Abstract
Myeloperoxidase (MPO) is reported to selectively bind to bacteria. The present study provides direct evidence of MPO binding selectivity and tests the relationship of selective binding to selective killing. The microbicidal effectiveness of H2O2 and of OCl− was compared to that of MPO plus H2O2. Synergistic microbicidal action was investigated by combining Streptococcus sanguinis, a H2O2-producing microbe showing low MPO binding, with high-MPO-binding Escherichia coli, Staphylococcus aureus, or Pseudomonas aeruginosa without exogenous H2O2, with and without MPO, and with and without erythrocytes (red blood cells [RBCs]). Selectivity of MPO microbicidal action was conventionally measured as the MPO MIC and minimal bactericidal concentration (MBC) for 82 bacteria including E. coli, P. aeruginosa, S. aureus, Enterococcus faecalis, Streptococcus pyogenes, Streptococcus agalactiae, and viridans streptococci. Both H2O2 and OCl− destroyed RBCs at submicrobicidal concentrations. Nanomolar concentrations of MPO increased H2O2 microbicidal action 1,000-fold. Streptococci plus MPO produced potent synergistic microbicidal action against all microbes tested, and RBCs caused only a small decrease in potency without erythrocyte damage. MPO directly killed H2O2-producing S. pyogenes but was ineffective against non-H2O2-producing E. faecalis. The MPO MICs and MBCs for E. coli, P. aeruginosa, and S. aureus were significantly lower than those for E. faecalis. The streptococcal studies showed much higher MIC/MBC results, but such testing required lysed horse blood-supplemented medium, thus preventing valid comparison of these results to those for the other microbes. E. faecalis MPO binding is reportedly weak compared to binding of E. coli, P. aeruginosa, and S. aureus but strong compared to binding of streptococci. Selective MPO binding results in selective killing.
The microbicidal activities of H2O2 and especially OCl− are potent, but such activities are best achieved in the absence of competing substrates. The reactivities of these agents are indiscriminate and capable of damaging mechanisms of immune defense as well as destroying microbes. Alexander Fleming appreciated this relationship, stating that “leukocytes are more sensitive to the action of chemical antiseptics than are the bacteria, and, in view of this, it is unlikely that any of these antiseptics have the power of penetrating into the tissues and destroying the bacteria without first killing the tissues themselves” (16).
Healthy human adults produce about 100 billion neutrophil leukocytes per day. After a circulating lifetime of about 10 h, these neutrophils leave the blood to enter the body tissues (8). Myeloperoxidase (MPO), a 145-kDa dimeric alpha-heme haloperoxidase present in azurophilic granules, makes up approximately 5% of the dry weight of the neutrophil (33). If a neutrophil is assumed to have a cell volume of 450 fl, a specific gravity of 1.1, and a cell water content of 84%, healthy human adults synthesize about 2.8 μmol (i.e., 0.4 g) of MPO per day. Furthermore, the production of neutrophils and the MPO content per neutrophil are markedly increased in states of inflammation and with granulocyte colony-stimulating factor (G-CSF) treatment (7).
MPO exerts potent and broad-spectrum microbicidal action against Gram-positive and Gram-negative bacteria, as well as yeast and fungi (23). Of the mammalian peroxidases, MPO is unique in its ability to catalyze the H2O2-dependent oxidation of Cl− to OCl−. Such haloperoxidase activity is required for effective microbe killing (22). In addition to the requirement of H2O2 for OCl− production, H2O2 also directly reacts with OCl− to produce singlet molecular oxygen (1O2*), a potent electrophilic oxygenating agent (21). The microbicidal action of MPO involves highly exergonic oxygenation reactions. These wet combustion reactions yield excited carbonyl products that relax with chemiluminescence (2, 3).
After leaving the blood, neutrophils migrate into cavities such as the mouth (38) and vagina (11). These spaces are characterized by acidic pH and the presence of flora rich in lactic acid bacteria (LAB). It is reasonable to assume that the neutrophils that have migrated into the acid milieu of the mouth or vagina eventually disintegrate, releasing their MPO content into these environments, and that this released MPO is available to interact with microbes of the resident flora.
MPO selectively binds to Gram-negative and many Gram-positive bacteria. However, streptococci, especially viridans streptococci, show little MPO binding (4, 6). Viridans streptococci are a major constituent of normal mouth flora (20). As members of the LAB, streptococci are cytochrome deficient and catalase negative and produce lactic acid and H2O2 as metabolic products (24). The rates of H2O2 production by Streptococcus sanguinis and Streptococcus mitis are reported to be in the range of 12 to 67 nanomoles/min/mg of dry weight (10). Another study of Streptococcus oralis and S. sanguinis reported a range of 1 to 5 nmol/min/106 CFU (18). These rates of H2O2 production are adequate to drive MPO oxidation of Cl− to OCl− and for H2O2 reaction with OCl− to produce 1O2*. At relatively low MPO concentrations, a H2O2-producing LAB showing low MPO binding might escape damage. Furthermore, the streptococcus-generated H2O2 would be available to drive combustive reactions against competing microbes showing higher MPO binding.
The present report compares the microbicidal capacities of H2O2, OCl−, and nanomolar MPO plus low concentrations of H2O2. The potency and selectivity of MPO microbicidal action are illustrated using either streptococci or glucose oxidase (GO) to generate H2O2. The selectivity of microbicidal action was conventionally assessed by measuring the MIC and minimal bactericidal concentration (MBC) of MPO against a large spectrum of clinical bacterial isolates.
(Portions of this research were presented at the 2008 International Conference on Gram Positive Pathogens, Omaha, NE, 5 to 8 October 2008, and at the 48th Interscience Conference on Antimicrobial Agents-Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 25 to 28 October 2008, poster A1-3497).
MATERIALS AND METHODS
Reagents and enzymes.
Stock H2O2 and NaOCl solutions were prepared and quantified. The concentration of the H2O2 stock solution was verified by measuring its UV absorbance spectrum using a 240-nm extinction coefficient (ɛ240) of 43.6 M−1·cm−1 and a 300-nm extinction coefficient (ɛ300) of 1.0 M−1· cm−1. The concentration of the NaOCl stock solution was verified by measuring its absorbance spectrum using a 292-nm extinction coefficient (ɛ292) of 350 M−1·cm−1.
Porcine MPO, EC 1.11.1.7, was produced by ExOxEmis, Inc. The absorbance extinction coefficient at 430 nm (ɛ430) for MPO is 178 mM−1·cm−1(1). The RZ (rheinheitzahl or purity number) value, i.e., the ratio of 430-nm to 280-nm absorbance (A430/280), estimates the purity of MPO relative to total protein. The A430/280 RZ for the MPO was 0.7 in the initial binding studies and 0.8, i.e., crystalline purity, for the MIC/MBC studies. One picomole of MPO equals 0.145 μg. The guaiac activity of MPO was 380 guaiac units (GU)/mg of MPO. Spectrophotometric measurements were performed using a DW2000 UV-visible light spectrophotometer (SLM Instruments Co.).
Glucose oxidase from Aspergillus niger, EC 1.1.3.4, was used as a H2O2 generator for the MIC/MBC studies. High-purity GO with minimal catalase activity was prepared by ExOxEmis, Inc. One picomole of GO equals 0.16 μg. The activity of GO was 320 units/mg measured using horseradish peroxidase with o-dianisidine as a substrate. For the MIC/MBC studies, MPO and GO were used at a molar ratio of 4.4. The solution also contained 5.6 mM l-alanine, 7.2 mM l-proline, 7.2 mM glycine, and 300 mM d-glucose as a substrate.
Microbes.
The initial descriptive investigation used six bacteria, i.e., Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 11303, Pseudomonas aeruginosa ATCC 9027, viridans streptococcus, Streptococcus pyogenes, and Enterococcus faecalis, and a yeast, i.e., Candida albicans ATCC 10231. Viridans group streptococcus, S. pyogenes, and E. faecalis were clinical isolates. S. pyogenes produce H2O2 and show β-hemolysis (34). E. faecalis does not produce H2O2 and showed no hemolysis on blood agar (15). The viridans streptococcus used was an oropharyngeal isolate selected for its high alpha-hemolytic activity, i.e., viridans character. This alpha-hemolytic activity reflects H2O2 generation (10, 24). This viridans group Streptococcus isolate was further typed to be S. sanguinis.
The bacteria were grown overnight (about 16 h) in Trypticase soy broth (TSB) at 35°C. The yeast was grown for about 16 h in Sabouraud's dextrose broth (SDB) at 35°C. The cultures were centrifuged at 3,000 rpm for 15 min, and the supernatants were discarded. The microbial pellets were resuspended and diluted with sterile 0.85% normal saline (NS) to an absorbance of 0.1 at a wavelength of 540 nm, i.e., about 108 bacteria CFU per ml and about 107 yeast CFU per ml.
Preparation and use of erythrocytes (RBCs).
Blood was collected by venipuncture from a healthy human volunteer (R. C. Allen) using lithium heparin as an anticoagulant. The whole blood was centrifuged at 1,500 rpm for 15 min, and the plasma and leukocyte buffy coat were removed by aspiration, leaving the packed erythrocytes. These erythrocytes were suspended in NS, and following mixing, the red blood cells (RBCs) were again centrifuged, aspirated, and suspended in NS. The erythrocyte suspension was then passed through sterile cotton gauze to remove remaining leukocytes. The centrifugation and aspiration steps were repeated, and the erythrocyte pellet was suspended and diluted with NS to a concentration of 108 RBCs per ml by hemocytometer count.
