Abstract
A defining facet of tick-Rickettsia symbioses is the molecular strategy employed by each partner to ensure its own survival. Ticks must control rickettsial colonization to avoid immediate death. In the current study, we show that rickettsial abundance in the tick midgut increases once the expression of a Kunitz-type serine protease inhibitor from the American dog tick (Dermacentor variabilis) (DvKPI) is suppressed by small interfering RNA (siRNA). A series of in vitro invasion assays suggested that DvKPI limits rickettsial colonization during host cell entry. Interestingly, we observed that DvKPI associates with rickettsiae in vitro as well as in the tick midgut. Collectively, our data demonstrate that DvKPI limits host cell invasion by Rickettsia montanensis, possibly through an association with the bacterium.
Spotted fever group (SFG) Rickettsia spp. have a worldwide distribution that is inextricably linked to their ixodid tick vectors. SFG rickettsiae range in degree of mammalian virulence from pathogenic to nonpathogenic. We use a closely related nonpathogenic rickettsia, Rickettsia montanensis, as a surrogate model for the virulent R. rickettsii, the etiologic agent for Rocky Mountain spotted fever (RMSF). RMSF was originally described in Snake River Valley, ID, and Bitterroot Valley, MT, as having a 62% fatality rate during the epidemic years from 1887 to 1941 (36). Since that time, the distribution of R. rickettsii has been recognized to include both North and South American regions, where case fatality rates have been reported to reach 1.4% (1997 to 2002) and 40 to 95%, respectively (35, 38).
Ticks are exposed to microorganisms that infect or colonize their mammalian hosts by way of hematophagy. A number of tick genes putatively involved with adhesion/invasion and stress/defense responses are differentially expressed in response to R. montanensis infection (26). Being obligate intracellular bacteria, rickettsiae may evade host immune pressures, leading to a successful infection. However, transcript abundance studies conducted in our laboratory indicated that rickettsiae are recognized as foreign and that ticks respond physiologically (5, 6, 26). Since rickettsiae represent a potential threat, an immune response is sure to be activated. The tick's immune response may control R. rickettsii infection, increasing survival and rendering the tick a competent vector. To this end, immune control of rickettsia abundance may be responsible for the observed low prevalence of tick infection in nature (2, 33, 44).
The immune response in ticks is defined by both early antimicrobial peptide (AMP) expression and, later, a cell-mediated response that may involve AMPs and/or encapsulation (walling off) of microorganisms (5-8, 12, 16-19, 21, 23, 30-32, 39, 40, 42). From an immunological standpoint, the tick midgut holds a great deal of interest because it is the first point of active contact between the tick and microbes. Indeed, controlling infections in the midgut may reduce pathogen load within the tick. To date, immune activity within the midgut is defined specifically by defensin and lysozyme expression (5, 6, 31, 39) as well as by host blood meal digestion by-products such as β-hemoglobin (11, 29, 43). Recently, we characterized DvKPI, a rickettsiostatic Kunitz-type serine protease inhibitor from the R. rickettsii vector tick, Dermacentor variabilis (6).
KPIs are conventionally characterized as anticoagulants that facilitate feeding in ticks (13, 14). We found that DvKPI does in fact possess anticoagulant and trypsin inhibitory properties (6). Our investigations into DvKPI's role as a rickettsiostatic protein began with the observation that transcript abundance is sustained over a 72-h period in midguts from ticks infected with R. montanensis (6). Additionally, we observed a 60% reduction in rickettsial abundance when L929 fibroblasts expressing DvKPI were infected with R. montanensis (6). These data suggest to us that DvKPI may function to control rickettsial colonization, a phenomenon well documented in legumes, where colonization by Rhizobium spp. is controlled in part by a Kunitz-type protease inhibitor, presumably to prevent physiological stress or host death (25, 27). In this study, we hypothesize that rickettsial abundance is controlled in the tick by DvKPI.
MATERIALS AND METHODS
Ticks, rickettsiae, and cell culture.
Four-day partially fed or unfed female D. variabilis ticks were provided by Daniel Sonenshine (Department of Biological Sciences, Old Dominion University). Immature ticks were fed on rats (Rattus norvegicus), and the adults were fed on New Zealand White rabbits (Oryctolagus cunniculus). Tick colony maintenance and animal husbandry were carried out according to approved protocols of the Old Dominion University's Institutional Animal Care and Use Committee. Vero 76 (ATCC CRL-1587) cells were used for routine propagation of R. montanensis. Vero cells were maintained at 34°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS). Unless otherwise noted, cells were grown in T-150 flasks (Corning, Corning, NY). For propagation, rickettsia-infected Vero 76 cells were grown to 80% infection, at which time the rickettsiae were purified from host cells by a Renografin procedure. Briefly, infected cells were washed with fresh medium, scraped, and lysed by five passages through a 3-ml syringe fitted with a 27-gauge needle. Large particulates of host material were removed by low-speed centrifugation at 500 × g for 5 min at 4°C. The clarified supernatant was layered onto a 25% Renografin solution (in SPG [218 mM sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM l-glutamate, pH 7.2]) at a ratio of supernatant to Renografin of 1:1. Each sample was centrifuged at 17,000 × g for 10 min at 4°C. The supernatant-Renografin was removed from the pelleted rickettsiae. Rickettsiae were resuspended in fresh medium and counted using the BacLight live-dead assay (Molecular Probes, Carlsbad, CA) on a hemocytometer at a magnification of ×400. Rickettsiae were stored in aliquots containing 1 × 106 to 1 × 107 rickettsiae at −80°C until use.
Tick infection.
Renografin-purified rickettsiae were resuspended in diluted sheep's blood (diluted 125-fold in 0.9% NaCl) to a final concentration of 30,000 rickettsiae/μl. Eight microliters of rickettsia suspension was drawn up into a glass capillary and placed over the tick's mouthparts. Each tick was allowed to imbibe the entire solution and then incubated at 22°C and 95 to 100% humidity until used.
Collection of midgut luminal contents.
Midguts from 4-day fed females were dissected and washed three times in 50 μl of phosphate-buffered saline (PBS). The gut was opened up to expose the lumen in 100 μl of PBS and transferred, with the PBS, to an Eppendorf tube on ice. The guts were incubated on ice for approximately 2 h, with gentle vortexing once every hour. Each sample was centrifuged at 2,000 × g for 5 min at room temperature. The supernatant was removed to a fresh Eppendorf tube and stored at 4°C until analysis by Western blotting. To detect DvKPI, the blots were incubated with anti-DvKPI (1:500). As a control, each blot was also probed with rabbit preimmune serum. To demonstrate that DvKPI was of tick origin, both rabbit and sheep sera were processed for Western blotting and probed using anti-DvKPI.
