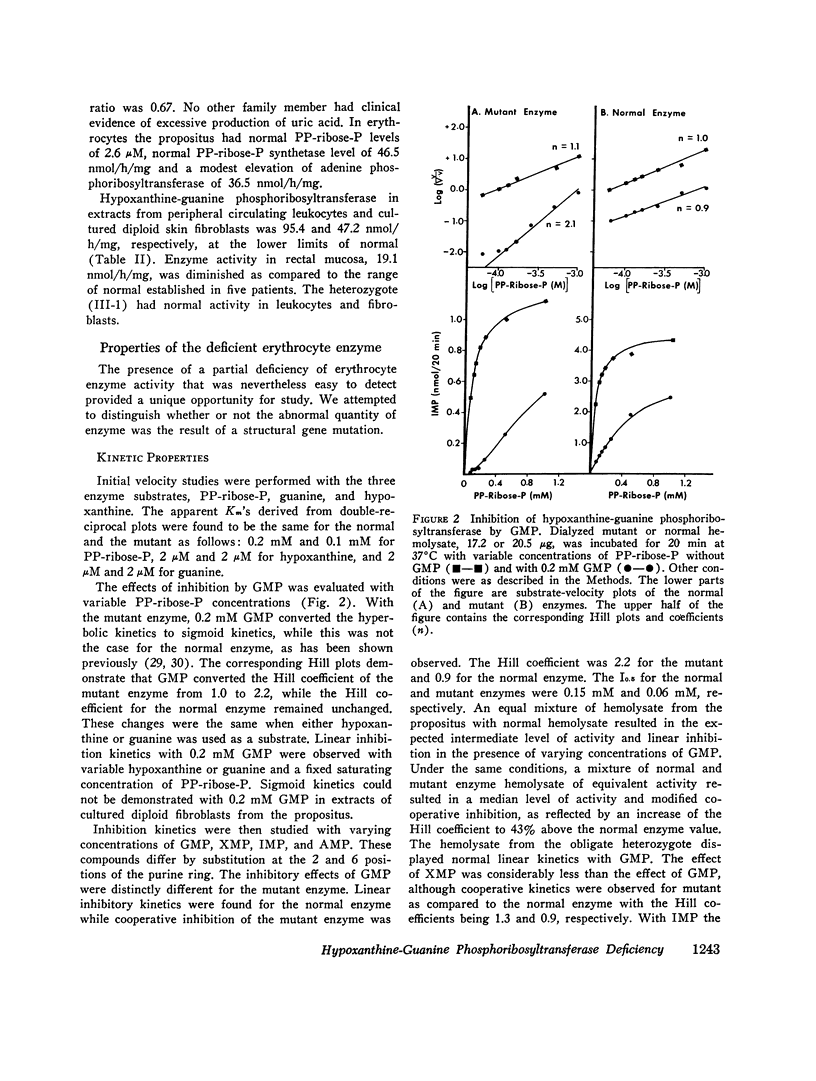
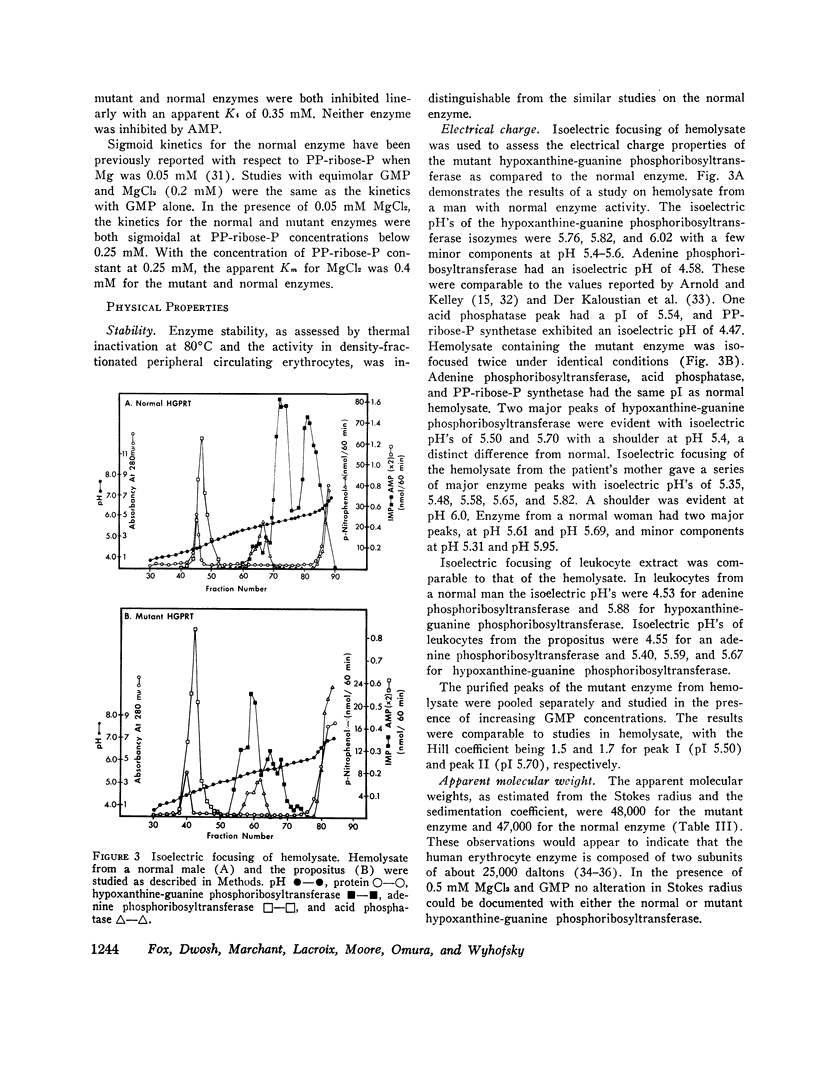
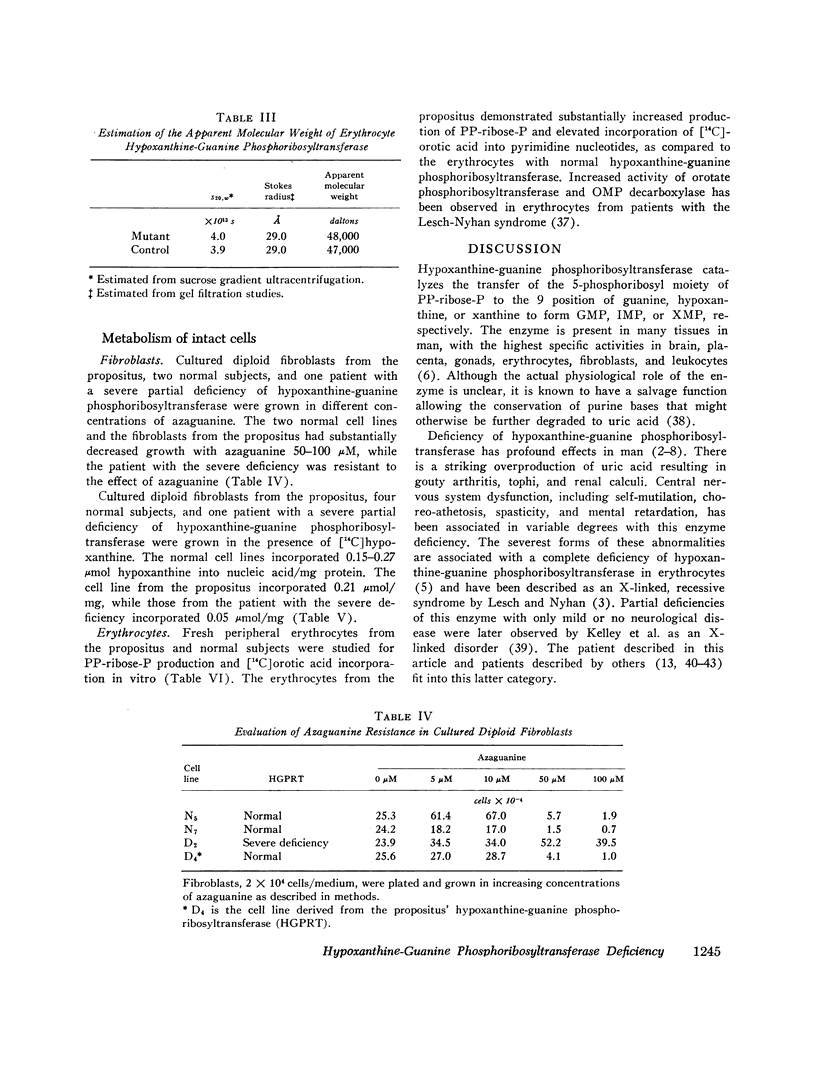
Abstract
The mutation in a young gouty male with a partial deficiency of hypoxanthine-guanine phosphoribosyltransferase has been evaluated. The serum uric acid was 11.8 mg/100 ml, and the urinary uric acid excretion was 1,279 mg/24 h. Erythrocyte hypoxanthine-guanine phosphoribosyltransferase was 34.2 nmol/h/mg, adenine phosphoribosyltransferase was 36.5 nmol/h/mg and phosphoribosylpyrophosphate was 2.6 muM. Hypoxanthine-guanine phosphoribosyltransferase from peripheral leukocytes and cultured diploid skin fibroblasts was within the normal range, but enzyme activity in rectal mucosa was below the normal range. Initial velocity studies of the normal enzyme and the mutant enzyme from erythrocytes with the substrates hypoxanthine, guanine, or phosphoribosylpyrophosphate showed that the Michaelis constants were similar. Product inhibition studies distinguished the mutant enzyme from the normal enzyme. Hyperbolic kinetics with increasing phosphoribosylpyrophosphate were converted to sigmoid kinetics by 0.2 mM GMP with the mutant enzyme but not with the normal enzyme. The mutant erythrocyte hypoxanthine-guanine phosphoribosyltransferase was inactivated normally at 80 degrees C and had a normal half-life in the peripheral circulation. The mol wt of 48,000 was similar to the normal enzyme mol wt of 47,000. With isoelectric focusing, the mutant erythrocyte enzyme had two major peaks with isoelectric pH's of 5.50 and 5.70, in contrast to the isoelectric pH's of 5.76, 5.82, and 6.02 of the normal isozymes. Isoelectric focusing of leukocyte extracts from the patient revealed the presence of the mutant enzyme. Cultured diploid fibroblasts from the propositus appeared to function normally, as shown by the inability to grow in 50-100 muM azaguanine and by the normal incorporation of [14C]hypoxanthine into nucleic acid. In contrast, erythrocytes from the patient displayed abnormal properties, including the increased synthesis of phosphoribosylphyrophosphate and elevated functional activity of orotate phosphoribosyltransferase and orotidylic decarboxylase. These unique kinetic, physical, and functional properties provide support for heterogeneous structural gene mutations in partial deficiencies of hypoxanthine-guanine phosphoribosyltransferase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., DeMars R. Diploid azaguanine-resistant mutants of cultured human fibroblasts. Science. 1970 Jul 31;169(3944):482–485. [PubMed] [Google Scholar]