Erythrocytes (0.1 ml containing 107 RBCs) were added to the microbe suspensions where indicated. Human RBCs contain catalase, i.e., H2O2:H2O2 oxidoreductase, an enzyme that catalyzes the destruction of H2O2 producing H2O and O2. The mechanism for removal of H2O2 in human erythrocytes is more than 90% dependent on catalase action (28). In addition to competitive destruction of H2O2, erythrocytes can directly react with oxidizing agents and, thus, compete with microbes for available reactants. RBCs are susceptible to damage by high concentrations of H2O2 and lower concentrations of OCl−. Introducing RBCs into the reaction mixture provides a means for assessing the specificity of the reactants for the microbe relative to the erythrocyte.
Following microbe-RBC incubation and centrifugation, the hemoglobin (Hgb) that remains in the pellet is proportional to the remaining intact erythrocytes, i.e., to RBC survival. Loss of Hgb from the pellet and its appearance in the supernatant indicate hemolysis, i.e., the destruction of RBC membrane integrity and release of cytoplasmic Hgb into the medium. The degree of hemolysis can be assessed by the distribution of Hgb in the pellet and supernatant. Complete hemolysis with Hgb destruction results in the disappearance of Hgb from both the pellet and supernatant. In addition to the oxidative reactants tested, microbial toxins can also produce hemolysis and Hgb destruction.
The concentrations of Hgb in the pellet and supernatant were measured by a derivative spectroscopic modification of the hemiglobincyanide (Drabkin) method (27). A suspension of 107 RBCs per ml yielded 0.2 to 0.3 mg of Hgb. The total Hgb of the supernatant and pellet was measured in the control and was expressed as unity, i.e., 1.0. The distribution of Hgb between the pellet and supernatant was expressed as a proportion of 1.0. This approach provides a rough but useful gauge of hemolytic activity. Small losses of Hgb from the pellet to the supernatant are an expected consequence of the preparation and experimental treatment, but large losses to the supernatant indicate active hemolysis. Complete destruction of Hgb is indicated by a total loss from the pellet and supernatant.
Measuring direct microbicidal action of H2O2, OCl−, H2O2-MPO, and MPO alone.
Using a sterile technique, 0.1 ml of the test microbe suspension (about 106 bacteria or 105 yeasts) and 0.1 ml of NS or RBC suspension were added to 12- by 75-mm polystyrene tubes. The reaction was initiated by adding the various dilutions of the H2O2 or OCl− prepared in NS to a final volume 1 ml. The reaction suspension was mixed and incubated for 30 min at 23°C. After remixing to suspend the cellular components, a 0.1-ml aliquot of the suspension was added to 0.9 ml of NS, and this suspension was serially (10n) diluted out to 10−3. Then 0.1 ml of each dilution was uniformly spread on agar plates using the “glass hockey stick” technique. Trypticase soy agar (TSA) was used to culture the bacteria and Sabouraud's dextrose agar (SDA) was used to culture C. albicans. The plates were then incubated at 36°C for about 24 h. Microbe colonies were counted, and the number of CFU/test was derived by multiplying the number of colonies counted by the initial dilution factor plus the plate dilution factor.
S. sanguinis-MPO synergistic killing of S. aureus, E. coli, P. aeruginosa, and C. albicans.
The bacteria, yeast, RBCs, and MPO were prepared and quantified as described above, and 0.1 ml of S. sanguinis (viridans group streptococcus) suspension (about 107 streptococci based on the optical density at 540 nm; 3.3 × 107 CFU), 0.1 ml of the target microbe suspension (about 106 microbes), 0.1 ml of NS or the MPO dilution indicated, 0.1 ml of glucose (1 mg), 0.5 ml of NS, and where indicated, either 0.1 ml of erythrocyte suspension (107 RBCs) or NS were added to each tube for a 1-ml final volume. No H2O2 was added. The contents were gently mixed, and the tubes were incubated undisturbed for 30 min at 23°C. Microbe killing was measured by the agar plate dilution technique as described above except that the colonies were grown for an additional day to increase the visibility of the small streptococcal colonies.
MPO is directly microbicidal against S. pyogenes but not against E. faecalis.
Bacteria, RBCs, and MPO were prepared and quantified as described above. A 0.1-ml suspension (about 106 microbes) of either S. pyogenes (Lancefield group A) or E. faecalis, 0.1 ml of NS or the MPO dilution as indicated, 0.1 ml of glucose (1 mg), 0.1 ml of NS or erythrocyte suspension (107 RBCs), and 0.6 ml of NS were added to each tube for a final volume of 1.0 ml. No H2O2 was added. The contents were gently mixed, and the tubes were incubated for 30 min at 23°C. Microbe killing was measured by the agar plate dilution method.
MIC and minimum bactericidal concentration of MPO.
In addition to the bacterial strains described above, a total of 78 clinical isolates were tested to determine sensitivity and consistency of MPO MICs and MPO MBCs. Eurofins Medinet Anti-Infective Services (Chantilly, VA) was contracted to perform these MIC and MBC studies on their collection of clinical isolates from diverse geographical regions within the United States. Fourteen S. aureus clinical isolates were tested; six isolates were methicillin-sensitive (MSSA), six isolates were methicillin-resistant (MRSA), and two isolates were vancomycin-resistant (VRSA). Five clinical isolates of E. coli were tested; one E. coli isolate was ceftazidime sensitive (CEF-S), and four were ceftazidime resistant (CEF-R). Five clinical isolates of P. aeruginosa were tested; two P. aeruginosa isolate were CEF-S, and three were CEF-R. Seven clinical isolates of E. faecalis were tested; five of the E. faecalis isolates were vancomycin sensitive (VSE), and two were vancomycin resistant (VRE). Twenty clinical isolates of pathogenic Lancefield group A and B streptococci were tested; 10 isolates were S. pyogenes (group A), and 10 clinical isolates were Streptococcus agalactiae (group B). Twenty-seven isolates of viridans group streptococci were tested; nine were S. mitis, nine were S. oralis, and nine were S. sanguinis. In addition, S. aureus ATCC 29213 was used as the quality control strain to validate the modified Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (12, 29).
Conventional MIC and MBC testing is designed to measure the inhibitory effect of an antibiotic on bacterial protein synthesis or cell wall formation. Such testing is not well suited to measuring the oxidative microbicidal action of MPO. For example, the pH of cation-adjusted Mueller-Hinton broth (CAMHB) is mildly alkaline, pH 7.3, but the optimum pH for MPO action is in the range of 5 to 6, i.e., the pH of the neutrophil phagolysosomal space, the skin, the mouth, and the vaginal space. In addition to the nonoptimum pH imposed by CAMHB, supplementation of CAMHB with 5% lysed horse blood (CAMHB-5% LHB) is required for MIC and MBC testing of the streptococci. Lysed horse blood contains significant catalase activity that destroys H2O2, thus removing the substrate required for MPO production of OCl− and 1O2*. This lysed horse blood also contains Hgb and other molecular substrates that can reactively compete with microbes for available OCl− and 1O2*. Lysed horse blood consumption of MPO-generated oxidants competitively inhibits microbicidal action.
MPO activity was tested by mixing a solution containing MPO and GO with a solution containing glucose. The enzyme and substrate solutions were mixed together in various proportions just prior to microbe exposure to produce the desired concentration of MPO. The molar ratio of MPO to GO was 4.4. Thus, GO production of H2O2 was in proportion to the available MPO. Broth MIC and MBC determinations were performed according to CLSI procedure specifications with modifications to accommodate the rapid in vitro activity of MPO. The enzyme solution was diluted in double-strength CAMHB and dispensed in microdilution trays. All streptococcal species were tested in double-strength CAMHB supplemented with 5% lysed horse blood (CAMHB-5% LHB) according to CLSI procedural specifications. Isolates were prepared by suspending colonies from an overnight culture on Trypticase soy agar with 5% sheep blood into sterile saline, and the bacterial suspension density was adjusted to a 0.5 McFarland standard (∼108 CFU/ml). Standardized bacterial suspensions were further diluted in double-strength substrate solution so that approximately 5 × 105 CFU/ml were mixed with serial MPO-GO dilutions. Contact with glucose activated the enzyme system. The microdilution trays were incubated in ambient air at 35°C for 18 to 24 h.
The MIC was the lowest concentration of MPO observed to completely inhibit microbe growth. The MBC was determined using the same modified broth microdilution method. The last MPO well on the microdilution tray showing visible growth and each of the clear wells were sampled by removing a 10-μl sample per well that was plated onto TSA with 5% sheep blood and incubated in ambient air for 24 h before examination for growth and colony counts. The MBC was determined as the lowest antimicrobial concentration demonstrating a >99.9% reduction in the number of CFU relative to the starting inoculum. The inhibitory effect of 5% lysed horse blood was documented by simultaneously retesting the MPO-dependent MIC and MBC activities of the same seven strains of E. faecalis in CAMHB and in CAMHB-5% LHB.