RNA isolation and quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was isolated from tissues or cells by use of an ALLPrep DNA/RNA Mini kit according to the manufacturer's procedures (Qiagen, Valencia, CA). For expression analysis, transcripts were amplified using a Brilliant II Sybr green QRT-PCR master mix kit according to the manufacturer's instructions (Agilent Technologies, Santa Clara, CA). For analysis of rickettsial abundance, rickettsial and host transcripts were amplified using a SuperScript III one-step RT-PCR kit with Platinum Taq polymerase according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Cycling was performed on a Stratagene Mx3005P real-time thermal cycler. Amplification curves from each experiment for each primer pair were collected and exported to Excel-based LinReg PCR software to calculate primer pair efficiencies. The primer efficiencies were imported into Excel-based QGene software, where the cycle threshold values for each target gene were normalized to the cycle threshold for its respective internal housekeeping gene for calculation of normalized expression (37). Primers used for amplification are listed in Table 1.
TABLE 1.
Primers and siRNA oligonucleotides
| Primer | Sequence (5′-3′)a | Expt |
|---|---|---|
| DvKPI forward | CGAAGAATCAGAGTGCTGGAGAAC | DvKPI expression (siRNA) |
| DvKPI reverse | CCGAGGTGGTTTTTAGGTCCTG | DvKPI expression (siRNA) |
| Actin forward | CCGGTTCAGCCCTCGTTCT | DvKPI expression (tick endogenous control) |
| Actin reverse | TTGAGGCCAGGGATGGAGC | DvKPI expression (tick endogenous control) |
| LUX set gltA forward | CAGTCCGAATTGCCAGCTCA | Measurement of R. montanensis burden |
| LUX set gltA reverse | CGGGCCAAAGTGAGGCAATACC-FAM-G | Measurement of R. montanensis burden |
| LUX set actin forward | GGAAGGACCTGTACGCCAACAC | Measurement of R. montanensis burden (tick endogenous control) |
| LUX set actin reverse | CGCCGATCTTCATGGTGGAAGG-FAM-G | Measurement of R. montanensis burden (tick endogenous control) |
| DvKPI sense oligonucleotide | UUGAGUGCAACAAGAAGUGUU | siRNA |
| DvKPI antisense oligonucleotide | UUAACUCACGUUGUUCUUCAC | siRNA |
| Control sense oligonucleotide | CUUUUACGGGUAGACGAUAUU | siRNA |
| Control antisense oligonucleotide | UUGAAAAUGCCCAUCUGCUAU | siRNA |
FAM, 6-carboxyfluorescein.
Rickettsial invasion of host cells.
Generation of bulk stable L929 cells expressing DvKPI was described previously (6). To generate conditioned medium, six-well plates were plated with 4 × 105 nontransfected (control) or DvKPI-expressing L929 cells and incubated for 72 h at 34°C and 5% CO2 in DMEM supplemented with 10% FBS without Geneticin. Medium was harvested from each cell type after 72 h of growth, filtered through a 0.22-mm filter (Millipore, Billerica, MA), and stored at −80°C until use. Pilot experiments demonstrated that increases in recombinant DvKPI (rDvKPI) concentrations in the medium are negligible after 72 h of growth (6). The presence of rDvKPI in the medium was determined by Western blotting with rabbit anti-DvKPI.
The day before the experiment, 1 × 104 Vero 76 cells were plated in 8-well glass LabTek slides in DMEM (with 10% FBS) for incubation at 34°C and 5% CO2. On the day of the experiment, 1 × 107 Renografin-purified rickettsiae were incubated with 50 μl of L929 cell- and DvKPI-conditioned medium or DvKPI-conditioned medium preabsorbed with 4 mg/ml preimmune or DvKPI IgG (IgG was purified using a MelonG IgG purification kit [Thermo Scientific]) for 30 min at 30°C with shaking at 220 rpm. The rickettsiae were washed three times by centrifugation at 16,000 × g for 10 min and were resuspended in fresh serum-free DMEM. Vero cells were washed in serum-free DMEM, infected at a multiplicity of infection (MOI) of ∼10 to 20 treated rickettsiae, centrifuged at 200 × g for 5 min, and allowed to incubate at 34°C and 5% CO2 for 1 h. At 1 h postinfection, Vero cells were washed five times in 1× PBS to remove nonadherent rickettsiae and then fixed in freshly prepared 4% paraformaldehyde (in 1× PBS) for 20 min at room temperature, followed by three washes for 5 min each in 1× PBS at room temperature. All subsequent staining was performed at room temperature. To stain extracellular rickettsiae, the samples were incubated with mouse anti-R. montanensis polyclonal sera for 1 h and then washed three times for 5 min each in 1× PBS, followed by a 30-min incubation with goat anti-mouse-Alexa Fluor 594 secondary antibody (Invitrogen). Vero cells were permeabilized by incubation with 0.1% Triton X-100 (in 1× PBS) for 5 min and then washed three times for 5 min each in 1× PBS. To stain total rickettsiae, the cells were stained as described for detection of extracellular rickettsiae, except for the use of goat anti-mouse-Alexa Fluor 488 secondary antibody. Samples were mounted under VectaShield with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) and visualized under oil at a magnification of ×1,000 on a Nikon Eclipse E600 microscope. Images were captured with a Qimaging digital camera using QCapture Pro software and processed with Adobe Illustrator CS3. Rickettsiae that fluoresced green only were considered intracellular, and those Vero cells containing intracellular rickettsiae were counted as invaded cells. Percent invasion was taken as the number of invaded Vero cells divided by the total number of rickettsia-associated cells. Two separate experiments were performed in duplicate, where at least 200 individual cells were counted. The results from both experiments were combined for statistical analysis. Results are expressed as means ± standard deviations (SD).
siRNA.
