Abstract
Since the 23-valent pneumococcal polysaccharide vaccine (PPV23) is less effective for older adults than for young adults, it is important to investigate the immunologic basis for the reduced efficacy of PPV23 among older adults. We determined the effectiveness of PPV23 among young (n = 55) and older (n = 44) adults by measuring the serum IgG, IgM, and IgA concentrations and opsonic capacities against serotypes 14, 18C, and 23F. While young and older adults showed no difference in levels of IgG antibodies against pneumococcal polysaccharide (PPS), older adults had lower IgA and IgM antibody levels than young adults for all three serotypes. In both age groups, anti-PPS IgA or IgM antibody levels were much lower than anti-PPS IgG antibody levels. Young adults showed higher opsonic capacities than older adults for serotypes 14 and 23F. In order to determine the effects of anti-PPS IgA or IgM antibodies on the functional difference between young and older adults, anti-PPS IgA or IgM antibodies were removed from immune sera by affinity chromatography. The difference in opsonic capacity between young and older adults disappeared for serotypes 14 and 23F (but not for serotype 18C) when IgM antibody was removed. However, there was no significant difference between the two age groups when IgA antibody was removed. In conclusion, even though anti-PPS IgG antibody levels are high compared with anti-PPS IgM antibody levels, the low levels of anti-PPS IgM antibody alone can explain the functional difference observed between young and older adults immunized with PPV23 with regard to some pneumococcal serotypes.
Streptococcus pneumoniae (pneumococcus) is a significant cause of morbidity and mortality due to bacterial meningitis and sepsis, particularly in young children and elderly adults (12). Also, it is receiving increased attention as the pathogen responsible for a large number of deaths among influenza-infected persons during influenza epidemics (2, 18, 22, 26, 45). To control pneumococcal infections, a 23-valent pneumococcal polysaccharide vaccine (PPV23) and three conjugate vaccines are currently available for immunizing old adults and children, respectively (1, 8, 43). Although conjugate vaccines have been shown to be highly effective among children (11, 31), the protective efficacy of PPV23 is less clear among adults, and its efficacy decreases dramatically with increased age (14, 34, 36, 37).
To investigate the immunologic basis for its reduced efficacy for the aged, the immunogenicity of PPV23 in old adults has been studied extensively (32, 33, 37). Previous immunogenicity studies of immune sera have focused on measuring both opsonic capacity and levels of IgG antibodies against pneumococcal polysaccharide (PPS), since anti-PPS antibodies provide protection primarily by opsonizing pneumococci, and anti-PPS IgG antibodies account for the majority of anti-PPS antibodies in the immune sera (5, 24, 29, 44). Several studies by different investigators and with different analytical approaches have shown that old adults elicit anti-PPS IgG antibody levels equivalent to those of young adults but that old adults' sera are less opsonic than young adults' sera (32, 34, 37).
The findings of the immunogenicity studies have often been interpreted as showing a reduction in antibody affinity or avidity due to aging, since animal studies have shown that aging is associated with changes in antibody V-gene expression and fewer somatic mutations (4, 20, 41). However, the role of anti-PPS IgM or IgA antibodies, which are made in smaller amounts than IgG antibodies, has not been investigated previously. It is important to clarify their role, since IgA antibodies have been shown to inhibit the opsonic capacity of IgG antibodies (16, 17, 35), and IgM antibody molecules are more efficient than IgG antibodies in opsonizing pneumococci (28, 42). Therefore, we have investigated the roles of anti-PPS IgM and IgA antibodies produced in response to PPV23 in both young and old adults.
MATERIALS AND METHODS
Serum samples.
Two groups of anonymous serum samples were obtained. One group was obtained from L. Jackson (Seattle, WA) and contained sera from 45 old adults, 70 through 79 years of age (mean age ± standard deviation [SD], 74.9 ± 2.4 years), who were immunized with PPV23 (Pneumovax; Merck & Co., Inc., Whitehouse Station, NJ) 4 weeks prior to phlebotomy (15). The exclusion criteria for this study population were nursing home residence, immunocompromise, chronic anticoagulation or a known bleeding disorder, asplenia, active cancer, liver or renal failure, known hypersensitivity to any pneumococcal vaccine component, and receipt of a diphtheria toxoid-containing vaccine in the previous 6 months. All elderly adults received one dose of PPV23 at least 5 years prior to enrollment (15). The other group was from M. Blake (Bethesda, MD) and contained sera from 55 young college students who were bled 4 weeks after immunization with PPV23. These students had not received PPV23 previously.
ELISA for anti-PS antibodies.