SPSS, version 17.0, and SigmaPlot, version 11, software were used for exploratory data analysis (37), one-way analysis of variance (ANOVA), an independent t test, and graphic presentation.
RESULTS
Antimicrobial action of H2O2 in the absence and presence of MPO.
The direct microbicidal action of H2O2 and the protective effect of human erythrocytes (RBCs) were measured using E. coli, S. aureus, P. aeruginosa, and C. albicans. The results are presented in Table 1.
TABLE 1.
Direct microbicidal action of hydrogen peroxide in the absence and presence of human erythrocytes
| Microbe | H2O2 concn (mM) | No. of CFU/mla |
Hemoglobin contenta,b |
||
|---|---|---|---|---|---|
| Without RBCs | With RBCs (107) | Supernatant | Pellet | ||
| E. coli | 0.0000 | 1,600,000 | 1,300,000 | 0.0 | 0.9 |
| 700.0000 | 0 | 10,000 | 0.0 | 0.0 | |
| 70.0000 | 0 | 1,100,000 | 0.0 | 0.4 | |
| 7.0000 | 670,000 | 1,300,000 | 0.1 | 0.9 | |
| 0.7000 | 1,600,000 | 1,200,000 | 0.1 | 0.9 | |
| 0.0700 | 1,300,000 | 1,700,000 | 0.1 | 0.9 | |
| 0.0070 | 1,600,000 | 1,200,000 | 0.1 | 0.9 | |
| 0.0007 | 1,200,000 | 1,300,000 | 0.0 | 1.0 | |
| S. aureus | 0.0000 | 1,200,000 | 1,300,000 | 0.0 | 0.9 |
| 700.0000 | 0 | 280,000 | 0.0 | 0.0 | |
| 70.0000 | 0 | 1,600,000 | 0.0 | 0.4 | |
| 7.0000 | 0 | 1,600,000 | 0.1 | 0.9 | |
| 0.7000 | 880,000 | 1,500,000 | 0.1 | 0.9 | |
| 0.0700 | 1,400,000 | 1,400,000 | 0.1 | 0.9 | |
| 0.0070 | 1,400,000 | 1,200,000 | 0.1 | 0.9 | |
| 0.0007 | 1,400,000 | 1,200,000 | 0.0 | 1.0 | |
| P. aeruginosa | 0.0000 | 2,000,000 | 2,000,000 | 0.0 | 0.9 |
| 700.0000 | 0 | 0 | 0.0 | 0.0 | |
| 70.0000 | 0 | 2,200,000 | 0.1 | 0.2 | |
| 7.0000 | 4,500 | 1,700,000 | 0.1 | 0.9 | |
| 0.7000 | 2,000,000 | 1,800,000 | 0.1 | 0.9 | |
| 0.0700 | 1,600,000 | 1,700,000 | 0.1 | 0.9 | |
| 0.0070 | 1,200,000 | 2,000,000 | 0.1 | 0.9 | |
| 0.0007 | 1,400,000 | 1,700,000 | 0.1 | 0.9 | |
| C. albicans | 0.0000 | 190,000 | 350,000 | 0.0 | 1.0 |
| 700.0000 | 78,000 | 150,000 | 0.0 | 0.0 | |
| 70.0000 | 230,000 | 300,000 | 0.0 | 1.0 | |
| 7.0000 | 150,000 | 380,000 | 0.0 | 1.0 | |
| 0.7000 | 180,000 | 330,000 | 0.0 | 1.0 | |
| 0.0700 | 170,000 | 280,000 | 0.0 | 0.9 | |
| 0.0070 | 160,000 | 290,000 | 0.0 | 0.9 | |
| 0.0007 | 270,000 | 320,000 | 0.0 | 1.0 | |
Values in boldface are less than 3 SDs from the expected mean and therefore indicate significant killing or significant hemolysis.
RBC damage was assessed by measuring the hemoglobin (Hgb) retained in the pelleted intact RBCs, lost to the medium as a consequence of hemolysis, or completely destroyed by oxidation. The yield of 107 RBCs is 0.25 to 0.34 mg of Hgb. The total Hgb distributed in the pellet and supernatant is expressed as proportions of unity (i.e., 1.0).
Erythrocytes provide a means for assessing the relative specificity of oxidative action. In the absence of RBCs, 700 mM H2O2 was effective in destroying all bacteria tested but was only partially effective against C. albicans. However, the microbicidal capacity of 700 mM H2O2 was markedly inhibited by the presence of RBCs. Only P. aeruginosa bacteria were completely killed. Note that 700 mM H2O2 produced complete erythrocyte destruction and complete Hgb destruction; i.e., Hgb completely disappeared from the pellets and the supernatants. As such, H2O2 activity was nonspecific. The RBCs served as a competitive substrate and were destroyed in the process.
Without RBCs, H2O2 in the range of 7.0 to 70 mM showed bactericidal action but was unable to kill C. albicans. In the presence of RBCs, H2O2 was essentially ineffective as a microbicidal agent at concentrations of 70 mM or lower. At 70 mM H2O2, hemolysis and Hgb destruction were present but incomplete. No significant hemolysis or Hgb destruction was detected at or below H2O2 concentrations of 7.0 mM. For all microbes tested, the H2O2 concentration required for microbicidal action was greater than the H2O2 concentration producing complete RBC destruction. Erythrocytes are more susceptible to destruction by H2O2 than are microbes.
The data of Table 2 illustrate the microbicidal action of MPO at H2O2 concentrations incapable of directly damaging RBCs or microbes. In the absence of exogenous H2O2, 5 nM (∼ 0.7 μg/ml) MPO did not show significant microbicidal action against these catalase-positive microbes. However, when MPO was presented with H2O2 concentrations several orders of magnitude lower than required for direct H2O2 microbicidal action, all microbes tested were successfully killed. MPO-dependent microbe killing was effective at H2O2 concentrations below 100 μM.
TABLE 2.
Microbicidal action of hydrogen peroxide in the absence and presence of MPO
| Microbe | H2O concn (mM) | No. of CFU/mla |
|
|---|---|---|---|
| Without MPO | With 5 nM MPO | ||
| E. coli | 0.0000 | 3,200,000 | 2,500,000 |
| 0.5600 | 2,800,000 | 0 | |
| 0.1120 | 3,200,000 | 0 | |
| 0.0045 | 2,100,000 | 0 | |
| 0.0009 | 3,000,000 | 19,000 | |
| S. aureus | 0.0000 | 2,400,000 | 1,700,000 |
| 0.5600 | 1,600,000 | 0 | |
| 0.1120 | 2,000,000 | 0 | |
| 0.0045 | 2,700,000 | 30,000 | |
| 0.0009 | 2,300,000 | 2,300,000 | |
| P. aeruginosa | 0.0000 | 2,100,000 | 2,500,000 |
| 0.5600 | 1,800,000 | 0 | |
| 0.1120 | 2,700,000 | 0 | |
| 0.0045 | 1,800,000 | 220,000 | |
| 0.0009 | 2,600,000 | 2,500,000 | |
| C. albicans | 0.0000 | 620,000 | 760,000 |
| 0.5600 | 460,000 | 0 | |
| 0.1120 | 420,000 | 0 | |
| 0.0045 | 440,000 | 180,000 | |
| 0.0009 | 420,000 | 520,000 | |
Values in boldface are less than 3 SDs from the expected mean and therefore indicate significant killing.
Microbicidal action of hypochlorite and the protective effect of RBCs.
The microbicidal potency of hypochlorite is well established (9, 14, 17, 25, 26). This set of experiments examined the microbicidal action of OCl− and the protective effect of RBCs. As for the previous H2O2 experiments, E. coli, S. aureus, P. aeruginosa, and C. albicans were tested, and the results presented in Table 3.
TABLE 3.
