Abstract
Pseudomonas aeruginosa-derived large extracellular protease (LepA) and hemolytic phospholipase C (PlcH) are considered to play an important role in the pathogenicity of this organism. Although bacterial growth appears to be closely related to virulence, little is known about whether LepA and PlcH participate in the growth and virulence of P. aeruginosa. In this study, we investigated whether LepA and PlcH contribute to the virulence and growth of P. aeruginosa using a wild-type strain and mutants. The growth rate of the isogenic lepA single mutant was lower than that of the wild-type strain in a minimal medium containing serum albumin or hemoglobin as the sole carbon and nitrogen source. Furthermore, the growth rate of the lepA plcH double mutant decreased greatly compared with that of the wild-type strain in a minimal medium containing erythrocytes as a sole nutrient source for growth. Thus, these results indicate that cooperation between LepA and PlcH would contribute to the utilization of erythrocytes as a sole nutrient source for the growth of P. aeruginosa. In addition, mouse infection experiments demonstrated that the virulence of the lepA and plcH single mutants was attenuated, and the numbers of the mutants were lower than the numbers of the wild-type strain in peritoneal lavage fluid and whole-blood specimens. In particular, the virulence and growth rate of the lepA plcH double mutant were markedly lower than those of the wild-type strain. Collectively, these results suggest that LepA and PlcH contribute to the in vivo virulence and growth of P. aeruginosa.
Pseudomonas aeruginosa has a wide environmental and ecological distribution and a remarkable ability to adapt to hostile environments with sparse nutrients. This versatility can probably be attributed to a comprehensive arsenal of enzymes combined with fitness genes (24, 49). P. aeruginosa is an opportunistic pathogen able to cause both local and disseminated infections, especially in patients with cancer, cystic fibrosis, and burns (26). The major virulence factors produced by this pathogen include secreted proteases that damage host tissues. Several P. aeruginosa proteases have been isolated and shown to be involved in pathogenesis. Of the proteases analyzed, alkaline protease (AprA) (21), elastase A (LasA) (9, 41), elastase B (LasB) (38, 44, 50), protease IV (PrpL) (11, 34), small protease (PasP) (27, 52), and large extracellular protease (LepA) (22) have been characterized extensively.
One of the functions of proteases is to hydrolyze proteins and peptides for nutrient acquisition either by degrading host enzymes or even by causing tissue damage to further the survival of the bacterium. For example, mucin degradation by AprA and LasB of P. aeruginosa leads to the acquisition of nutrients for growth (1). In addition, the Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin (16). Thus, bacterial proteases are considered to play an important role in the utilization of proteins and peptides as sources of nutrients. Despite extensive studies of P. aeruginosa-derived proteases, little is known about the involvement of proteases other than AprA and LasB in the acquisition of nutrients for growth.
In humans, the majority of iron is located in intracellularly complexed ferritin, hemoglobin, and heme proteins. Hemoglobin and heme, when released by lysis of erythrocytes, are bound by the plasma proteins haptoglobin and hemopexin, respectively. The small quantities of extracellular iron are complexed to carrier proteins like transferrin, present in serum, and lactoferrin, present within mucosal surfaces (31, 37). Therefore, hemoglobin release from erythrocytes by hemolytic action and degradation of iron-binding proteins by proteolytic action are considered to play an important role in the utilization of heme iron by bacteria. P. aeruginosa has been shown to secrete a heat-labile phospholipase C known as the hemolytic phospholipase C (PlcH) (25). PlcH has been demonstrated to be a virulence determinant of P. aeruginosa in a variety of infection models in mammals (7, 17, 36). Moreover, purified PlcH is also cytotoxic in a variety of eukaryotic cells (30) and it suppresses neutrophil respiratory bursts by interfering with a protein kinase C-dependent, non-p38 kinase-dependent pathway (53). Although PlcH is considered to participate in the pathogenicity of P. aeruginosa, little is known about whether PlcH contributes to the acquisition of nutrients from erythrocytes.
We previously reported that P. aeruginosa LepA induces inflammatory responses through protease-activated receptors (PARs) in a human bronchiole cell line, EBC-1 (22). LepA, with a molecular mass of 100 kDa, belongs to the two-partner-secretion (TPS) exoprotein (TpsA) family of molecules, which are exoproteins secreted in a TPS manner. The TpsAs are large proteins that range in size from 100 kDa to more than 500 kDa, and many of them are associated with virulence (18, 19, 29). For instance, a TpsA of enterotoxigenic E. coli, EtpA, mediates adhesion between flagella and host cells, thereby promoting colonization in the intestine (13, 42, 43). In addition, a TPS system of Neisseria meningitidis, HrpB-HrpA, contributes to the interaction of meningococci with epithelial cells and is essential for intracellular survival and escape from infected cells (45, 51). Although a large number of genes encoding potential TPS systems have been identified through DNA sequencing of microbial genomes, only a limited number of TPS molecules have been characterized so far. As described above, LepA appears to play an important role in the pathogenicity of P. aeruginosa. However, whether LepA functions as a virulence factor of P. aeruginosa is poorly understood. Hence, we hypothesized that cooperation between LepA and PlcH would be involved in the growth of P. aeruginosa in the presence of limited nutrients, thereby contributing to in vivo virulence and growth. In this study, we examined the virulence and growth of a wild-type strain and mutants using a mouse model of acute systemic infection by P. aeruginosa. Herein, we report that LepA and PlcH contribute to the in vivo virulence and growth of P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. All bacterial strains were grown in Luria-Bertani (LB) medium (LB-Miller; Nacalai tesque, Kyoto, Japan) unless otherwise noted. The growth medium was supplemented with antibiotics at the following concentrations: ampicillin, 100 μg/ml (Escherichia coli); carbenicillin, 500 μg/ml (P. aeruginosa); kanamycin, 1 mg/ml (P. aeruginosa); and tetracycline, 100 μg/ml (P. aeruginosa).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | LasB- and AprA-producing strain | 55 |
| KU2 | Clinical isolate, LepA-producing strain | 22 |
| KU2ΔlepA | Isogenic lepA mutant, Kanr | 22 |
| KU2ΔplcH | Isogenic plcH mutant, Tetr | This study |
| KU2ΔlepAΔplcH | Isogenic lepA and plcH mutant, Kanr Tetr | This study |
| E. coli strains | ||
| DH5α | Cloning strain | Toyobo |
| S17-1λpir | Mobilizer strain | Biomedal |
| Plasmids | ||
| pUC18Not | Ampr; pUC18 with two NotI sites | Biomedal |
| pUTmini-Tn5 Tc | Ampr Tetr; source of Tetr cassette | Biomedal |
| pYK1-T | sacB oriT Ampr; suicide vector | 22 |
| pYK4 | plcH Ampr; pUC18Not with a 4.4-kb PCR fragment containing plcH | This study |
| pYK4-Tc | ΔplcH::Tetr; pYK4 with 2.8-kb BglII-SmaI deletion in plcH and insertion of Tetr | This study |
| pYK5 | pYK1-T with a 3.0-kb NotI fragment containing ΔplcH::Tetr of pYK4-Tc | This study |
Preparation of inocula for infection.
Each P. aeruginosa strain was cultured in LB broth to stationary phase at 37°C with rotary shaking at 150 rpm (AT-12R shaker; Thomas, Tokyo, Japan). The culture was centrifuged at 10,000 × g for 5 min, and the bacterial pellet was washed twice with saline. The pellet was resuspended in an adequate volume using saline, and the optical density at 600 nm (OD600) adjusted to give the approximate desired inocula (OD600 of 1 ≅ 5 × 108 CFU/ml). The inocula were verified by serial 10-fold dilutions of the suspensions and plating on cetrimide agar (Nissui Pharmaceutical, Tokyo, Japan).