- Arnold W. J., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and subunit structure. J Biol Chem. 1971 Dec 10;246(23):7398–7404. [PubMed] [Google Scholar]
- Arnold W. J., Meade J. C., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase: characteristics of the mutant enzyme in erythrocytes from patients with the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jul;51(7):1805–1812. doi: 10.1172/JCI106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L., Fawcett N., Kogut M. D. Isoenzymes of hypoxanthine-guanine-phosphoribosyl transferase in a family with partial deficiency of the enzyme. Biochem Genet. 1972 Aug;7(1):73–85. doi: 10.1007/BF00487011. [DOI] [PubMed] [Google Scholar]
- Beardmore T. D., Meade J. C., Kelley W. N. Increased activity of two enzymes of pyrimidine biosynthesis de novo in erythrocytes from patients with the Lesch-Nyhan syndrome. J Lab Clin Med. 1973 Jan;81(1):43–52. [PubMed] [Google Scholar]
- Beaudet A. L., Roufa D. J., Caskey C. T. Mutations affecting the structure of hypoxanthine: guanine phosphoribosyltransferase in cultured Chinese hamster cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):320–324. doi: 10.1073/pnas.70.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke P. J., Herrick N., Hebert A. Hypoxanthine-guanine phosphoribosyltransferase variant associated with accelerated purine synthesis. J Clin Invest. 1973 Sep;52(9):2234–2240. doi: 10.1172/JCI107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod J., Sirota J. H. THE RENAL CLEARANCE OF ENDOGENOUS "CREATININE" IN MAN. J Clin Invest. 1948 Sep;27(5):645–654. doi: 10.1172/JCI102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- DAVIDSON J. D., BRADLEY T. R., ROOSA R. A., LAW L. W. Purine nucleotide pyrophosphorylases in 8-azaguanine-sensitive and-resistant P388 leukemias. J Natl Cancer Inst. 1962 Oct;29:789–803. [PubMed] [Google Scholar]
- Dancis J., Yip L. C., Cox R. P., Piomelli S., Balis M. E. Disparate enzyme activity in erythocytes and leukocytes. A variant of hypoxanthine phosphoribosyl-transferase deficiency with an unstable enzyme. J Clin Invest. 1973 Aug;52(8):2068–2074. doi: 10.1172/JCI107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., Held K. R. The spontaneous azaguanine-resistant mutants of diploid human fibroblasts. Humangenetik. 1972;16(1):87–110. doi: 10.1007/BF00393992. [DOI] [PubMed] [Google Scholar]
- Emmerson B. T., Thompson L. The spectrum of hypoxanthine-guanine phosphoribosyltransferase deficiency. Q J Med. 1973 Apr;42(166):423–440. [PubMed] [Google Scholar]
