Abstract
Aspergillus fumigatus, a ubiquitous airborne fungus, can cause invasive infection in immunocompromised individuals but also triggers allergic bronchopulmonary aspergillosis in a subset of otherwise healthy individuals repeatedly exposed to the organism. This study addresses a critical gap in our understanding of the immunoregulation in response to repeated exposure to A. fumigatus conidia. C57BL/6 mice were challenged intranasally with A. fumigatus conidia weekly, and leukocyte composition, activation, and cytokine production were examined after two, four, and eight challenges. Approximately 99% of A. fumigatus conidia were cleared within 24 h after inoculation, and repeated exposure to A. fumigatus conidia did not result in hyphal growth or accumulation of conidia with time. After 2 challenges, there was an early influx of neutrophils and regulatory T (Treg) cells into the lungs but minimal inflammation. Repeated exposure promoted sustained expansion of the draining lymph nodes, while the influx of eosinophils and other myeloid cells into the lungs peaked after four exposures and then decreased despite continued A. fumigatus challenges. Goblet cell metaplasia and low-level fibrosis were evident during the response. Repeated exposure to A. fumigatus conidia induced T cell activation in the lungs and the codevelopment by four exposures of TH1, TH2, and TH17 responses in the lungs, which were maintained through eight exposures. Changes in CD4 T cell polarization or Treg numbers did not account for the reduction in myeloid cell numbers later in the response, suggesting a non-T-cell regulatory pathway involved in dampening inflammation during repeated exposure to A. fumigatus conidia.
Allergic bronchopulmonary aspergillosis (ABPA) is characterized by early allergic and late-phase lung injury in response to repeated exposures to Aspergillus antigens, which are the consequence of persistent fungal colonization of the lungs (47, 60). The disease occurs primarily in patients who have skewed pulmonary immune responses, such as those found in atopic asthma or cystic fibrosis. The pulmonary immune response in these patients includes a strong T helper 2 (TH2) response to the colonizing fungus. The underlying mechanism(s) by which Aspergillus induces TH2 responses in some patients, but not others, is presently unknown. If undiagnosed, ABPA can result in progressive lung damage, pulmonary fibrosis, and death (47, 60).
Aspergillus fumigatus conidia are frequently inhaled into airways at a rate of several thousand a day (41), and pulmonary exposure to large numbers of conidia is not uncommon (24). Upon reaching the warm, moist environment of the lungs, the conidia lose their hydrophobic properties and begin to germinate (34). Following conidial swelling and germ tube extension, the fungus develops invasive hyphae (54), and the cycle repeats itself. Although A. fumigatus can pose a serious threat to immunocompromised individuals, even relatively large doses of conidia pose little danger to immunocompetent hosts.
The immune response to inhaled A. fumigatus in healthy individuals is characterized by a complex interaction between innate and adaptive immune responses, both of which are activated upon exposure to the fungus (18, 31, 56, 58). Macrophages and neutrophils efficiently phagocytize inhaled conidia in the lungs, with neutrophils being absolutely essential, and conidial clearance can occur with minimal inflammation (7, 33, 44, 63, 66, 67, 70). Invasive infection of humans is associated with decreased gamma interferon (IFN-γ) production and poor T cell proliferation (27), and inhibition of IFN-γ or tumor necrosis factor alpha (TNF-α) enhances fungal invasion (2, 14, 43). A single inhalation of aerosolized A. fumigatus spores by mice can induce the expression of TNF-α, IFN-γ, interleukin 12 (IL-12), and IL-18 (10). In an adoptive transfer model, A. fumigatus-specific CD4+ T cells are rapidly primed in lung-associated lymph nodes by CCR2+ Ly6Chi monocytes/dendritic cells, and these CD4+ T cells differentiate fully into IFN-γ-producing TH1 cells upon arrival in the airways (30, 62).
Most studies have focused on the host response following one exposure or a very limited number of exposures to A. fumigatus conidia. While repeated exposure to A. fumigatus conidia is common, little is known about the evolution and regulation of the host response to repeated exposure to A. fumigatus conidia. There is a significant body of literature on the pulmonary allergic response to a conidial challenge of mice previously sensitized by intraperitoneal injection of Aspergillus antigen extracts in an adjuvant (6, 40), which has provided much information about the mechanisms underlying TH2-mediated pathological changes in the lungs. In addition, we have previously reported that two intranasal exposures to A. fumigatus conidia without a sensitizing event do not result in pulmonary allergic inflammation (51, 52). Our current study addresses a critical gap in our understanding of the regulation and evolution of adaptive immune responses to repeated exposures to A. fumigatus conidia. We set out to test the hypothesis that while two airway exposures to A. fumigatus conidia stimulate an innate response (neutrophils and macrophages) and begin priming for a Th1 response to the fungus, repeated exposures also stimulate the development of both TH2 and TH17 cells, which coincides with the development of a robust inflammatory response in the lungs.
MATERIALS AND METHODS
Mice.
Wild-type (C57BL/6J) mice obtained from the Jackson Laboratories (Bar Harbor, ME) were housed under pathogen-free conditions in enclosed filter-top cages. Clean food and water were given ad libitum. The mice were handled and maintained using microisolator techniques, with daily veterinarian monitoring. All studies involving mice were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Aspergillus fumigatus.
Strain ATCC 13073 was grown on Sabouraud dextrose agar (SDA; Difco) for 14 days. Conidia were harvested by washing plates with sterile phosphate-buffered saline (PBS; pH 7.4) with 0.1% Tween 80 (PBS-Tween), followed by filtration of the suspension through two layers of sterile gauze to remove hyphae. Conidia were washed in PBS-Tween, counted with a hemocytometer, diluted to 108 spores/ml in sterile PBS-Tween, and stored at 4°C for as long as 4 months. The conidial preps consisted of >99.9% resting conidia and did not contain appreciable numbers of swollen conidia. The viability of the conidial stocks, as assessed by dilution plating, remained reproducible and high.
Intranasal challenge.
To achieve sedation, mice were injected intraperitoneally with 0.4 mg/ml xylazine (Lloyd Laboratories, Shenandoah, IA) and 10 mg/ml ketamine (Fort Dodge Animal Health, Fort Dodge, IA) in sterile saline (Hospira, Inc., Lake Forest, IL) based on weight. Following sedation, 20 μl of an Aspergillus fumigatus suspension was administered intranasally for a total of 2 × 106 conidia per mouse per challenge.
Lung histology.
Lungs were fixed by inflation with 10% neutral buffered formalin (Sigma). After paraffin embedding, 5-μm-thick sections were cut and stained with either hematoxylin and eosin (H&E) for histological analysis, periodic acid-Schiff stain (PAS) for the detection of mucus and goblet cell metaplasia, Masson's trichrome stain for the detection of collagen deposition, or Grocott's methenamine silver (GMS) stain for the detection of conidia and hyphae (McClinchey Histology Lab, Stockbridge, MI).
Lung digestion for whole-lung leukocyte enrichment.
Lungs from each mouse were excised, washed in PBS, minced, and digested enzymatically for 30 min in 15 ml/lung of digestion buffer (RPMI medium, 5% fetal calf serum, 1 mg/ml collagenase [Boehringer Mannheim Biochemical, Chicago, IL], and 30 μg/ml DNase [Sigma Chemical Co., St. Louis, MO]) as previously described (50). After erythrocyte lysis using NH4Cl buffer (0.83% NH4Cl, 0.1% KHCO3, 0.037% disodium EDTA [pH 7.4]), cells were washed, resuspended in RPMI medium with 5% fetal calf serum and 20% Percoll (Sigma), and centrifuged for 30 min at 2,000 × g to separate leukocytes from cell debris and epithelial cells. Total-lung leukocyte numbers were enumerated in the presence of trypan blue by using a hemocytometer.
In vivo quantification of viable conidia.
Following digestion of the lung, an aliquot was taken for analysis prior to centrifugation and erythrocyte lysis. The sample was serially diluted and plated onto SDA in duplicate. Individual mycelial colonies were counted to determine the number of CFU per dilution, which was then multiplied to yield the total number of viable conidia in the lungs of each mouse 24 h after inoculation.
Isolation of lymph node cells.