Cell culture.
A human monocytic cell line, THP-1, and a human T cell line, Jurkat, were maintained in RPMI-1640 medium (Nissui Pharmaceutical) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (endotoxin contents, <0.1 ng per milliliter of serum; Gibco, Rockville, MD), 2 mM l-glutamine, 0.15% sodium bicarbonate.
Construction of mutants.
Allele replacement of plcH was performed with a modification of the method of Schweizer (46). In brief, a 4.4-kb PCR fragment containing plcH was amplified from P. aeruginosa PAO1 genomic DNA by using primers U/plc (5′-GGAAACGAATTCGCGAAGCGGCCGGTATCCGCCATGTGGTCTTC-3′; underline indicates EcoRI restriction site) and D/plc (5′-GGAAACGGATCCGTCAGCGGGCCGAAGCCGTAGTGCTCGCTG-3′; underline indicates BamHI restriction site). After digestion with EcoRI and BamHI, the resulting fragments were cloned into pUC18Not, producing plasmid pYK4. A 1.4-kb PCR fragment containing a Tetr cassette was amplified from pUTmini-Tn5 Tc by using primers Tet-F (5′-GGAAACAGATCTCCGAGATGCGCCGCGTGCGGCTGCTGGAG-3′; underline indicates BglII restriction site) and Tet-R (5′-GGAAACAGTACTTAAGCTTTAATGCGGTAGTTTATCACAG-3′; underline indicates ScaI restriction site). After digestion with BglII and ScaI, the resulting fragments were cloned into pYK4, which was digested with BglII and SmaI to yield plasmid pYK4-Tc. The ΔplcH::Tetr fragment was then subcloned into the NotI site of the suicide vector pYK1-T, which has the oriT for conjugative transfer and the counter-selectable marker sacB, producing plasmid pYK5. This plasmid was used for allelic exchange and conjugated from E. coli S17-1λpir into P. aeruginosa KU2 or KU2ΔlepA on LB agar using filters.
Merodiploid single-crossover mutants were selected from the conjugation mixture by plating on LB agar containing 100 μg/ml tetracycline. Purified single-crossover mutants were cultured overnight in LB broth without antibiotics. This culture was then serially diluted in saline and plated on LB agar containing 100 μg/ml tetracycline and 7% sucrose to select against the sacB marker present on the pYK5 vector and, hence, select for strains which had undergone a second homologous recombination event resulting in loss of the pYK5 vector. This was confirmed by the loss of the vector-encoded carbenicillin resistance. In addition, the double-crossover mutants were confirmed by PCR using the primers U/plc and D/plc (data not shown).
Minimal media and growth conditions.
The bovine serum albumin (catalogue no. A2934; Sigma-Aldrich, St. Louis, MO) used in this study was electrophoretically 98% pure (endotoxin contents, <0.1 ng per milligram of protein) and essentially gamma globulin free. The bovine hemoglobin (catalogue no. H2625; Sigma-Aldrich) was prepared from washed, lysed, and dialyzed erythrocytes. Albumin and hemoglobin media were prepared as follows: basal buffer (10 mM KCl, 10 mM MgCl2, and 10 mM NaH2PO4 [pH 7.0]) was supplemented with bovine serum albumin (10 mg/ml) or bovine hemoglobin (2 mg/ml), respectively. To prepare trypsin-treated albumin medium, albumin medium was supplemented with trypsin (100 μg/ml) and incubated at 37°C for 4 h prior to use. To prepare iron-limited hemoglobin medium, hemoglobin medium was supplemented with 1.5 mM 2,2′-dipyridyl (Nacalai tesque) or 4,4′-dipyridyl (Nacalai tesque). To examine the effect of the addition of iron on growth under iron-limiting conditions, iron-limited hemoglobin medium was supplemented with 2.5 mM FeCl3. Each P. aeruginosa strain was cultured in LB broth to stationary phase at 37°C with rotary shaking at 150 rpm (AT-12R shaker; Thomas) and then diluted 50-fold with a minimal medium and incubated at 37°C with rotary shaking at 150 rpm. Bacterial growth was monitored by measuring the optical density at 600 nm using a DU730 spectrophotometer (Beckman Coulter, Brea, CA).
Use of erythrocytes as a sole nutrient by P. aeruginosa.
Sheep erythrocytes (Japan lamb, Hiroshima, Japan) were washed three times with saline and resuspended at 1 × 1010 cells/ml in saline. The erythrocytes were seeded into 96-well flat-bottom tissue culture plates (Becton Dickinson, NJ) at a density of 5 × 108 cells/well. Subsequently, 50 μl of the inoculum of each P. aeruginosa strain prepared as described above was added to yield a final concentration of 1 × 105 CFU/ml and the mixture incubated at 37°C for 24 h. Then, the growth of P. aeruginosa was measured using the Alamar blue assay (39). The redox activity related to growth changes Alamar blue from the oxidized form to the reduced form. Briefly, 10 μl of Alamar blue (Invitrogen, Carlsbad, CA) was added to the above-described mixture and incubated at 37°C for 8 h. After the incubation, the absorbance of Alamar blue at 570 and 595 nm was measured using a model 680 microplate reader (Bio-Rad Laboratories, Hercules, CA). The growth level of P. aeruginosa was expressed as the absorbance of reduced Alamar blue at 570 nm minus the absorbance of oxidized Alamar blue at 595 nm.
Cytotoxicity assays.
The cytotoxicity of wild-type P. aeruginosa KU2 and the mutants for human cell lines was estimated by the lactate dehydrogenase (LDH) release method. Briefly, an inoculum of each P. aeruginosa strain was prepared as described above. THP-1 or Jurkat cells (5 × 104 cells/well; viability of >98% as determined by trypan blue dye exclusion) in RPMI-1640 supplemented with 10% FBS was mixed with the inoculum (5 × 104 CFU/well) in 96-well flat-bottom tissue culture plates (Becton Dickinson). After 4 h of incubation at 37°C, the amount of LDH in the supernatant was determined using a cytotoxicity detection kit (Roche, Basel, Switzerland) according to the vendor's instructions. Treatment of cells with 2% Triton X-100 and treatment of cells with saline alone were used as positive and negative controls, respectively. The level of cell lysis was expressed as the percentage of the maximal cell lysis obtained by Triton X-100 treatment.
Animals.
Four- to 6-week-old male ddY mice weighing 18 to 20 g (Kyudo, Saga, Japan) were used. Leukopenia was induced by treatment with a single intraperitoneal (i.p.) dose of 250 mg/kg of body weight of cyclophosphamide (Sigma-Aldrich), given 4 days before bacterial challenge (32). All experimental procedures were reviewed and approved by the Kurume University School of Medicine Institutional Animal Care and Use Committee. Experimental procedures were performed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (8).
LD50 determinations.
Leukopenic ddY mice, in groups of eleven, were challenged with a single i.p. injection of 0.2 ml of an inoculum of each P. aeruginosa strain prepared as described above. Eight dilutions, containing different numbers of viable bacteria, were used to determine 50% lethal doses (LD50s), and mortality was assessed daily for 7 days after infection. The LD50s of the bacteria were calculated by probit analysis from survival rates of the mice after 7 days of infection (2).
In vivo growth of P. aeruginosa in mice.