The amount of anti-PPS IgA, IgG, or IgM antibody was determined by a “sandwich”-type third-generation pneumococcal antibody enzyme-linked immunosorbent assay (ELISA) (48). Briefly, the wells of microtiter plates were coated at 37°C with capsular polysaccharide (PS; usually 1 to 10 μg/ml) (ATCC, Rockville, MD) for 5 h in phosphate-buffered saline (PBS) with 0.02% NaN3. Next, the plates were washed with Tris-buffered saline (10 mM Tris with 150 mM NaCl and 2 mM KCl at pH 7.4) containing 0.1% Brij 35 (Sigma-Aldrich, St. Louis, MO). All serum samples were preabsorbed with 5 μg of cell wall polysaccharide (C-PS) (Statens Serum Institut, Copenhagen, Denmark) and 5 μg of 22F PS in a total volume of 1 ml of PBS containing 0.05% Tween 20 for 30 min at room temperature (RT). The reference serum, pool 89-SF, from the Food and Drug Administration (Bethesda, MD), was absorbed only with C-PS and was used as the standard. The preabsorbed serum samples and 89-SF were serially diluted and added to the microtiter plates. After 2 h of incubation at RT and after washing as described above, an alkaline phosphatase-conjugated goat antibody specific for human IgA, IgG, or IgM (Southern Biotech, Birmingham, AL) in PBS with 0.02% NaN3 was added to the plates. After 2 h of incubation at RT, the plates were loaded with a p-nitrophenyl phosphate substrate (Sigma-Aldrich, St. Louis, MO) in diethanolamine buffer and were incubated for 2 h at RT. The reactions were stopped with NaOH, and the optical density at 405 nm was measured using an ELISA microplate reader. The amount of antibody was determined by comparing the optical densities for each of the diluted serum samples with the optical density curve constructed with the standard sample (89-SF) at multiple dilutions.
MOPA.
A multiplexed opsonophagocytic killing assay (MOPA) based on antibiotic-resistant target bacteria was performed for three serotypes (serotypes 14, 18C, and 23F) as previously described (9). Briefly, frozen aliquots of each target pneumococcus were thawed, washed twice, diluted to the proper bacterial density (∼2 × 105 CFU/ml for each serotype), and pooled by mixing equal volumes. All serum samples were incubated at 56°C for 30 min, and serial dilutions were prepared. Serially diluted serum (20 μl/well) was mixed with 10 μl of the bacterial mixture in each well of round-bottom 96-well plates (Corning Inc., Corning, NY). After 30 min of incubation at RT with shaking, 10 μl of complement from 3- to 4-week-old rabbits (PelFreez Biologicals, Rogers, AR) and 40 μl of differentiated HL60 cells (4 × 105 cells) were added to each well. HL60 cells were differentiated with dimethylformamide as previously described (9). After 45 min in an incubator (37°C, 5% CO2) with shaking, an aliquot of the final reaction mixture (10 μl) was spotted onto three different Todd-Hewitt yeast (THY) agar plates (Todd-Hewitt broth with 0.5% yeast extract [THY broth] and 1.5% agar). When the fluid was absorbed into the agar, an equal volume of an overlay agar was applied to the THY agar plate. The overlay agar was THY broth with 0.75% agar, 25 mg/liter of 2,3,5-triphenyltetrazolium chloride, and an antibiotic. Streptomycin, optochin, or trimethoprim was chosen for the plates designed to detect colonies expressing serotype 14, 18C, or 23F, respectively (9). After overnight incubation at 37°C, the bacterial colonies on the agar plates were counted. The opsonization index was defined as the serum dilution that kills 50% of bacteria. A detailed protocol is posted at www.vaccine.uab.edu.
Affinity chromatography for absorption of IgA or IgM from immune sera.
To remove IgA or IgM antibodies from immune sera, each serum sample was mixed with agarose beads conjugated with an antibody specific for anti-human IgA or IgM (Sigma-Aldrich, St. Louis, MO) for 2 h at RT. For mock absorption, unconjugated agarose beads were used instead of antibody-conjugated beads. To recover the absorbed serum from the agarose beads, the mixture was centrifuged in a Bio-Rad chromatography spin column (Bio-Rad Laboratories, Inc., Hercules, CA) at 2,000 rpm for 1 min at 4°C. The completeness of absorption was monitored by using a third-generation pneumococcal antibody ELISA to determine the level of anti-PPS antibody in the absorbed sera.
Statistical analysis.
The geometric mean concentrations (GMC) of anti-PPS IgG, IgM, and IgA antibodies were calculated for each group. The geometric mean opsonophagocytic index (GMI) was also calculated for each group. Young and old adults were compared using a two-sample, unpaired Student t test after logarithmic transformation. P values less than 0.05 were considered to be significant. Statistical calculations were performed by use of JMP software, version 8.0 (SAS Institute Inc., Cary, NC).
