Abstract
The vector-borne protozoan Leishmania infantum chagasi causes minimal inflammation after inoculation into skin but disseminates to cause fatal visceral leishmaniasis. To define the inflammatory response at the parasite inoculation site, we introduced metacyclic L. infantum chagasi promastigotes intradermally into BALB/c mouse ears and studied inflammatory cells over 7 days. Ly6G+ neutrophils rapidly infiltrated the dermis, peaking after 6 to 24 h. Macrophages and NK cells next infiltrated the dermis, and NK followed by B cells expanded in draining lymph nodes. Parasite-containing phagocytes were tracked with fluorescent mCherry-labeled L. infantum chagasi. Ly6G+ neutrophils contained the greatest proportion of intracellular parasites 6 to 24 h after inoculation, whereas dermal macrophages harbored the majority of intracellular parasites after 2 to 7 days. These observations were validated microscopically. Low doses of antibody transiently depleted mice of neutrophils, leaving other cells intact. Combined results of in vivo imaging, flow cytometry, and quantitative PCR showed that neutrophil depletion slowed the clearance of extracellular (luciferase-positive) promastigotes during the first 24 h after inoculation yet decreased the numbers of leukocytes containing intracellular (mCherry-positive) parasites. From 3 days onward, total L. infantum chagasi-containing dermal leukocytes and total L. infantum chagasi parasites in draining lymph nodes were similar in both groups. Nonetheless, a second wave of L. infantum chagasi-containing neutrophils occurred 7 days after parasite inoculation into neutrophil-depleted mice, corresponding to the time of neutrophil recovery. Thus, neutrophils were recruited to the dermis even late after inoculation, and L. infantum chagasi trafficked through neutrophils in both neutrophil-depleted and control mice, albeit with different kinetics. Recruitment of neutrophils and transient parasite residence in neutrophils may play a role in nonulcerative forms of leishmaniasis.
Parasites belonging to the genus Leishmania cause a spectrum of human diseases, the most deadly of which is visceral leishmaniasis. Leishmania infantum chagasi is one of the two most common etiologic agents of visceral leishmaniasis in humans. During natural infection, a bolus of metacyclic promastigotes is delivered into a hemorrhagic dermal lesion formed by a feeding female phlebotomine sand fly (5). Parasites quickly encounter soluble and cellular microbicidal immune elements. Rather than succumb, many are taken up by phagocytic host cells, where they transform to intracellular amastigotes, a form that can multiply and survive in phagolysosomes (9, 48).
Although the majority of host cells harboring Leishmania sp. amastigotes are macrophages, intracellular amastigotes have been observed in other mammalian cell types as well, including dendritic cells (DCs), fibroblasts, and neutrophils (6, 21, 28, 33). Recent studies suggest that neutrophils can promote the early establishment of intradermal infection with Leishmania major, a cause of human cutaneous leishmaniasis (30, 31). Cutaneous leishmaniasis is characterized by a cutaneous nodule at the infection site, which eventually ulcerates. In contrast, infections with the visceralizing Leishmania spp. (L. infantum chagasi or Leishmania donovani) start with either no lesion or a nonulcerating nodular lesion at the site of the sand fly bite, after which parasites can disseminate and replicate in visceral organs. Thus, the different Leishmania species have an inherent propensity to induce either a pathogenic inflammatory response, as in the case of L. major, or an immunosuppressive phenotype in visceralizing disease (38, 48).
Published studies indicate that the T cell phenotypic response is molded during the first few days of L. major infection (39). Recent studies showed that the first cells responding at the infection site are neutrophils (10, 30, 31). Neutrophils can, in turn, release chemokines, such as CCL3, that recruit other cell types (e.g., monocytes and dendritic cells) to the inflammatory site (11). In the context of L. major infection, most parasites in the skin are contained within inflammatory cutaneous dendritic cells which produce inducible nitric oxide synthase (iNOS) in genetically resistant mice as effectors of the type I response. This response is suppressed in susceptible mice (15).
In contrast to the response to L. major infection, the early local inflammatory response to the Leishmania species causing visceral leishmaniasis, such as L. infantum chagasi, is not well characterized. Given their very different forms of local pathogenesis at the site of skin inoculation, we hypothesized that different inflammatory cells would be recruited to the local site, guiding distinct downstream outcomes. We therefore investigated the inflammatory cell types that infiltrate the local skin inoculation site and the draining lymph nodes (dLN) during the first hours to days after L. infantum chagasi inoculation. We adopted an intradermal BALB/c mouse model of chronic Leishmania infantum infection. Transgenic L. infantum chagasi promastigotes expressing either firefly luciferase or the fluorescent marker mCherry allowed us to track the total parasite population using in vivo imaging and the phagocytic cells harboring intracellular parasites by flow cytometry during the first few days of infection (2). Our data showed that neutrophils are the first cells to phagocytose L. infantum chagasi at the site of parasite inoculation, but the parasite load was quickly transferred to macrophages.
MATERIALS AND METHODS
Mice and parasites.
Female BALB/c mice (4 to 6 weeks old) were purchased from Harlan Breeders. Studies were approved by the Animal Care and Use Committees of the University of Iowa and the Iowa City Veterans Affairs Medical Center.
Intradermal introduction of parasites.
A Brazilian strain of wild-type L. infantum chagasi (MHOM/BR/00/1669) was maintained in hamsters by serial intracardiac injection of amastigotes. Parasites were grown as promastigotes at 26°C in liquid hemoflagellate-modified minimal essential medium (4). Parasite subcultures were grown to stationary phase, and metacyclic promastigotes were enriched on a density gradient as described previously (49).
Transgenic parasites were generated by transfection of the wild-type strain with an integrating construct leading to stable mCherry or luciferase expression. Briefly, the gene encoding mCherry or firefly luciferase was cloned into the XmaI site of pIR1SAT, an integrating vector that was kindly provided to us by Stephen M. Beverley of Washington University, St. Louis, MO (8). After electroporation (12) and selection on semisolid medium, correct insertion was verified by Southern blotting (data not shown). BALB/c mice were anesthetized and inoculated intradermally (i.d.) in the ear pinna with 10 μl of L. infantum chagasi parasites at various doses, using an insulin syringe.
Tissue processing.