Leukopenic ddY mice, in groups of 12, were challenged by i.p. injection with 0.2 ml (1 × 106 CFU) of the inoculum of each P. aeruginosa strain prepared as described above. Four hours after i.p. injection, the mice were anesthetized with diethyl ether and sacrificed by exsanguination. Simultaneously, whole-blood samples were collected in tubes containing 2 mg of disodium dihydrogen EDTA as an anticoagulant. Subsequently, glycerol was added to the whole-blood specimens at a final concentration of 15% and stored at −80°C until use. Then, the peritoneal cavities were lavaged with 5 ml of saline, glycerol was added to the lavage fluid at a final concentration of 15%, and the lavage fluid was stored at −80°C until use. These samples were diluted appropriately in saline, plated in duplicate onto cetrimide agar (Nissui Pharmaceutical), and incubated at 37°C to determine the numbers of viable P. aeruginosa in the samples. The bacterial viability did not change after the cryopreservation of the specimen.
Statistical analysis.
Statistical comparisons of more than two groups were performed using one-way analysis of variance followed by Tukey's multiple-comparison posttest. Data with P values of <0.05 were considered significant.
RESULTS
Growth of P. aeruginosa in albumin or hemoglobin medium.
P. aeruginosa-derived large extracellular protease (LepA) is considered to play an important role in the pathogenicity of this organism (22). However, it is not known whether LepA participates in the proliferation of P. aeruginosa. Therefore, we examined the growth of a lepA-deficient P. aeruginosa mutant in a minimal medium containing bovine serum albumin or bovine hemoglobin as the sole carbon and nitrogen source. As shown by the results in Fig. 1, the growth rate of KU2ΔlepA was lower than that of the LepA-producing wild-type KU2 in albumin or hemoglobin medium, indicating that LepA is essential for the assaccharolytic growth of wild-type KU2. Furthermore, the poor growth of KU2ΔlepA was recovered by incubation in trypsin-treated albumin medium (Fig. 2). Therefore, these results indicate that LepA functions in the degradation of bovine serum albumin or bovine hemoglobin to create peptide pools for the growth of P. aeruginosa.
FIG. 1.
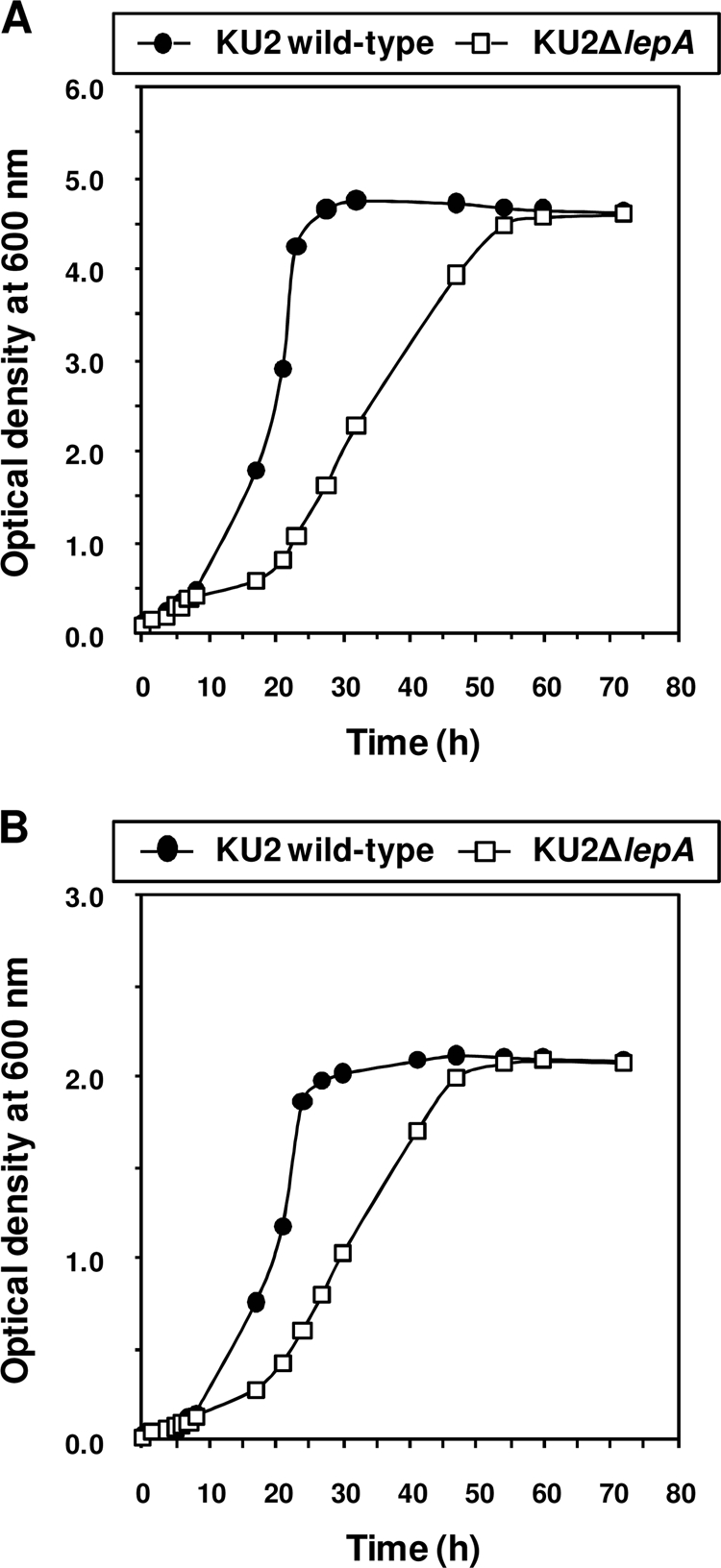
Utilization of protein as the sole carbon and nitrogen source for growth of wild-type P. aeruginosa KU2 and the lepA mutant. (A) Growth in albumin medium. (B) Growth in hemoglobin medium. The growth rate of each P. aeruginosa strain was monitored sequentially by measuring the optical density at 600 nm. Values represent the mean results from duplicate determinations. The data from a representative experiment are presented, and similar results were obtained in three independent experiments.
FIG. 2.
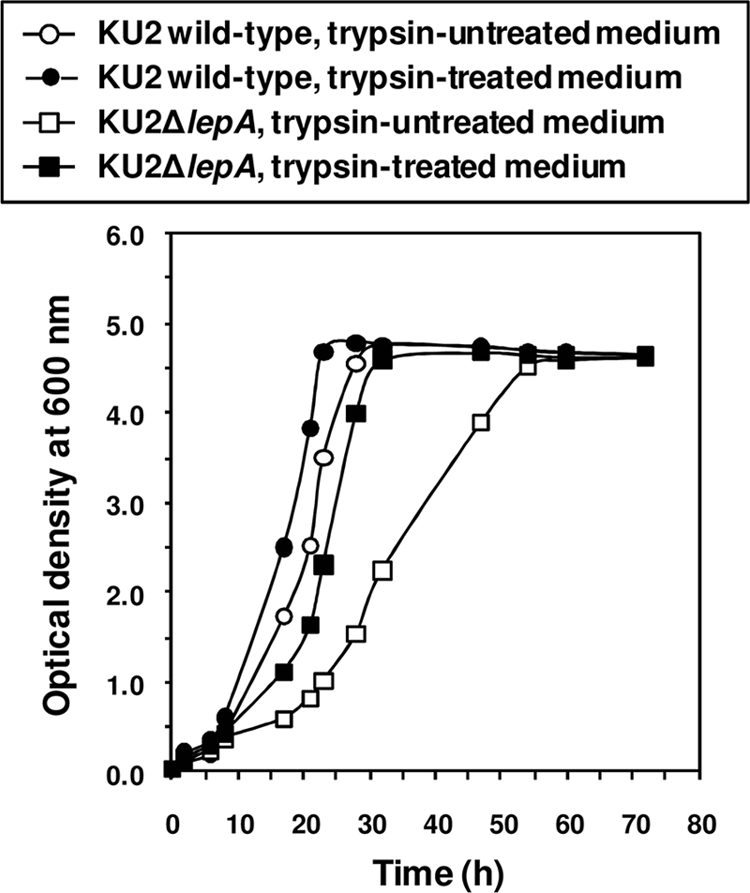
Growth of wild-type P. aeruginosa KU2 and the lepA mutant in trypsin-treated albumin medium. The growth rate of each P. aeruginosa strain was monitored sequentially by measuring the optical density at 600 nm. Values represent the mean results from duplicate determinations. The data from a representative experiment are presented, and similar results were obtained in three independent experiments.