Direct microbicidal action of hypochlorite in the absence and presence of human erythrocytes
| Microbe | OCl− concn (μM) | No. of CFU/mla |
Hemoglobin contenta,b |
||
|---|---|---|---|---|---|
| Without RBCs | With RBCs (107) | Supernatant | Pellet | ||
| E. coli | 0.0000 | 1,700,000 | 1,500,000 | 0.0 | 0.9 |
| 6,300.0000 | 0 | 0 | 0.0 | 0.0 | |
| 630.0000 | 0 | 1,400,000 | 0.3 | 0.1 | |
| 63.0000 | 0 | 1,400,000 | 0.7 | 0.0 | |
| 6.3000 | 0 | 1,700,000 | 0.0 | 1.0 | |
| 0.6300 | 1,600,000 | 1,500,000 | 0.0 | 0.9 | |
| 0.0630 | 1,500,000 | 1,500,000 | 0.0 | 1.0 | |
| 0.0063 | 1,500,000 | 1,600,000 | 0.0 | 1.0 | |
| 0.0006 | 1,400,000 | 1,600,000 | 0.0 | 1.0 | |
| S. aureus | 0.0000 | 1,500,000 | 1,400,000 | 0.0 | 1.0 |
| 6,300.0000 | 0 | 0 | 0.0 | 0.0 | |
| 630.0000 | 0 | 71,000 | 0.3 | 0.1 | |
| 63.0000 | 0 | 1,200,000 | 1.0 | 0.0 | |
| 6.3000 | 0 | 1,500,000 | 0.0 | 1.0 | |
| 0.6300 | 1,300,000 | 1,400,000 | 0.0 | 0.9 | |
| 0.0630 | 1,400,000 | 1,500,000 | 0.0 | 1.0 | |
| 0.0063 | 1,300,000 | 1,200,000 | 0.0 | 1.0 | |
| 0.0006 | 1,500,000 | 1,300,000 | 0.0 | 1.0 | |
| P. aeruginosa | 0.0000 | 1,800,000 | 2,700,000 | 0.1 | 0.9 |
| 6,300.0000 | 0 | 0 | 0.0 | 0.0 | |
| 630.0000 | 0 | 2,000,000 | 1.0 | 0.0 | |
| 63.0000 | 0 | 2,300,000 | 0.0 | 1.0 | |
| 6.3000 | 400 | 2,100,000 | 0.0 | 1.0 | |
| 0.6300 | 5,000 | 2,300,000 | 0.1 | 0.9 | |
| 0.0630 | 2,100 | 2,300,000 | 0.0 | 1.0 | |
| 0.0063 | 1,200 | 2,900,000 | 0.1 | 0.9 | |
| 0.0006 | 2,100,000 | 2,500,000 | 0.0 | 0.9 | |
| C. albicans | 0.0000 | 360,000 | 340,000 | 0.1 | 1.0 |
| 6,300.0000 | 0 | 0 | 0.0 | 0.0 | |
| 630.0000 | 0 | 350,000 | 0.2 | 0.0 | |
| 63.0000 | 0 | 350,000 | 0.9 | 0.1 | |
| 6.3000 | 240,000 | 250,000 | 0.1 | 0.9 | |
| 0.6300 | 250,000 | 310,000 | 0.1 | 0.7 | |
| 0.0630 | 280,000 | 360,000 | 0.1 | 0.8 | |
| 0.0063 | 240,000 | 340,000 | 0.1 | 0.9 | |
| 0.0006 | 290,000 | 290,000 | 0.1 | 0.9 | |
Values in boldface are less than 3 SDs from the expected mean and therefore indicate significant killing or significant hemolysis.
RBC damage was assessed by measuring the hemoglobin (Hgb) retained in the pelleted intact RBCs, lost to the medium as a consequence of hemolysis, or completely destroyed by oxidation. Hgb was calculated as described in footnote b of Table 1.
In the absence of RBCs, 63 μM OCl− completely destroyed all microbes tested, and 6.3 μM OCl− was microbicidal for all bacteria tested. Compared to findings reported in Table 1, the microbicidal action of OCl− is 1,000-fold more potent than that of H2O2 on a molar basis. These findings are consistent with the range of 2 to 2.0 ppm, i.e., 4 to 40 μM OCl−, previously reported to show effective bactericidal action (9).
The presence of RBCs completely inhibited the microbicidal action of 630 μM OCl− for all microbes tested, except S. aureus, which was inhibited at 63 μM OCl−. Note that 6.3 mM (i.e., 6,300 μM) OCl− completely destroyed all RBCs and Hgb; i.e., no Hgb was detected in the pellets or supernatants. At 630 μM, OCl− caused RBC hemolysis, liberating Hgb into the supernatant with partial destruction of Hgb. At 63 μM, OCl− caused hemolysis and release of Hgb into the supernatant for all microbes except P. aeruginosa. As such, OCl− oxidative activity was nonspecific; i.e., the RBCs served as a competitive substrate and were destroyed. As previously described for H2O2, OCl− is a potent microbicidal agent, but it is more effective at destroying host cells, e.g., RBCs, than killing microbes (14, 16).
Selectivity of MPO binding.
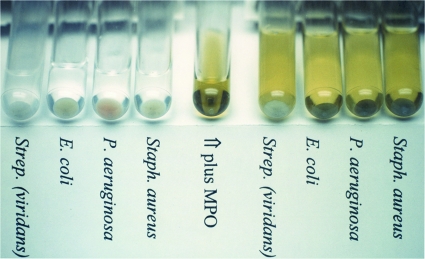
Selectivity of MPO binding is illustrated in Fig. 1. Exposure of bacterial suspensions to MPO resulted in dark coloration of most but not all of the bacterial pellets formed when the suspensions were centrifuged. Note that the pellets of E. coli, P. aeruginosa, and S. aureus show dark MPO coloration, but that the pellet of the viridans streptococcus S. sanguinis does not.
FIG. 1.
Photographic demonstration of MPO binding and coloration of S. aureus, E. coli, and P. aeruginosa. Note the absence of MPO binding to S. sanguinis (viridans group). The four tubes to the left show the centrifuged microbe pellets in the absence of MPO exposure. The center tube contains 2 mg/ml MPO without microbes. The four tubes to the right show the centrifuged microbe pellets exposed to MPO.
In the picture, the center tube contains 2 mg/ml MPO, the amount of MPO added to each bacterial suspension. The suspensions were then centrifuged to concentrate the pellets, as shown to the right in Fig. 1. For comparison, the untreated bacteria pellets are shown in the tubes to the left in the photograph. Visual comparison of the pellets shows the degree of MPO binding, i.e., MPO staining. Except for viridans group streptococci, all bacteria tested showed MPO binding.
Synergistic S. sanguinis-MPO microbicidal action and effect of RBCs.
The rate of H2O2 production by S. sanguinis and other viridans streptococci is reported to be in the range of 12 to 67 nmol/min/mg of dry weight (10) and 1 to 5 nmol/min/106 CFU (18). As such, S. sanguinis produces sufficient H2O2 to drive MPO oxidation of Cl− to OCl− and to drive H2O2 reaction with OCl− to produce 1O2*. Singlet oxygen is a reactive oxygenating agent with a finite reactive lifetime. It is an electronically excited molecule with a microsecond half-life that restricts reactivity to the proximity of its generation (4, 30, 35). As such, combustive microbicidal activity is essentially confined to sites of MPO binding. Presenting a small quantity of MPO to a mixture of two bacteria, i.e., a low-MPO-binding microbe, such as S. sanguinis, and any of the high-MPO-binding microbes previously described, provides a model for testing the relationship of selective MPO binding to selective MPO killing. The results of such testing, presented in Table 4, illustrate the synergistic S. sanguinis-MPO killing of E. coli, S. aureus, P. aeruginosa, and C. albicans.
TABLE 4.
Viridans group S. sanguinis-myeloperoxidase synergistic microbicidal action in the absence and presence of human erythrocytes
| Microbe added to S. sanguinis culturea | MPO concn (nM) | No. of CFU/mlb |
Hemoglobin contentc |
||
|---|---|---|---|---|---|
| Without RBCs | With RBCs (107) | Supernatant | Pellet | ||
| E. coli | 0.0 | 2,000,000 | 2,600,000 | 0.0 | 1.0 |
| 50.0 | 0 | 0 | 0.0 | 1.0 | |
| 16.7 | 0 | 0 | 0.0 | 1.0 | |
| 5.6 | 0 | 10,000 | 0.0 | 1.0 | |
| 1.9 | 10,000 | 930,000 | 0.0 | 1.1 | |
| 0.6 | 650,000 | 2,600,000 | 0.0 | 1.0 | |
| 0.2 | 1,900,000 | 2,500,000 | 0.0 | 1.0 | |
| S. aureus | 0.0 | 1,600,000 | 1,800,000 | 0.0 | 1.0 |
| 50.0 | 0 | 8,000 | 0.0 | 0.9 | |
| 16.7 | 0 | 740,000 | 0.0 | 1.0 | |
| 5.6 | 820,000 | 930,000 | 0.0 | 1.0 | |
| 1.9 | 430,000 | 1,900,000 | 0.0 | 1.0 | |
| 0.6 | 1,600,000 | 1,700,000 | 0.1 | 1.0 | |
| 0.2 | 1,700,000 | 1,900,000 | 0.0 | 1.0 | |
| P. aeruginosa | 0.0 | 2,000,000 | 3,400,000 | 0.0 | 1.0 |
| 50.0 | 0 | 0 | 0.1 | 1.0 | |
| 16.7 | 0 | 10,000 | 0.0 | 0.9 | |
| 5.6 | 0 | 7,200 | 0.0 | 0.9 | |
| 1.9 | 0 | 6,200 | 0.0 | 1.0 | |
| 0.6 | 0 | 520,000 | 0.1 | 1.0 | |
| 0.2 | 10,000 | 4,500,000 | 0.0 | 1.0 | |
| C. albicans | 0.0 | 200,000 | 240,000 | 0.0 | 1.0 |
| 50.0 | 0 | 4,000 | 0.0 | 0.8 | |
| 16.7 | 0 | 250,000 | 0.0 | 0.9 | |
| 5.6 | 0 | 180,000 | 0.0 | 1.0 | |
| 1.9 | 0 | 240,000 | 0.0 | 1.0 | |
| 0.6 | 6,000 | 250,000 | 0.0 | 1.0 | |
| 0.2 | 160,000 | 210,000 | 0.0 | 1.0 | |
Each suspension contained approximately 107 S. sanguinisbacteria plus the additional microbe, as indicated. Microbe concentration was estimated by the optical density measurement at 540 nm. No H2O2 was added.
Values in boldface are less than 3 SDs from the expected mean and therefore indicate significant killing.