Mice were euthanized, and ears and the draining lymph nodes were removed. Ears were processed as previously described (25). Briefly, dermal sheets were separated and placed in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 0.2 mg/ml Liberase CI (Roche Diagnostic Systems), 100 U/ml penicillin, and 100 μg/ml streptomycin for 2 h at 37°C. Tissues were processed into single-cell suspensions by gentle agitation with frosted microscope slides and placed on ice. Cells were passed through a 70-μm-pore-size nylon mesh and then processed for flow cytometry or DNA isolation.
Transient neutrophil depletion.
Mice were treated with an antibody ([Ab] RB6-8C5 or 1A8) to deplete neutrophils or with a control antibody (rat Ig) (BD Pharmingen). Antibodies were delivered intravenously with a single dose (25 μg/animal in 200 μl of saline). Mock-treated animals received a saline injection. Parasites were inoculated intradermally into ear pinna the following day. The efficacy of neutrophil depletion was analyzed in peripheral blood obtained by tail bleeding and collected in microcentrifuge tubes containing heparin. Peripheral blood was processed for flow cytometry.
Flow cytometry.
Single-cell suspensions were suspended in staining buffer (1× phosphate-buffered saline [PBS], 0.5% heat-inactivated fetal bovine serum [HI-FBS], 0.01% sodium azide) at ∼106 cells/sample and kept on ice. Fc receptors were blocked with 10 μl of normal rat serum and 0.5 μg of anti-mouse CD16/32 (purified from 2.4G2 hybridoma supernatant) for 15 min and incubated with fluorochrome-conjugated antibodies. Cells were subsequently washed two times and fixed in 2% paraformaldehyde (PFA). Leukocytes were stained with the following fluorochrome-conjugated monoclonal antibodies (MAb): anti-Ly6G (1A8; BD Pharmingen), anti-CD11b (M1/70; BD Pharmingen), anti-CD49b (HMα2; BD Pharmingen), anti-CD11c (HL3; BD Pharmingen), F4/80 (A3-1; AbD Serotec), CD45 (30-F11; BD Pharmingen), CD4 (GK-1.5; BD Pharmingen), CD8 (53-6.7; BD Pharmingen), CD3 (145-2C11; BD Pharmingen), and CD19 (1D3; eBioscience). Samples were analyzed by flow cytometry (LSRII and FACSDiva; Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed by FlowJo software (Tree Star, Inc., Ashland, OR).
Microscopic examination of infiltrating cells.
Dermal cells were recovered from the site of parasite inoculation and applied to microscopic slides using a cytocentrifuge. After cells were stained with Wright Giemsa equivalent (Diff Kwik; Fischer), the mononuclear phagocytes, neutrophils, and other cell types harboring intracellular Leishmania parasites on replicate slides were quantified microscopically by two independent observers.
Assessment of parasite load by in vivo imaging.
Relative burdens of luciferase-expressing L. infantum chagasi parasites at the inoculation site were determined as previously described (43). Briefly, 1 day after inoculation of neutrophil-depleting or control antibody, metacyclic L. infantum chagasi promastigotes expressing luciferase were introduced into female BALB/c mice intradermally in the ear. Mice were inoculated with luciferin intraperitoneally and anesthetized with isoflurane prior to imaging.
Assessment of parasite load by real-time PCR.
Tissue parasite burden in draining lymph nodes was quantified using real-time PCR of total DNA extracted from lymph nodes. Genomic DNA was isolated from mouse tissue using a Gentra Puregene DNA purification system (Qiagen) according to the manufacturer's instructions. Primers and probes for quantitative PCR (qPCR) were used to amplify the mouse tumor necrosis factor alpha (TNF-α) gene as a positive control and Leishmania DNA polymerase kinetoplastid DNA to quantify parasites. qPCR primers and probes were previously described (7, 13, 23). TaqMan probes were purchased from Applied Biosystems Inc. (ABI), and primers were purchased from Integrated DNA Technologies (IDT). Triplicate measurements of samples from three mice were performed. Data were analyzed using a standard curve generated from L. infantum chagasi promastigotes (22).
Statistical analysis.
Statistical significance was assessed using two-way analysis of variance (ANOVA) or a Student's t test in Prism (GraphPad) software.
RESULTS
Leukocyte dynamics at the site of intradermal L. infantum chagasi inoculation.
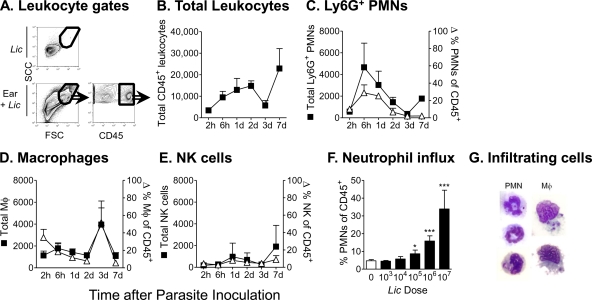
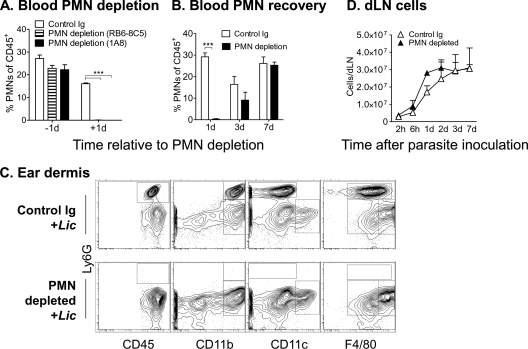
We investigated the nature of the earliest inflammatory cell response at the site of parasite introduction in a mouse model of infection. BALB/c mice were inoculated intradermally with 106 metacyclic L. infantum chagasi promastigotes in the ear pinna. Leukocyte subsets in the ear dermis were analyzed by flow cytometry between 2 h and 7 days later (Fig. 1). The leukocyte gating strategy excluded the vast majority of free parasites in the light scatter plot. This is shown for unstained L. infantum chagasi parasites in Fig. 1A but was also observed with parasites stained with the fluorescent marker mCherry or carboxyfluorescein succinimidyl ester (CFSE) (data not shown). Leukocytes were identified and gated based on CD45 expression (Fig. 1A, lower right). The total number of leukocytes increased over time after parasite inoculation (Fig. 1B). Leukocytes were identified by high levels of CD45 expression (CD45high), and leukocyte subsets were classified based on their surface antigen profiles at different times after parasite inoculation and are shown as both the total numbers of the cell subset (closed symbols) and the percentage of total leukocytes (open symbols) in the infiltrate (Fig. 1C to E). There was a rapid and transient increase in the number of cells expressing the neutrophil surface antigen Ly6G (Ab clone 1A8), reaching a peak at 6 h. A second rise in Ly6G+ neutrophils was observed after 7 days although the proportion of neutrophils in the inflammatory focus remained low at this late time point (Fig. 1C). In contrast, the numbers and proportions of macrophages in the infiltrate increased after 3 days of parasite exposure (Fig. 1C). The NK cell count remained fairly stable but increased somewhat after 7 days of infection (Fig. 1E). The prominent neutrophil infiltrate observed 6 h after parasite inoculation was responsive to different doses of metacyclic promastigotes (Fig. 1F). Microscopic examination of the inflammatory cells 24 h after parasite inoculation demonstrated visually that some infiltrating neutrophils and macrophages contained intracellular parasites (Fig. 1G).