Unfed ticks were used for small interfering RNA (siRNA) experiments to alleviate confounding effects of feeding on DvKPI expression. siRNA oligonucleotides were designed using Ambion's siRNA Target Finder and suggested guidelines (Applied Biosciences/Ambion, Austin, TX). The experimental oligonucleotides (sense and antisense) (Table 1) were designed to target DvKPI. A scrambled control oligonucleotide was used to control for off-target effects. Each oligonucleotide was tested by the BLAST algorithm to determine the amount of complementarity between the experimental or control oligonucleotide and the target sequence. Oligonucleotides were synthesized by the University of Maryland Biopolymer Core Facility. Oligonucleotides were hybridized by mixing a 20 μM concentration of each oligonucleotide with annealing buffer (10 mM Tris, pH 8.0, 20 mM NaCl) and heating the solution to 90°C for 1 min, followed by incubation at 37°C for 1 h. The hybridized oligonucleotides were precipitated using isopropanol precipitation, resuspended in nuclease-free water, and quantified using a Thermo Nanodrop spectrophotometer (Thermo Scientific, Jessup, MD). The hybridized experimental or control oligonucleotide solutions were diluted to 2 μg/μl, and 0.5 to 1 μl was injected into the 4th coxal-trochanter joint of each tick, using a 33-gauge needle fitted on a 5-μl syringe (Hamilton, Reno, NV). After incubation at 22°C and 95 to 100% humidity for 48 h, ticks were either dissected to test for protein or transcript knockdown or fed 8 μl of a rickettsial solution containing 30,000 rickettsiae/μl as described above and incubated at 22°C and 95 to 100% humidity for 24 h. In short, transcript and protein levels for DvKPI were assessed 48 h after delivery of the siRNA, after which ticks were fed the rickettsial suspension. At 24 h postinfection, tick midguts were dissected for measurement of rickettsial abundance only. This methodology ensures that rickettsiae enter the midgut when DvKPI levels are suppressed. Measurement of DvKPI levels and abundance was not performed at the same time post-rickettsial infection because these studies were focused on the effect of DvKPI suppression as it relates to rickettsial entry. Tick midguts were dissected, placed in RLT extraction buffer (Qiagen), and stored at −80°C until used for RNA isolation. Alternatively, midguts were dissected in 1× PBS, homogenized in 100 μl of NP-40 lysis buffer (1% NP-40, 20 mM Tris, pH 8.0, 150 mM NaCl, 10% glycerol, HALT protease inhibitor cocktail [Thermo Scientific]), and stored at 4°C until used for protein analysis. Quantitative RT-PCR was used to determine transcript and rickettsial abundance as described above. Western blotting was used to assess knockdown at the protein level. Briefly, 15 to 25 μg of midgut protein lysate was analyzed by Western blotting, using rabbit anti-DvKPI (1:500), to measure knockdown of DvKPI. Rabbit anti-actin (1:500; Sigma) was used to normalize protein loads on Western blots. Ten ticks were designated for each treatment for each experiment. Two separate experiments were performed. The results from both experiments were combined for statistical analysis. The means were plotted within a scatterplot representing all tick replicates.
Bacterial affinity pull-down assay.
Assays were run as described previously, with modification (10, 28, 41, 46). Renografin-purified rickettsiae (1 × 107) were pelleted by centrifugation at 17,000 × g for 10 min at 4°C and then resuspended in 30 μl of NP-40 lysis/interaction/wash buffer (1% NP-40, 20 mM Tris, pH 8.0, 150 mM NaCl, 10% glycerol). Thirty to 40 μg of DvKPI was added to the rickettsiae along with a micro-stir bar, and the sample was incubated for 2 h at 4°C with stirring. The sample was centrifuged at 14,000 × g for 10 min at 4°C, and the supernatant containing unbound DvKPI was stored at −20°C for analysis. The pellet was then resuspended in 30 μl of NP-40 lysis/interaction/wash buffer. This sequence was repeated once more with NP-40 lysis/interaction/wash buffer and twice with wash buffer 2 (1% NP-40, 20 mM Tris, pH 8.0, 300 mM NaCl, 10% glycerol). The pellet was resuspended in elution buffer (1% NP-40, 20 mM Tris, pH 8.0, 1.15 M NaCl, 10% glycerol) and incubated for 1 h at 4°C with stirring. The rickettsial pellet was collected by centrifugation and resuspended in 1× SDS sample buffer. To control for potential nonspecific binding of recombinant DvKPI conferred by the 6× His tag, we performed this assay using 40 to 50 μg of recombinant green fluorescent protein (GFP; U.S. Biologicals, Swampscott, MA). To assess nonspecific binding of DvKPI and GFP to any remaining host cell components in the rickettsial preparations, we performed each assay with a Renografin mock preparation (uninfected host cells). The entire sample for each wash and elution and the rickettsial pellet were analyzed by Western blotting for DvKPI, using anti-V5 antibodies (Invitrogen), or for GFP, using anti-His (Novagen, Gibbstown, NJ). Each assay was repeated twice.
In vitro colocalization.
In vitro colocalization studies were performed similarly to the invasion assays, except that R. montanensis was incubated with DvKPI-conditioned medium or purified recombinant DvKPI before being placed onto host cells. DvKPI was purified as described previously (6). After fixation, Vero cells were washed three time for 5 min each with 1× PBS and incubated with rabbit anti-DvKPI (1:200 in 1× PBS-5% bovine serum albumin [BSA]) for 30 min at 37°C or for 1 h at room temperature in a humidity chamber. The cells were washed three times for 5 min each in 1× PBS, followed by an incubation with mouse anti-R. montanensis polyclonal serum. The cells were washed as described above and then incubated with goat anti-rabbit-Alexa Fluor 594 and goat anti-mouse-Alexa Fluor 488 secondary antibodies, each at 1:500 (in 1× PBS-5% BSA), for 30 min in the dark at room temperature (Invitrogen). The samples were mounted under DAPI with VectaShield and stored at 4°C until viewed. Images were viewed and captured on a Zeiss LSM 510 Meta inverted confocal microscope (Zeiss, Inc., Thornwood, NY). Figures were composed in Adobe Illustrator CS5 (Adobe, San Jose, CA). Percent colocalization was determined by dividing the number of DvKPI-associated rickettsiae by the total number of rickettsiae counted. Results from two separate experiments are expressed as means ± SD.
Electron microscopy.