The draining mediastinal lymph node was excised from the thoracic cavity, placed in 1 ml of RPMI medium (with 5% fetal calf serum) in a six-well plate (Corning Incorporated, Corning, NY), and ground with the flat edge of a 1-ml syringe. The cell suspension was then transferred through a 100-μm-pore-size screen and was washed with 2 ml of RPMI medium. After erythrocyte lysis using NH4Cl buffer, cells were washed, resuspended in RPMI medium (with 5% fetal calf serum), and counted with a hemocytometer prior to analysis.
Flow cytometry.
Cells were washed and resuspended at a concentration of 106/25 μl FA buffer (Difco) plus 0.1% NaN3, and Fc receptors were blocked by the addition of unlabeled anti-CD16/32 (Fc Block; BD Pharmingen, San Diego, CA). After Fc receptor blocking, 0.5 × 106 to 1 × 106 cells were stained in a final volume of 50 μl in 96-well round-bottom plates (Corning Incorporated, Corning, NY) for 30 min at 4°C. Cells were washed twice with FA buffer, resuspended in 120 μl of 4% formalin (Sigma), and transferred to 12- by 75-mm polystyrene tubes (Becton Dickinson, Franklin Lakes, NJ). A minimum of 100,000 events were acquired on a FACSCanto flow cytometer (BD Pharmingen) using CellQuest software (BD Pharmingen). The data acquired were analyzed with FlowJo software (Tree Star, Stanford, CA). Fluorochrome-conjugated antibodies directed against the following antigens were obtained: CD45 (BioLegend, San Diego, CA); CD3, CD4, CD8, CD11c, CD19, CD25, CD44, CD49b, CD69, Gr1, Siglec F, FcɛRI, IFN-γ, IL-4, IL-10, and IL-17 (BD Pharmingen); and Foxp3 (eBioscience, San Diego, CA).
Differential analysis.
Cells from whole-lung digests were analyzed as follows. First, lung leukocytes were identified by CD45 expression. The following leukocyte subsets were then identified within this gate: (i) neutrophils, identified using a CD11c-versus-Gr1 plot as cells expressing little CD11c but large amounts of Gr1; (ii) mature eosinophils, identified as cells expressing moderate amounts of CD11c and Gr1 and further expressing large amounts of Siglec F; and (iii) lymphocytes, identified within the population of cells displaying low forward and side scatter and then subdivided into CD4, CD8, or B cells (CD19), based on cell surface staining. Basophils were identified as FSClow SSClow cells that were Gr1− CD11c− CD3− CD19− CD49b+ FcɛRI+ as described previously (53). Monocytes/macrophages/dendritic cells (DC) represent a continuum of myeloid differentiation, and the cell surface markers required to fully differentiate specific myeloid populations were not used in this study. These cells were analyzed in aggregate and were identified based on their forward and side scatter characteristics, lack of lymphocyte markers, and exclusion of the neutrophil and eosinophil populations (described above). Where possible, changes in the cell differentials were also confirmed by Wright-Giemsa stains of cytospin slides.
CD4 T cell activation.
CD4 T cells in the lungs and lymph nodes were identified by CD45 and CD4 staining. Cells were additionally stained with fluorescently labeled antibodies specific for CD44 and CD69, both of which are markers of T cell activation. CD4 T cells that were CD44high CD69+ were counted as activated.
Intracellular flow staining.
Prior to intracellular cytokine staining, cells were stimulated in vitro for 6 h with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (1 μg/ml) in the presence of brefeldin A (BD Pharmingen) to promote the intracellular accumulation of cytokines. After stimulation, cells were washed twice prior to surface molecule staining. Subsequently, intracellular molecules were stained using the BD Cytofix/Cytoperm kit according to the manufacturer's instructions (BD Pharmingen).
Blood collection and serum separation.
Blood was collected from mice at the time of harvest. Serum was collected after centrifugation for 1 min at 6,000 rpm in Microtainer tubes (BD Pharmingen).
Enzyme-linked immunosorbent assay.
IgE in serum was measured by a sandwich enzyme-linked immunosorbent assay (sandwich ELISA) using the manufacturer's instructions supplied with the specific kits (BD Pharmingen).
Bronchoalveolar lavage (BAL) for cell recovery (intermittent challenge experiment).
Airway contents were recovered by the instillation and retrieval of 1 ml of sterile PBS through a tracheotomy tube. Cells collected by three total lavages were pooled. After erythrocyte lysis using NH4Cl buffer, cells were washed, resuspended in complete medium (RPMI 1640, 10% fetal calf serum, 2 mmol/liter l-glutamine, 50 μmol/liter 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate), and enumerated under a light microscope in the presence of trypan blue by using a hemocytometer.
Statistical analyses.
At least four separate experiments (Fig. 2 to 8) or two separate experiments (Fig. 9) with 3 to 4 mice per group per experiment were performed. The quantitative data in each graph are from the cumulative analysis across the multiple experiments (i.e., the graphs do not show results for a single representative data set). All values are reported as means ± standard errors of the means. Differences between groups were evaluated by analysis of variance (ANOVA) with a posthoc test; a P value of <0.05 was considered statistically significant.
RESULTS
Cellular infiltrate and airway remodeling following repeated pulmonary exposure to Aspergillus conidia.
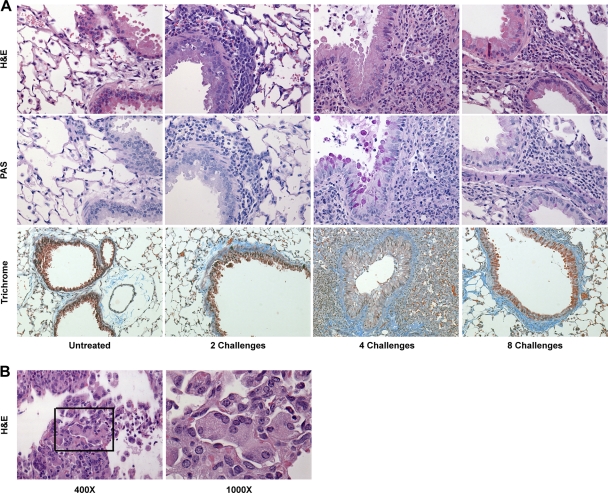
To determine the host response to repeated exposure to A. fumigatus conidia, mice were challenged intranasally once a week for 2, 4, or 8 weeks with 2 × 106 live A. fumigatus conidia and were analyzed 24 h after the final conidial challenge. This intranasal exposure dose is 5- to 100-fold lower than that typically used by labs in studies of invasive aspergillosis (43, 46, 63, 76). We prepared histological sections of the lungs and analyzed pulmonary inflammation (H&E), goblet cell metaplasia (PAS), and fibrosis (trichrome). Following two challenges, granulocytic infiltrates were beginning to become evident around the airways, with minimal changes in goblet cells or collagen deposition (Fig. 1 A). After four challenges, the size of the inflammatory infiltrate had increased markedly around the airways and in the parenchyma, including large numbers of eosinophils and neutrophils and the formation of multinucleated giant cells (Fig. 1B). In addition, goblet cell metaplasia in the epithelium was now evident, along with low-level fibrotic changes (Fig. 1A). Following eight challenges, inflammatory infiltrates, goblet cell metaplasia, and fibrotic changes were still histologically evident, but all were either at the same level or a lower level than those observed at four challenges, though still greater than those observed at two challenges (Fig. 1A). Despite repeated challenges and diminished pulmonary inflammation, the survival rate over the course of 8 weeks was 100% (data not shown). Thus, repeated exposure of C57BL/6 mice to A. fumigatus conidia induced a marked pulmonary inflammatory response between 2 and 4 weeks of conidial challenge; however, the magnitude of the pulmonary inflammatory response did not continue to amplify despite continued A. fumigatus challenges.
FIG. 1.
(A) Cellular infiltrate around the airways, goblet cell metaplasia, and collagen deposition following repeated intranasal exposure to Aspergillus fumigatus conidia. Lungs from nonchallenged mice (Untreated) and from mice challenged two, four, or eight times were fixed in formalin and embedded in paraffin blocks. Histological slices were then stained with either H&E, PAS, or Masson's trichrome stain. Magnifications, ×400 for H&E and PAS; ×200 for trichrome. (B) Multinucleated giant cells were observed in the lungs of mice challenged four times with conidia. Magnifications are given below the images.
Fungal clearance and expansion of cellular populations in the lungs and lymph nodes following two, four, and eight conidial challenges.