FIG. 1.
Composition of the early cellular infiltrate in the ear dermis at the local site of intradermal L. infantum chagasi (Lic) inoculation. BALB/c mice were inoculated intradermally in the ear pinna with 106 metacyclic L. infantum chagasi promastigotes. Cells draining the site of inoculation were isolated from dermal tissue and examined by flow cytometry at times indicated in the figure. (A) Light scatter profiles are shown of the L. infantum chagasi parasites alone (top left panel) or the cells in the inoculated ear (bottom left panel). Leukocytes were identified by high expression levels of the pan-leukocyte marker CD45 (bottom right panel). (B) The number of leukocytes in the inflammatory focus is shown at the indicated times after intradermal inoculation of promastigotes. (C, D, and E) Total numbers (closed symbols) or percentages (open symbols) of CD45+ polymorphonuclear leukocytes (PMN) staining for the neutrophil marker Ly6G+, macrophage characteristics (CD45+ CD11b+ Ly6G− CD11c−), or the NK cell marker (CD49b). (F) The proportion of total CD45+ leukocytes staining with the Ly6G neutrophil marker at 6 h after inoculation of the indicated doses of metacyclic L. infantum chagasi promastigotes is shown. Results are expressed as means ± standard deviations from a representative of four separate experiments, each containing three mice/group. (G) Bright-field images of cells prepared from cytospins of dermal infiltrates 24 h after inoculation of L. infantum chagasi. Microscope slides were stained with Wright Giemsa to illustrate intracellular parasites. Data indicate the means ± standard deviations for six mice in panels B through F. d, day.
Leukocyte dynamics in the lymph nodes draining the L. infantum chagasi inoculation site.
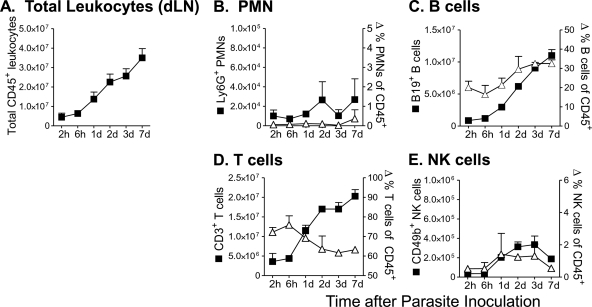
Similar to the above data, the identity and the kinetics of inflammatory cells responding to L. infantum chagasi were determined in the lymph nodes draining the site of parasite inoculation. Observations were made between 2 h and 7 days after inoculation of 106 metacyclic L. infantum chagasi promastigotes. The total number of leukocytes increased progressively over time (Fig. 2 A). Unlike the dermal inoculation site, there was not an early peak in neutrophils in the draining nodes (Fig. 2B). Both cells expressing the B cell marker CD19 and T cells expressing CD3 increased between 2 and 7 days after parasite inoculation, but the CD19+ cells increased to a greater extent, resulting in a proportionally greater increase in B than T cells in the draining nodes (Fig. 2C and D). NK cell numbers expanded 2 to 3 days after parasite inoculation and then declined (Fig. 2E).
FIG. 2.
Composition of the early cellular response in lymph nodes draining the site of L. infantum chagasi metacyclic promastigote inoculation. BALB/c mice were inoculated intradermally in the ear pinna with 106 metacyclic L. infantum chagasi promastigotes. Single-cell suspensions from draining lymph nodes were stained and examined by flow cytometry at the indicated times after inoculation. The total numbers (closed symbols) or percentages (open symbols) of CD45+ leukocytes of dLN cells staining for the pan-leukocyte marker CD45 (A), the neutrophil marker Ly6G+ (B), the B cell marker CD19 (C), the T cell marker CD3 (D), or the NK cell marker (CD49b) (E) are shown. Results represent the means ± standard deviations for six mice.
The above data indicate that intradermal introduction of L. infantum chagasi induced rapid infiltration of neutrophils followed by macrophages at the local the site of inoculation. There was a corresponding influx of NK cells and preferential expansion of B cells in the dLN.
Leukocytes harboring intracellular L. infantum chagasi.