Growth of P. aeruginosa under iron-limiting conditions.
In general, iron is an important element to support the growth of bacteria (57). We therefore tested whether wild-type KU2 and the lepA mutant show a sensitivity to iron limitation in hemoglobin medium under iron-limiting conditions. As shown by the results in Fig. 3 A, the growth rate of LepA-producing wild-type KU2 in the presence of 1.5 mM 2,2′-dipyridyl was 60 to 70% lower than that in the presence of 1.5 mM 4,4′-dipyridyl, a non-chelating agent that is structurally similar to 2,2′-dipyridyl, after 48 to 72 h of growth. On the other hand, the growth of KU2ΔlepA was inhibited more strongly than that of wild-type KU2 in the presence of 2,2′-dipyridyl. No inhibition of growth was observed when 4,4′-dipyridyl was added, indicating that the inhibitory effect of 2,2′-dipyridyl was a consequence of iron limitation and not due to a direct toxic effect of these heterocyclic compounds. In fact, the addition of 2.5 mM FeCl3 at the beginning of the incubation restored normal growth of each strain in the presence of 1.5 mM 2,2′-dipyridyl (Fig. 3B). Thus, the results suggest that LepA plays an important role in the degradation of hemoglobin to acquire heme iron for the growth of P. aeruginosa.
FIG. 3.
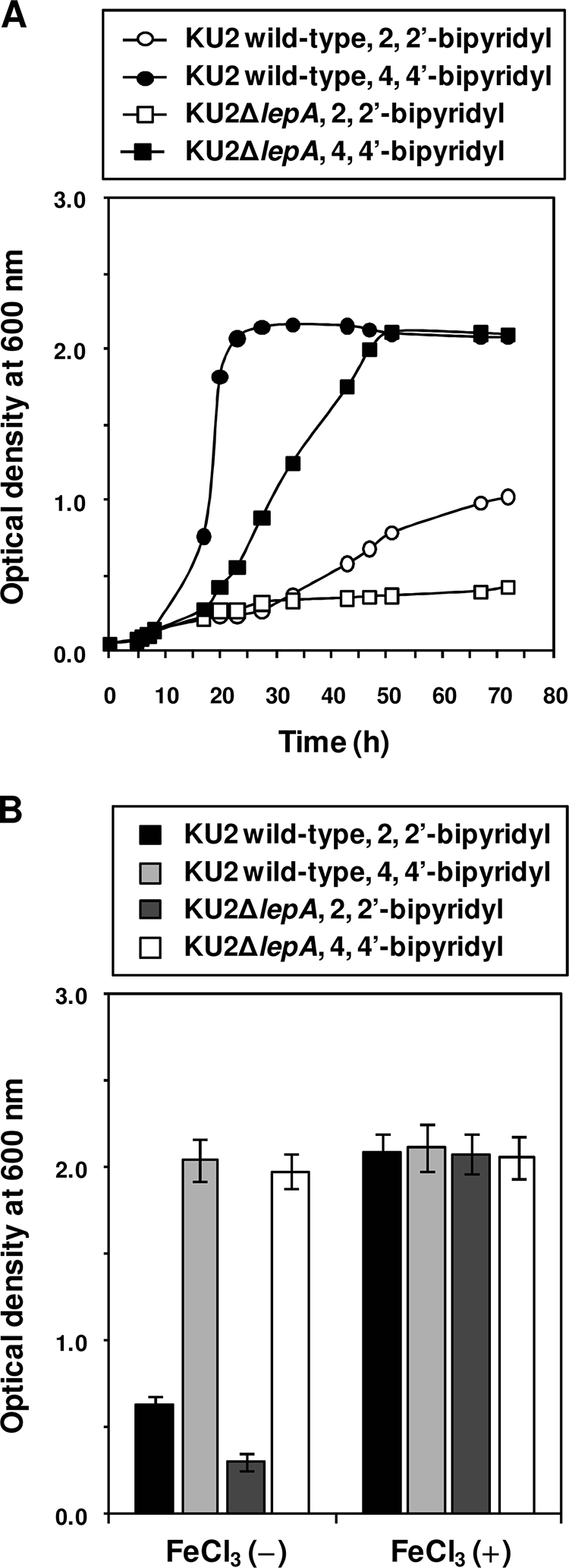
Sensitivity of wild-type P. aeruginosa KU2 and the lepA mutant to iron limitation in hemoglobin medium. (A) Effect of iron limitation by 2,2′-dipyridyl on growth. (B) Restoration of growth by the addition of FeCl3. The growth rate of each P. aeruginosa strain was monitored sequentially (A) or after 48 h of incubation (B) by measuring the optical density at 600 nm. Values represent the mean results from duplicate (A) or triplicate (B) determinations. Error bars indicate the plus-or-minus standard deviation. The data from a representative experiment are presented, and similar results were obtained in three independent experiments.
Utilization of erythrocytes as a sole nutrient source for growth of P. aeruginosa.
P. aeruginosa has been shown to secrete a hemolysin known as hemolytic phospholipase C (PlcH) (25). Hemolysins have been postulated to be related to bacterial iron metabolism, because these molecules cause the release of heme iron and hemoglobin by lysis of erythrocytes (40). Accordingly, we speculated that PlcH would also play an important role in the acquisition of nutrients from erythrocytes. We therefore examined whether wild-type P. aeruginosa KU2 and the plcH mutant have the ability to utilize sheep erythrocytes as a sole nutrient source for growth. As shown by the results in Fig. 4, the growth rate of KU2ΔplcH decreased greatly compared with that of wild-type KU2 (P < 0.01), similar to that of KU2ΔlepA. In contrast, the growth rates of KU2ΔlepA and KU2ΔplcH were significantly higher than that of the lepA plcH double mutant. Thus, these results indicate that cooperation between LepA and PlcH would contribute to the utilization of erythrocytes as a sole nutrient source for the growth of P. aeruginosa.
FIG. 4.