- Emmerson B. T., Wallace D. C., Thompson C. J. Partial deficiency of hypoxanthine-guanine phosphoribosyltransferase: intermediate enzyme deficiency in heterozygote red cells. Ann Intern Med. 1972 Feb;76(2):285–287. doi: 10.7326/0003-4819-76-2-285. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971 Sep 25;246(18):5739–5748. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Phosphoribosylpyrophosphate in man: biochemical and clinical significance. Ann Intern Med. 1971 Mar;74(3):424–433. doi: 10.7326/0003-4819-74-3-424. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Wyngaarden J. B., Kelley W. N. Depletion of erythrocyte phosphoribosylpyrophosphate in man. N Engl J Med. 1970 Nov 26;283(22):1177–1182. doi: 10.1056/NEJM197011262832201. [DOI] [PubMed] [Google Scholar]
- Harris J. F., Whitmore G. F. Chinese hamster cells exhibiting a temperature dependent alteration in purine transport. J Cell Physiol. 1974 Feb;83(1):43–51. doi: 10.1002/jcp.1040830107. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Brox L. W., Kelley W. N., Rosenbloom F. M., Seegmiller J. E. Kinetic studies of hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 May 25;243(10):2514–2522. [PubMed] [Google Scholar]
- Henderson J. F. Possible functions of hypoxanthine-guanine phosphoribosyltransferase and their relation to the biochemical pathology of the Lesch-Nyhan syndrome. Fed Proc. 1968 Jul-Aug;27(4):1075–1077. [PubMed] [Google Scholar]
- Hershko A., Hershko C., Mager J. Increased formation of 5-phosphoriboxyl-1-pyrophosphate in red blood cells of some gouty patients. Isr J Med Sci. 1968 Sep-Oct;4(5):939–944. [PubMed] [Google Scholar]
- Hughes S. H., Wahl G. M., Capecchi M. R. Purification and characterization of mouse hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1975 Jan 10;250(1):120–126. [PubMed] [Google Scholar]
- JONES O. W., Jr, ASHTON D. M., WYNGAARDEN J. B. Accelerated turnover of phosphoribosylpyrophosphate, a purine nucleotide precursor, in certain gouty subjects. J Clin Invest. 1962 Sep;41:1805–1815. doi: 10.1172/JCI104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaloustian VM der, Awdeh Z. L., Hallal R. T., Wakid N. W. Analysis of human hypoxanthine-guanine phosphoribosyl transferase isozymes by isoelectric focusing in polyacrylamide gel. Biochem Genet. 1973 May;9(1):91–95. doi: 10.1007/BF00485594. [DOI] [PubMed] [Google Scholar]