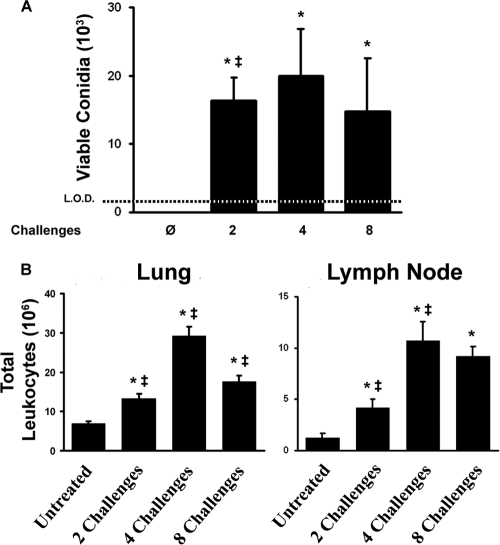
We next addressed the question of whether the changes in the pulmonary inflammatory response with increasing exposures were accompanied by changes in conidial clearance. The numbers of viable conidia in the lungs were quantified at each time point, as described in Materials and Methods. Twenty-four hours after inoculation, the initial number of conidia (2 × 106) administered to the lungs had been reduced to less than 2 × 104 viable conidia; levels of conidial clearance at 24 h postinoculation were identical for the mice in the two-, four-, and eight-challenge groups, regardless of differences in the inflammatory response between these time points (Fig. 1 and 2 A). By silver staining, we could occasionally identify very small numbers of conidia but never observed hyphal masses. Most commonly, we could not identify any significant amount of fungal material at sites of inflammation or other uninvolved regions of the lungs by silver staining (data not shown). We did occasionally observe rare germinating conidia in some mice (data not shown), which is worth noting because germinating conidia are metabolically active and reorganize the contents of their cell walls to expose immunostimulatory glucans (32, 70). Thus, approximately 99% of A. fumigatus conidia were cleared within 24 h of inoculation, and repeated exposure to A. fumigatus conidia did not result in hyphal growth or accumulation of conidia with time.
FIG. 2.
Fungal clearance and pulmonary inflammation following intranasal challenges. (A) Twenty-four hours after the final challenge, lungs were digested; an aliquot of the digest was serially diluted and plated onto SDA medium; and mycelial colonies were counted. Bars show the average numbers of viable conidia per lung detected 24 h after no challenge or two, four, or eight challenges. (B) Leukocyte influx into the lungs during the inflammatory response and expansion of lymphocytes in the mediastinal (draining) lymph node following repeated exposure to A. fumigatus conidia. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
To provide a quantitative analysis of the kinetics of the pulmonary inflammatory response, leukocytes were isolated from enzymatically dispersed lungs of challenged mice 24 h after final challenge at each time point. Two challenges stimulated the influx of a small number of leukocytes into the lungs and expansion of the draining mediastinal lymph nodes (Fig. 2B). Repeated challenges augmented pulmonary inflammation and lymph node lymphocyte numbers through 4 weeks. By 8 weeks, pulmonary inflammation had begun to wane with the additional challenges, although the numbers of lymphocytes in the draining lymph nodes remained elevated (Fig. 2B). Thus, repeated exposure promoted sustained expansion of the draining lymph nodes, while the pulmonary inflammatory response peaked and waned.
We next used flow cytometry to identify specific myeloid cell populations in the lungs and to further delineate the dynamics of the inflammatory response through eight weekly challenges with A. fumigatus conidia. Consistent with our histological analysis, we observed a significant difference in the number of granulocytes between untreated mice and those challenged twice (Fig. 3). However, mice challenged four times with conidia had 6-fold more eosinophils in the lungs than mice challenged twice (Fig. 3). By eight challenges, there were significantly fewer neutrophils and a trend toward fewer eosinophils in the lungs. The numbers of basophils in the lungs increased significantly between two and four challenges and then decreased slightly by eight challenges (Fig. 4). The monocyte/macrophage/DC population was numerically the largest cell population in the lungs. This heterogeneous population followed kinetics similar to those of the other myeloid cells, with a significant peak at four challenges and a significant decline by eight challenges. These data provide quantitative analyses that are consistent with the histological observations described above and confirm that, after peaking at four challenges, the influx of eosinophils and other myeloid cells did not continue to grow despite continued A. fumigatus challenges.
FIG. 3.
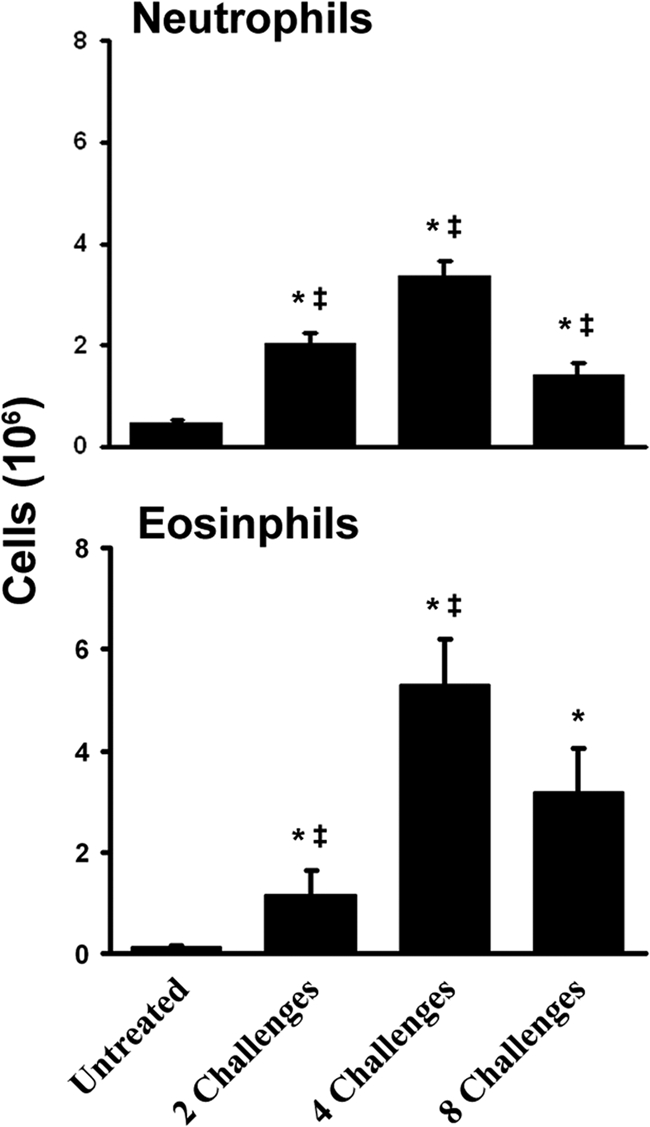
Neutrophil and eosinophil influx into the lungs during the inflammatory response. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
FIG. 4.
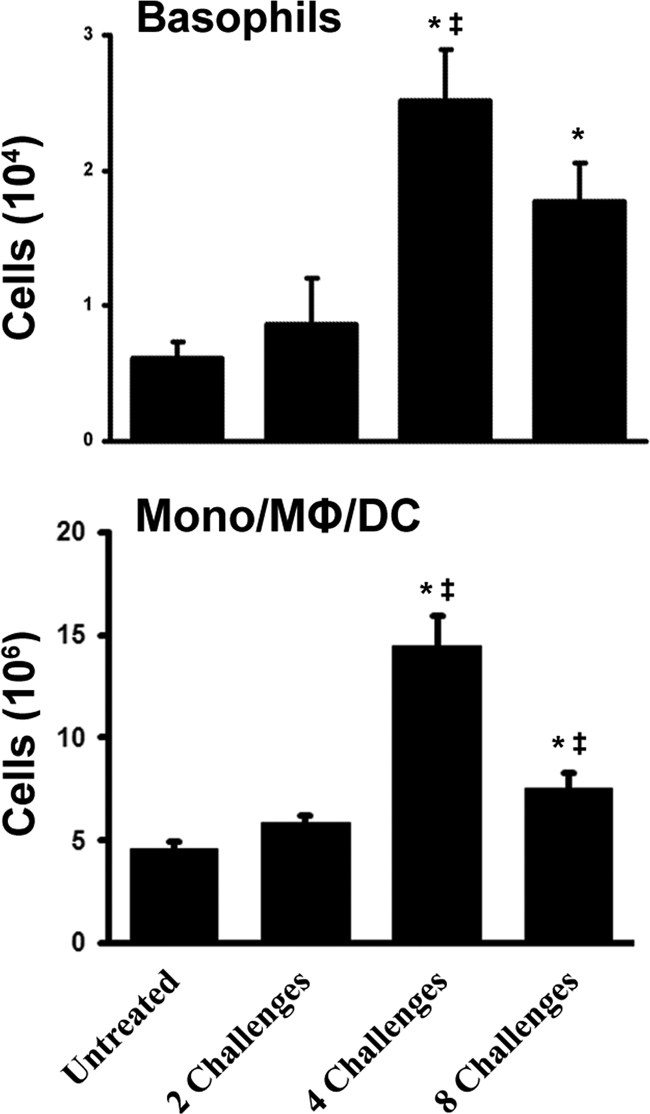
Basophil and monocyte/macrophage/DC influx into the lungs during the inflammatory response. Mφ, macrophages. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
Activation of CD4 T cells in the lungs and lymph node in response to repeated pulmonary challenges with conidia.