Midgut tissues from infected ticks were dissected, immediately fixed in 4F1G (a mixture of 4% formaldehyde and 1% glutaraldehyde in 0.05 M NaPO4 buffer adjusted to pH 7.2) for 1 h, and then placed in 0.05 M NaPO4 buffer. Tissues were stained en bloc with 1% uranyl acetate in 0.05 M NaPO4 buffer, dehydrated in a graded series of ethanol, and embedded in LR White resin (Structure Probe, Inc., West Chester, PA). Ultrathin sections were cut on an ultramicrotome (Ultracut S; Leica, Deerfield, IL). LR White sections were placed on Formvar-coated nickel grids (Electron Microscopy Sciences, Hatfield, PA) and stained with rabbit anti-DvKPI and mouse anti-R. montanensis immune serum. Following primary antibody incubations, the grids were incubated on drops of goat anti-rabbit IgG(H+L) labeled with 15-nm gold particles and goat anti-mouse IgG(H+L) labeled with 5-nm gold particles (Ted Pella, Inc., Redding, CA). The dilution was 1:20 for each antibody. The diluent for all reactions was 1% BSA in 0.05 M Tris-buffered saline (TBS) (pH 7.2). Ultrathin sections were incubated on blocking buffer (1% BSA containing 0.01 M glycine in 0.05 M TBS). Sections were incubated with primary antibodies for 1 h at room temperature and then overnight at 4°C. The sections were incubated with secondary antibodies for 1 h at room temperature. Sections were washed between antibody incubations on drops of 0.05 M TBS (pH 7.2). The sections were fixed with 2% aqueous glutaraldehyde and stained with 2% aqueous uranyl acetate (5 min). The grids were examined in a JEOL JEM-1200EX transmission electron microscope (JEOL, Peabody, MA) at 80 kV.
Antibody production.
To generate anti-R. montanensis polyclonal sera, BALB/c mice were injected with whole rickettsiae. Prebleed and immune sera were collected on a conventional schedule as described previously (15). Antibodies to DvKPI were produced by Primm Biotech, Inc. (Cambridge, MA) according to the company's procedures. A 10×-His-tag fusion protein including amino acid residues 178 to 325 from DvKPI was expressed from plasmid pN2 in Escherichia coli. Two rabbits were given four immunizations by a standard schedule. Enzyme-linked immunosorbent assay (ELISA) was used to test the reactivity of both the preimmune and immune sera to the recombinant protein. Preimmune bleeds from each rabbit were provided as a control to use for Western blots and immunofluorescence assays.
Statistical analyses.
If the data failed to follow a normal distribution, as determined using the Shapiro-Wilk test, the data were normalized by log transformation before we performed parametric statistical analyses. Data were screened for outliers by use of the interquartile range and were removed from the analysis if necessary. One-tailed Student's t test was used to look for statistical differences between two treatment groups. Graphing was performed in Excel or Sigmaplot. Statistical analyses were performed using Excel software.
RESULTS
Rickettsial colonization of tick midgut is limited by DvKPI.
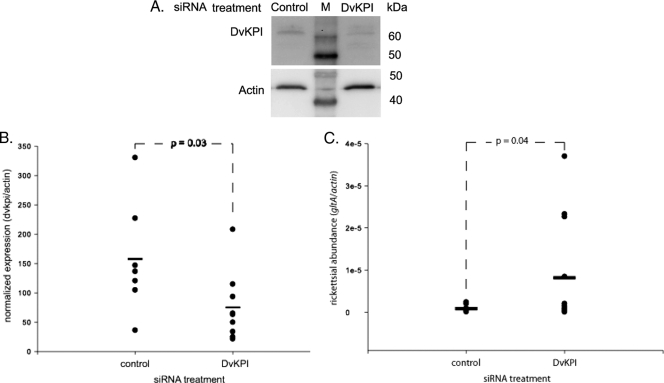
Rickettsial infection of DvKPI-expressing L929 host cells was reduced approximately 60% compared to that of LacZ-expressing or nontransfected L929 cells (6). We wanted to address the biological significance of this finding by using siRNA in vivo. Unfed female ticks were used to address the potential confounding effects of feeding (6). Injection of siRNA through the 4th coxal-trochanter joint of unfed ticks reduced DvKPI protein levels in the midgut (Fig. 1 A). DvKPI transcript abundance was suppressed 52% (P = 0.03) in ticks that received DvKPI siRNA oligonucleotide compared to that in ticks that received a control siRNA oligonucleotide (Fig. 1B). Correspondingly, rickettsial abundance increased 90% (P = 0.04) above the respective control levels in ticks receiving DvKPI siRNA oligonucleotide (Fig. 1C).
FIG. 1.
DvKPI limits rickettsial colonization of the tick midgut. Forty-eight hours after unfed female ticks were injected with DvKPI siRNA or a control siRNA, R. montanensis was delivered per os. Midguts were dissected 24 h after delivery of rickettsiae for measurement of burden. (A) Protein levels for the DvKPI siRNA treatment group were reduced compared to those for the siRNA control group. (B) Transcript levels in the DvKPI siRNA-treated ticks (n = 9) were reduced 52% from levels observed in the control siRNA-treated ticks (n = 7). (C) Accordingly, we observed a 90% increase in rickettsial abundance for the DvKPI siRNA-treated ticks (n = 12) compared to the control siRNA-treated ticks (n = 8). Transcript and rickettsial abundance averages represent individual tick replicates. Each experiment was run at least twice. P values were derived using one-tailed Student's t test. Horizontal bars represents the means.
R. montanensis invasion of host cells is limited during early infection.
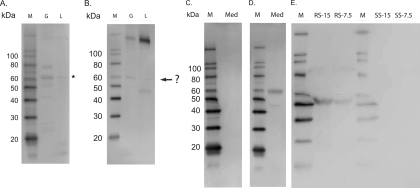
Our aim was to determine if DvKPI affects invasion of host cells by R. montanensis. In previous studies, we showed that DvKPI transcript abundance increases during feeding and in response to rickettsial infection (6). Accordingly, we detected DvKPI in the luminal contents of midguts from partially fed ticks (Fig. 2 A). It is important that anti-DvKPI serum does not recognize a band consistent with the molecular size of DvKPI in either rabbit or sheep blood (Fig. 2B), indicating that DvKPI is not of host origin. The thick band for each rabbit blood sample was a result of the reaction with the goat anti-rabbit secondary antibody. Likewise, protein bands appearing in the midgut samples that were probed with anti-DvKPI or preimmune serum were the results of nonspecific cross-reactions. Native DvKPI appeared in the midgut as an approximately 62-kDa protein (Fig. 1A and 2A), as indicated by an asterisk in Fig. 2A, which consistently ran higher than the 60-kDa band (highlighted by a question mark) in the same sample probed with preimmune serum (Fig. 2B). We also note that recombinant DvKPI appeared at 60 kDa (Fig. 2D), which is a lower apparent molecular mass than that of native DvKPI in the midgut. We speculate that the discrepancy in molecular size between recombinant and native DvKPI may be due to the posttranslational modifications (e.g., glycosylation) that occur in the midgut.