RBC damage was assessed by measuring the hemoglobin (Hgb) retained in the pelleted intact RBCs, lost to the medium as a consequence of hemolysis, or completely destroyed by oxidation as described in footnote b of Table 1.
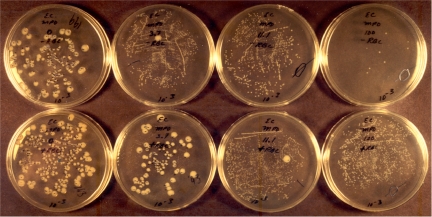
Figure 2 is a photograph of the actual 10−3 CFU dilution petri plates of the E. coli study. The top row of plates presents the findings in the absence of RBCs; reading from left to right, the final MPO concentrations were 0, 1.9, 5.6, and 50 nM. Note that in the absence of MPO, only the large E. coli colonies are observed; there is no evidence of the small S. sanguinis colonies. MPO at a concentration of 1.9 nM, i.e., 0.28 μg/ml, killed more than 99% of the E. coli and allowed the emergence of numerous small S. sanguinis colonies that are visible in Fig. 2. At 5.6 nM MPO, killing of E. coli was complete, and S. sanguinis was completely spared. Note the numerous small streptococci colonies, i.e., ∼ 2 × 107 S. sanguinis CFU/ml, visible on the 1.9 and 5.6 nM MPO plates.
FIG. 2.
Photograph of petri plates at the 10−3 CFU dilution used to measure S. sanguinis-MPO synergistic action against E. coli. The top and bottom rows of plates present the findings in the absence and presence of RBCs, respectively. From left to right, the final MPO concentrations were 0, 1.9, 5.6, and 50 nM. The plates were incubated for about 48 h to better visualize the smaller streptococcal colonies.
At 50 nM, MPO killing of S. sanguinis was also observed; the S. sanguinis colony count dropped to ∼80,000 CFU, a decrease of more than 100-fold. At a relatively high MPO concentration, streptococcal H2O2 was directed against the streptococci. Such observations strongly support the proposition that low concentrations of MPO provide selective advantage to the viridans streptococci in their competition with MPO-binding microbes such as E. coli for dominance in the mouth flora. Thus, in addition to its role in neutrophil antimicrobial action, MPO can serve the host by providing a selective advantage to beneficial flora and by controlling the population density of such flora.
Erythrocytes show little or no MPO binding (4). At relatively low H2O2 concentrations, the presence of erythrocytes allows assessment of the relative specificity of MPO-dependent oxidative activity. When oxidative activity is nonspecific, the RBCs serve as a competitive substrate and are damaged or destroyed in the process. If the oxidative activity is specifically directed, erythrocyte catalase can decrease the concentration of H2O2 with no or minimal damage to the RBCs. The bottom plates in Fig. 2 show the effect of RBCs on S. sanguinis-MPO synergistic microbicidal action. Reading from left to right, the final MPO concentrations were 0, 1.9, 5.6, and 50 nM. In the presence on 107 RBCs, 1.9 nM MPO produced modest microbicidal action, killing about two-thirds of the E. coli and allowing the emergence of very small streptococcus colonies that are visible on the plate. Despite the presence of erythrocytes at an RBC/E. coli ratio of about 5:1, MPO at a concentration of 5.6 nM killed more than 99% of the E. coli bacteria while sparing S. sanguinis. With RBCs present, 50 nM MPO produced complete killing of E. coli and spared S. sanguinis (about 107 CFU). There was no evidence of RBC hemolysis and, therefore, no significant erythrocyte damage at any concentration of MPO tested. MPO oxidative activity was specific. Erythrocyte catalase is expected to decrease H2O2 and protect streptococci. There was no evidence of hemolysis or Hgb destruction. The consumption of the streptococcal H2O2 by RBC catalase was apparently sufficient to protect both the erythrocytes and the streptococci from bystander injury associated with unbound MPO.
Comparison of the data of Tables 1, 3, and 4 confirms that the inhibition of S. sanguinis-MPO synergistic microbicidal action by RBCs was minuscule relative to the inhibition of H2O2 and OCl− microbicidal action by RBCs. No significant hemolysis or Hgb destruction was observed in the S. sanguinis-MPO synergistic microbicidal studies. These findings are consistent with selective MPO microbe binding, the short reactive lifetime of 1O2*, and the competitive consumption of H2O2 by erythrocyte catalase. Confining oxygenation activity to the site of MPO binding concentrates combustive action and limits bystander injury.
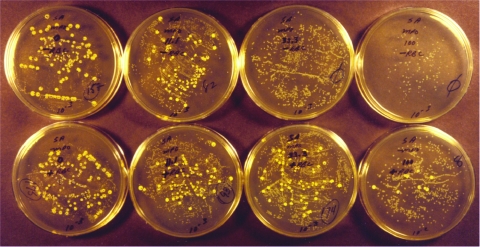
The photograph in Fig. 3 shows CFU dilution plates of the S. sanguinis-MPO and S. aureus study. The top row of 10−3 dilution plates presents the results of testing in the absence of RBCs. Reading from left to right, the final MPO concentrations were 0, 5.6, 16.7 and 50 nM.
FIG. 3.
Photograph of petri plates at the 10−3 CFU dilution (top four and bottom left three plates) and 10−2 CFU dilution (bottom far right plate) used to measure S. sanguinis-MPO synergistic action against S. aureus. The top and bottom rows of plates present the findings in the absence and presence of RBCs, respectively. From left to right, the final MPO concentrations were 0, 5.6, 16.7, and 50 nM. The plates were incubated for about 48 h to better visualize the smaller streptococcal colonies.
In the absence of MPO, both the larger yellow S. aureus colonies and the smaller S. sanguinis (viridans group) colonies are visible. An MPO concentration of 16.7 nM produced complete killing of S. aureus and spared the viridans streptococcus; i.e., the S. sanguinis count was ∼107 CFU. At a concentration of 50 nM, MPO produced complete killing of S. aureus but also decreased the viridans streptococcus count by 10-fold; i.e., the S. sanguinis count was ∼106 CFU. Low concentrations of MPO provided a selective advantage to S. sanguinis in its competition with MPO-binding S. aureus.
The dishes on the bottom row of Fig. 3 show the S. aureus findings in the presence of RBCs. Reading from left to right, the final MPO concentrations were 0, 5.6, 16.7, and 50 nM. The plates are shown at the 10−3 dilution except for the 50 nM plate, which is shown at the 10−2 dilution; no S. aureus colony was observed on the 10−3 plate. The presence of RBCs provided somewhat more protection to S. aureus than previously observed with E. coli. In the presence of RBCs, greater than 99.5% of the S. aureus bacteria were killed with 50 nM MPO, but this concentration of MPO also decreased the S. sanguinis count. Erythrocyte inhibition of S. sanguinis-MPO synergistic microbicidal action against S. aureus is consistent with, but slightly less impressive than, that observed for E. coli. No significant hemolysis or Hgb destruction was observed.
Synergistic microbicidal action of MPO-viridans group Streptococcus against P. aeruginosa was consistent with the E. coli results. In the absence of RBCs, MPO exerted potent microbicidal action against P. aeruginosa at all concentrations tested. In the presence of RBCs, killing of P. aeruginosa was extensive but incomplete. In the presence of RBCs, 1.9 nM MPO was sufficient to kill 99.9% of the P. aeruginosa bacteria. No hemolysis or Hgb destruction was observed.
In the absence of RBCs, the S. sanguinis-MPO synergistic microbicidal action against C. albicans was consistent with the P. aeruginosa results, but the presence of RBCs provided strong protection against MPO microbicidal action. In the presence of RBCs, 50 nM MPO killed 98% of the C. albicans, but no significant microbicidal action was observed at the lower MPO concentrations. The binding of MPO to C. albicans is considerably less than that observed for the bacteria tested, but MPO binding to C. albicans is greater than MPO binding to S. sanguinis (4, 6). There was no evidence of significant bystander damage to RBCs.
MPO without exogenous H2O2 kills S. pyogenes but not E. faecalis.
Table 5 presents the results of MPO microbicidal action against S. pyogenes and E. faecalis in the absence of H2O2 and in absence and presence of RBCs. In the absence of the protective effect of RBCs, S. pyogenes bacteria were directly killed by MPO without exogenous H2O2. Erythrocyte catalase protected S. pyogenes from its metabolic product, H2O2, thus limiting MPO action. Previous binding studies have reported that E. faecalis showed stronger MPO binding than S. pyogenes (group A) or S. agalactiae (group B) and that S. pyogenes and S. agalactiae showed stronger MPO binding than S. sanguinis (4).
TABLE 5.