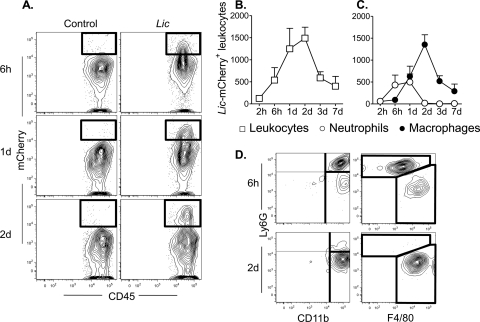
We used L. infantum chagasi stably expressing the fluorescent marker mCherry to track intracellular parasites in the inflammatory site. CD45high leukocytes containing intracellular mCherry-expressing (mCherry+) L. infantum chagasi parasites were evident by flow cytometry (Fig. 3 A). Parasite-laden leukocytes in the dermis were detected as early as 2 h and peaked 1 to 2 days after parasite inoculation (Fig. 3B). Surface staining of CD45+ mCherry+ events distinguished two major leukocyte subpopulations containing intracellular L. infantum chagasi. Ly6G+ neutrophils constituted the majority of cells with intracellular L. infantum chagasi parasites 6 h after inoculation of promastigotes, peaking between 6 and 24 h and declining by 2 days after inoculation. Neutrophils were replaced by macrophages harboring the largest numbers of intracellular mCherry+ L. infantum chagasi parasites 2 days after inoculation (Fig. 3C and D). Fig. 3 shows example plots of data of Ly6G versus F4/80 or CD11b. Macrophages were identified as F4/80+ CD11b+ Ly6G− CD11c−. Very few of the F4/80+ CD11b+ cells, either with or without intracellular L. infantum chagasi, were dermal DCs coexpressing CD11c that would correspond to the recently described TNF- and iNOS-producing (TiP) DCs (3, 40) (data not shown). In particular, TiP DCs were not the major cells harboring intracellular L. infantum chagasi, and in this manner L. infantum chagasi infection differs from models of L. major infection (15).
FIG. 3.
Progression of L. infantum chagasi parasites through different inflammatory cell types during the first 7 days after introduction into host tissues. BALB/c mice were inoculated intradermally in the ear with 106 transgenic metacyclic L. infantum chagasi promastigotes expressing the fluorescent protein mCherry and with saline as a control. Dermal inflammatory cells harboring intracellular parasites were characterized by flow cytometry with antibodies to surface markers. (A) Representative contour plot from three time points after parasite inoculation are shown. There is a progression of CD45+ cells harboring fluorescent L. infantum chagasi in inflammatory cells from mouse ears inoculated with parasites but not saline controls. The total numbers of L. infantum chagasi-containing leukocytes that stain for CD45+ are shown in panel B, and the CD45+ cells that costain for markers of neutrophils (Ly6G) or macrophages (CD11b+ CD11c− Ly6G−) are indicated in panel C. (D) Cells were gated on dermal inflammatory CD45+ leukocytes that costain for mCherry. Plots show subpopulations of these L. infantum chagasi-harboring cells that express surface markers for neutrophils and/or macrophages, as in panel C.
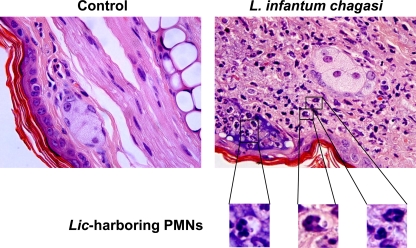
Flow cytometric observations were validated by microscopic examination of cells recovered from the inflammatory site and applied to slides by cytospin centrifugation. Enumeration of Wright Giemsa-stained cells recovered 24 h after parasite inoculation revealed that 44.0% ± 5.1% of cells were neutrophils, 36.0% ± 8.4% were mononuclear phagocytic cells, and 23.6% ± 3.3% were other cell types (lymphocytes and other mononuclear cells). A total of 20.2% ± 4.8% of the polymorphonuclear and 12.3% ± 5.7% of the mononuclear phagocytes contained intracellular amastigotes. To address the possibility that leukocytes containing intracellular L. infantum chagasi had taken up the parasites in vitro after isolation from tissues (15), microscopic sections were prepared from ears 24 h after inoculation of promastigotes. After samples were embedded in paraffin and stained with hematoxylin and eosin (H&E) (Fig. 4) or tissue Giemsa (not shown), neutrophils containing intracellular L. infantum chagasi could be observed in the infected tissues (Fig. 4).
FIG. 4.
Histologic stain of ears inoculated intradermally with metacyclic L. infantum chagasi promastigotes. BALB/c mouse ears were inoculated with either saline (control) or 106 metacyclic L. infantum chagasi promastigotes. After 24 h, ears were removed, fixed in paraformaldehyde, paraffin embedded, and processed for histologic stains. Shown are hematoxylin and eosin stains of these sections. Insets show magnifications of neutrophils with intracellular amastigote forms.
The above data suggest that Ly6G+ neutrophils rapidly migrated to the site of parasite inoculation, where they internalized L. infantum chagasi within a few hours after inoculation. Neutrophils were only transiently the dominant cell type containing intracellular parasites, however, and macrophages rapidly replaced neutrophils as the predominant host cell harboring intracellular L. infantum chagasi parasites starting 2 days after exposure to the parasite.
Leukocyte dynamics after neutrophil depletion.
Several reports address the functional importance of neutrophils by introducing parasites into mice treated with antibody to deplete neutrophils systemically (24, 35, 37, 41). Treatment with 200 μg of RB6-8C5 has been reported to deplete other cell types in addition to neutrophils (45). Accordingly, we chose to transiently deplete mice of neutrophils with a single low-dose (25 μg) injection of either RB6-8C5 (anti-Ly6G/Ly6C) or 1A8 (anti-Ly6G). According to flow cytometry, treatment with either Ab effectively depleted neutrophils from the blood (Fig. 5 A and B), ear, and dLN (Table 1). Neutrophils returned to detectable levels by 7 days after treatment. In contrast to Ly6G+ neutrophils, other leukocyte subsets in the dermis or draining lymph nodes were not affected by low-dose antibody treatment (Table 1, PMN depletion). In particular, macrophage and DC populations remained intact after neutrophil depletion (Fig. 5D). Importantly, neither infection nor neutrophil depletion led to a significant change in the numbers of CD11c+ CD11b+ cells, a population that should include TiP DCs (3). Nonetheless, parasite inoculation into the neutrophil-depleted environment still drove an expansion of the total leukocyte population in draining lymph nodes (Fig. 5D).
FIG. 5.
Leukocyte analysis after antibody treatment induced polymorphonuclear leukocyte (PMN) depletion, without or with subsequent inoculation of L. infantum chagasi. (A) Mice were inoculated with 25 μg of either MAb RB6-8C or MAb 1A8 intravenously to deplete neutrophils or with control rat Ig. Total CD45+ leukocytes from peripheral blood were analyzed by flow cytometry for the proportion of Ly6G+ neutrophils either 1 day before or 1 day after depletion. (B) The proportion of Ly6G+ neutrophils in peripheral blood CD45+ leukocytes was quantified at the indicated number of days after inoculation of 25 μg of neutrophil-depleting MAb RB6-8C or control Ig. (C) BALB/c mice were treated with depleting or control Ab as described in panel B, and 24 h later metacyclic L. infantum chagasi promastigotes were inoculated intradermally in the ear. Contour plots show total CD45+ leukocytes or CD45+ leukocytes staining for markers that distinguish different populations of macrophages or dendritic cells. Results show the means ± standard deviations of three mice/group/time point from a representative experiment of three. Two-way ANOVA was performed to test significance. ***, P < 0.001.