RESULTS
PPV23 induces more-effective antibody responses in young adults than in old adults.
To confirm previous results indicating that young adults produce more-effective anti-capsule antibodies than old adults (34), we obtained immune sera from new groups of young and old vaccinees and then determined their anti-PPS IgG antibody concentrations, opsonic indices (OI), and antibody potency for serotypes 14, 18C, and 23F. As was found previously, young and old adults showed similar levels of IgG antibodies to serotypes 14, 18C, and 23F (P, 0.14, 0.8, and 0.47, respectively) (Table 1). However, young adults had higher OI than did old adults for serotypes 14 and 23F (P, <0.01 for both serotypes), and as a result, young adults had higher antibody potency than did old adults for these serotypes (P, <0.01 for both serotypes) (Table 1). Even though the difference was not statistically significant (P, 0.18), young adults showed an approximately 2-fold higher GMI than old adults for serotype 18C (1,149 [young] versus 698 [old]) (Table 1).
TABLE 1.
IgA, IgG, and IgM antibodies against serotypes 14, 18C, and 23F among young and elderly adults
| Parameter | Valuea |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype 23F |
Serotype 18C |
Serotype 14 |
|||||||
| Young | Old | P | Young | Old | P | Young | Old | P | |
| Concn of antibodyb | |||||||||
| IgG | 3.17 (2.34-4.30) | 2.71 (1.93-3.81) | 0.47 | 4.67 (3.45-6.33) | 4.92 (3.50-6.91) | 0.8 | 11.85 (8.74-16.06) | 7.00 (4.98-9.84) | 0.14 |
| IgA | 0.54 (0.40-0.73) | 0.27 (0.19-0.38) | <0.01 | ND | ND | ND | ND | <0.01 | |
| IgM | 0.2 (0.15-0.27) | 0.09 (0.06-0.13) | <0.01 | 0.46 (0.34-0.62) | 0.19 (0.13-0.27) | <0.01 | 1.04 (0.77-1.41) | 0.35 (0.24-0.5) | <0.01 |
| Opsonic indexc | 752 (554-1,019) | 186 (132-260) | <0.01 | 1,149 (848-1,558) | 698 (497-981) | 0.18 | 4,939 (3,643-6,695) | 1,102 (784-1,548) | <0.01 |
| Antibody potencyd | 250.73 (184.95-339.89) | 80.23 (57.09-112.73) | <0.01 | 243.35 (179.01-330.82) | 157.22 (111.89-220.93) | 0.16 | 381.29 (280.48-518.35) | 160.24 (114.03-225.17) | <0.01 |
Values in parentheses are 95% confidence intervals. ND, not determined.
Geometric mean concentration, in micrograms per milliliter.
Geometric mean opsonophagocytic index.
Geometric mean antibody potency (OI/[IgG]).
A significant number of immune sera from young adults are more opsonic than are other sera from young or old adults.
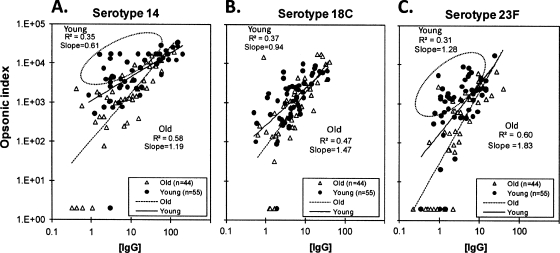
To investigate the observed difference in antibody potency between the two populations, the OI of sera were plotted against their anti-PPS IgG antibody concentrations (Fig. 1). The slope of the best-fit line was close to 1 for old adults but less than 1 for young adults. Also, the correlation between the OI and the anti-PPS IgG antibody levels was significantly higher for old adults than for young adults for all serotypes (0.58 [old] versus 0.35 [young] for serotype 14, 0.60 [old] versus 0.31 [young] for serotype 23F, and 0.47 [old] versus 0.37 [young] for serotype 18C). Interestingly, for serotypes 14 and 23F, many samples were away from the 45° trend line (shown by the dotted circles in Fig. 1). For instance, in serotype 23F, a significant proportion of sera from young adults (24 out of 55 [43.6%]) had high opsonic activities even when their anti-PPS IgG levels were low (less than 5.5 μg/ml), whereas only a few such sera from old adults (5 out of 44 [11.4%]) were observed (P, 0.001 by Fisher's exact test). The poor correlation and low slope for young adults observed with serotypes 14 and 23F could be due to those samples away from the trend line, which may have non-IgG antibodies that are opsonic.
FIG. 1.