- Kaufman J. M., Greene M. L., Seegmiller J. E. Urine uric acid to creatinine rtio--a screening test for inherited disorders of purine metabolism. Phosphoribosyltransferase (PRT) deficiency in X-linked cerebral palsy and in a variant of gout. J Pediatr. 1968 Oct;73(4):583–592. doi: 10.1016/s0022-3476(68)80274-4. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Arnold W. J. Human hypoxanthine-guanine phosphoribosyltransferase: studies on the normal and mutant forms of the enzyme. Fed Proc. 1973 Jun;32(6):1656–1659. [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Meade J. C. Studies on hypoxanthine-guanine phosphoribosyltransferase in fibroblasts from patients with the Lesch-Nyhan syndrome. Evidence for genetic heterogeneity. J Biol Chem. 1971 May 10;246(9):2953–2958. [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Miller J., Seegmiller J. E. An enzymatic basis for variation in response to allopurinol. Hypoxanthine-guanine phosphoribosyltransferase deficiency. N Engl J Med. 1968 Feb 8;278(6):287–293. doi: 10.1056/NEJM196802082780601. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Seegmiller J. E. The effects of azathioprine (imuran) on purine synthesis in clinical disorders of purine metabolism. J Clin Invest. 1967 Sep;46(9):1518–1529. doi: 10.1172/JCI105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinenberg J. R., Goldfinger S., Bradley K. H., Seegmiller J. E. An enzymatic spectrophotometric method for the determination of xanthine and hypoxanthine. Clin Chem. 1967 Oct;13(10):834–846. [PubMed] [Google Scholar]
- Kogut M. D., Donnell G. N., Nyhan W. L., Sweetman L. Disorder of purine metabolism due to partial deficiency of hypoxanthine-guanine phosphoribosyltransferase. A study of a family. Am J Med. 1970 Feb;48(2):148–161. doi: 10.1016/0002-9343(70)90111-7. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Papaioannou R., Elion G. B. Human hypoxanthine phosphoribosyltransferase. I. Purification, properties, and specificity. J Biol Chem. 1969 Mar 10;244(5):1263–1270. [PubMed] [Google Scholar]
- Krenitsky T. A., Papaioannou R. Human hypoxanthine phosphoribosyltransferase. II. Kinetics and chemical modification. J Biol Chem. 1969 Mar 10;244(5):1271–1277. [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971 Feb 19;171(3972):689–691. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Chinese hamster hypoxanthine-guanine phosphoribosyltransferase. Purification, structural, and catalytic properties. J Biol Chem. 1974 Jul 10;249(13):4030–4037. [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Subunit molecular weight of human hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1974 Jul 10;249(13):4038–4040. [PubMed] [Google Scholar]