We next examined the dynamics of the CD4 T cell response to repeated challenges with A. fumigatus conidia. First, we quantified CD4, CD8, and B lymphocyte levels in the lungs by flow cytometry as described in Materials and Methods. The number of CD4 T cells did not increase significantly following two challenges, but four challenges induced a 5-fold increase in the total number of CD4 T cells in the lungs, which remained elevated through eight challenges (Fig. 5). There was a similar influx of B cells into the lungs; however, CD8 T cell numbers remained relatively low throughout. In the draining lymph nodes, the CD4 T and B cell populations expanded as early as two challenges and continued to expand through four challenges but then leveled off and remained elevated through eight challenges (Fig. 5), concurrently with the increase in serum IgE levels at four and eight challenges over those for unchallenged controls (see Fig. 9A).
FIG. 5.
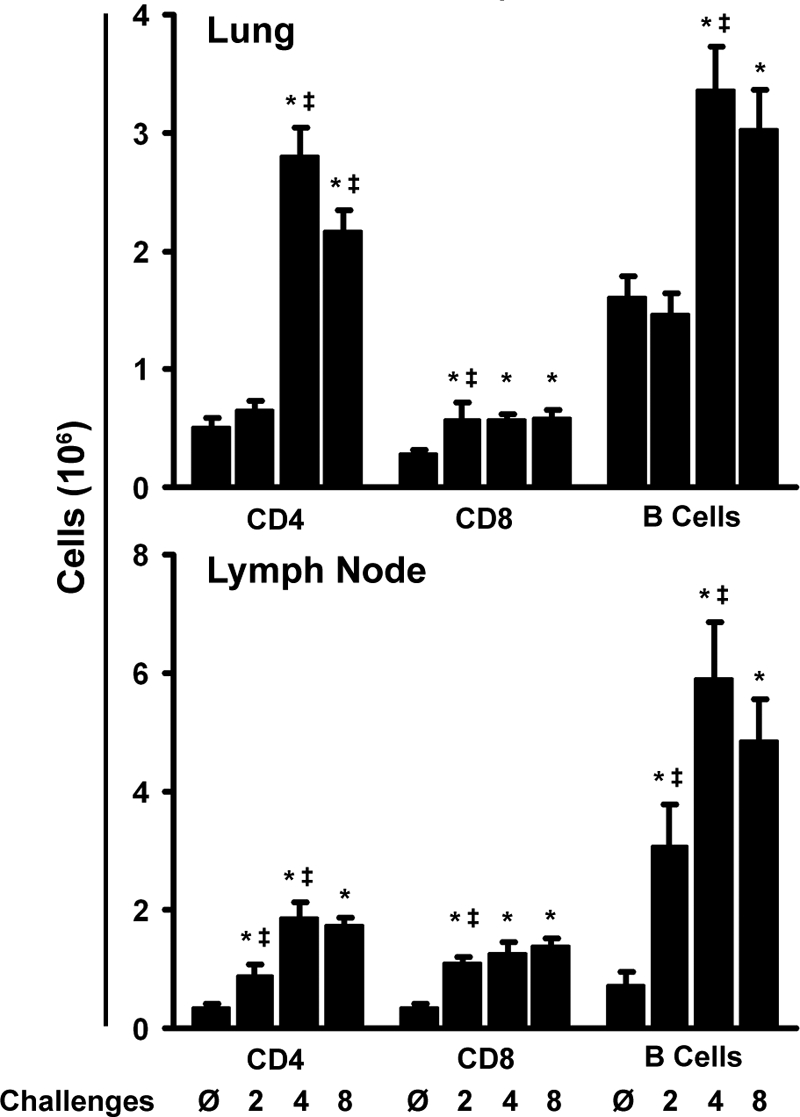
Expansion of the lymphocyte populations in both the lung and the mediastinal lymph node. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
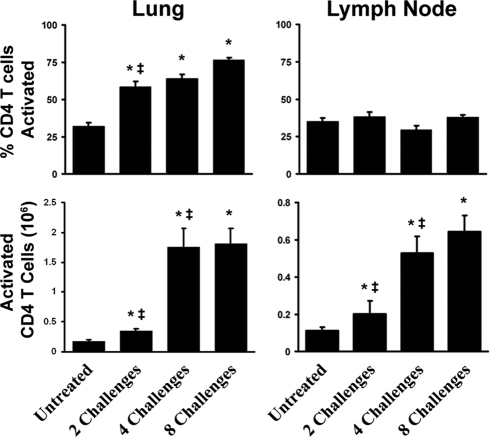
We hypothesized that differences in CD4 T cell activation might account for the development and waning of the pulmonary inflammatory response. Just two exposures to conidia resulted in an increase in the percentage of pulmonary CD4 T cells that were activated (CD44high CD69+), even though the number of CD4 T cells in the lungs did not increase significantly (Fig. 6). The proportion of activated CD4 T cells did not change significantly between two, four, and eight challenges (remaining high, approximately 70%). In the lymph node, the fraction of activated CD4 T cells was lower than that in the lungs and remained constant throughout the course of exposure, although the number of activated CD4 T cells increased coincidently with the increase in the total number of CD4 T cells in the lymph nodes (Fig. 6). The activation marker analysis supports the concept that an adaptive immune response begins to develop between two to four challenges and is sustained, despite the presence of less pulmonary inflammation, through eight exposures.
FIG. 6.
CD4 T cell activation in response to A. fumigatus conidia in the lung and lymph nodes. Lung and lymph node CD4 T cells were isolated via gating, and those that were CD44high CD69+ were counted as activated. Both the percentage and the total number of activated CD4 T cells in the lung and lymph nodes were calculated for each mouse and averaged for each time point. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
Polarization of the CD4 T cell response during repeated challenges with conidia.
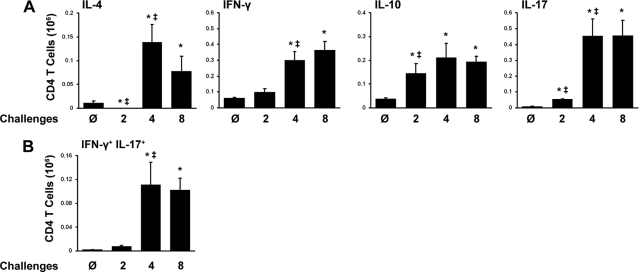
We next investigated whether changes in the polarization of the CD4 TH cell response accounted for the development of the pulmonary inflammatory response by four challenges and its subsequent waning by eight challenges. Using intracellular flow cytometry, we observed that after two challenges, the only notable change was an increase in the number of IL-10+ CD4 T cells (Fig. 7 A). By four challenges, there were marked increases in the numbers of IL-4+, IL-17+, and IFN-γ+ CD4 T cells. After eight challenges, the numbers of IL-4+, IL-17+, IFN-γ+, and IL-10+ T cells all remained elevated in the lungs. Repeated A. fumigatus exposure also resulted in the recruitment of IFN-γ+ IL-17+ CD4 T cells, although this was a minor population (Fig. 7B). These data demonstrate that repeated exposure to A. fumigatus conidia induces the codevelopment of TH1, TH2, and TH17 responses in the lungs by four exposures, that these responses are maintained through eight exposures, and that changes in CD4 T cell polarization do not account for the reduction in myeloid cell numbers later in the response.
FIG. 7.