Scatter plots evaluating associations between the anti-pneumococcal polysaccharide IgG concentration (in micrograms per milliliter) and the opsonic index for serotypes 14 (A), 18C (B), and 23F (C). Circled points (A and C) represent subgroups of serum samples that have a higher opsonic index with a lower IgG concentration. Solid and broken lines are the trend lines for each group.
Anti-PPS IgM antibodies affect the functional difference between young and old adults immunized with PPV23.
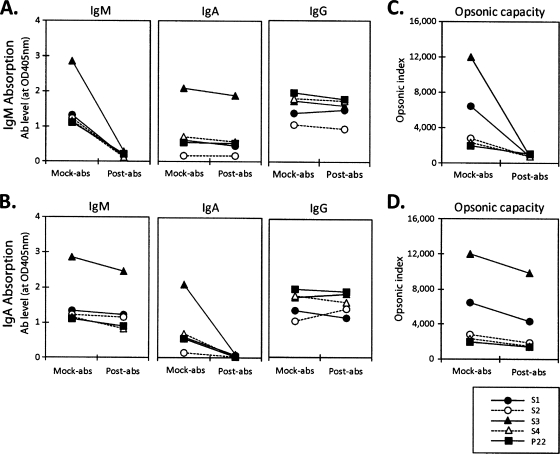
To directly investigate the contribution of anti-PPS IgM or IgA antibodies to the opsonic capacity of the immune sera, we selected four sera with low levels of anti-23F IgG antibody but with high opsonic indices against serotype 23F (circled in Fig. 1C), selectively removed their IgM or IgA by affinity chromatography, and determined their abilities to opsonize serotype 23F. The measured opsonic capacity was complement dependent: the OI was reduced by more than 95% when the rabbit serum (complement) was heat inactivated (data not shown). Also, nonspecific removal of antibody during the absorption was minimal (<10%), since mock absorption of serum samples with plain agarose beads reduced antibody concentrations and opsonic indices by less than 10%. Our affinity chromatography selectively removed more than 80% of IgM or IgA from the four immune sera but removed IgG minimally (less than 10%) and nonspecifically (Fig. 2 A and B). When we determined the opsonophagocytic killing capacities of these absorbed sera, the opsonic capacities of the sera almost completely disappeared after IgM depletion (Fig. 2C) but decreased only minimally (18 to 34%) after IgA depletion (Fig. 2D). These studies strongly suggested that anti-PPS IgM antibodies are responsible for the opsonic activity observed for these four serum samples.
FIG. 2.
Absorption of IgA or IgM from sera immunized with PPV23. Four samples (S1, S2, S3, and S4) were selected from the subgroup circled in Fig. 1C. Pooled control sera (P22) obtained from several healthy persons either left unimmunized or immunized with PPV23 were also used. The levels of IgA and IgM were measured at an 80-fold sample dilution, and the level of IgG was measured at a 400-fold sample dilution. (A) Absorption of IgM from immune sera. IgA, IgM, or IgG levels are shown for four immune sera and P22 after mock absorption (Mock-abs) and IgM absorption (Post-abs). (B) Absorption of IgA from immune sera. IgA, IgM, or IgG levels are shown for four immune sera and P22 after mock absorption and IgA absorption. (C) Opsonic indices of IgM-absorbed fractions for four immune sera and P22 after mock absorption and IgM absorption. (D) Opsonic indices of IgA-absorbed fractions for four immune sera and P22 after mock absorption and IgA absorption.