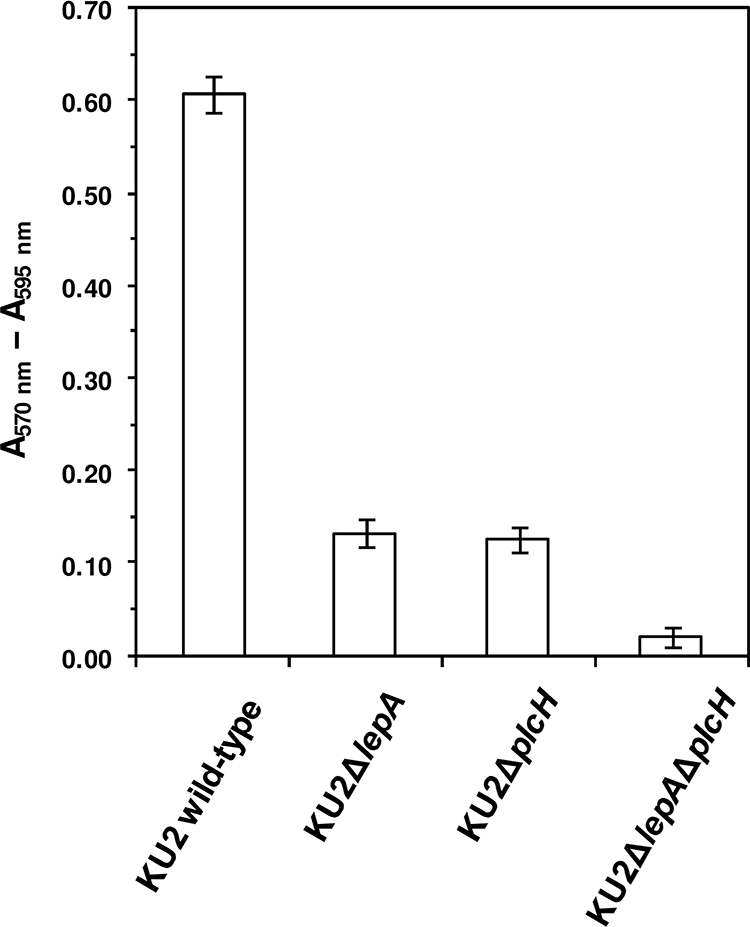
Utilization of erythrocytes as a sole nutrient source for growth of wild-type P. aeruginosa KU2 and the lepA and plcH mutants. The growth of each P. aeruginosa strain was measured after 24 h of incubation at 37°C using an Alamar blue reagent. Alamar blue was added to the sample and incubated at 37°C for 8 h. After the incubation, the absorbance of Alamar blue at 570 and 590 nm was measured. The growth level of each P. aeruginosa strain was expressed as the absorbance of reduced Alamar blue at 570 nm minus the absorbance of oxidized Alamar blue at 595 nm. Values represent the mean results ± standard deviations from triplicate determinations. The data from a representative experiment are presented, and similar results were obtained in three independent experiments. The difference between the growth of KU2ΔlepA and KU2ΔplcH was not significant. The differences of all the other pairings were significant with P values of <0.01.
Cytotoxicity of P. aeruginosa against human cell lines.
The above-described observations suggest that LepA and PlcH play an important role in the growth of P. aeruginosa in the presence of the limited nutrient source. Since bacterial growth is considered to be closely relevant to its virulence (6, 12), the effect of the inactivation of protease (lepA) and hemolysin (plcH) genes on the ability of P. aeruginosa to lyse THP-1 and Jurkat cells in culture was determined by the LDH release assay. Inoculation of the parent strain KU2 resulted in lysis of approximately 95% of the cells (Fig. 5). The lysis values obtained with the isogenic lepA (30%) or plcH (45%) mutant were significantly lower (P < 0.01) than that of the parent strain under the same experimental conditions. The cytotoxicity of the lepA single mutant decreased significantly compared to that of the plcH single mutant (P < 0.05). The decrease in cytotoxicity was more pronounced when the cells were inoculated with the lepA plcH double mutant; after 4 h of incubation, only 5% of the cells were lysed, as estimated by the extent of LDH release. This value was significantly (P < 0.01) lower than the lysis value obtained with either of the single mutants. Therefore, these results indicate that the functions of LepA and PlcH are independent of each other but additive in that together they contribute to the cytotoxicity of P. aeruginosa.
FIG. 5.
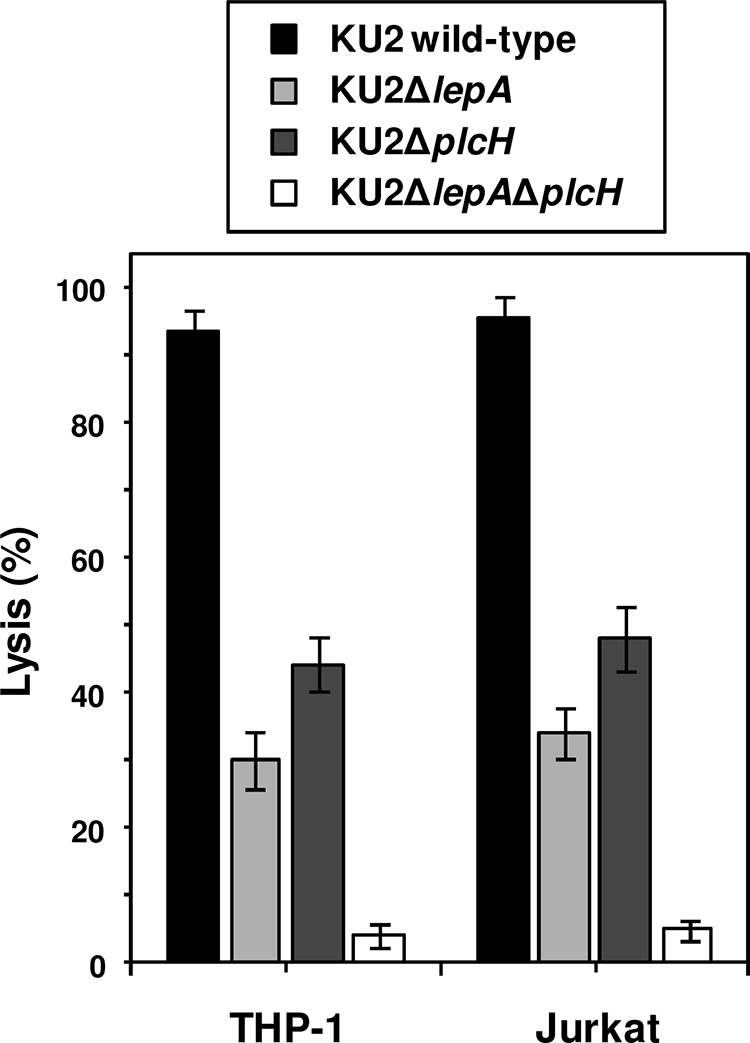
Cytotoxicity of wild-type P. aeruginosa KU2 and the lepA and plcH mutants for human cell lines. THP-1 or Jurkat cells (5 × 104 cells) were cocultured with each P. aeruginosa strain (5 × 104 CFU) for 4 h. The cytotoxicity of each P. aeruginosa strain was evaluated by an assay measuring LDH release from the cells. Values represent the mean results ± standard deviations from triplicate determinations. The data from a representative experiment are presented, and similar results were obtained in three independent experiments. The difference between KU2ΔlepA and KU2ΔplcH was significant with a P value of <0.05. The differences of all the other pairings were significant with P values of <0.01.
Virulence and growth of P. aeruginosa KU2 and mutants in mice.