FIG. 2.
DvKPI is secreted into the lumen of the midgut. The midgut luminal contents from 4-day fed ticks were collected and electrophoresed on SDS-PAGE gels. (A) DvKPI was detected in both the gut and gut luminal contents. The asterisk denotes DvKPI. (B) Gut and luminal contents probed with rabbit preimmune serum. The question mark denotes an unknown protein. (C) DvKPI-conditioned medium probed with rabbit preimmune serum. (D) DvKPI-conditioned medium probed with rabbit anti-DvKPI. (E) DvKPI was not detected in the blood from either tick host. M, molecular size marker; G, midgut; L, luminal contents; Med, DvKPI-conditioned medium; RS-15, rabbit serum at 15 μg; RS-7.5, rabbit serum at 7.5 μg; SS-15, sheep serum at 15 μg; SS-7.5, sheep serum at 7.5 μg.
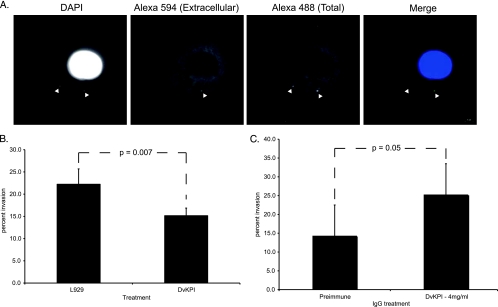
We demonstrate in Fig. 1 that DvKPI limits rickettsial colonization within a 24-h time frame post-tick infection. Thus, we hypothesized that rickettsiae interact with and are vulnerable to DvKPI before they invade host cells. To test this hypothesis, we performed a series of invasion assays. An example of staining results is found in Fig. 3 A. When rickettsiae were treated with DvKPI-conditioned medium, invasion was reduced 31.8% (Fig. 3B) (P = 0.007) compared to that in Vero cells infected by rickettsiae treated with conditioned medium from nontransfected L929 cells. To provide some dimension of specificity to the assay, we compared the invasion capacities of rickettsiae treated with DvKPI-conditioned medium that was preabsorbed with either rabbit preimmune IgG or anti-DvKPI IgG. Invasion increased 42% above the control level (preimmune IgG-absorbed medium) (Fig. 3C) (P = 0.05) when rickettsiae were treated with DvKPI-conditioned medium preabsorbed with anti-DvKPI IgG. Collectively, these data suggest that DvKPI reduces the invasion capacity of R. montanensis within the tick midgut.
FIG. 3.
DvKPI limits rickettsial invasion early in the infection cycle. (A) Alexa 594 secondary antibodies were applied before permeabilization, and Alexa 488 secondary antibodies were applied after permeabilization. Invaded Vero cells were associated with Alexa 594 (extracellular)-stained and/or Alexa 488 (total)-stained rickettsiae. Rickettsiae that fluoresced upon excitation for Alexa 488 but not for Alexa 594 were considered intracellular rickettsiae, and the host cell was counted as invaded. (B) R. montanensis was incubated with conditioned medium from L929 cells or DvKPI-expressing L929 cells and then used to infect Vero cells. (C) Cell-free conditioned medium from DvKPI-expressing L929 cells was preabsorbed with rabbit preimmune IgG or anti-DvKPI IgG at 4 mg/ml. Treated rickettsiae were used to infect Vero cells. Percent invasion for panels B and C was calculated as follows: (number of invaded cells/number of rickettsia-associated cells) × 100. Assays were run at least twice in duplicate. Values represent the means ± SD. P values were derived using Student's t test.
DvKPI associates with rickettsiae.
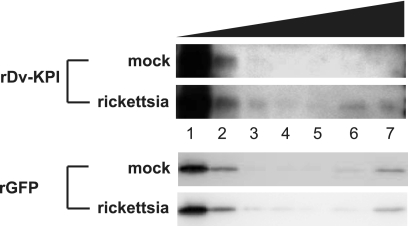
Bacterial affinity pull-down assays have been used extensively to demonstrate protein-microbe interactions (10, 28, 41, 46). Similar to results reported by other researchers, recombinant DvKPI associated with R. montanensis in our study, as indicated by the amount of protein that eluted from (Fig. 4, top panels, lane 6) and remained bound to (Fig. 4, top panels, lane 7) the rickettsial pellet. The GFP control protein appeared to bind nonspecifically to the host material remaining in a Renografin-purified rickettsial sample, as evidenced by similar elution patterns for both the mock and rickettsial samples (Fig. 4, bottom panels). We note, however, that DvKPI did not bind to the mock sample (Fig. 4, top panels).
FIG. 4.
Recombinant DvKPI associates with rickettsiae. Recombinant proteins were incubated with either mock (top) or rickettsial (bottom) preparations. Mock preparations were performed to account for any binding to background host material in each rickettsial preparation (see Materials and Methods). Bacterial affinity pull-down assays using recombinant DvKPI demonstrated binding to R. montanensis but not to host material in the mock preparation. Recombinant GFP bound both mock and R. montanensis preparations in a similar manner, suggesting nonspecific binding to background host material. The results are representative of two separate experiments. Lanes: lane 1, unbound supernatant (DvKPI or GFP); lane 2, wash 1 with buffer 1; lane 3, wash 2 with buffer 1; lane 4, wash 1 with buffer 2; lane 5, wash 2 with buffer 2; lane 6, eluate; lane 7, rickettsial or mock pellet.