TABLE 1.
Cell composition in draining lymph nodes or at the dermal inoculation site 24 h after inoculation with L. infantum chagasi of mice treated with control or neutrophil-depleting Ig
| Sample type and treatmenta | No. of cellsb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Leukocytes | PMN | Mφ | DCs | B cells | T cells | NK cells | CD4+ cells | |
| Draining lymph nodes | ||||||||
| Control Ig | (2.5 ± 1.2) × 106 | (4.7 ± 0.02) × 103 | (6.1 ± 2.8) × 104 | (2.2 ± 1.1) × 104 | (1.1 ± 0.1) × 106 | (1.2 ± 0.6) × 106 | (3.5 ± 0.9) × 104 | (7.6 ± 4.9) × 105 |
| PMN depletion | (2.2 ± 1.1) × 106 | (3.2 ± 1.6) × 101 | (3.0 ± 1.6) × 104c | (1.9 ± 1.2) × 104 | (9.4 ± 1.0) × 105 | (1.1 ± 0.6) × 106 | (1.6 ± 0.9) × 104 | (8.5 ± 4.9) × 105 |
| Ear dermis | ||||||||
| Control Ig | (6.7 ± 1.4) × 103 | (2.5 ± 0.4) × 103 | (3.8 ± 0.8) × 103 | (8.9 ± 2.0) × 102 | (3.4 ± 0.7) × 101 | (6.5 ± 0.6) × 101 | (1.3 ± 0.5) × 102 | (2.2 ± 1.4) × 101 |
| PMN depletion | (7.4 ± 1.3) × 103 | (1.1 ± 0.5) × 101 | (5.8 ± 9.7) × 103 | (8.1 ± 3.9) × 102 | (5.3 ± 1.7) × 101 | (2.1 ± 0.2) × 102 | (5.1 ± 1.3) × 102 | (3.1 ± 0.7) × 101 |
All mice were inoculated with metacyclic L. infantum chagasi promastigotes.
Cells were quantified at 24 h postinoculation by flow cytometry using markers described in the Materials and Methods section. PMN, polymorphonuclear leukocytes.
Not statistically significant.
Parasite burden in a neutrophil-depleted host.
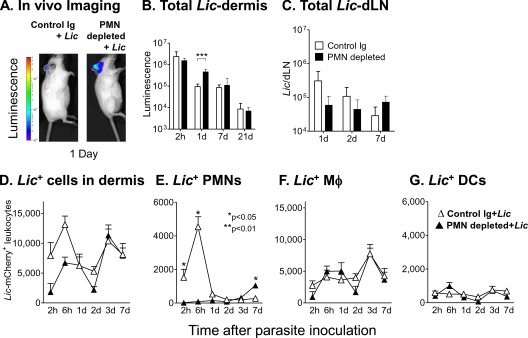
One day after treatment with low-dose depleting antibody or saline, metacyclic L. infantum chagasi parasites stably expressing either luciferase or mCherry were introduced intradermally in the ear pinna, and the parasite load was quantified in the local site and draining lymph nodes between 2 h and 7 days after parasite inoculation (Fig. 6). The total numbers of parasites in tissues were quantified by in vivo imaging (Xenogen IVIS 200 system) (Fig. 6A) (43). The numbers of parasites in the draining lymph nodes were quantified by qPCR (Fig. 6C), and numbers of leukocytes harboring intracellular mCherry-expressing parasites were determined by flow cytometry (Fig. 6D to G).
FIG. 6.
Parasite burden in neutrophil-depleted or control mice after L. infantum chagasi inoculation. BALB/c mice were treated with low-dose (25 μg) RB6-8C5, 1A8, or rat Ig control antibody. Mice were inoculated with transgenic L. infantum chagasi metacyclic promastigotes 1 day after antibody administration. Data are shown for RB6-8C5 depletion; similar results were obtained using 1A8. (A and B) Control or depleted mice were inoculated intradermally in the ear with 106 luciferase-expressing metacyclic L. infantum chagasi promastigotes. Parasite burdens were visualized by in vivo imaging on a Xenogen IVIS 200 system as shown in panel A. Total parasite numbers were quantified using in vivo imaging over 21 days and are shown graphically in panel B. Data show the mean number (± standard deviation) of pixels from five replicate mice. (C) DNA extracted from the lymph nodes draining the inoculated ear was used as template for a qPCR assay to quantify the total L. infantum chagasi parasites in the node. Data show mean ± standard deviations of parasites in five mice per time point. (D to G) Control or depleted mice were inoculated intradermally in the ear with 106 metacyclic L. infantum chagasi promastigotes expressing the fluorescent marker mCherry. Leukocytes were extracted at the indicated times from the dermis, and flow cytometric gates were set on CD45+ mCherry+ leukocytes. Cells were stained for markers of neutrophils (E), macrophages (F), or dendritic cells (G) as described in the legends of Fig. 1 and 2. The total L. infantum chagasi-harboring cell numbers in the dermis are expressed as means ± standard deviations from six mice in a representative experiment. Statistical analyses were done with a Student's t test.
Twenty-four hours after inoculation of parasites i.d. in the ear pinna, a majority of the parasites were removed from tissues (Fig. 6A and B). We assume that the majority of parasites in the inoculum were removed from the site, possibly by innate immune factors. Indeed, parasite clearance was significantly slowed in mice depleted of neutrophils, suggesting that neutrophils participate in the initial parasite clearance. The opposite pattern was observed in draining lymph nodes 1 day after parasite inoculation. That is, there was a trend toward more parasite-containing cells in the lymph nodes draining inoculation sites in intact mice than in neutrophil-depleted mice. This suggested the hypothesis that the excess luminescent parasites in tissues (viewed in Fig. 6A) were extracellular and not contained in leukocytes that were likely to drain in lymph vessels (Fig. 6C).