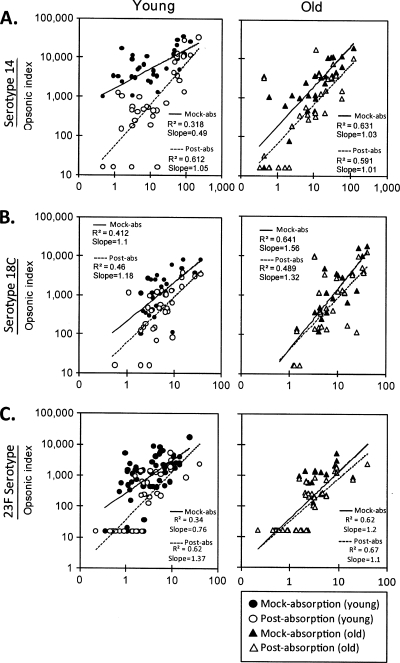
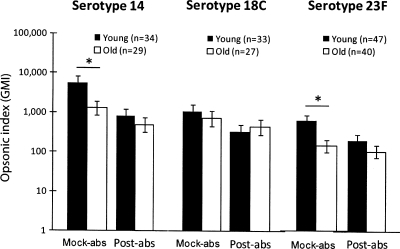
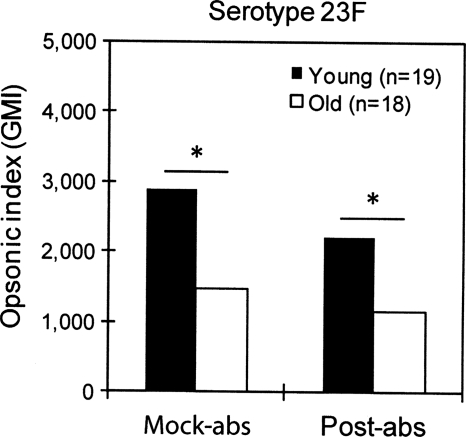
To extend this observation, we removed IgM from immune sera from both young and old vaccinees and determined the capacities of the sera to opsonize serotypes 14, 18C, and 23F (Fig. 3) (see the table in the supplemental material). Since we had technical difficulties with some samples, we could not analyze some serum samples (see the table in the supplemental material), and the numbers of samples analyzed are given in the legend to Fig. 3. The removal of IgM increased the correlation between the serum IgG concentration and the opsonic indices for young adults for all three serotypes. Following the removal, the correlation coefficient increased from 0.32 to 0.61 for serotype 14 (Fig. 3A) and from 0.34 to 0.62 for serotype 23F (Fig. 3C), but it did not change for serotype 18C (Fig. 3B). The individual sera that showed higher opsonic titers than expected on the basis of the levels of their anti-PPS IgG antibodies (shown as circles in Fig. 1) were exactly matched with the sera whose opsonic titers were decreased significantly after IgM depletion (data not shown). For old adults, IgM absorption did not increase the correlation coefficient for any of the three serotypes (Fig. 3). IgM antibody depletion also reduced the differences in the geometric means of OI observed between young and old adults (Fig. 4). When the OI was compared with the IgM antibody concentration, they showed a modest correlation (r, 0.4 to 0.7) (see the figure in the supplemental material). We also analyzed the effect of IgA absorption on the opsonic titers of young and old adults for serotype 23F, but no changes were observed (Fig. 5). Thus, anti-PPS IgM antibodies are responsible for the high potency of the antibodies from young adults.
FIG. 3.
Correlation between IgG concentration (x axis) and opsonic index (y axis) after mock absorption (filled symbols) and IgM absorption (open symbols) from immune sera from young (circles) or old (triangles) adults immunized with PPV23 for three serotypes: serotypes 14 (A), 18C (B), and 23F (C). For each serotype, samples from young versus old adults were compared: 34 young and 29 old adults for serotype 14, 33 young and 27 old adults for serotype 18C, and 47 young and 40 old adults for serotype 23F. Straight lines represent immune sera after mock absorption (solid lines) or IgM absorption (broken lines).
FIG. 4.
Comparisons of geometric means of the opsonic index (GMI) between young (filled bars) and old (open bars) adults after mock absorption (Mock-abs) and IgM absorption (Post-abs) for serotypes 14, 18C, and 23F. *, P < 0.05 by the Student t test. Error bars represent 2 standard deviations of the mean of the logarithm of the opsonic index.
FIG. 5.
Comparisons of geometric means of the opsonic index (GMI) between young (filled bars) and old (open bars) adults after mock absorption (Mock-abs) and IgA absorption (Post-abs) for serotype 23F. *, P < 0.05 by the Student t test.
Anti-PPS IgM antibody levels are small compared with anti-PPS IgG antibody levels.
In view of the large impact of anti-PPS IgM antibodies on opsonization indices, we studied the amounts of those antibodies in the immune sera from young and old vaccinees. The level of anti-PPS IgM antibody was relatively low (only about 3 to 10%) compared with the level of anti-PPS IgG antibody (Table 1). Thus, although anti-PPS IgM antibodies are present in small quantities, they make an important contribution to the opsonic capacity of immune serum.
DISCUSSION
Consistent with the results of previous studies (32, 34, 37), we found higher opsonic capacities in young adults than in old adults for serotypes 14 and 23F, with no significant difference in IgG levels between the two groups. However, anti-PPS IgA and IgM antibody levels, in contrast to IgG, are lower in older adults than in young adults. Furthermore, the opsonic capacity differences between the two groups disappeared after the removal of IgM (but not IgA) antibodies from immune sera for all three serotypes. The anti-PPS IgA antibody levels of the immune sera studied here are likely too low to influence the opsonic activity of IgG antibodies (16). In contrast, low levels of anti-PPS IgM antibodies can account for the functional difference observed between young and old adults immunized with PPV23, since IgM is more efficient at fixing complement than IgG. Indeed, studies with human monoclonal antibodies have shown that IgM antibodies can be 10- to 100-fold more effective than IgG antibodies at opsonizing bacteria or protecting animals from infections (30, 39).