Distinct CD4 cytokine profiles in the lung following each stage of the immune response to A. fumigatus. (A) Cells taken from the lung were stimulated for 6 h with PMA and ionomycin and were then stained with fluorescently labeled antibodies specific for CD45 and CD4. Following permeabilization, cells were stained for intracellular IFN-γ, IL-4, IL-10, and IL-17 expression. The mean total number of CD4 T cells expressing each cytokine is shown. (B) The mean number of CD4 T cells expressing multiple cytokines is shown. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
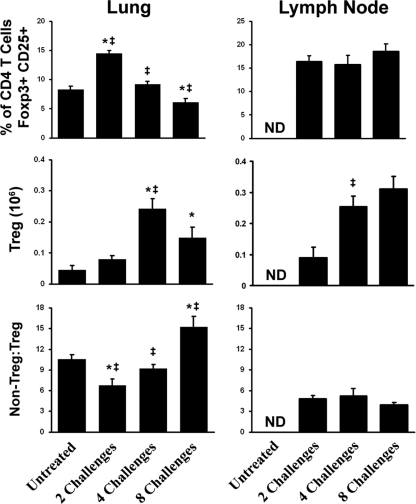
We next investigated whether an increase in the ratio of regulatory to effector CD4 T cells could account for the waning of the inflammatory response. In the draining lymph nodes, the percentage of regulatory T (Treg) cells within the pool of CD4 T cells was constant over the course of exposure to A. fumigatus conidia. In the lungs, the percentage of Treg (CD25+ Foxp3+) cells increased nearly 50% after two exposures, consistent with the observed increase in the number of IL-10+ CD4 T cells, suggesting a higher regulatory/nonregulatory ratio early in the response (Fig. 8). As the number of challenges increased, the total number of Treg cells also increased. However, the relative proportion of Treg cells dropped (i.e., an increase in the non-Treg/Treg cell ratio) concurrently with the development of the inflammatory response. Despite the presence of less inflammation, the ratio of non-Treg cells to Treg cells remained high at eight challenges, ruling out an expansion of the Treg population as a major factor in modulating the inflammatory response.
FIG. 8.
Regulatory CD4 T cells in the lung and lymph node during repeated A. fumigatus exposure. Cells taken from both the lung and the mesenchymal lymph node were stained for CD45, CD4, and CD25. Then, following permeabilization, cells were stained for intracellular Foxp3 expression. The non-Treg/Treg ratio was determined by comparing the percentage of CD4 T cells that were double positive for CD25 and Foxp3 to the percentage of those that were double negative. Each bar represents the mean for mice at a single time point, and error bars represent standard errors of the means. Numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure; ‡, P < 0.05 for comparison to the previous “challenge” time point.
Immune recall response to repeated pulmonary Aspergillus challenges.
In our final set of studies, we addressed whether the inflammatory response would progress or resolve after four challenges if there were no additional conidial challenges. Since the numbers of inflammatory cells in the bronchoalveolar lavage fluid were determined in these studies, they also provided quantitative data to address whether inflammation was similar in the airway and parenchymal pulmonary compartments. Both mice challenged four times and mice challenged eight times had significant leukocytic infiltrates in the airways (Fig. 9 B). However, the infiltrate in the mice challenged four times was dominated by eosinophils, while that in the mice challenged eight times was dominated by macrophages (Fig. 9C). These results are similar to those seen for the whole-lung digests except that the diminished eosinophilia was more pronounced in the airways. If the mice were challenged four times and then left to rest for 4 weeks, the inflammatory response resolved to near baseline, except for the number of lymphocytes, which remained slightly elevated. We also elicited a recall response during the resolution phase by challenging a group of mice previously exposed four times after 2 weeks of resolution (Fig. 9B and C). The recall response was similar to the peak allergic response noted after 4 weeks in terms of airway eosinophilia (Fig. 9C), suggesting that continued exposure throughout 8 weeks, in contrast to intermittent exposure, resulted in the attenuation of the allergic response in spite of the presence of primed cells capable of promoting a vigorous hypersensitivity response.
FIG. 9.
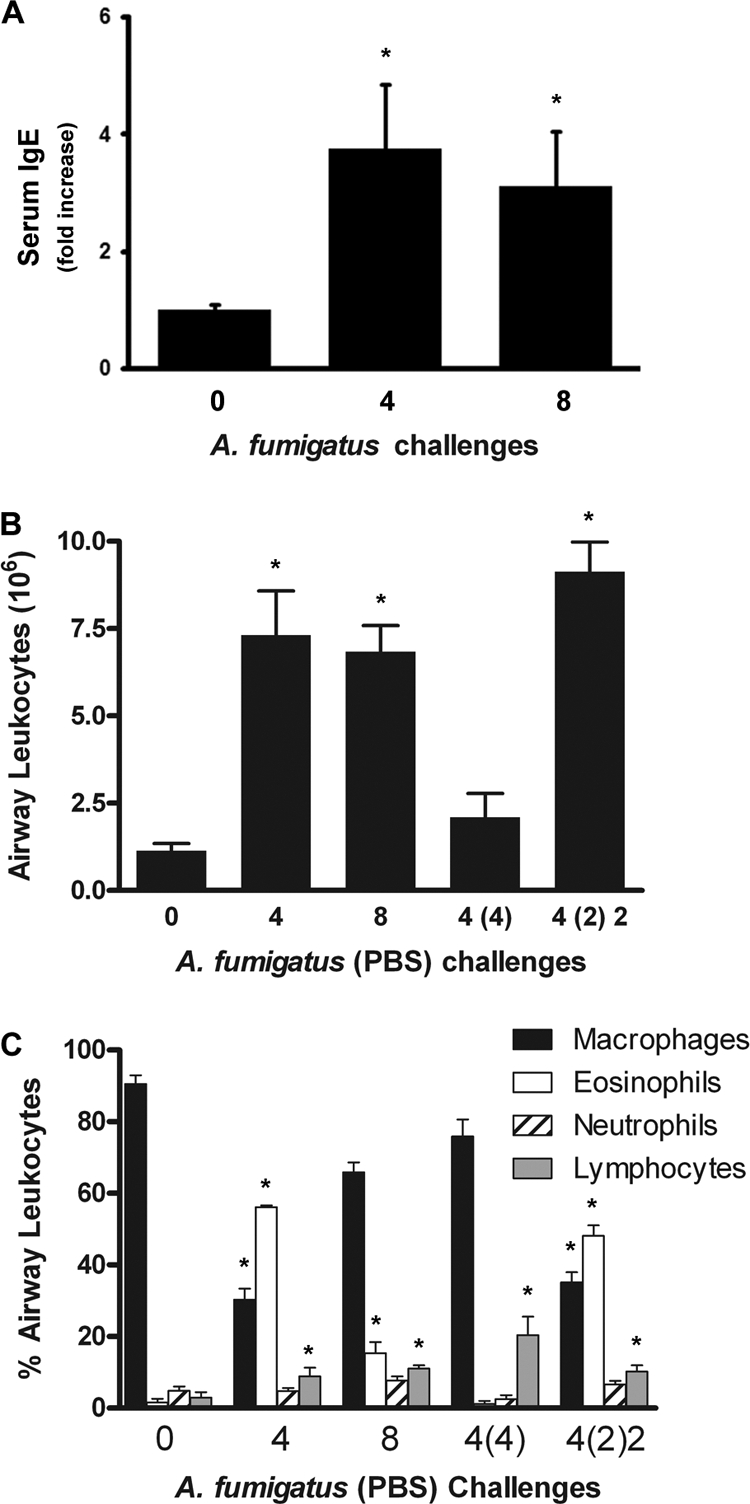
Characterization of the inflammatory response to prolonged continuous or intermittent A. fumigatus inhalational exposure. (A) Serum IgE was measured for each mouse in two independent experiments. Bars represent mean relative serum IgE levels in mice challenged four and eight times. *, P < 0.05 versus no challenge. (B) After mice were treated with variable numbers of weekly exposures to A. fumigatus (for which the PBS vehicle was sometimes substituted), airway leukocytes were recovered by BAL and were counted under a light microscope. The numbers of exposures to the PBS vehicle are given in parentheses, and the order of the numbers indicates the sequence of the exposures [e.g., “4 (2) 2” indicates 4 weekly A. fumigatus exposures followed by 2 weekly PBS exposures, which, in turn, were followed by 2 weekly A. fumigatus exposures]. Each bar represents the mean of data compiled from two independent experiments, except for “0” and “4 (4),” for each of which data were from one experiment (≥4 mice per group per experiment). (C) After mice were treated with variable numbers of weekly exposures to A. fumigatus (for which control exposures to PBS vehicle were sometimes substituted), flow cytometric leukocyte differential analyses were performed on BAL fluid cells. Data are means ± standard errors of the means; numbers of animals and replicates are provided in Materials and Methods. *, P < 0.05 for comparison to no exposure.