- Richardson B. J., Ryckman D. L., Komarnicki L. M., Hamerton J. L. Heterogeneity in the biochemical characteristics of red blood cell hypoxanthine-guanine phosphoribosyl transferase from two unrelated patients with the Lesch-Nyhan syndrome. Biochem Genet. 1973 Jun;9(2):197–202. doi: 10.1007/BF00487450. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Rosenbloom F. M., Kelley W. N., Miller J., Henderson J. F., Seegmiller J. E. Inherited disorder of purine metabolism. Correlation between central nervous system dysfunction and biochemical defects. JAMA. 1967 Oct 16;202(3):175–177. doi: 10.1001/jama.202.3.175. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Ruddle F. H. Microscale isoelectric focusing studies of mouse and human hypoxanthine-guanine phosphoribosyl transferases. Biochem Genet. 1973 Jun;9(2):175–185. doi: 10.1007/BF00487447. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEGMILLER J. E., GRAYZEL A. I., LASTER L., LIDDLE L. Uric acid production in gout. J Clin Invest. 1961 Jul;40:1304–1314. doi: 10.1172/JCI104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi T., Sekiguchi F. Interallelic complementation in hybrid cells derived from Chinese hamster diploid clones deficient in hypoxanthine-guanine phosphoribosyl-transferase activity. Exp Cell Res. 1973 Mar 15;77(1):391–403. doi: 10.1016/0014-4827(73)90593-4. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. I. Nature of mutations conferring resistance to 8-azaguanine in mouse cell lines. J Cell Sci. 1974 Mar;14(2):235–251. doi: 10.1242/jcs.14.2.235. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sperling O., Boer P., Eilam G., de Vries A. Evidence for molecular alteration of erythrocyte hypoxanthine-guanine phosphoribosyltransferase in a gouty family with partial deficiency of the enzyme. Rev Eur Etud Clin Biol. 1972 Jan;17(1):72–75. [PubMed] [Google Scholar]
- Sweetman L., Nyhan W. L. Excretion of hypoxanthine and xanthine in a genetic disease of purine metabolism. Nature. 1967 Aug 19;215(5103):859–860. doi: 10.1038/215859a0. [DOI] [PubMed] [Google Scholar]
- Thomas C. B., Arnold W. J., Kelley W. N. Human adenine phosphoribosyltransferase. Purification, subunit structure, and substrate specificity. J Biol Chem. 1973 Apr 10;248(7):2529–2535. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Yü T. F., Balis M. E., Krenitsky T. A., Dancis J., Silvers D. N., Elion G. B., Gutman A. B. Rarity of X-linked partial hypoxanthine-guanine phosphoribosyltransferase deficiency in a large gouty population. Ann Intern Med. 1972 Feb;76(2):255–264. doi: 10.7326/0003-4819-76-2-255. [DOI] [PubMed] [Google Scholar]