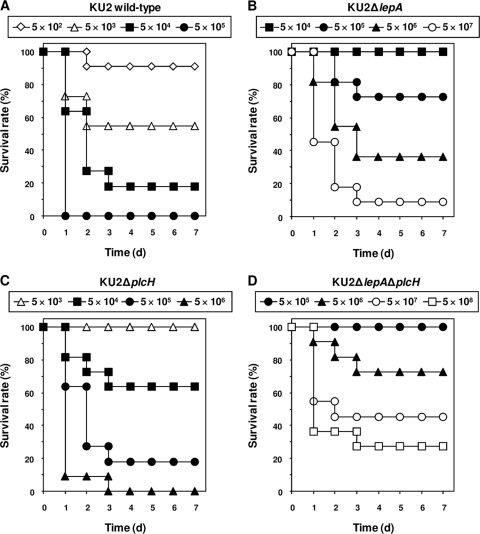
To investigate the roles of LepA and PlcH in in vivo virulence of P. aeruginosa, we compared the virulence of wild-type KU2 and the lepA and plcH mutants using a mouse model of acute systemic infection by P. aeruginosa. The survival of mice, monitored after the infections, is depicted in Fig. 6. The LD50 of each P. aeruginosa strain in leukopenic mice was as follows: wild-type KU2, 6.70 × 103 CFU/mouse; KU2ΔlepA, 2.46 × 106 CFU/mouse; KU2ΔplcH, 1.06 × 105 CFU/mouse; and KU2 ΔlepAΔplcH, 5.37 × 107 CFU/mouse. The results of these experiments demonstrated that the virulence of KU2ΔlepA and KU2ΔplcH was decreased compared with that of wild-type KU2. In accordance with the in vitro results (Fig. 5), the virulence of KU2ΔlepA was lower than that of KU2ΔplcH. In particular, the virulence of KU2ΔlepAΔplcH was attenuated greatly compared to that of wild-type KU2. Therefore, these results indicate that the functions of LepA and PlcH play an important role in the in vivo virulence of P. aeruginosa.
FIG. 6.
Survival of leukopenic ddY mice infected intraperitoneally with different doses of wild-type P. aeruginosa KU2 and the lepA and plcH mutants. (A) Infection with 5 × 102 to 5 × 105 CFU/mouse of wild-type P. aeruginosa KU2. (B) Infection with 5 × 104 to 5 × 107 CFU/mouse of P. aeruginosa KU2ΔlepA. (C) Infection with 5 × 103 to 5 × 106 CFU/mouse of P. aeruginosa KU2ΔplcH. (D) Infection with 5 × 105 to 5 × 108 CFU/mouse of P. aeruginosa KU2ΔlepAΔplcH. Eleven mice were used in each group.
To evaluate the roles of LepA and PlcH in in vivo growth of P. aeruginosa, we determined the bacterial numbers in the peritoneal cavity and whole blood in infected mice. The bacterial numbers in the peritoneal lavage fluid and whole-blood samples taken at 4 h after infection were enumerated. As shown by the results in Fig. 7, the bacterial numbers in the specimens from mice infected with KU2ΔplcH (mean log10 CFU/ml ± standard deviation: peritoneal lavage fluid, 6.19 ± 0.33, and whole blood, 4.18 ± 0.31) were significantly (P < 0.01) lower than the numbers in specimens from mice infected with wild-type KU2 (peritoneal lavage fluid, 7.18 ± 0.22, and whole blood, 5.85 ± 0.36). In addition, the bacterial numbers in the specimens from mice infected with KU2ΔlepA (peritoneal lavage fluid, 4.79 ± 0.25, and whole blood, 2.81 ± 0.28) were significantly (P < 0.01) lower than the numbers in specimens from mice infected with KU2ΔplcH. In particular, the bacterial numbers in the specimens in KU2ΔlepAΔplcH-infected mice (peritoneal lavage fluid, 3.43 ± 0.26, and whole blood, 1.78 ± 0.32) were markedly lower than the numbers in specimens from mice infected with wild-type KU2. Thus, these results correlate well with the LD50 of each P. aeruginosa strain, suggesting that the functional activities of LepA and PlcH are highly relevant to the in vivo growth of P. aeruginosa.
FIG. 7.
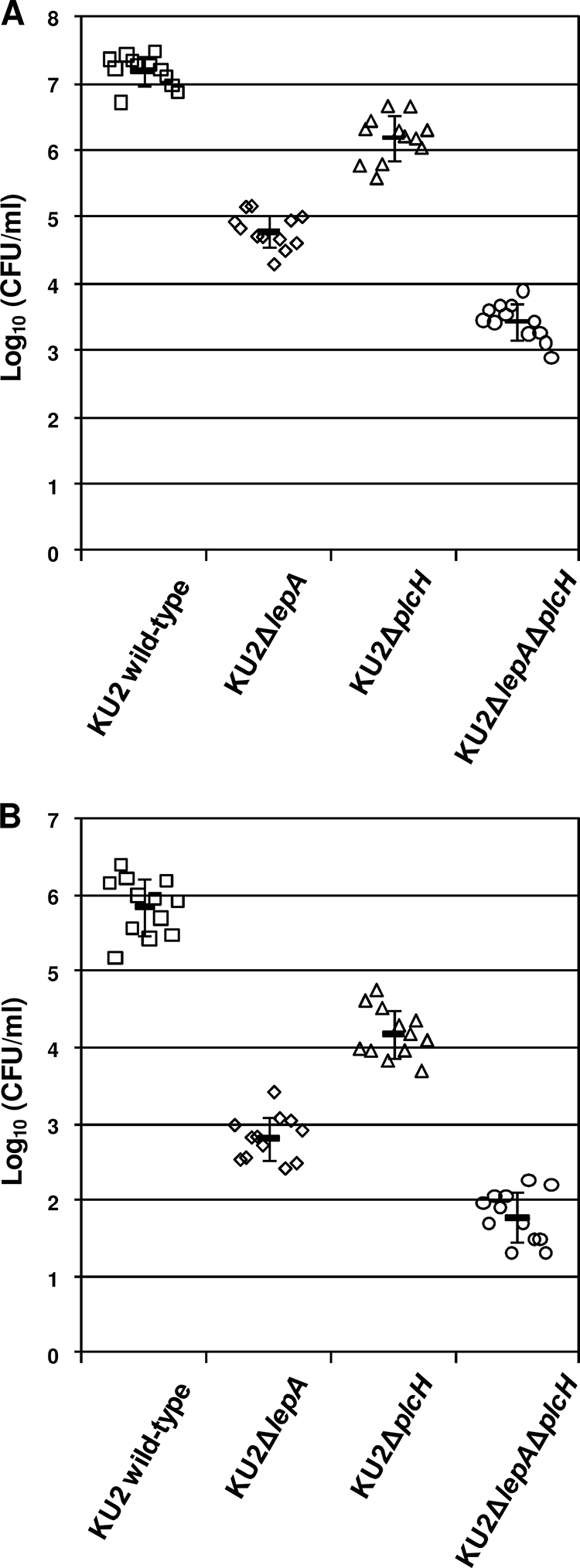
In vivo growth of wild-type P. aeruginosa KU2 and the lepA and plcH mutants in leukopenic ddY mice. (A) Bacterial count of peritoneal lavage fluid. (B) Bacterial count of whole blood. Leukopenic ddY mice were challenged by i.p. injection with 1 × 106 CFU/mouse of each P. aeruginosa strain. Four hours after i.p. injection, the samples were collected. Each symbol represents the log10 CFU/ml of peritoneal lavage fluid or whole blood from an individual mouse. The horizontal lines with error bars represent the means ± standard deviations. Twelve mice were used in each group. The difference of each strain pairing was significant with a P value of <0.01.