DISCUSSION
We have demonstrated that repeated exposure of an immunocompetent host to A. fumigatus conidia does not result in invasive aspergillosis or fatal disease but does result in the development of chronic pulmonary inflammation. Repeated exposure to A. fumigatus conidia induces the coevolution of TH1, TH2, and TH17 responses in the lungs by four exposures, and these responses are maintained through eight exposures. We observed striking increases in the numbers of IFN-γ- and IL-17-producing CD4 T cells between two and four challenges. IFN-γ IL-17 double-positive T cells are associated with inflammation in several models of autoimmunity and graft rejection (11, 15, 45, 48), and their presence suggests a role for IL-17 in maintaining the inflammatory response to Aspergillus. However, by eight exposures, myeloid cell recruitment is diminished, including that of airway eosinophils, and changes in CD4 T cell polarization or Treg numbers do not account for the waning inflammatory response with continued exposure. The total time of exposure is not a factor, because if, during the same 8-week period, exposures are stopped for 2 weeks, then inflammation resolves and a robust recall response occurs.
Repeated exposure to viable A. fumigatus conidia induced both CD4+ TH2 and TH1 cells. Several previous reports have demonstrated that a single exposure to A. fumigatus conidia leads primarily to the priming of CD4+ TH1 cells (9, 31) and that exposure to A. fumigatus hyphae or antigen extracts leads primarily to the priming of CD4+ TH2 cells (9, 36). Systemic priming and airway exposure to A. fumigatus antigens establish a TH2-mediated allergic airway disease (AAD) that can subsequently be augmented by exposure to conidia, indicating that previously expanded CD4+ TH2 cells will respond to conidia (6). Differences in the CD4+ T cell response to conidia may depend on the dosing and frequency used in the various studies. In the studies mentioned above that resulted primarily in CD4+ TH1 priming, 5 to 100 times the number of conidia used in our study were administered (9, 31). The differential effect of dose and frequency of exposure to A. fumigatus conidia has clinical relevance, because TH1-associated hypersensitivity pneumonitis results from very high dose exposure, generally in an occupational setting (1, 20, 57, 65).
It is interesting that in spite of the presence of TH2 and TH1 cells, the CD4+ T cell-dependent hypersensitivity disease that developed after repeated exposure to conidia had many features of AAD, a TH2 disease. TH1 cells can abrogate TH2 cell-mediated AAD (35); however, TH1 responses to viral infections are known to exacerbate asthma symptoms (4, 42). Furthermore, it has been demonstrated in mice that a TH1-promoting rhinovirus infection can augment the TH2 response in AAD and that a preexisting TH2 response augmented the TH1 response to rhinovirus infection (3). The data generated in the present study support the emerging understanding that allergic diseases are not simply the result of an imbalance in counterregulatory TH1 and TH2 responses (73). Rather, current evidence suggests that both TH2 and TH1 responses are inflammatory and that both are subject to control by myriad regulatory mechanisms, such as those mediated by dedicated regulatory T cells.
Viable conidia could be detected in lung homogenates 24 h after each challenge, providing a sustained source of antigen or inflammatory stimuli during the evolving CD4 T cell response despite a lack of hyphal formation. Neutrophils, a major cell type during the early phases of the response, are well known to play a central role in the host defense against Aspergillus (5, 29, 43, 44, 64) and were seen at all stages of the response, particularly at early stages prior to the engagement of the adaptive response. Levels of lung eosinophils, serum IgE, and IL-4+ CD4 T cells, all hallmarks of a TH2 response, were elevated at four and eight exposures. The chronic inflammatory response included the recruitment of antigen-presenting cells, such as basophils, dendritic cells, macrophages, CD4 T cells, and B cells, all of which are capable of driving TH2, TH1, TH17, or Treg responses.
Expansion of the regulatory T cell population is important for limiting disease, because Treg cells ultimately aid in the clearance of fungi by limiting TH1 inflammation (17) or dampening TH2 hypersensitivity reactions (8, 16). Exposure to A. fumigatus conidia or fungal glucan has been reported to induce regulatory responses via Toll-like receptor 2 (TLR2) and dectin-1 (19, 46). However, in aspergillosis studies using high doses of conidia (108 spores per mouse), a strong TH1 response occurs 1 week after the initial exposure (32), rather than the Treg response that we have reported here during repeated exposure to A. fumigatus conidia, and there is no subsequent TH2 reaction.
There are several possible explanations, then, for the transition from tolerance to inflammation that we observed during repeated exposure to A. fumigatus conidia. One possibility is that the accumulation of innate cells eventually overwhelms the regulatory response. Chitin—which is generated following conidial germination—can drive an accumulation of innate cells, stimulating inflammation that ultimately leads to an allergic response (61). We describe here that viable conidia are still present 24 h after challenge and that germinating fungi can be detected in the lungs even when low concentrations of conidia are used. Thus, it is possible that chitin production results in an innate response that outpaces the tolerance response and eventually results in the engagement of TH2 adaptive immunity. Another possibility is that suppression of the TH1 response by Treg cells facilitates the development of the TH2 response. The TH1 response can be responsible for tissue damage (69), so it is possible that the immune system dampens the TH1 response to a dose of conidia that can easily be cleared by the innate immune system. Such TH1 control could, in turn, allow the expansion of a TH2 response, since TH1 and TH2 responses are often reciprocally regulated. The latter hypothesis is supported by our studies demonstrating increased numbers of IL-17-producing CD4 T cells during the response, because the TH17 response can be negatively regulated by IFN-γ (21).
Like the development of the TH2 response, the TH17 adaptive immune response during repeated exposure to A. fumigatus conidia may result from a combination of factors. The initial TH17 adaptive immune response may be triggered simply by the presence of conidia: like regulatory T cells, TH17 cells are promoted by fungal cell wall components via interaction with dectin-1 (23, 71). In addition, Treg cells can facilitate the differentiation of TH17 cells (37, 74), and regulatory T cells themselves can be converted to TH17 cells (59), a process that is facilitated by DC (55). Thus, the initial TH17 response seen following two challenges may be driven by the conversion of regulatory T cells to TH17 cells. This process would be aided by the dampened TH1 response, since the development of TH17 cells is inhibited by TH1 and TH2 cytokines. On the other hand, CD4 T cells already committed to the TH17 lineage are resistant to TH1- or TH2-mediated suppression (26), which could explain why we observed that IL-17-producing CD4 T cells continue to expand even during the TH2-driven inflammatory response that follows four challenges. The TH17 T cells in our study are also likely playing an active role in shaping the reaction to conidia, since TH17 regulates TH1 differentiation (49, 75) and dampens the production of indoleamine-2,3-dioxygenase (IDO). While IDO can inhibit TH1 activity, it also suppresses the cytotoxic potential of neutrophils. This would be consistent with the persistent number of viable conidia seen in the lungs even after eight challenges, because TH17-induced IDO reduction and subsequent neutrophil inhibition have been shown to inhibit fungal clearance (17).
We observed that TH17 cell levels increased during repeated exposures to A. fumigatus conidia, while TH2 cell levels remained steady or decreased. Previous studies have indicated that TH2 airway inflammation is enhanced by TH17 cells (72). However, in the context of repeated A. fumigatus exposures, it may be that the TH17 response, rather than suppressing the TH2 response, becomes the dominant adaptive response through attrition. Repeated exposure to an antigen leads to restimulation-induced death of CD4 T cells, but it has been reported in autoimmune disease models that TH17 cells are resistant to this form of apoptosis (30). To our knowledge, there have been no reports demonstrating that the TH17 response directly regulates TH2 responses, so the presence of TH17 cells does not explain the dampening of the inflammatory response between four and eight challenges. However, multiple reports have shown that IL-17 both exacerbates and attenuates inflammatory TH2 responses and that the function of IL-17 is timing dependent (28, 68).