Some IgM antibodies that are protective against bacterial infections bind many unrelated antigens, such as single-stranded DNA, thyroglobulin, and β-galactosidase (27). Such IgM antibodies, which are called polyreactive or natural antibodies, are found in preimmune sera (3, 27, 49, 50) and have been isolated as hybridomas producing anti-PPS antibody (6). Also, the level of natural antibodies has been shown to decrease with age (10). Nevertheless, the absorption of several postimmune sera that are rich in IgM antibody with a mixture of single-stranded DNA, thyroglobulin, and β-galactosidase did not reduce the opsonic activities of the sera (unpublished observation). Thus, the highly opsonic anti-PPS IgM antibody present in our postimmune sera is not likely to be polyreactive but specific for PPS produced in response to vaccination.
A previous study reported that following immunization with PPV23, less anti-PPS IgM antibody appeared in old adults than in young adults (38). Another study reported that naturally acquired (in nonvaccinated persons) anti-PPS IgM antibody levels decreased with aging (40) for five out of six different capsule types. However, these studies used a nonspecific ELISA and did not investigate the functional significance of the reduced anti-PPS IgM antibodies. Consequently, to our knowledge, our report may be the first linking anti-PPS IgM antibodies with the observed functional difference between sera from young and old adults immunized with PPV23. Nevertheless, one must be aware that there could be additional explanations, since our studies are limited to several pneumococcal capsule types, and immune responses to some pneumococcal PSs may differ.
The relative deficiency of anti-PPS IgM antibodies observed among older adults may be a result of their deficient IgM memory B cells. Shi et al. reported a significant reduction in the number of IgM+ CD27+ B cells with aging (38). Even though Moens et al. reported that IgM+ CD27+ B lymphocytes are also involved in IgG antibody production in response to PPV23, IgM+ CD27+ B lymphocytes are generally accepted to be IgM memory B cells and to produce anti-PPS IgM antibody in response to TI-2 antigens, including PPS (25). Also, common variable immunodeficiency (CVID) patients with an IgM+ CD27+ B cell deficiency are poorly responsive to pneumococcal vaccines and thus suffer from frequent pneumococcal infections (21). Therefore, future work should further investigate the impact of aging on IgM+ CD27+ B cell populations and their role in pneumococcal vaccine responses.
The deficiency in anti-PPS IgM antibody responses may explain the reduced effectiveness of pneumococcal vaccines in populations other than old adults. For instance, HIV+ persons and splenectomized patients have deficient IgM memory B cells (13, 21). Interestingly, HIV+ persons are known to have normal levels of IgG antibodies against pneumococcal capsular PS but to have reduced OPA titers (23). Similar observations have been made for patients with certain forms of CVID (21). Thus, the immunogenicity of pneumococcal vaccines for these populations should be reassessed by investigating the impact of anti-PPS IgM antibodies. Also, it is possible that a new pneumococcal vaccine may elicit anti-PPS IgM antibodies better than current vaccines.
Despite our observation, the deficiency of anti-PPS IgM antibodies is not likely to be the only aging- or HIV-related immune change. Patients with HIV infections have fewer B cells expressing the VH3 subtype (7). Studies of old adults have suggested an aging-associated reduction in isotype switching and somatic hypermutation and a loss of antibody oligoclonality (20). Some studies with human antibodies suggested age-related changes in the V regions of pneumococcal antibodies (20, 41, 46, 47), but another study failed to find such changes (19). In our study, an OPA showed no evidence that anti-PPS IgG antibodies from older adults are less effective than those from young adults. This finding may suggest that the V regions of anti-PPS IgG antibodies may be similar in young and old adults. However, our studies were not designed to investigate V regions and thus should not be interpreted to mean that there are no aging-associated V region differences.
In conclusion, we show that anti-PPS IgM antibodies can provide significant immune protection despite their low levels of expression. In addition to differences in fixing complement, IgM and IgG antibody responses have different time courses; consequently, their relative levels may differ during the course of a vaccinee's response to a vaccination. For instance, IgM levels were several times higher than IgG levels and correlated with opsonic capacity better than IgG levels among toddlers who received only one dose of a 9-valent pneumococcal conjugate vaccine (B. Simell, B. A. Nurkka, K. Jousimies, S. Gronholm, N. Givon-Lavi, H. Kayhty, and R. Dagan, presented at ISPPD-7, Tel Aviv, Israel, 2010). We propose that both anti-PPS IgM and IgG levels should be used in the future to monitor responses to pneumococcal vaccines as well as to vaccines that are similar to pneumococcal vaccines (e.g., meningococcus vaccines).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant R01-AI-69509 from the National Institute of Allergy and Infectious Diseases to M.H.N.