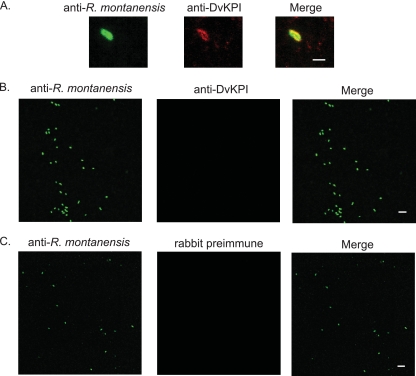
Our aim in use of the in vitro colocalization assay was to provide a visual and quantitative assay for what we suspect is an association between DvKPI and R. montanensis. When Renografin-purified rickettsiae were treated with DvKPI-conditioned medium, we observed that DvKPI associated with 6.0% ± 2.0% of the R. montanensis organisms counted (Fig. 5). Background association was significantly lower (0.8% ± 0.7%; P = 0.0002) for DvKPI-treated R. montanensis stained with rabbit preimmune serum. No association was observed in samples where rickettsiae were incubated with conditioned medium from nontransfected L929 cells and stained with anti-DvKPI sera. To demonstrate the specificity of our anti-R. montanensis serum, we cytocentrifuged untreated Renografin-purified rickettsiae onto glass slides for immunofluorescence assays (IFAs). Our mouse anti-R. montanensis serum strongly recognized untreated Renografin-purified rickettsiae (Fig. 5B). To demonstrate the specificity of our anti-DvKPI serum, we cytocentrifuged DvKPI-treated R. montanensis onto a slide and probed the sample with rabbit preimmune serum. We observed no cross-reactivity between rabbit preimmune serum and DvKPI-treated R. montanensis (Fig. 5C).
FIG. 5.
DvKPI colocalizes with rickettsiae in vitro. Renografin-purified R. montanensis was incubated with DvKPI-conditioned medium or purified recombinant DvKPI. R. montanensis was cytocentrifuged onto a glass specimen slide, and slides were allowed to air dry before fixation with 4% paraformaldehyde (in 1× PBS). Slides were probed sequentially with the indicated primary sera and anti-mouse-Alexa 488 or anti-rabbit-Alexa 594 secondary antibody. Each experiment was run twice in duplicate. (A) R. montanensis incubated with DvKPI and probed with anti-R. montanensis and anti-DvKPI. Bar, 1 μm. (B) R. montanensis (untreated) probed with anti-R. montanensis and anti-DvKPI. Bar, 5 μm. (C) R. montanensis incubated with DvKPI and probed with anti-R. montanensis and preimmune sera. Bar, 5 μm. Magnification, ×1,000.
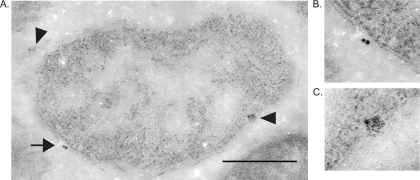
Ultrastructural examination of R. montanensis-infected tick midguts proved to be less subjective and more definitive than our in vitro colocalization assay. Most importantly, we observed that DvKPI localized to the cell walls of rickettsiae (Fig. 6). This finding is consistent with the results of both the bacterial affinity pull-down assay (Fig. 4) and the in vitro colocalization assay (Fig. 5).
FIG. 6.
DvKPI associates with R. montanensis in the tick midgut. (A) Electron photomicrograph of R. montanensis in the tick midgut demonstrating the association of tick DvKPI on the cytoplasmic membrane. The arrow indicates anti-DvKPI recognition (15-nm gold particles). Arrowheads indicate anti-R. montanensis recognition (6-nm gold particles). (B) Detail of anti-DvKPI recognition. (C) Detail of anti-R. montanensis recognition. Total magnification, ×62,700. Bar, 0.5 μm.
DISCUSSION
Since rickettsiae are imbibed with the tick's blood meal, they encounter the antibacterial activity of the midgut. Previously, we reported that DvKPI transcript abundance in the tick midgut is sustained above the control level in response to infection with R. montanensis (6). We also demonstrated that rickettsial abundance is reduced in cultured cells expressing DvKPI (6). In this report, we tested the biological significance of this finding by using siRNA. When we suppressed DvKPI expression, we observed a corresponding increase in rickettsial abundance. These observations suggest that DvKPI's activity reduces rickettsial colonization in the tick midgut.
Our Western blot of the midgut luminal contents suggests that DvKPI is present in the midgut lumen. The influx of rickettsiae that are imbibed with a host blood meal is temporally in sync with the secretion of DvKPI into the lumen of the midgut. It follows that DvKPI will interact with rickettsiae in the midgut lumen before they invade. Collectively, our results led to the hypothesis that DvKPI limits invasion of the tick midgut. Indeed, we demonstrated that a reduction in rickettsial invasion occurred if rickettsiae were pretreated with conditioned medium containing DvKPI. These data were strengthened by the observation that rickettsial invasion was restored to pretreatment levels (compare Fig. 3B and C) if DvKPI-conditioned medium was first preabsorbed using anti-DvKPI IgG and then used to treat rickettsiae. A pattern of conserved function is emerging for select serine protease inhibitors as immune molecules that target obligate intracellular microbes, endosymbionts, and extracellular microbes. Serine protease inhibitors inhibit Plasmodium parasite survival in Anopheles stephensi and Anopheles gambiae (1). In plants, KPIs are thought to limit infiltration of uninfected root system tissue by nodulating bacteria (25, 27). Similar trends exist for Kazal-type serine protease inhibitors (Kazal SPIs) from the hydra, Hydra magnipapillata, and the crayfish, Procambarus clarkii, which are reported to inhibit the growth of Staphylococcus aureus and Bacillus spp., respectively (3, 24). With regard to arthropod vectors, serine protease inhibitors may protect both the mosquito and the tick from a level of infection that would lead to premature death and, ultimately, interruption of zoonoses. To this end, serine protease inhibitors may be considered intrinsic factors that define vector competency.
The mechanism that underlies the bacteriostatic properties of DvKPI is unknown but may be rooted in an association between the protease inhibitor and the bacterium, as exemplified by the Rhizobium-legume symbiosis (27). Additional examples are found in the freshwater crayfish, P. clarkii, in which a Kazal-type serine protease inhibitor associates with various Gram-positive and Gram-negative bacteria (24). We show evidence of an association between DvKPI and extracellular rickettsiae both in vitro and in vivo. The level of association in our in vitro colocalization assay may at first seem low, but we argue that it is consistent with our previous in vitro abundance assays (6) as well as with our results from the siRNA and invasion experiments in this report. In all instances, DvKPI did not abrogate colonization/invasion completely. Thus, we do not expect every rickettsiae to be associated with DvKPI. Even though the infected midguts for electron microscopy analysis were sampled at 18 to 24 h postinfection, we still observed DvKPI associated with R. montanensis. We note that our in vitro colocalization and invasion assays suggest that DvKPI limits invasion of host cells by R. montanensis.