Thus, the emergent TH17 response may arise as an imperfect immune compromise when an individual is dealing with repeated exposure to low levels of conidia. The TH17 response hinders TH1-mediated clearance of fungi and can promote severe neutrophil-mediated tissue inflammatory pathology associated with infection (17), but on its own the TH17 response has antifungal properties (22, 25, 38, 39). A persistent TH1 response would result in damage to the tissue of the lung, but its suppression facilitates an emergent TH2 response that does little to aid in the clearance of nonhyphal A. fumigatus. Therefore, it is likely that repeated pulmonary exposure to A. fumigatus conidia eventually leads to immune homeostasis and the induction of non-T cell regulatory pathways that result in the least possible tissue damage while still controlling conidial germination (12, 13).
Acknowledgments
We thank the members of the Young lab for feedback regarding data. We also thank Gwo-Hsiao Chen at the University of Michigan Veterans Affairs Healthcare System for aid in the identification of cellular populations, Nicole Falkowski for support in laboratory operations and submission of the manuscript, and Vincent Young, John Erb-Downward, and John Kao for helpful discussions on this project.
This work was supported in part by grants R01AI064479 (to G.B.H.), R21AI083473-01 (to G.B.H.), and T32AI007413 (to B.J.M.) from the National Institute of Allergy and Infectious Diseases and by grant R01HL085083 (to E.S.W.) from the National Heart, Lung, and Blood Institute, as well as by funding from the Drews Sarcoidosis Research Fund at the University of Michigan.
We have no conflicting financial interests in this project.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 1 November 2010.
REFERENCES
- 1.Aebischer, C. C., U. Frey, and M. H. Schoni. 2002. Hypersensitivity pneumonitis in a five-year-old boy: an unusual antigen source. Pediatr. Pulmonol. 33:77-78. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong-James, D. P., S. A. Turnbull, I. Teo, J. Stark, N. J. Rogers, T. R. Rogers, E. Bignell, and K. Haynes. 2009. Impaired interferon-gamma responses, increased interleukin-17 expression, and a tumor necrosis factor-alpha transcriptional program in invasive aspergillosis. J. Infect. Dis. 200:1341-1351. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, N. W., R. P. Walton, M. R. Edwards, J. Aniscenko, G. Caramori, J. Zhu, N. Glanville, K. J. Choy, P. Jourdan, J. Burnet, T. J. Tuthill, M. S. Pedrick, M. J. Hurle, C. Plumpton, N. A. Sharp, J. N. Bussell, D. M. Swallow, J. Schwarze, B. Guy, J. W. Almond, P. K. Jeffery, C. M. Lloyd, A. Papi, R. A. Killington, D. J. Rowlands, E. D. Blair, N. J. Clarke, and S. L. Johnston. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 14:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beasley, R., E. D. Coleman, Y. Hermon, P. E. Holst, T. V. O'Donnell, and M. Tobias. 1988. Viral respiratory tract infection and exacerbations of asthma in adult patients. Thorax 43:679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellocchio, S., S. Moretti, K. Perruccio, F. Fallarino, S. Bozza, C. Montagnoli, P. Mosci, G. B. Lipford, L. Pitzurra, and L. Romani. 2004. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 173:7406-7415. [DOI] [PubMed] [Google Scholar]
- 6.Blease, K., B. Mehrad, T. J. Standiford, N. W. Lukacs, J. Gosling, L. Boring, I. F. Charo, S. L. Kunkel, and C. M. Hogaboam. 2000. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J. Immunol. 165:2603-2611. [DOI] [PubMed] [Google Scholar]
- 7.Bonnett, C. R., E. J. Cornish, A. G. Harmsen, and J. B. Burritt. 2006. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect. Immun. 74:6528-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudousquié, C., C. Pellaton, N. Barbier, and F. Spertini. 2009. CD4+CD25+ T cell depletion impairs tolerance induction in a murine model of asthma. Clin. Exp. Allergy 39:1415-1426. [DOI] [PubMed] [Google Scholar]
- 9.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362-1371. [DOI] [PubMed] [Google Scholar]
- 10.Brieland, J. K., C. Jackson, F. Menzel, D. Loebenberg, A. Cacciapuoti, J. Halpern, S. Hurst, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2001. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrell, B. E., K. Csencsits, G. Lu, S. Grabauskiene, and D. K. Bishop. 2008. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J. Immunol. 181:3906-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, A., and L. A. Pirofski. 2003. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 1:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall, A., and L. A. Pirofski. 2000. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 68:6511-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cenci, E., A. Mencacci, C. Fe d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y., C. L. Langrish, B. McKenzie, B. Joyce-Shaikh, J. S. Stumhofer, T. McClanahan, W. Blumenschein, T. Churakovsa, J. Low, L. Presta, C. A. Hunter, R. A. Kastelein, and D. J. Cua. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curotto de Lafaille, M. A., N. Kutchukhidze, S. Shen, Y. Ding, H. Yee, and J. J. Lafaille. 2008. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29:114-126. [DOI] [PubMed] [Google Scholar]
- 17.De Luca, A., C. Montagnoli, T. Zelante, P. Bonifazi, S. Bozza, S. Moretti, C. D'Angelo, C. Vacca, L. Boon, F. Bistoni, P. Puccetti, F. Fallarino, and L. Romani. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 179:5999-6008. [DOI] [PubMed] [Google Scholar]
- 18.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 19.Dillon, S., S. Agrawal, K. Banerjee, J. Letterio, T. L. Denning, K. Oswald-Richter, D. J. Kasprowicz, K. Kellar, J. Pare, T. van Dyke, S. Ziegler, D. Unutmaz, and B. Pulendran. 2006. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116:916-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enríquez-Matas, A., S. Quirce, E. Hernandez, A. Vereda, J. Carnes, and J. Sastre. 2007. Hypersensitivity pneumonitis caused by domestic exposure to molds. J. Investig. Allergol. Clin. Immunol. 17:126-127. [PubMed] [Google Scholar]
- 21.Feng, G., W. Gao, T. B. Strom, M. Oukka, R. S. Francis, K. J. Wood, and A. Bushell. 2008. Exogenous IFN-γ ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur. J. Immunol. 38:2512-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gafa, V., R. Lande, M. C. Gagliardi, M. Severa, E. Giacomini, M. E. Remoli, R. Nisini, C. Ramoni, P. Di Francesco, D. Aldebert, R. Grillot, and E. M. Coccia. 2006. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect. Immun. 74:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerosa, F., B. Baldani-Guerra, L. A. Lyakh, G. Batoni, S. Esin, R. T. Winkler-Pickett, M. R. Consolaro, M. De Marchi, D. Giachino, A. Robbiano, M. Astegiano, A. Sambataro, R. A. Kastelein, G. Carra, and G. Trinchieri. 2008. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 205:1447-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodley, J. M., Y. M. Clayton, and R. J. Hay. 1994. Environmental sampling for aspergilli during building construction on a hospital site. J. Hosp. Infect. 26:27-35. [DOI] [PubMed] [Google Scholar]
- 25.Happel, K. I., P. J. Dubin, M. Zheng, N. Ghilardi, C. Lockhart, L. J. Quinton, A. R. Odden, J. E. Shellito, G. J. Bagby, S. Nelson, and J. K. Kolls. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 27.Hebart, H., C. Bollinger, P. Fisch, J. Sarfati, C. Meisner, M. Baur, J. Loeffler, M. Monod, J. P. Latgé, and H. Einsele. 2002. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 100:4521-4528. [DOI] [PubMed] [Google Scholar]
- 28.Hellings, P. W., A. Kasran, Z. Liu, P. Vandekerckhove, A. Wuyts, L. Overbergh, C. Mathieu, and J. L. Ceuppens. 2003. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28:42-50. [DOI] [PubMed] [Google Scholar]
- 29.Hohl, T. M., and M. Feldmesser. 2007. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot. Cell 6:1953-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohl, T. M., A. Rivera, L. Lipuma, A. Gallegos, C. Shi, M. Mack, and E. G. Pamer. 2009. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6:470-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohl, T. M., A. Rivera, and E. G. Pamer. 2006. Immunity to fungi. Curr. Opin. Immunol. 18:465-472. [DOI] [PubMed] [Google Scholar]