Direct myeloperoxidase microbicidal action against streptococcal species in the absence and presence of human erythrocytes
| Microbea | MPO concn (nM) | No. of CFU/mlb |
Hemoglobin contentc |
||
|---|---|---|---|---|---|
| Without RBC's | With RBCs (107) | Supernatant | Pellet | ||
| S. pyogenes beta-hemolytic group A | 0.00 | 2,500,000 | 970,000 | 0.1 | 0.9 |
| 50.00 | 0 | 290,000 | 0.1 | 0.9 | |
| 16.67 | 0 | 280,000 | 0.1 | 0.9 | |
| 5.56 | 0 | 260,000 | 0.3 | 0.7 | |
| 1.85 | 0 | 300,000 | 0.1 | 0.9 | |
| 0.62 | 0 | 130,000 | 0.2 | 0.8 | |
| 0.21 | 600 | 750,000 | 0.1 | 0.9 | |
| 0.07 | 1,300,000 | 970,000 | 0.0 | 1.0 | |
| E. faecalis nonhemolytic group D | 0.00 | 1,900,000 | 1,700,000 | 0.2 | 0.8 |
| 50.00 | 1,600,000 | 1,500,000 | 0.2 | 0.8 | |
| 16.67 | 1,900,000 | 1,300,000 | 0.4 | 0.6 | |
| 5.56 | 1,700,000 | 1,500,000 | 0.2 | 0.8 | |
| 1.85 | 1,500,000 | 1,400,000 | 0.1 | 0.9 | |
| 0.62 | 1,500,000 | 1,600,000 | 0.1 | 0.9 | |
| 0.21 | 1,700,000 | 1,700,000 | 0.2 | 0.8 | |
| 0.07 | 1,800,000 | 1,500,000 | 0.2 | 0.8 | |
Each suspension contained either S. pyogenes (Lancefield group A) or E. faecalis (group D) alone. No H2O2 was added.
Values in boldface are less than 3 SDs from the expected mean and therefore indicate significant killing.
RBC damage was assessed by measuring the hemoglobin (Hgb) retained in the pelleted intact RBCs, lost to the medium as a consequence of hemolysis, or completely destroyed by oxidation, as described in footnote b of Table 1.
Like S. sanguinis, beta-hemolytic S. pyogenes is an LAB and produces the H2O2 (34) required for MPO haloperoxidase activity. Consequently, S. pyogenes is susceptible to MPO microbicidal action in the absence of RBCs. Consistent with MPO binding, MPO killing of S. pyogenes was greater than that observed against S. sanguinis in the previous experiments. In the absence of RBCs, killing of S. sanguinis was insignificant below 50 nM MPO.
E. faecalis does not excrete appreciable amounts of H2O2 and shows no hemolysis on blood agar. E. faecalis contains NADH peroxidase, i.e., NADH:H2O2 oxidoreductase. This nonheme flavoprotein catalyzes the direct reduction of H2O2 to H2O, which is a pseudo-catalase activity (15). Consequently, any H2O2 generated by E. faecalis metabolism is destroyed by reduction, thus depriving MPO of a substrate. Despite significant MPO binding (4), no microbicidal action was observed at any of the MPO concentrations tested in the absence or in the presence of RBCs. As shown in the MIC and MBC results, E. faecalis is highly susceptible to MPO when a source of H2O2 is provided.
MIC and MBC of MPO against diverse bacteria.
MPO selectively binds to bacteria (4, 6), and when a source of H2O2 is present, selective MPO binding results in selective microbe killing. The following conventional MIC and MBC studies were conducted to expand the range of observations in order to better quantify and statistically analyze the selectivity of MPO microbicidal action across a broad selection of bacteria. The previously described ATCC strains plus the control S. aureus ATCC 29213 and an additional 78 clinical isolates from diverse geographic areas of the United States were tested to establish the MICs and MBCs of MPO. In these studies MPO haloperoxidase activity was driven using glucose plus glucose oxidase (GO) as the H2O2 generator. The molar ratio of MPO to GO was 4.4 to 1. The range of MPO tested was 0.028 to 55 nM, or 0.004 to 8.0 μg MPO/ml. The MIC/MBC studies followed CLSI guidelines (12, 29). Such testing is designed to measure conventional antibiotics that inhibit bacterial protein synthesis or cell wall formation and is far from optimum for measuring MPO activity. The cation-adjusted Mueller-Hinton broth (CAMHB) used has a pH of 7.3. This hydrogen ion concentration is 10-fold to 100-fold less than optimum for MPO haloperoxidase activity (3). However, despite this limitation, the GO-MPO system showed good microbicidal action, as measured by MIC and MBC.
As described in Table 6, the lowest MIC and MBCs for MPO were observed for S. aureus. Methicillin-sensitive S. aureus (MSSA) showed no significant difference from methicillin-resistant S. aureus (MRSA) or vancomycin-intermediate/resistant S. aureus (VISA/VRSA) with regard to either MIC or MBC. Consistent with the results of the MPO binding studies (4, 6) and the empirical findings presented above, the MIC and MBC for Gram-negative bacteria were also relatively low, although higher than observed for S. aureus. With regard to ceftazidime sensitivity, E. coli and P. aeruginosa showed no significant differences in either MICs or MBCs. The MIC and especially the MBC of MPO were higher for E. faecalis than for the other bacteria tested in CAMHB. Vancomycin-sensitive and vancomycin-resistant E. faecalis (VSE and VRE, respectively) showed no significant difference in MIC and MBC results.
TABLE 6.
MIC and MBC of MPO against a spectrum of bacteria
| Medium and microbea | Resistance phenotypeb | No. of isolates | MPO MIC (nM)c |
MPO MBC (nM)c |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | |||
| CAMHB | ||||||||
| E. coli | CEF-S | 2 | 1.276 | 0.634 | 1.276 | 1.276 | 0.634 | 1.276 |
| CEF-R | 4 | 1.500 | 0.448 | 1.724 | 2.793 | 2.768 | 1.724 | |
| P. aeruginosa | CEF-S | 3 | 0.345 | 0.010 | 0.414 | 0.690 | 0.239 | 0.828 |
| CEF-R | 3 | 0.345 | 0.010 | 0.414 | 1.862 | 1.522 | 1.724 | |
| S. aureus | MSSA | 8 | 0.129 | 0.048 | 0.104 | 0.142 | 0.054 | 0.104 |
| MRSA | 6 | 0.129 | 0.048 | 0.104 | 0.142 | 0.054 | 0.104 | |
| VISA/VRSA | 2 | 0.155 | 0.073 | 0.155 | 0.155 | 0.073 | 0.155 | |
| E. faecalis (group D) | VSE | 5 | 2.759 | 0.944 | 3.449 | 6.207 | 4.496 | 3.449 |
| VRE | 2 | 1.276 | 0.634 | 1.276 | 5.173 | 2.439 | 5.173 | |
| CAMHB—5% LHB | ||||||||
| S. pyogenes (group A) | NA | 10 | 2.497 | 1.038 | 2.586 | 4.656 | 1.999 | 3.449 |
| S. agalactiae (group B) | NA | 10 | 1.973 | 1.091 | 1.724 | 2.842 | 1.731 | 2.586 |
| S. mitis (viridans group) | NA | 9 | 8.813 | 3.898 | 6.897 | 13.028 | 8.754 | 6.897 |
| S. oralis (viridans group) | NA | 9 | 12.261 | 6.703 | 13.794 | 15.327 | 7.538 | 13.794 |
| S. sanguinis (viridans group) | NA | 9 | 6.131 | 3.351 | 6.897 | 9.579 | 4.145 | 6.897 |
The bacteria included ATCC stains of E. coli (one), P. aeruginosa (one), and S. aureus (two) and 78 clinical isolates collected from diverse geographical areas in the United States. CAMHB, cation-adjusted Mueller-Hinton broth; LHB, lysed horse blood.
CEF-S, ceftazidime sensitive; CEF-R, ceftazidime resistant; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; VISA/VRSA, vancomycin intermediate/resistant S. aureus; VSE, vancomycin-sensitive E. faecalis; VRE vancomycin-resistant E. faecalis; NA, not applicable.
Glucose oxidase (GO) generated the H2O2 required for MPO action. The molar ratio of MPO to GO was 4.4.
The CLSI guidelines for MIC/MBC testing of streptococcal species required the use of cation-adjusted Mueller-Hinton broth supplemented with 5% lysed horse blood (CAMHB-5% LHB) for growth (12). In addition to the nonoptimal pH of CAMHB, the 5% LHB contained abundant catalase activity capable of consuming the H2O2 required as a substrate for MPO generation of OCl− and 1O2*. This lysed horse blood also presents Hgb and additional molecular substrates capable of reacting with the MPO-generated oxidants and competitively inhibiting microbicidal action.
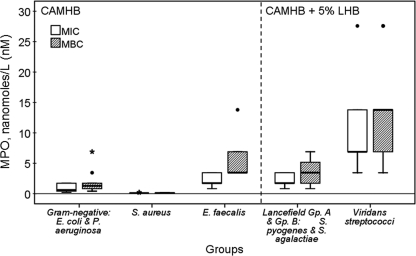
The effect of 5% lysed horse blood on the MICs and MBCs of GO-driven MPO was investigated by retesting the same seven E. faecalis isolates in both CAMHB and CAMHB-5% LHB. The mean MIC ± standard deviation (SD) was 1.4357 ± 0.4772 nM in CAMHB and 45.3264 ± 13.7190 nM in CAMHB-5% LHB. The mean MBC ± SD was 3.4493 ± 1.1730 nM in CAMHB and 53.2093 ± 7.3737 nM in CAMHB-5% LHB. Legitimate comparison of the MIC/MBC results requires that the condition of testing be essentially the same. Thus, the results based on CAMHB and CAMHB-5% LHB were considered separately. The left portion of Fig. 4 presents the Tukey box plots of MPO MIC and MBC results for the CAMHB testing groups, i.e., Gram-negative E. coli and P. aeruginosa, S. aureus, and E. faecalis. The right portion of Fig. 4 presents plots for the CAMHB-5% LHB testing groups, i.e., the Lancefield group A S. pyogenes and group B S. agalactiae and the viridans group streptococci S. mitis, S. oralis, and S. sanguinis.