Although the nature of the association between DvKPI and R. montanensis is unclear, our data and those of others lead us to hypothesize that DvKPI neutralizes a protein(s) present on the surface of rickettsiae that mediates, to some extent, host cell invasion. Given that DvKPI possesses trypsin-inhibitory activity (6), we hypothesize that the ligand is a protease. Limited empirical data exist for the use of proteases by rickettsiae during host colonization. Synthetic amidine trypsin inhibitors were shown to reduce cytopathic effects in vitro and to delay the onset of symptoms associated with R. rickettsii infection in a guinea pig model (45). However, utilization of membrane-bound serine proteases for invasion is a common theme among other intracellular pathogens, especially apicomplexan parasites (4, 34) and the agent of bubonic plague, Yersinia pestis (22). DvKPI may also associate with another tick protein recruited to the surface of rickettsiae. The Lyme disease bacterium, Borrelia burgdorferi, recruits host-derived factors such as those belonging to the mammalian plasminogen activation system to facilitate transmigration of the tick midgut epithelium and, ultimately, perpetuation of the zoonotic cycle (9, 20).
We are currently running experiments to describe further the nature of the DvKPI-rickettsia association. Regardless of the factors that underlie the molecular interaction, we have described a tick protein that can function outside its conventional definition as an anticoagulant to limit R. montanensis invasion, possibly through association with the bacterium. Because invasion is an absolute requirement for obligate intracellular bacteria, modes of entry will be conserved, at least for bacteria within the genus Rickettsia. To this end, we hypothesize that DvKPI function parallels those of KPIs observed in plants and other invertebrates, as part of the acute defense response in ticks, and will be bacteriostatic for nonpathogenic and pathogenic rickettsiae alike.
Acknowledgments
The research presented in this article was supported by funds from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01AI043006 and R01AI017828).
The content of this report is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 18 October 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abraham, E. G., S. B. Pinto, A. Ghosh, D. L. Vanlandingham, A. Budd, S. Higgs, F. C. Kafatos, M. Jacobs-Lorena, and K. Michel. 2005. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc. Natl. Acad. Sci. U. S. A. 102:16327-16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammerman, N. C., K. I. Swanson, J. M. Anderson, T. R. Schwartz, E. C. Seaberg, G. E. Glass, and D. E. Norris. 2004. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg. Infect. Dis. 10:1478-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustin, R., S. Siebert, and T. C. Bosch. 2009. Identification of a Kazal-type serine protease inhibitor with potent anti-staphylococcal activity as part of hydra's innate immune system. Dev. Comp. Immunol. 33:830-837. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers, V. B., and M. J. Blackman. 2005. A new release on life: emerging concepts in proteolysis and parasite invasion. Mol. Microbiol. 55:1617-1630. [DOI] [PubMed] [Google Scholar]
- 5.Ceraul, S. M., S. M. Dreher-Lesnick, J. J. Gillespie, M. S. Rahman, and A. F. Azad. 2007. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect. Immun. 75:1973-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceraul, S. M., S. M. Dreher-Lesnick, A. Mulenga, M. S. Rahman, and A. F. Azad. 2008. Functional characterization and novel rickettsiostatic effects of a Kunitz-type serine protease inhibitor from the tick Dermacentor variabilis. Infect. Immun. 76:5429-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceraul, S. M., D. E. Sonenshine, and W. L. Hynes. 2002. Resistance of the tick Dermacentor variabilis (Acari: Ixodidae) following challenge with the bacterium Escherichia coli (Enterobacteriales: Enterobacteriaceae). J. Med. Entomol. 39:376-383. [DOI] [PubMed] [Google Scholar]
- 8.Ceraul, S. M., D. E. Sonenshine, R. E. Ratzlaff, and W. L. Hynes. 2003. An arthropod defensin expressed by the hemocytes of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Insect Biochem. Mol. Biol. 33:1099-1103. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 10.Dong, Y., H. E. Taylor, and G. Dimopoulos. 2006. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 4:e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fogaca, A. C., P. I. da Silva, Jr., M. T. Miranda, A. G. Bianchi, A. Miranda, P. E. Ribolla, and S. Daffre. 1999. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J. Biol. Chem. 274:25330-25334. [DOI] [PubMed] [Google Scholar]
- 12.Fogaca, A. C., D. M. Lorenzini, L. M. Kaku, E. Esteves, P. Bulet, and S. Daffre. 2004. Cysteine-rich antimicrobial peptides of the cattle tick Boophilus microplus: isolation, structural characterization and tissue expression profile. Dev. Comp. Immunol. 28:191-200. [DOI] [PubMed] [Google Scholar]
- 13.Francischetti, I. M., T. N. Mather, and J. M. Ribeiro. 2004. Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thromb. Haemost. 91:886-898. [DOI] [PubMed] [Google Scholar]
- 14.Francischetti, I. M., J. G. Valenzuela, J. F. Andersen, T. N. Mather, and J. M. Ribeiro. 2002. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood 99:3602-3612. [DOI] [PubMed] [Google Scholar]
- 15.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 16.Hynes, W. L., S. M. Ceraul, S. M. Todd, K. C. Seguin, and D. E. Sonenshine. 2005. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med. Vet. Entomol. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 17.Johns, R., D. E. Sonenshine, and W. L. Hynes. 1998. Control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae): evidence for the existence of antimicrobial proteins in tick hemolymph. J. Med. Entomol. 35:458-464. [DOI] [PubMed] [Google Scholar]
- 18.Johns, R., D. E. Sonenshine, and W. L. Hynes. 2001. Identification of a defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect Biochem. Mol. Biol. 31:857-865. [DOI] [PubMed] [Google Scholar]