A flow cytometric study of cells containing intracellular L. infantum chagasi in the dermis supported the above hypothesis. That is, there were significantly lower numbers of L. infantum chagasi-containing leukocytes in the skin during the first few hours after parasite inoculation into depleted mice than in skin of the controls. This is consistent with the idea that neutrophils are the initial host cells at the site scavenging the invading microbes. Nonetheless, at 24 h or later times, the numbers of L. infantum chagasi-containing leukocytes were similar in depleted and control animals. As would be predicted, neutrophils were the main cells containing mCherry+ L. infantum chagasi early after parasite exposure in control mice, and this population was absent in neutrophil-depleted mice. Both conditions demonstrated an increase in L. infantum chagasi-containing macrophages after 3 days. An unanticipated observation was a significant increase in the numbers of neutrophils containing intracellular L. infantum chagasi occurring 7 days after L. infantum chagasi inoculation in depleted but not control mice (Fig. 6E). Thus, neutrophils were recruited to the local site containing parasites in mice depleted of neutrophils, but this recruitment was delayed until after neutrophil counts recovered systemically (Fig. 5B). As such, even in mice transiently depleted of neutrophils, many parasites trafficked through the intracellular neutrophil environment at an early time after parasite inoculation.
DISCUSSION
Different forms of leishmaniasis result in distinct clinical syndromes, roughly divided into tegumentary (including cutaneous and mucosal) and visceralizing leishmaniasis. Species of Leishmania that classically cause human cutaneous leishmaniasis often induce an inflammatory response leading to ulceration at the site of parasite infection. Species causing human visceral leishmaniasis, in contrast, usually cause no or minimal cutaneous evidence at the infection site (48). Even a newly described cutaneous form of leishmaniasis due to the visceralizing species Leishmania infantum chagasi most often manifests as nonulcerating cutaneous lesions, termed nonulcerative cutaneous leishmaniasis (32). One would surmise that the inflammatory cellular response to the different Leishmania species, therefore, might also differ.
The purpose of this study was to define the early cells infiltrating the cutaneous site of infection with L. infantum chagasi, a cause of human visceral leishmaniasis. The importance lies in the fact that the skin is the usual route of natural infection, and often the downstream immune responses to an invading pathogen are guided by the initial cellular responses at the invasion site. Using an intradermal ear model of infection, we observed that experimental inoculation of BALB/c mice with metacyclic L. infantum chagasi promastigotes led to a rapid influx of Ly6G+ CD11b+ neutrophils at the dermal inoculation site, peaking during the first day after parasite introduction. This was followed by expansion of macrophages and moderate expansion of NK cells at the local inoculation site and by transient expansion of NK cells followed by B cells in the draining lymph nodes. Paralleling the cellular predominance, most intracellular mCherry-labeled promastigotes were observed in neutrophils during the first 24 h of infection. The neutrophil predominance subsided by 2 days after parasite inoculation, and Ly6G− dermal macrophages carried the bulk of internalized L. infantum chagasi in the skin at time points later than 48 h.
In an effort to discern a potential functional consequence of the early transit of parasites through neutrophils at the dermal site, mice were experimentally depleted of neutrophils with monoclonal antibodies. Published studies of neutrophil-depleted mice have primarily employed high-dose treatment with MAb RB6-8C5 directed at Ly6G. This Ab binds to other cell types that express small amounts of Ly6C (17, 27), and it has been shown that high-dose RB6-8C5 treatment depletes plasmacytoid dendritic cells, among others (14, 45). We overcame this problem by using a single low-dose antibody treatment that effectively depleted neutrophils but left other leukocyte populations intact. Low-dose Ab effectively depleted neutrophils for 3 days, i.e., longer than the duration of L. infantum chagasi parasite transit through neutrophils after their introduction into murine tissues. Using transgenic L. infantum chagasi parasites expressing luciferase or the fluorescent marker mCherry, we were able to track both total luminescent parasites and L. infantum chagasi-harboring leukocytes at the inoculation site. The data suggested that the number of viable luciferase-expressing L. infantum chagasi parasites diminished dramatically during the first 24 h after inoculation, but this decrease was largely due to a loss of extracellular parasites. According to depletion studies, the clearance of free parasites was partially but not wholly accounted for by the presence of neutrophils. In contrast to free luminescent L. infantum chagasi, there was a decrease in the number of host cells harboring intracellular mCherry-labeled parasites in mice depleted of neutrophils. This decrease was mostly accounted for by a decrease in L. infantum chagasi-containing neutrophils, with a minor contribution from lower numbers of infected macrophages. Once macrophages infiltrated, the numbers of total parasites and the numbers of parasite-laden cells equalized between the groups. The final unanticipated observation was an influx of neutrophils harboring intracellular L. infantum chagasi 7 days after parasite inoculation into mice that had received neutrophil-depleting antibody. This influx occurred after neutrophil counts had fully recovered to the control level. A similar “wave” of infiltrating neutrophils 7 days after parasite inoculation was evident in Fig. 1C, but in this case and in the case of control mice for Fig. 6, the neutrophils did take up discernible numbers of parasites. We conclude that L. infantum chagasi was taken up by neutrophils in both the control and the neutrophil-depleted groups of mice. The timing of this transit was delayed but not prevented by neutrophil depletion.
These observations raise a number of interesting possibilities. First, it is difficult to determine what would happen during an infection in which parasites did not reside at least transiently inside neutrophils since, clearly, this occurred in both groups of mice. Second, the wave of infiltrating neutrophils on day 7 in depleted mice occurred after the effects of trauma due to needles, sand fly bite, or the direct effects of salivary components might be expected to dissipate. This suggests that the parasites themselves, or chemokines/cytokines released in response to the parasites, provide a sufficient stimulus for neutrophil recruitment. Host-derived immunomodulators such as interleukin-8 (IL-8), macrophage inflammatory protein 2 (MIP-2), CXCL1, IL-17, or TNF-α could account for neutrophil recruitment if they were produced by other cell types as a result of the parasite exposure. It is also possible that the parasite itself induced neutrophil recruitment via the described but ill-characterized Leishmania chemotactic factor (LCF) (46). Finally, somehow the parasites were able to parasitize equal numbers of macrophages at 6 to 24 h after inoculation, despite the lack of neutrophils to harbor a majority of promastigotes early in infection of neutrophil-depleted mice. Whether these parasites remained extracellular in host tissues for this length of time is not clear.