FIG. 4.
The composite results of MPO MIC and MBC testing with the data presented as Tukey box plots. The results are grouped by the medium used, i.e., CAMHB and CAMHB-5% LHB. Five groups of bacteria were tested. As indicated on the x axis, the groups (number of isolates) are as follows: Gram-negative E. coli (n = 6) and P. aeruginosa (n = 6), S. aureus (n = 16), E. faecalis (n = 7), Lancefield group A S. pyogenes (n = 10) and group B S. agalactiae (n = 10), and viridans group streptococci (n = 27). The bottom and top portions (hinges) of each box are the lower and upper quartiles, respectively, and the hinge or H-spread (i.e., the interquartile range) is the distance between the bottom and top of the box. The heavy horizontal band shows the median. The whiskers are the lines drawn from the upper hinge to the upper adjacent value and from the lower hinge to the lower adjacent value within 1.5× the H-spread. An outlier is marked with a circular dot if it is between the inner fences (i.e., 1.5× the H-spread) and outer fence (i.e., 3× the H-spread). An extreme outlier is marked by an asterisk if it is beyond the outer fences.
Independent t tests were used to compare the results for the independent bacterial groups tested with CAMHB and separately for those tested with CAMHB-5% LHB. For the first two CAMHB groups, i.e., Gram-negative bacteria group (mean MIC ± SD of 0.9024 ± 0.6317 nM and mean MBC ± SD of 1.7817 ± 1.8176 nM) and S. aureus (MIC of 0.1229 ± 0.0417 nM and MBC of 0.1293 ± 0.0463 nM), the t value with 11 df [t(11)] was 4.267 with a P value of 0.0013 for the MIC, and t(11) was 3.148 with a P value of 0.0093 for the MBC. Analysis of Gram-negative bacteria and E. faecalis (MIC of 2.3351 ± 1.0886 nM and MBC of 5.9117 ± 3.8371 nM) gave a t(8) of −3.183 with a P value of 0.1232 for the MIC and a t(17) of −3.207 with a P value of 0.0052 for the MBC*. The asterisk indicates that this MBC analysis had a Levene's test for equality of variances of >0.05, and, as such, equal variance was assumed. The Levene's test was <0.05 for all other analyses, and consequently, equal variance was not assumed. Analysis of S. aureus and E. faecalis gave a t(6) of −5.375 with a P value of 0.0017 for MIC and t(6) of −3.987 with a P value of 0.0072 for the MBC.
For the two CAMHB-5% LHB groups, i.e., Lancefield group A S. pyogenes and group B S. agalactiae (MIC of 2.2346 ± 1.0709 nM and MBC of 3.7485 ± 2.0442 nM) and viridans group streptococci (MIC of 9.0683 ± 5.3380 nM and MBC of 12.6445 ± 7.2210 nM), the t(28) was −6.478 with a P value of <0.0001 for the MIC and the t(31) was −6.081 with a P value of <0.0001 for the MBC. The composite MIC and MBC results are consistent with, and conclusively document, the mixed culture results described earlier.
DISCUSSION
Members of the lactic acid family of bacteria (LAB) are common to the normal flora of humans. Viridans group streptococci, such as S. sanguinis, S. mitis, and S. oralis, are indigenous flora of the healthy human mouth (20). These LAB do not synthesize heme and, consequently, lack respiratory cytochromes and catalase. As such, streptococcal redox metabolism is dependent on flavoenzymes generating lactic acid and, in many cases, H2O2, as metabolic products (10, 18, 24). As described by Rosebury, “Wherever in nature two or more species of microorganisms grow in intimate association, each will interact with the others; and if the microorganisms grow upon a host organism, then the host will be in some way influenced by the interaction” (31). The status of LAB in the normal flora of humans suggests the probability of a symbiotic host-microbe interaction. According to Sanders, “The interaction of man's indigenous microflora and exogenously acquired pathogens has been the subject of sporadic investigation and continuous speculation for more than 5 decades. However, only recently has it been demonstrated conclusively that antagonistic interactions may enhance man's capacity to resist infection” (32).
Microbial antagonism was demonstrated by Colebrook in his 1915 report that pneumococcus and viridans group streptococcus kill meningococcus and other Gram-negative bacteria (13). The importance of streptococci in suppressing the growth of potential pathogens is also implied by the phenomenon of superinfection following antibiotic therapy. The significance of viridans group streptococci in this regard was described by Sprunt et al. “Members of the viridans group of streptococci, the predominant strains of the oropharyngeal flora in most individuals, can inhibit the growth of enteric Gram-negative bacilli, the organisms that commonly overgrow at this site following therapy with massive doses of penicillin. It was proposed that suppression (or elimination) of these streptococci by massive doses of antibiotics suppresses (or eliminates) their inhibitory action and permits multiplication of the previously inhibited (or newly introduced) bacilli” (36).
The results presented herein support the conclusion that low MPO binding to viridans streptococci provides these LAB with a competitive advantage over high-MPO-binding microbes. LAB-generated H2O2 is the substrate for the MPO-catalyzed oxidation of Cl− to OCl− and for the reaction of H2O2 with OCl− to produce singlet oxygen, 1O2* (2, 3, 19). When H2O2 is limiting, OCl− can directly participate in dehydrogenations and chlorination of amines, but when sufficient H2O2 is available, reaction with OCl− produces 1O2* (19). The microsecond reactive lifetime of 1O2* restricts combustive oxygenation activity to within a radius of about 0.2 μm of its point of generation (4, 30, 35). Thus, combustive oxygenations are focused to the site of MPO binding with relatively little bystander injury to microbes or erythrocytes showing no or low MPO binding.
The migration of neutrophil leukocytes from the blood to body spaces such as the mouth and vagina (11, 38) guarantees delivery of MPO to these sites and the possibility for MPO interaction with the microbes present. In the competition among microbes for a place within the flora, the presence of low concentrations of MPO favors non-MPO-binding LAB. The role of MPO in such competition is demonstrated by S. sanguinis-MPO synergistic microbicidal action against E. coli, S. aureus, P. aeruginosa, and C. albicans.
When oxidative activity is nonspecific, RBCs serve as a competitive substrate and are destroyed. When oxidative activity is specific and focused and the concentration of H2O2 is relatively low, erythrocyte catalase can effectively consume excess H2O2 with little or no erythrocyte hemolysis. RBCs show essentially no MPO binding (4).
The results for the H2O2 and OCl− studies demonstrate nonspecific oxidative action. RBCs competitively inhibit antimicrobial activity and are destroyed in the process. RBCs are consumed before microbicidal action can begin; i.e., erythrocytes are more susceptible than microbes to H2O2 and to OCl−. The S. sanguinis-MPO synergistic microbicidal model demonstrates that MPO-dependent killing is focused and specific and without bystander damage to the RBCs. Erythrocyte catalase, by consuming streptococcal H2O2, prevents significant erythrocyte injury and minimizes damage to the low-MPO-binding streptococci that are the source of H2O2.
MPO binding to S. pyogenes is stronger than binding to S. sanguinis (4, 6). In the absence of RBCs, S. pyogenes and, to a lesser extent, S. sanguinis are directly killed by MPO. Both streptococci generate H2O2 metabolically, and, as such, exogenous H2O2 is not necessary for killing. Conventional MIC and MBC testing confirms that S. pyogenes and S. agalactiae are significantly (P values of less than 0.00001) more susceptible to the microbicidal action of the GO-driven MPO system than are the viridans group streptococci.
Although weaker than for E. coli, P. aeruginosa, and S. aureus, MPO binding to E. faecalis is relatively stronger than MPO binding to S. pyogenes and S. agalactiae (4, 6), but in the absence of added H2O2, MPO is not directly microbicidal. E. faecalis contains a pseudo-catalase, i.e., NADH peroxidase, that prevents metabolically generated H2O2 from accumulating. This NADH peroxidase uses NADH to reduce H2O2 to H2O (15). As demonstrated in the MPO MIC and MBC studies, E. faecalis is susceptible to MPO microbicidal action when GO is present as a H2O2 generator. Consistent with MPO binding activities, the MPO concentrations required for MIC and MBC action against E. faecalis were significantly higher than those for E. coli, P. aeruginosa, and S. aureus. The MIC and MBC studies further demonstrate that selective MPO binding results in selective microbicidal action.
The relationship of bacterial metabolism to MPO microbicidal action is clinically illustrated by chronic granulomatous disease (CGD), a disorder resulting from defective neutrophil NADPH oxidase function and characterized by severe and recurrent staphylococcal, coliform, and fungal infections (5). The neutrophils of CGD patients are capable of phagocytosis and azurophilic granule fusion producing an MPO-rich phagolysosomal space surrounding the phagocytized microbe. However, the NADPH oxidase of CGD neutrophils is defective and incapable of H2O2 production. Consequently, there is no H2O2 to drive MPO generation of OCl− and 1O2* as required for combustive microbicidal action and chemiluminescence. Pertinent to this discussion, streptococcal infections are not increased in CGD patients. Phagocytosis of viable streptococci by CGD neutrophils results in microbicidal action and chemiluminescence (5).