DISCUSSION
In this article, we describe findings showing that LepA functions in the degradation of hemoglobin to acquire peptide pools and heme iron for the growth of P. aeruginosa (Fig. 1 to 3). Furthermore, we demonstrate that cooperation between LepA and PlcH contributes to the utilization of erythrocytes as a sole nutrient source for its growth (Fig. 4). Inorganic iron (Fe3+) is one of the growth factors that are obtained from the environment, and bacteria generally need 0.05 to 0.5 μM free iron (Fe3+) to grow (28). The concentration of free iron in the human body is in the order of 1 × 10−12 μM, and almost all iron exists as heme (5). The total amount of iron in an adult human is roughly 3 to 4 g, and approximately 2.5 g of the iron exists as hemoglobin in erythrocytes (37). Extracellular iron is strongly conjugated to glycoprotein, transferrin, and lactoferrin, thereby decreasing the concentration of free iron in the body to extremely low levels (31, 37). Thus, the concentration of free iron is much lower than that required for bacterial growth. Therefore, hemoglobin release from erythrocytes by hemolytic action and degradation of iron-binding proteins by proteolytic action would contribute to the utilization of heme iron for bacterial growth. Hence, it is possible that LepA and PlcH would play an important role in the growth of P. aeruginosa. Unexpectedly, strain KU2ΔplcH was found to be able to utilize erythrocytes as a sole nutrient source, which was similar to the results for strain KU2ΔlepA (Fig. 4). P. aeruginosa was shown to produce a heat-stable glycolipid (known as rhamnolipid) as a hemolysin distinct from PlcH (14, 20). Therefore, it is feasible that strain KU2ΔplcH may utilize rhamnolipids to release hemoglobin from erythrocytes, leading to the degradation of hemoglobin by LepA for the growth of the strain.
As shown by the results in Fig. 5, in the in vitro experimental system, both LepA and PlcH contribute to the expression of virulence, and an additive effect by these factors was also observed. The in vitro virulence of P. aeruginosa KU2ΔlepA appeared to be attenuated compared to that of strain KU2ΔplcH (Fig. 5). P. aeruginosa was reported to produce, in addition to proteases and hemolysins, virulence factors such as exotoxins and exoenzymes (15). Since bacterial growth is considered to be closely related to virulence (6, 12), KU2ΔlepA is likely to have reduced levels of production of such factors relative to their levels in KU2ΔplcH, thereby decreasing its virulence.
Subsequently, we examined the roles of LepA and PlcH in in vivo virulence and growth of P. aeruginosa using a mouse model of leukopenia. This model has been developed to study the pathogenesis of P. aeruginosa infection under immunosuppressed conditions and to evaluate therapeutic agents (10, 56). Challenge of leukopenic mice by i.p. injection with P. aeruginosa was reported to cause sepsis by acute systemic infection, leading to the death of mice within 1 to 3 days after infection (32, 56). In our in vivo model, leukopenic mice infected i.p. with each P. aeruginosa strain started to die within 1 to 3 days after infection (Fig. 6). The results of survival experiments suggested a hierarchy of virulent strains: wild-type KU2 strain > KU2ΔplcH strain > KU2ΔlepA strain > KU2ΔlepAΔplcH strain. Similar to the in vitro results, the virulence of P. aeruginosa KU2ΔlepA was somewhat lower than that of KU2ΔplcH. Accordingly, the results of the in vivo survival experiments are consistent with those of the in vitro cytotoxicity assay. Furthermore, it is of note that the results of the in vivo growth experiments using each P. aeruginosa strain reflect those of the in vivo survival experiments (Fig. 6 and 7). Thus, our data suggest that LepA, rather than PlcH, may play an important role in the expression of virulence during P. aeruginosa infection. To further investigate whether LepA participates in the pathogenesis of P. aeruginosa, the construction of recombinant LepA molecules is now in progress in our laboratory.
As for another virulence factor, an extracellular DNase of P. aeruginosa has been demonstrated to play a role in the utilization of DNA as a nutrient source (33). In other bacteria, such as Staphylococcus aureus and Streptococcus pyogenes, extracellular DNase has been hypothesized to facilitate the dissemination of infecting bacteria by lysing pus (47, 54). Earlier studies demonstrated a role for extracellular DNases in host immune evasion by the degradation of neutrophil extracellular traps (3, 4). We also previously reported that Serratia marcescens serralysin and P. aeruginosa LepA induce inflammatory responses through protease-activated receptors (PARs) in a human bronchiole cell line, EBC-1 (22, 23). In the mammalian body, PAR activation contributes to a variety of physiological and pathophysiological functions, including immunity, inflammation, and tumor cell growth (35, 48). Therefore, it is possible that the DNases and proteases of pathogenic microorganisms function not only to acquire nutrients from the host but also as modulators of host immune responses.
In summary, to investigate the roles of LepA and PlcH in in vivo virulence and growth of P. aeruginosa, we compared the virulence and growth of a wild-type strain and its mutants using a mouse model of acute systemic infection by P. aeruginosa. The results of mouse infection experiments demonstrated that the virulence of the isogenic lepA or plcH single mutant was attenuated, and the numbers of the mutants were lower than the numbers of the wild-type strain in peritoneal lavage fluid and whole blood. In particular, the virulence and growth rate of the lepA plcH double mutant were markedly lower than those of the wild-type strain. Taken together, these results suggest that LepA and PlcH contribute to the in vivo virulence and growth of P. aeruginosa.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 November 2010.
REFERENCES
- 1.Aristoteli, L. P., and M. D. Willcox. 2003. Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro. Infect. Immun. 71:5565-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss, C. I. 1934. The method of probits. Science 79:38-39. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D. S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, J. T., A. J. Simpson, R. K. Aziz, G. Y. Liu, S. A. Kristian, M. Kotb, J. Feramisco, and V. Nizet. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396-400. [DOI] [PubMed] [Google Scholar]
- 5.Bullen, J. J. 1981. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarty, A. M. 1998. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol. Microbiol. 28:875-882. [DOI] [PubMed] [Google Scholar]
- 7.Chin, J. C., and J. E. Watts. 1988. Biological properties of phospholipase C purified from a fleecerot isolate of Pseudomonas aeruginosa. J. Gen. Microbiol. 134:2567-2575. [DOI] [PubMed] [Google Scholar]
- 8.Clark, J. D., Institute of Laboratory Animal Resources (Washington, DC), and National Research Council (United States) Committee to Revise the Guide for the Care and Use of Laboratory Animals. 1996. Guide for the care and use of laboratory animals, rev. ed. National Academic Press, Washington, DC.