The University of Alabama at Birmingham has applied for the intellectual property rights for some of the methods and reagent used in this research.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 1 November 2010.
Supplemental data for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Anonymous. 2010. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59:258-261. [PubMed] [Google Scholar]
- 2.Anonymous. 2009. Update: influenza activity—United States, August 30-October 31, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1236-1241. [PubMed] [Google Scholar]
- 3.Avrameas, S. 1991. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton.’ Immunol. Today 12:154-159. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, M., R. Mehr, A. Belelovsky, J. Spencer, and D. K. Dunn-Walters. 2002. Age- and tissue-specific differences in human germinal center B cell selection revealed by analysis of IgVH gene hypermutation and lineage trees. Eur. J. Immunol. 32:1947-1957. [DOI] [PubMed] [Google Scholar]
- 5.Bardardottir, E., S. Jonsson, I. Jonsdottir, A. Sigfusson, and H. Valdimarsson. 1990. IgG subclass response and opsonization of Streptococcus pneumoniae after vaccination of healthy adults. J. Infect. Dis. 162:482-488. [DOI] [PubMed] [Google Scholar]
- 6.Baxendale, H. E., M. Johnson, R. C. Stephens, J. Yuste, N. Klein, J. S. Brown, and D. Goldblatt. 2008. Natural human antibodies to pneumococcus have distinctive molecular characteristics and protect against pneumococcal disease. Clin. Exp. Immunol. 151:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berberian, L., Y. Valles-Ayoub, N. Sun, O. Martinez-Maza, and J. Braun. 1991. A VH clonal deficit in human immunodeficiency virus-positive individuals reflects a B-cell maturational arrest. Blood 78:175-179. [PubMed] [Google Scholar]
- 8.Bryant, K. A., S. L. Block, S. A. Baker, W. C. Gruber, D. A. Scott, and the PCV13 Infant Study Group. 2010. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics 125:866-875. [DOI] [PubMed] [Google Scholar]
- 9.Burton, R. L., and M. H. Nahm. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Z. J., C. J. Wheeler, W. Shi, A. J. Wu, C. H. Yarboro, M. Gallagher, and A. L. Notkins. 1998. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur. J. Immunol. 28:989-994. [DOI] [PubMed] [Google Scholar]
- 11.Darkes, M. J., and G. L. Plosker. 2002. Pneumococcal conjugate vaccine (Prevnar; PNCRM7): a review of its use in the prevention of Streptococcus pneumoniae infection. Paediatr. Drugs 4:609-630. [DOI] [PubMed] [Google Scholar]
- 12.Haglund, L. A., G. R. Istre, D. A. Pickett, D. F. Welch, D. P. Fine, and the Pneumococcus Study Group. 1993. Invasive pneumococcal disease in central Oklahoma: emergence of high-level penicillin resistance and multiple antibiotic resistance. J. Infect. Dis. 168:1532-1536. [DOI] [PubMed] [Google Scholar]
- 13.Hart, M., A. Steel, S. A. Clark, G. Moyle, M. Nelson, D. C. Henderson, R. Wilson, F. Gotch, B. Gazzard, and P. Kelleher. 2007. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 178:8212-8220. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, L. A., and E. N. Janoff. 2008. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin. Infect. Dis. 47:1328-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, L. A., K. M. Neuzil, M. H. Nahm, C. G. Whitney, O. Yu, J. C. Nelson, P. T. Starkovich, M. Dunstan, B. Carste, D. K. Shay, J. Baggs, and G. M. Carlone. 2007. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 25:4029-4037. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis, G. A., and J. M. Griffiss. 1991. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J. Immunol. 147:1962-1967. [PubMed] [Google Scholar]
- 17.Jarvis, G. A., and J. M. Griffiss. 1989. Human IgA1 initiates complement-mediated killing of Neisseria meningitidis. J. Immunol. 143:1703-1709. [PubMed] [Google Scholar]
- 18.Klugman, K. P., and S. A. Madhi. 2007. Pneumococcal vaccines and flu preparedness. Science 316:49-50. [DOI] [PubMed] [Google Scholar]
- 19.Kolar, G. R., D. Mehta, P. C. Wilson, and J. D. Capra. 2006. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand. J. Immunol. 64:314-324. [DOI] [PubMed] [Google Scholar]
- 20.Kolibab, K., S. L. Smithson, B. Rabquer, S. Khuder, and M. A. Westerink. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect. Immun. 73:7465-7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruetzmann, S., M. M. Rosado, H. Weber, U. Germing, O. Tournilhac, H. H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, I. Quinti, and R. Carsetti. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhi, S. A., and K. P. Klugman. 2004. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10:811-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhi, S. A., L. Kuwanda, C. Cutland, A. Holm, H. Kayhty, and K. P. Klugman. 2005. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr. Infect. Dis. J. 24:410-416. [DOI] [PubMed] [Google Scholar]