A role for neutrophils in modifying the course of immunity and infection with several Leishmania spp. has been documented (10, 35). Neutrophils can kill Leishmania in vitro through neutrophil elastases and Toll-like receptor 4 (TLR4)-mediated activation of infected macrophages to kill intracellular Leishmania parasites (34). Leishmania sp. parasites are taken up by neutrophils in vitro and in vivo early after introduction into a host. Intracellular Leishmania spp. affect neutrophil functions such as release of chemokines and consequent recruitment of macrophages and immature dendritic cells to the infection site (11, 35). Neutrophil-like polymorphonuclear CD11b+ Gr1+ leukocytes can also function in antigen presentation and cross-priming, with consequent cytotoxic T lymphocyte (CTL) activation (44).
In vitro, phagocytosis of Leishmania spp. by neutrophils is hypothesized to provide a mechanism of “silent” entry into macrophages (19, 47). In this context, Leishmania parasites are taken up by neutrophils, where they can be killed, or if phagocytosis proceeds through a non-opsonin-dependent, nonlytic pathway, the parasites survive and suppress apoptosis (18, 20). Neutrophils harboring intracellular Leishmania eventually undergo apoptosis and phagocytosis by macrophages, a pathway that could avoid macrophage activation through ligating phosphatidylserine receptors, with consequent release of transforming growth factor β (TGF-β) (16). Neutrophils also act to release MIP-1β and consequently recruit macrophages to inflammatory sites (26). The above observations led to a “Trojan horse” hypothesis that neutrophils serve as the transport vehicle through which Leishmania spp. can silently enter macrophages (1, 19, 47).
Studies of different mouse strains and different Leishmania spp. suggest that the role of neutrophils in prolonging or shortening the survival of the parasite in a host is dramatically different in different environments (24, 30, 34, 37, 42). Functional studies have used neutrophil depletion as a means of investigating the role of neutrophils in leishmaniasis. Such studies of the visceralizing Leishmania spp. have indicated that depletion results in an increased parasite load either acutely (L. infantum chagasi infection of BALB/c mice) or later in the visceral organs (L. donovani in C57BL/6 mice) (24, 37). Using a different inoculation route (intradermal rather than intravenous), the data reported herein are in agreement with Rousseau et al. in the observation that the acute, but perhaps not the chronic, parasite load was greater when neutrophils were absent from the inoculation site during the first few days. It cannot be discerned from published studies of visceralizing infections whether parasites underwent a late passage through neutrophils after the cells had recovered from acute depletion, similarly to our observations.
The model utilized in this report mimics some, but not by any means all, factors influencing the early inflammatory response to natural L. infantum chagasi infection. In nature, sand fly-derived virulent promastigotes, selected and purified by the sand fly gut environment, are introduced in low numbers into mammalian skin. Along with these highly purified promastigotes, the sand fly introduces components from its own saliva and secreted proteophosphoglycan derived from the promastigote. These substances have been shown to influence the nature and function of inflammatory cells recruited to the infection site (29, 30, 36). Nonetheless, the model herein has enabled us to discern a role for neutrophils in acute “cleanup” of promastigotes from the infection site, and a potential role of L. infantum chagasi in chemo-attracting neutrophils that may be independent of sand fly-derived factors.
Acknowledgments
This work was supported by NIH grants R01 AI045540, R01 AI067874, and R01 AI076233 and Merit Review and Persian Gulf RFA grants from the Department of Veterans' Affairs. The work was performed in part during support of C.J.T. by NIH T32 AI076233.
We are grateful to Melissa Miller for maintenance and preparation of parasite isolates and to Stephen M. Beverly for providing the pIR1SAT expression vector used to make mCherry- and luciferase-transgenic parasite lines.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. va Zandbergen, H. Laufs, B. Hansen, K. Muller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, S., M. Colmenares, L. Soong, K. Goldsmith-Pestana, L. Mustnermann, R. Molina, and D. McMahon-Pratt. 2003. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect. Immun. 71:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge, J. R., C. E. Moseley, D. A. Boltz, N. J. Negovetich, C. Reynolds, J. Franks, S. A. Brown, P. C. Doherty, R. G. Webster, and P. G. Thomas. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. U. S. A. 106:5306-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berens, R. L., R. Brun, and S. M. Krassner. 1976. A simple monophasic medium for axenic culture of hemoflagellates. J. Parasitol. 62:360-365. [PubMed] [Google Scholar]
- 5.Blackwell, J. M., et al. 2009. Genetics and visceral leishmaniasis: of mice and man. Parasite Immunol. 31:254-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniasis. J. Exp. Med. 194:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretagne, S., R. Durand, M. Olivi, J.-F. Garin, A. Sulahian, D. Rivollet, M. Vidaud, and M. Deniau. 2001. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin. Diagn. Lab. Immunol. 8:828-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capul, A. A., T. Barron, D. E. Dobson, S. J. Turco, and S. M. Beverley. 2007. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J. Biol. Chem. 282:14006-14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, K.-P. 1983. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int. Rev. Cytol. Suppl. 14:267-303. [PubMed] [Google Scholar]
- 10.Charmoy, M., F. Auderset, C. Allenbach, and F. Tacchini-Cottier. 25 October 2009, posting date. The prominent role of neutrophils during the initial phase of infection by Leishmania parasites. J. Biomed. Biotechnol. doi: 10.1155/2010/719361. [DOI] [PMC free article] [PubMed]
- 11.Charmoy, M., S. Brunner-Agten, D. Aebischer, F. Auderset, P. Launois, G. Milon, A. E. Proudfoot, and F. Tacchini-Cottier. 2010. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS. Pathog. 6:e1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coburn, C. M., K. M. Otteman, T. McNeely, S. J. Turco, and S. M. Beverley. 1991. Stable DNA transfection of a wide range of trypanosomatids. Mol. Biochem. Parasitol. 46:169-180. [DOI] [PubMed] [Google Scholar]