- 32.Hohl, T. M., H. L. Van Epps, A. Rivera, L. A. Morgan, P. L. Chen, M. Feldmesser, and E. G. Pamer. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim-Granet, O., G. Jouvion, T. M. Hohl, S. Droin-Bergere, F. Philippart, O. Y. Kim, M. Adib-Conquy, R. Schwendener, J. M. Cavaillon, and M. Brock. 2010. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol. 10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibrahim-Granet, O., B. Philippe, H. Boleti, E. Boisvieux-Ulrich, D. Grenet, M. Stern, and J. P. Latgé. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irifune, K., A. Yokoyama, K. Sakai, A. Watanabe, H. Katayama, H. Ohnishi, H. Hamada, M. Nakajima, N. Kohno, and J. Higaki. 2005. Adoptive transfer of T-helper cell type 1 clones attenuates an asthmatic phenotype in mice. Eur. Respir. J. 25:653-659. [DOI] [PubMed] [Google Scholar]
- 36.Kheradmand, F., A. Kiss, J. Xu, S. H. Lee, P. E. Kolattukudy, and D. B. Corry. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169:5904-5911. [DOI] [PubMed] [Google Scholar]
- 37.Kitani, A., and L. Xu. 2008. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 1(Suppl. 1):S43-S46. [DOI] [PubMed] [Google Scholar]
- 38.Kleinschek, M. A., U. Muller, S. J. Brodie, W. Stenzel, G. Kohler, W. M. Blumenschein, R. K. Straubinger, T. McClanahan, R. A. Kastelein, and G. Alber. 2006. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J. Immunol. 176:1098-1106. [DOI] [PubMed] [Google Scholar]
- 39.Kleinschek, M. A., A. M. Owyang, B. Joyce-Shaikh, C. L. Langrish, Y. Chen, D. M. Gorman, W. M. Blumenschein, T. McClanahan, F. Brombacher, S. D. Hurst, R. A. Kastelein, and D. J. Cua. 2007. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204:161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurup, V. P., S. Mauze, H. Choi, B. W. Seymour, and R. L. Coffman. 1992. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J. Immunol. 148:3783-3788. [PubMed] [Google Scholar]
- 41.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallia, P., and S. L. Johnston. 2006. How viral infections cause exacerbation of airway diseases. Chest 130:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 44.Mircescu, M. M., L. Lipuma, N. van Rooijen, E. G. Pamer, and T. M. Hohl. 2009. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J. Infect. Dis. 200:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momcilović, M., Z. Miljkovic, D. Popadic, D. Miljkovic, and M. Mostarica-Stojkovic. 2008. Kinetics of IFN-γ and IL-17 expression and production in active experimental autoimmune encephalomyelitis in Dark Agouti rats. Neurosci. Lett. 447:148-152. [DOI] [PubMed] [Google Scholar]
- 46.Montagnoli, C., F. Fallarino, R. Gaziano, S. Bozza, S. Bellocchio, T. Zelante, W. P. Kurup, L. Pitzurra, P. Puccetti, and L. Romani. 2006. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J. Immunol. 176:1712-1723. [DOI] [PubMed] [Google Scholar]
- 47.Moss, R. B. 2005. Pathophysiology and immunology of allergic bronchopulmonary aspergillosis. Med. Mycol. 43(Suppl. 1):S203-S206. [DOI] [PubMed] [Google Scholar]
- 48.Murphy, C. A., C. L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R. A. Kastelein, J. D. Sedgwick, and D. J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakae, S., Y. Iwakura, H. Suto, and S. J. Galli. 2007. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J. Leukoc. Biol. 81:1258-1268. [DOI] [PubMed] [Google Scholar]
- 50.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noverr, M. C., N. R. Falkowski, R. A. McDonald, A. N. McKenzie, and G. B. Huffnagle. 2005. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noverr, M. C., R. M. Noggle, G. B. Toews, and G. B. Huffnagle. 2004. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72:4996-5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obata, K., K. Mukai, Y. Tsujimura, K. Ishiwata, Y. Kawano, Y. Minegishi, N. Watanabe, and H. Karasuyama. 2007. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110:913-920. [DOI] [PubMed] [Google Scholar]
- 54.Osherov, N., and G. S. May. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199:153-160. [DOI] [PubMed] [Google Scholar]
- 55.Osorio, F., S. LeibundGut-Landmann, M. Lochner, K. Lahl, T. Sparwasser, G. Eberl, and C. Reis e Sousa. 2008. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 38:3274-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park, S. J., and B. Mehrad. 2009. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 22:535-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel, A. M., J. H. Ryu, and C. E. Reed. 2001. Hypersensitivity pneumonitis: current concepts and future questions. J. Allergy Clin. Immunol. 108:661-670. [DOI] [PubMed] [Google Scholar]
- 58.Phadke, A. P., and B. Mehrad. 2005. Cytokines in host defense against Aspergillus: recent advances. Med. Mycol. 43(Suppl. 1):S173-S176. [DOI] [PubMed] [Google Scholar]
- 59.Radhakrishnan, S., R. Cabrera, E. L. Schenk, P. Nava-Parada, M. P. Bell, V. P. Van Keulen, R. J. Marler, S. J. Felts, and L. R. Pease. 2008. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J. Immunol. 181:3137-3147. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Rapaka, R. R., and J. K. Kolls. 2009. Pathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis: current understanding and future directions. Med. Mycol. 47(Suppl. 1):S331-S337. [DOI] [PubMed] [Google Scholar]
- 61.Reese, T. A., H. E. Liang, A. M. Tager, A. D. Luster, N. Van Rooijen, D. Voehringer, and R. M. Locksley. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivera, A., G. Ro, H. L. Van Epps, T. Simpson, I. Leiner, D. B. Sant'Angelo, and E. G. Pamer. 2006. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 25:665-675. [DOI] [PubMed] [Google Scholar]
- 63.Rivera, A., H. L. Van Epps, T. M. Hohl, G. Rizzuto, and E. G. Pamer. 2005. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect. Immun. 73:7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez, T. E., N. R. Falkowski, J. R. Harkema, and G. B. Huffnagle. 2007. Role of neutrophils in preventing and resolving acute fungal sinusitis. Infect. Immun. 75:5663-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-Hornillos, F. J., M. De Barrio Fernandez, P. T. Molina, I. S. Marcen, G. D. Fernandez, M. R. Sotes, and M. L. de Ocariz. 2007. Occupational asthma due to esparto hypersensitivity in a building worker. Allergy Asthma Proc. 28:571-573. [DOI] [PubMed] [Google Scholar]
- 66.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Invest. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaffner, A., H. Douglas, A. I. Braude, and C. E. Davis. 1983. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect. Immun. 42:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnyder-Candrian, S., D. Togbe, I. Couillin, I. Mercier, F. Brombacher, V. Quesniaux, F. Fossiez, B. Ryffel, and B. Schnyder. 2006. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203:2715-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sitkovsky, M. V., D. Lukashev, S. Apasov, H. Kojima, M. Koshiba, C. Caldwell, A. Ohta, and M. Thiel. 2004. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22:657-682. [DOI] [PubMed] [Google Scholar]
- 70.Steele, C., R. R. Rapaka, A. Metz, S. M. Pop, D. L. Williams, S. Gordon, J. K. Kolls, and G. D. Brown. 2005. The β-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Beelen, A. J., Z. Zelinkova, E. W. Taanman-Kueter, F. J. Muller, D. W. Hommes, S. A. Zaat, M. L. Kapsenberg, and E. C. de Jong. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660-669. [DOI] [PubMed] [Google Scholar]
- 72.Wakashin, H., K. Hirose, Y. Maezawa, S. Kagami, A. Suto, N. Watanabe, Y. Saito, M. Hatano, T. Tokuhisa, Y. Iwakura, P. Puccetti, I. Iwamoto, and H. Nakajima. 2008. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 178:1023-1032. [DOI] [PubMed] [Google Scholar]
- 73.Wills-Karp, M., J. Santeliz, and C. L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69-75. [DOI] [PubMed] [Google Scholar]
- 74.Xu, L., A. Kitani, I. Fuss, and W. Strober. 2007. Regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol. 178:6725-6729. [DOI] [PubMed] [Google Scholar]
- 75.Yi, T., D. Zhao, C. L. Lin, C. Zhang, Y. Chen, I. Todorov, T. LeBon, F. Kandeel, S. Forman, and D. Zeng. 2008. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood 112:2101-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelante, T., A. De Luca, P. Bonifazi, C. Montagnoli, S. Bozza, S. Moretti, M. L. Belladonna, C. Vacca, C. Conte, P. Mosci, F. Bistoni, P. Puccetti, R. A. Kastelein, M. Kopf, and L. Romani. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695-2706. [DOI] [PubMed] [Google Scholar]