- 24.Messina, J. P., P. G. Hickox, M. L. Lepow, B. Pollara, and R. A. Venezia. 1985. Modification of a direct enzyme-linked immunosorbent assay for the detection of immunoglobulin G and M antibodies to pneumococcal capsular polysaccharide. J. Clin. Microbiol. 21:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moens, L., M. Wuyts, I. Meyts, K. De Boeck, and X. Bossuyt. 2008. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J. Immunol. 181:5306-5312. [DOI] [PubMed] [Google Scholar]
- 26.Morens, D. M., J. K. Taubenberger, and A. S. Fauci. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198:962-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Notkins, A. L. 2004. Polyreactivity of antibody molecules. Trends Immunol. 25:174-179. [DOI] [PubMed] [Google Scholar]
- 28.Pollack, M., N. L. Koles, M. J. Preston, B. J. Brown, and G. B. Pier. 1995. Functional properties of isotype-switched immunoglobulin M (IgM) and IgG monoclonal antibodies to Pseudomonas aeruginosa lipopolysaccharide. Infect. Immun. 63:4481-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quataert, S. A., C. S. Kirch, L. J. Q. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raff, H. V., C. Bradley, W. Brady, K. Donaldson, L. Lipsich, G. Maloney, W. Shuford, M. Walls, P. Ward, E. Wolff, and L. J. Harris. 1991. Comparison of functional activities between IgG1 and IgM class-switched human monoclonal antibodies reactive with group B streptococci or Escherichia coli K1. J. Infect. Dis. 163:346-354. [DOI] [PubMed] [Google Scholar]
- 31.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 32.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. F. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 33.Rubins, J. B., A. K. Puri, J. Loch, D. Charboneau, R. MacDonald, N. Opstad, and E. N. Janoff. 1998. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J. Infect. Dis. 178:431-440. [DOI] [PubMed] [Google Scholar]
- 34.Schenkein, J. G., S. Park, and M. H. Nahm. 2008. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 26:5521-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber, J. R., V. Barrus, K. L. Cates, and G. R. Siber. 1986. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J. Infect. Dis. 153:8-16. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 37.Shelly, M. A., H. Jacoby, G. L. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197 conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, Y., T. Yamazaki, Y. Okubo, Y. Uehara, K. Sugane, and K. Agematsu. 2005. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 175:3262-3267. [DOI] [PubMed] [Google Scholar]
- 39.Shyur, S., H. V. Raff, J. F. Bohnsack, D. K. Kelsey, and H. R. Hill. 1992. Comparison of the opsonic and complement triggering activity of human monoclonal IgG1 and IgM antibody against group B streptococci. J. Immunol. 148:1879-1884. [PubMed] [Google Scholar]
- 40.Simell, B., M. Lahdenkari, A. Reunanen, H. Kayhty, and M. Vakevainen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15:1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smithson, S. L., K. Kolibab, A. K. Shriner, N. Srivastava, S. Khuder, and M. A. Westerink. 2005. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable light chain repertoire. Infect. Immun. 73:7477-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 43.Vesikari, T., J. Wysocki, B. Chevallier, A. Karvonen, H. Czajka, J. P. Arsene, P. Lommel, I. Dieussaert, and L. Schuerman. 2009. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 28:S66-S76. [DOI] [PubMed] [Google Scholar]
- 44.Vidarsson, G., S. T. Sigurdardottir, T. Gudnason, S. Kjartansson, K. G. Kristinsson, G. Ingolfsdottir, S. Jonsson, H. Valdimarsson, G. Schiffman, R. Schneerson, and I. Jonsdottir. 1998. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect. Immun. 66:2866-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter, N. D., T. H. Taylor, D. K. Shay, W. W. Thompson, L. Brammer, S. F. Dowell, M. R. Moore, and the Active Bacterial Core Surveillance Team. 2010. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin. Infect. Dis. 50:175-183. [DOI] [PubMed] [Google Scholar]
- 46.Weksler, M. E. 2000. Changes in the B-cell repertoire with age. Vaccine 18:1624-1628. [DOI] [PubMed] [Google Scholar]
- 47.Weksler, M. E., and P. Szabo. 2000. The effect of age on the B-cell repertoire. J. Clin. Immunol. 20:240-249. [DOI] [PubMed] [Google Scholar]
- 48.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, Z. H., A. G. Tzioufas, and A. L. Notkins. 2007. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J. Autoimmun. 29:219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, Z. H., Y. Zhang, Y. F. Hu, L. M. Wahl, J. O. Cisar, and A. L. Notkins. 2007. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 1:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.