- 13.Cummings, K. L., and R. L. Tarleton. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 129:53-59. [DOI] [PubMed] [Google Scholar]
- 14.Daley, J. M., A. A. Thomay, M. D. Connolly, J. S. Reichner, and J. E. Albina. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64-70. [DOI] [PubMed] [Google Scholar]
- 15.De Trez, C., S. Magez, S. Akira, B. Ryffel, Y. Carlier, and E. Muraille. 2009. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS. Pathog. 5:e1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming, T. J., M. L. Fleming, and T. R. Malek. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399-2408. [PubMed] [Google Scholar]
- 18.Gueirard, P., A. Laplante, C. Rondeau, G. Milon, and M. Desjardins. 2008. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell. Microbiol. 10:100-111. [DOI] [PubMed] [Google Scholar]
- 19.Laskay, T., Z. G. van, and W. Solbach. 2008. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology 213:183-191. [DOI] [PubMed] [Google Scholar]
- 20.Laufs, H., K. Muller, J. Fleischer, N. Reiling, N. Jahnke, J. C. Jensenius, W. Solbach, and T. Laskay. 2002. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect. Immun. 70:826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon, B., M. Lopez-Bravo, and C. Ardavin. 2007. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26:519-531. [DOI] [PubMed] [Google Scholar]
- 22.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 23.Mary, C., F. Faraut, L. Lascombe, and H. Dumon. 2004. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 42:5249-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarlane, E., C. Perez, M. Charmoy, C. Allenbach, K. C. Carter, J. Alexander, and F. Tacchini-Cottier. 2008. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect. Immun. 76:532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon, J. N., and P. A. Bretscher. 1998. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur. J. Immunol. 28:4020-4028. [DOI] [PubMed] [Google Scholar]
- 26.Muller, K., Z. G. van, B. Hansen, H. Laufs, N. Jahnke, W. Solbach, and T. Laskay. 2001. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. 190:73-76. [DOI] [PubMed] [Google Scholar]
- 27.Nagendra, S., and A. Schlueter. 2004. Absence of cross-reactivity between murine Ly-6C and Ly-6G. Cytometry A 58:195-200. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, R. D., and R. T. Steigbigel. 1981. Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J. Immunol. 127:1438-1443. [PubMed] [Google Scholar]
- 29.Peters, N. C., N. Kimblin, N. Secundino, S. Kamhawi, P. Lawyer, and D. L. Sacks. 2009. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS. Pathog. 5:e1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters, N. C., and D. L. Sacks. 2009. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol. 11:1290-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters, N. C., D. L. Sacks, et al. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponce, C., E. Ponce, A. Morrison, A. Cruz, R. Kreutzer, D. McMahon-Pratt, and F. Neva. 1991. Leishmania donovani chagasi: new clinical variant of cutaneous leishmaniasis in Honduras. Lancet 337:67-70. [DOI] [PubMed] [Google Scholar]
- 33.Prina, E., S. Z. Abdi, M. Lebastart, E. Perret, N. Winter, and J.-C. Antoine. 2004. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J. Cell Sci. 117:315-325. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro-Gomes, F. L., M. C. A. Moniz-de-Souza, M. S. Alexandre-Moreira, W. B. Dias, M. F. Lopes, M. P. Nunes, G. Lungarella, and G. A. DosReis. 2007. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 179:3988-3994. [DOI] [PubMed] [Google Scholar]
- 35.Ritter, U., F. Frischknecht, and G. van Zandbergen. 2009. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 25:505-510. [DOI] [PubMed] [Google Scholar]
- 36.Rogers, M., P. Kropf, B. S. Choi, R. Dillon, M. Podinovskaia, P. Bates, and I. Muller. 2009. Proteophosophoglycans regurgitated by Leishmania-infected sand flies target the l-arginine metabolism of host macrophages to promote parasite survival. PLoS Pathog. 5:e1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rousseau, D., S. Demartino, B. Ferrua, J. F. Michiels, F. Anjuere, K. Fragaki, Y. Le Fichoux, and J. Kubar. 2001. In vivo involvement of polymorphonuclear neutrophils in Leishmania infantum infection. BMC Microbiol. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 39.Scott, P. 2003. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunol. Res. 27:489-498. [DOI] [PubMed] [Google Scholar]
- 40.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF-iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59-70. [DOI] [PubMed] [Google Scholar]
- 41.Smelt, S. C., S. E. J. Cotterell, C. R. Engwerda, and P. M. Kaye. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164:3681-3688. [DOI] [PubMed] [Google Scholar]
- 42.Tacchini-Cottier, F., C. Zweifel, Y. Belkaid, C. Mukankundiye, M. Vasei, P. Launois, G. Milon, and J. A. Louis. 2000. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 165:2628-2636. [DOI] [PubMed] [Google Scholar]
- 43.Thalhofer, C. J., J. W. Graff, L. Love-Homan, S. M. Hickerson, N. Craft, S. M. Beverley, and M. E. Wilson. 2010. In vivo imaging of transgenic Leishmania parasites in a live host. J. Vis. Exp. 41:1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomihara, K., M. Guo, T. Shin, X. Sun, S. M. Ludwig, M. J. Brumlik, B. Zhang, T. J. Curiel, and T. Shin. 2010. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b+ Gr-1+ cells. J. Immunol. 184:6151-6160. [DOI] [PubMed] [Google Scholar]
- 45.Tvinnereim, A. R., S. E. Hamilton, and J. T. Harty. 2004. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J. Immunol. 173:1994-2002. [DOI] [PubMed] [Google Scholar]
- 46.van Zandbergen, G., N. Hermann, H. Laufs, W. Solbach, and T. Laskay. 2002. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70:4177-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Zandbergen, G., M. Klinger, A. Mueller, S. Dannenberg, A. Gebert, W. Solbach, and T. Laskay. 2004. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 173:6521-6525. [DOI] [PubMed] [Google Scholar]
- 48.Wilson, M. E., S. M. B. Jeronimo, and R. D. Pearson. 2005. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 38:147-160. [DOI] [PubMed] [Google Scholar]
- 49.Yao, C., Y. Chen, B. Sudan, J. E. Donelson, and M. E. Wilson. 2008. Leishmania chagasi: homogenous metacyclic promastigotes isolated by buoyant density are highly virulent in a mouse model. Exp. Parasitol. 118:129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]