- 19.Johns, R., D. E. Sonenshine, and W. L. Hynes. 2000. Response of the tick Dermacentor variabilis (Acari: Ixodidae) to hemocoelic inoculation of Borrelia burgdorferi (Spirochetales). J. Med. Entomol. 37:265-270. [DOI] [PubMed] [Google Scholar]
- 20.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, and R. A. Rogers. 1996. Binding of human urokinase type plasminogen activator and plasminogen to Borrelia species. J. Infect. Dis. 174:97-104. [DOI] [PubMed] [Google Scholar]
- 21.Kopacek, P., R. Vogt, L. Jindrak, C. Weise, and I. Safarik. 1999. Purification and characterization of the lysozyme from the gut of the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 29:989-997. [DOI] [PubMed] [Google Scholar]
- 22.Lahteenmaki, K., M. Kukkonen, and T. K. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 504:69-72. [DOI] [PubMed] [Google Scholar]
- 23.Lai, R., L. O. Lomas, J. Jonczy, P. C. Turner, and H. H. Rees. 2004. Two novel non-cationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. Biochem. J. 379:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, X. C., X. W. Wang, Z. H. Wang, X. F. Zhao, and J. X. Wang. 2009. A three-domain Kazal-type serine proteinase inhibitor exhibiting domain inhibitory and bacteriostatic activities from freshwater crayfish Procambarus clarkii. Dev. Comp. Immunol. 33:1229-1238. [DOI] [PubMed] [Google Scholar]
- 25.Lievens, S., S. Goormachtig, and M. Holsters. 2004. Nodule-enhanced protease inhibitor gene: emerging patterns of gene expression in nodule development on Sesbania rostrata. J. Exp. Bot. 55:89-97. [DOI] [PubMed] [Google Scholar]
- 26.Macaluso, K. R., A. Mulenga, J. A. Simser, and A. F. Azad. 2006. Characterization of Dermacentor variabilis molecules associated with rickettsial infection. Ann. N. Y. Acad. Sci. 1078:384-388. [DOI] [PubMed] [Google Scholar]
- 27.Manen, J. F., P. Simon, J. C. Van Slooten, M. Osteras, S. Frutiger, and G. J. Hughes. 1991. A nodulin specifically expressed in senescent nodules of winged bean is a protease inhibitor. Plant Cell 3:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, J. J., S. Seveau, E. Veiga, S. Matsuyama, and P. Cossart. 2005. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 123:1013-1023. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima, Y., K. Ogihara, D. Taylor, and M. Yamakawa. 2003. Antibacterial hemoglobin fragments from the midgut of the soft tick, Ornithodoros moubata (Acari: Argasidae). J. Med. Entomol. 40:78-81. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima, Y., H. Saido-Sakanaka, D. Taylor, and M. Yamakawa. 2003. Up-regulated humoral immune response in the soft tick, Ornithodoros moubata (Acari: Argasidae). Parasitol. Res. 91:476-481. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima, Y., A. van der Goes van Naters-Yasui, D. Taylor, and M. Yamakawa. 2002. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol. Biol. 11:611-618. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima, Y., A. van der Goes van Naters-Yasui, D. Taylor, and M. Yamakawa. 2001. Two isoforms of a member of the arthropod defensin family from the soft tick, Ornithodoros moubata (Acari: Argasidae). Insect Biochem. Mol. Biol. 31:747-751. [DOI] [PubMed] [Google Scholar]
- 33.Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Donnell, R. A., and M. J. Blackman. 2005. The role of malaria merozoite proteases in red blood cell invasion. Curr. Opin. Microbiol. 8:422-427. [DOI] [PubMed] [Google Scholar]
- 35.Paddock, C. D., S. Fernandez, G. A. Echenique, J. W. Sumner, W. K. Reeves, S. R. Zaki, and C. E. Remondegui. 2008. Rocky Mountain spotted fever in Argentina. Am. J. Trop. Med. Hyg. 78:687-692. [PubMed] [Google Scholar]
- 36.Philip, R. N. 2000. Rocky Mountain spotted fever in Western Montana: anatomy of pestilence, p. 57. Bitter Root Valley Historical Society, Hamilton, MT.
- 37.Ramakers, C., J. M. Ruijter, R. H. L. Deprez, and A. F. M. Moorman. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339:62-66. [DOI] [PubMed] [Google Scholar]
- 38.Raoult, D., and P. Parola. 2008. Rocky Mountain spotted fever in the U. S. A.: a benign disease or a common diagnostic error? Lancet Infect. Dis. 8:587-589. [DOI] [PubMed] [Google Scholar]
- 39.Rudenko, N., M. Golovchenko, M. J. Edwards, and L. Grubhoffer. 2005. Differential expression of Ixodes ricinus tick genes induced by blood feeding or Borrelia burgdorferi infection. J. Med. Entomol. 42:36-41. [DOI] [PubMed] [Google Scholar]
- 40.Simser, J. A., K. R. Macaluso, A. Mulenga, and A. F. Azad. 2004. Immune-responsive lysozymes from hemocytes of the American dog tick, Dermacentor variabilis and an embryonic cell line of the Rocky Mountain wood tick, D. andersoni. Insect Biochem. Mol. Biol. 34:1235-1246. [DOI] [PubMed] [Google Scholar]
- 41.Smith, J. G., and G. R. Nemerow. 2008. Mechanism of adenovirus neutralization by human alpha-defensins. Cell Host Microbe 3:11-19. [DOI] [PubMed] [Google Scholar]
- 42.Sonenshine, D. E., S. M. Ceraul, W. E. Hynes, K. R. Macaluso, and A. F. Azad. 2002. Expression of defensin-like peptides in tick hemolymph and midgut in response to challenge with Borrelia burgdorferi, Escherichia coli and Bacillus subtilis. Exp. Appl. Acarol. 28:127-134. [DOI] [PubMed] [Google Scholar]
- 43.Sonenshine, D. E., W. L. Hynes, S. M. Ceraul, R. Mitchell, and T. Benzine. 2005. Host blood proteins and peptides in the midgut of the tick Dermacentor variabilis contribute to bacterial control. Exp. Appl. Acarol. 36:207-223. [DOI] [PubMed] [Google Scholar]
- 44.Walker, D. H., C. D. Paddock, and J. S. Dumler. 2008. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med. Clin. North Am. 92:1345-1361. [DOI] [PubMed] [Google Scholar]
- 45.Walker, D. H., R. R. Tidwell, T. M. Rector, and J. D. Geratz. 1984. Effect of synthetic protease inhibitors of the amidine type on cell injury by Rickettsia rickettsii. Antimicrob. Agents Chemother. 25:582-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warr, E., S. Das, Y. Dong, and G. Dimopoulos. 2008. The Gram-negative bacteria-binding protein gene family: its role in the innate immune system of Anopheles gambiae and in anti-Plasmodium defence. Insect Mol. Biol. 17:39-51. [DOI] [PubMed] [Google Scholar]