- 9.Coin, D., D. Louis, J. Bernillon, M. Guinand, and J. Wallach. 1997. LasA, alkaline protease and elastase in clinical strains of Pseudomonas aeruginosa: quantification by immunochemical methods. FEMS Immunol. Med. Microbiol. 18:175-184. [DOI] [PubMed] [Google Scholar]
- 10.Cryz, S. J., Jr., E. Furer, and R. Germanier. 1983. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect. Immun. 39:1067-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel, L. S., J. M. Hill, J. M. Moreau, L. C. Green, J. A. Hobden, and R. J. O'Callaghan. 1998. Pseudomonas aeruginosa protease IV produces corneal damage and contributes to bacterial virulence. Invest. Ophthalmol. Vis. Sci. 39:662-665. [PubMed] [Google Scholar]
- 12.Filiatrault, M. J., K. F. Picardo, H. Ngai, L. Passador, and B. H. Iglewski. 2006. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect. Immun. 74:4237-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleckenstein, J. M., K. Roy, J. F. Fischer, and M. Burkitt. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect. Immun. 74:2245-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita, K., T. Akino, and H. Yoshioka. 1988. Characteristics of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 56:1385-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman, A. L., and S. Lory. 2004. Analysis of regulatory networks in Pseudomonas aeruginosa by genomewide transcriptional profiling. Curr. Opin. Microbiol. 7:39-44. [DOI] [PubMed] [Google Scholar]
- 16.Harrington, S. M., J. Sheikh, I. R. Henderson, F. Ruiz-Perez, P. S. Cohen, and J. P. Nataro. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 77:2465-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollsing, A. E., M. Granstrom, M. L. Vasil, B. Wretlind, and B. Strandvik. 1987. Prospective study of serum antibodies to Pseudomonas aeruginosa exoproteins in cystic fibrosis. J. Clin. Microbiol. 25:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob-Dubuisson, F., R. Fernandez, and L. Coutte. 2004. Protein secretion through autotransporter and two-partner pathways. Biochim. Biophys. Acta 1694:235-257. [DOI] [PubMed] [Google Scholar]
- 19.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, M. K., and D. Boese-Marrazzo. 1980. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 29:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharazmi, A. 1991. Mechanisms involved in the evasion of the host defence by Pseudomonas aeruginosa. Immunol. Lett. 30:201-205. [DOI] [PubMed] [Google Scholar]
- 22.Kida, Y., Y. Higashimoto, H. Inoue, T. Shimizu, and K. Kuwano. 2008. A novel secreted protease from Pseudomonas aeruginosa activates NF-kappaB through protease-activated receptors. Cell. Microbiol. 10:1491-1504. [DOI] [PubMed] [Google Scholar]
- 23.Kida, Y., H. Inoue, T. Shimizu, and K. Kuwano. 2007. Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor 2. Infect. Immun. 75:164-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiewitz, C., and B. Tummler. 2000. Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J. Bacteriol. 182:3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116:481-489. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 27.Marquart, M. E., A. R. Caballero, M. Chomnawang, B. A. Thibodeaux, S. S. Twining, and R. J. O'Callaghan. 2005. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Invest. Ophthalmol. Vis. Sci. 46:3761-3768. [DOI] [PubMed] [Google Scholar]
- 28.Martinez, J. L., A. Delgado-Iribarren, and F. Baquero. 1990. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol. Rev. 6:45-56. [DOI] [PubMed] [Google Scholar]
- 29.Mazar, J., and P. A. Cotter. 2007. New insight into the molecular mechanisms of two-partner secretion. Trends Microbiol. 15:508-515. [DOI] [PubMed] [Google Scholar]
- 30.Meyers, D. J., K. C. Palmer, L. A. Bale, K. Kernacki, M. Preston, T. Brown, and R. S. Berk. 1992. In vivo and in vitro toxicity of phospholipase C from Pseudomonas aeruginosa. Toxicon 30:161-169. [DOI] [PubMed] [Google Scholar]
- 31.Mietzner, T. A., and S. A. Morse. 1994. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu. Rev. Nutr. 14:471-493. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki, S., T. Matsumoto, K. Tateda, A. Ohno, and K. Yamaguchi. 1995. Role of exotoxin A in inducing severe Pseudomonas aeruginosa infections in mice. J. Med. Microbiol. 43:169-175. [DOI] [PubMed] [Google Scholar]
- 33.Mulcahy, H., L. Charron-Mazenod, and S. Lewenza. 2010. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ. Microbiol. 12:1621-1629. [DOI] [PubMed] [Google Scholar]
- 34.O'Callaghan, R. J., L. S. Engel, J. A. Hobden, M. C. Callegan, L. C. Green, and J. M. Hill. 1996. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest. Ophthalmol. Vis. Sci. 37:534-543. [PubMed] [Google Scholar]
- 35.Ossovskaya, V. S., and N. W. Bunnett. 2004. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84:579-621. [DOI] [PubMed] [Google Scholar]
- 36.Ostroff, R. M., B. Wretlind, and M. L. Vasil. 1989. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect. Immun. 57:1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otto, B. R., A. M. Verweij-van Vught, and D. M. MacLaren. 1992. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit. Rev. Microbiol. 18:217-233. [DOI] [PubMed] [Google Scholar]
- 38.Pavlovskis, O. R., and B. Wretlind. 1979. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect. Immun. 24:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller, M. A., C. Grant, V. Morthland, and J. Rhine-Chalberg. 1994. Comparative evaluation of alternative methods for broth dilution susceptibility testing of fluconazole against Candida albicans. J. Clin. Microbiol. 32:506-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole, K., and V. Braun. 1988. Iron regulation of Serratia marcescens hemolysin gene expression. Infect. Immun. 56:2967-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston, M. J., P. C. Seed, D. S. Toder, B. H. Iglewski, D. E. Ohman, J. K. Gustin, J. B. Goldberg, and G. B. Pier. 1997. Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect. Immun. 65:3086-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy, K., D. Hamilton, K. P. Allen, M. P. Randolph, and J. M. Fleckenstein. 2008. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect. Immun. 76:2106-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy, K., G. M. Hilliard, D. J. Hamilton, J. Luo, M. M. Ostmann, and J. M. Fleckenstein. 2009. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 457:594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. W. Frank, D. B. Doroques, T. Long, M. A. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt, C., D. Turner, M. Boesl, M. Abele, M. Frosch, and O. Kurzai. 2007. A functional two-partner secretion system contributes to adhesion of Neisseria meningitidis to epithelial cells. J. Bacteriol. 189:7968-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 47.Sherry, S., W. S. Tillett, and L. R. Christensen. 1948. Presence and significance of deoxyribose nucleoprotein in the purulent pleural exudates of patients. Proc. Soc. Exp. Biol. Med. 68:179-184. [DOI] [PubMed] [Google Scholar]
- 48.Shpacovitch, V., M. Feld, N. W. Bunnett, and M. Steinhoff. 2007. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 28:541-550. [DOI] [PubMed] [Google Scholar]
- 49.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 50.Suter, S. 1994. The role of bacterial proteases in the pathogenesis of cystic fibrosis. Am. J. Respir. Crit. Care Med. 150:S118-S122. [DOI] [PubMed] [Google Scholar]
- 51.Tala, A., C. Progida, M. De Stefano, L. Cogli, M. R. Spinosa, C. Bucci, and P. Alifano. 2008. The HrpB-HrpA two-partner secretion system is essential for intracellular survival of Neisseria meningitidis. Cell. Microbiol. 10:2461-2482. [DOI] [PubMed] [Google Scholar]
- 52.Tang, A., M. E. Marquart, J. D. Fratkin, C. C. McCormick, A. R. Caballero, H. P. Gatlin, and R. J. O'Callaghan. 2009. Properties of PASP: a Pseudomonas protease capable of mediating corneal erosions. Invest. Ophthalmol. Vis. Sci. 50:3794-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terada, L. S., K. A. Johansen, S. Nowbar, A. I. Vasil, and M. L. Vasil. 1999. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect. Immun. 67:2371-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tillett, W. S., S. Sherry, and L. R. Christensen. 1948. Streptococcal deoxyribonuclease; significance in lysis of purulent exudates and production by strains of hemolytic streptococci. Proc. Soc. Exp. Biol. Med. 68:184-188. [DOI] [PubMed] [Google Scholar]
- 55.Twining, S. S., S. E. Kirschner, L. A. Mahnke, and D. W. Frank. 1993. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Invest. Ophthalmol. Vis. Sci. 34:2699-2712. [PubMed] [Google Scholar]
- 56.Uezumi, I., M. Terashima, T. Kohzuki, M. Kato, K. Irie, H. Ochi, and H. Noguchi. 1992. Effects of a human antiflagellar monoclonal antibody in combination with antibiotics on Pseudomonas aeruginosa infection. Antimicrob. Agents Chemother. 36:1290-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg, E. D. 1978. Iron and infection. Microbiol. Rev. 42:45-66. [DOI] [PMC free article] [PubMed] [Google Scholar]