The process of neutrophil phagocytosis obviates the necessity for selective MPO binding. Phagocytosis, phagosome formation, and fusion of the MPO-rich azurophilic granules producing the phagolysosome guarantee that MPO is confined to the proximity to the phagocytized microbe. There is no apparent necessity for MPO binding selectivity within the phagolysosomal space. However, the evidence presented herein suggests that specificity of microbe binding can affect flora composition when microbes contact MPO released from the neutrophils that have migrated into a body space such as the mouth.
In summary, MPO plus streptococci exert a synergistic microbicidal action against a range of MPO-binding microbes, suggesting a mechanistic explanation for the dominance of viridans group streptococci in the normal mouth flora. Streptococci belong to the heme-deficient lactic acid family of bacteria and generate lactic acid and H2O2. These metabolic products satisfy the pH and substrate requirements for MPO oxidation of chloride to hypochlorite and for reaction of H2O2 with hypochlorite to produce 1O2*, a potent oxygenating agent with a microsecond half-life. The short lifetime of 1O2* restricts reactivity to within a radius of about 0.2 μm from its point of generation. As such, no or low MPO binding serves to protect H2O2-producing viridans group streptococci and erythrocytes from MPO-generated OCl− and 1O2*. Microbes with strong MPO binding are targeted for MPO-dependent OCl− and 1O2* generation when H2O2 is available. Combustive destruction is concentrated on the MPO-bound microbes. MPO MIC and MBC studies provide statistical evidence that high MPO binding is related to low MPO MIC and MBC values. Selective MPO binding results in selective MPO killing. The composite findings suggest a role for MPO in the establishment and maintenance of the normal floras of the human mouth.
Acknowledgments
We thank Rebecca Bexar and Martha A. Mireles for their technical assistance in the conduct of this research. We thank Gerald A. Denys for his suggestions. The MIC and MBC studies were contracted to and conducted by Parveen Grover, Eurofins Medinet Anti-Infective Services, 13665 Dulles Technology Drive, Herndon, VA, under the direction of Chief Science Officer, Daniel F. Sahm.
The research was supported in full by ExOxEmis, Inc., Little Rock, AR.
Editor: A. Camilli
Footnotes
Published ahead of print on 25 October 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Agner, K. 1958. Crystalline myeloperoxidase. Acta Chem. Scand. 12:89-94. [Google Scholar]
- 2.Allen, R. C. 1975. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem. Biophys. Res. Commun. 63:675-683. [DOI] [PubMed] [Google Scholar]
- 3.Allen, R. C. 1975. The role of pH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem. Biophys. Res. Commun. 63:684-691. [DOI] [PubMed] [Google Scholar]
- 4.Allen, R. C. March 1999. Method for selectively inhibiting the growth of microbes using a haloperoxidase-halide-peroxide system. U.S. patent 5,888,505.
- 5.Allen, R. C., E. L. Mills, T. R. McNitt, and P. G. Quie. 1981. Role of myeloperoxidase and bacterial metabolism in chemiluminescence of granulocytes from patients with chronic granulomatous disease. J. Infect. Dis. 144:344-348. [DOI] [PubMed] [Google Scholar]
- 6.Allen, R. C. and J. T. Stephens, Jr. 9 February 2010, posting date. Reduced-oxidized difference spectral analysis and chemiluminescence-based Scatchard analysis demonstrate selective binding of myeloperoxidase to microbes. Luminescence. doi: 10.1002/bio.1210. [DOI] [PubMed]
- 7.Allen, R. C., P. R. Stevens, T. H. Price, G. S. Chatta, and D. C. Dale. 1997. In vivo effects of recombinant human granulocyte colony-stimulating factor on neutrophil oxidative functions in normal human volunteers. J. Infect. Dis. 175:1184-1192. [DOI] [PubMed] [Google Scholar]
- 8.Bainton, D. F. 1999. Developmental biology of neutrophils and eosinophils, p. 13-34. In J. I. Gallin and R. S. Snyderman (ed.), Inflammation, basic principles and clinical correlates, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 9.Butterfield, C. T., E. Wattie, S. Megregian, and C. W. Chambers. 1943. Influence of pH and temperature on the survival of coliforms and enteric pathogens when exposed to chlorine. Publ. Health Rep. 58:1837-1866. [PubMed] [Google Scholar]
- 10.Carlsson, J., Y. Iwami, and T. Yamada. 1983. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect. Immun. 40:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauci, S., S. Guaschino, D. de Aloysio, S. Driussi, D. De Santo, P. Penacchioni, and F. Quadrifoglio. 2003. Interrelationships of interleukin-8 with interleukin-1β and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol. Hum. Reprod. 9:53-58. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. CLSI M07-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 13.Colebrook, L. 1915. Bacterial antagonism, with particular reference to meningococcus. Lancet 186:1136-1138. [Google Scholar]
- 14.Dakin, H. D. 1915. The antiseptic action of hypochlorites. Br. Med. J. 2:809-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolin, M. I. 1956. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. III. Isolation and properties of a flavin peroxidase for reduced diphosphopyridine nucleotide. J. Biol. Chem. 225:557-573. [PubMed] [Google Scholar]
- 16.Fleming, A. 1919. The action of chemical and physiological antiseptics in a septic wound. Br. J. Surg. 7:99-129. [Google Scholar]
- 17.Friberg, L., and E. Hammarström. 1956. The action of free available chlorine on bacteria and bacterial viruses. Acta Pathol. Microbiol. Scand. 38:127-134. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Mendoza, A., J. Liebana, A. M. Castillo, A. de la Higuera, and G. Piedrola. 1993. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J. Med. Microbiol. 39:434-439. [DOI] [PubMed] [Google Scholar]
- 19.Held, A. M., D. J. Halko, and J. K. Hurst. 1978. Mechanism of chlorine oxidation by hydrogen peroxide. J. Am. Chem. Soc. 100:5732-5740. [Google Scholar]
- 20.Johnston, D. A., and G. P. Bodey. 1970. Semiquantitative oropharyngeal culture technique. Appl. Microbiol. 20:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasha, M., and A. U. Khan. 1970. The physics, chemistry, and biology of singlet molecular oxygen. Ann. N. Y. Acad. Sci. 171:5-23. [Google Scholar]
- 22.Klebanoff, S. J. 1968. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J. Bacteriol. 95:2131-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff, S. J., and R. A. Clark. 1978. The neutrophil: function and clinical disorders. North-Holland Publishing Co., Amsterdam, Netherlands.
- 24.McLeod, J. W., and J. Gordon. 1922. Production of hydrogen peroxide by bacteria. Biochem. J. 16:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks, H. C., O. Wyss, and F. B. Strandskov. 1945. Studies on the mode of action of compounds containing available chlorine. J. Bacteriol. 49:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercer, W. A., and I. I. Somers. 1957. Chlorine in food plant sterilization. Adv. Food Res. 7:129-160. [Google Scholar]
- 27.Morris, M. W., and F. R. Davey. 2001. Basic examination of blood, p. 480. In J. B. Henry (ed.), Clinical diagnosis and management by laboratory methods, 20th ed. W. B. Saunders Co., Philadelphia, PA.
- 28.Mueller, S., H. D. Riedel, and W. Stremmel. 1997. Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 90:4973-4978. [PubMed] [Google Scholar]
- 29.NCCLS. 1999. Methods for determining bacterial activity of antimicrobial agents. Approved standard M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 30.Redmond, R. W., and I. E. Kochevar. 2006. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 82:1172-1186. [DOI] [PubMed] [Google Scholar]
- 31.Rosebury, T. 1962. Microorganisms indigenous to man. McGraw-Hill Book Co., New York, NY.
- 32.Sanders, E. 1969. Bacterial interference. I. Its occurrence among the respiratory tract flora and characterization of inhibition of group A streptococci. J. Infect. Dis. 120:698-707. [DOI] [PubMed] [Google Scholar]
- 33.Schultz, J., and K. Kaminker. 1962. Myeloperoxidase of the leukocyte of normal human blood. I. Content and localization. Arch. Biochem. Biophys. 96:465-467. [DOI] [PubMed] [Google Scholar]
- 34.Seki, M., K. Iida, M. Saito, H. Nakayama, and S. Yoshida. 2004. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactate oxidase and coupling with aerobic utilization of lactate. J. Bacteriol. 186:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skovsen, E., J. W. Snyder, J. D. C. Lambert, and P. R. Ogilby. 2005. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 109:8570-8573. [DOI] [PubMed] [Google Scholar]
- 36.Sprunt, K., G. A. Leidy, and W. Redman. 1971. Prevention of bacterial overgrowth. J. Infect. Dis. 123:1-10. [DOI] [PubMed] [Google Scholar]
- 37.Tukey, J. W. 1977. Exploratory data analysis. Addison-Wesley, Reading, MA.
- 38.Wright, D. G., A. I. Meierovics, and J. M. Foxley. 1986. Assessing the delivery of neutrophils to tissues in neutropenia. Blood 67:1023-1030. [PubMed] [Google Scholar]