Abstract
A combinatorial immunoglobulin gene library was constructed from lymphocytes in peripheral blood of a patient with toxoplasmosis and screened for production of human monoclonal antibody Fab fragments to recombinant surface antigen 1 (SAG1) of Toxoplasma gondii. Two Fab clones, Tox203 and Tox1403, which consisted of a common heavy chain and different light chains, showed positive staining on the entire surface of tachyzoites in confocal microscopy. Sequence analysis of the heavy-chain gene revealed that the closest germ line V segments were VH3-23. The germ line D segment was D1-7, and the closest germ line J segment was JH4. In the light-chain genes, the closest germ line V segment was Vκ1-17 with the Jκ1 or Jκ4 segments. The dissociation constants of these Fab fragments with recombinant SAG1 were 3.09 × 10−9 M for Tox203 and 2.01 × 10−8 M for Tox1403, indicating that the affinity of Tox203 was 7 times higher than that of Tox1403. Preincubation of T. gondii tachyzoites with Tox203 significantly inhibited their attachment to cultured MDBK cells. Passive immunization of mice with Tox203 also significantly reduced mortality after challenge with T. gondii tachyzoites. This is the first report of bacterial expression of human monoclonal antibody Fab fragments to SAG1 of T. gondii. These results also demonstrate that human Fab fragments to SAG1 might be applicable for immunoprophylaxis of toxoplasmosis.
Toxoplasma gondii is an obligate intracellular parasite in the phylum Apicomplexa and infects a variety of warm-blooded domestic and wild animals worldwide. It is an important food-borne parasite transmitted primarily from animals to humans through meat, as well as through oocysts shed by cats into the environment (25). Infection in humans is usually asymptomatic in immunocompetent hosts. However, primary infection during pregnancy can result in severe neonatal malformations and ocular complications in the fetus. In immunocompromised hosts such as patients with human immunodeficiency virus/AIDS, reactivation of the latent infection results in symptomatic diseases such as toxoplasmic encephalitis (14, 22). Transmission of T. gondii by organ transplantation from a seropositive donor to a seronegative recipient is also an important potential cause of disease in heart, heart-lung, kidney, liver, and liver-pancreas transplant patients (25).
The surface of T. gondii is the first component to contact the host cells. The T. gondii surface is coated by closely related antigens that belong to the surface antigen 1 (SAG1)-related sequences (SRS) superfamily (13, 16). SAG1 is the most abundant and immunogenic of these antigens and is important for the process of invasion. Treatment of T. gondii with mouse monoclonal or rabbit polyclonal antibodies to SAG1 inhibits parasite attachment to host cells (24). Fab fragments derived from a mouse monoclonal antibody also showed dose-dependent inhibition of parasite attachment (23). Therefore, human monoclonal antibodies to SAG1 may be applicable for prevention of transmission and reactivation of T. gondii in immunocompromised hosts.
Hybridoma technology has been relatively unsuccessful for generation of human monoclonal antibodies. However, several methods for preparation of human monoclonal antibodies have been developed through recent advances in molecular biology (3, 5, 36). Here, we report the production of neutralizing human monoclonal antibody Fab fragments to SAG1. We also evaluated the protective effect of the Fab fragments by passive immunization in experimental T. gondii-infected mice.
MATERIALS AND METHODS
Parasites.
The RH strain of T. gondii was maintained by intraperitoneal passages in BALB/c or ICR mice. Briefly, 102 to 103 tachyzoites in 500 μl of phosphate-buffered saline (PBS) were intraperitoneally (i.p.) injected into each mouse. Tachyzoites were obtained from peritoneal exudates of the mice 4 to 7 days after injection. The exudates were diluted with PBS and forcibly extruded through a 27-gauge needle twice to release tachyzoites from host cells. The exudates were then passed twice through a Nuclepore polycarbonate membrane filter (8.0-μm pore size; Costar Corp., Cambridge, MA) to separate parasites from host cell debris and were washed twice with PBS. The harvested tachyzoites were used for further experiments within 2 h. The parasites were also maintained in vitro in HeLa cells cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum at 37°C with 5% CO2.
Preparation of recombinant SAG1.
Total RNA from T. gondii tachyzoites was isolated by using a RNeasy Plus minikit (Qiagen GmbH, Hilden, Germany). The nucleotide sequence encoding amino acids 61 to 289 of SAG1 was amplified from the RNA by using a GeneAmp RNA PCR kit (Perkin-Elmer Cetus, Norwalk, CT). According to the sag1 sequence (GenBank accession no. AAO61460), sense (5′-CCC ATA TGT TCA CTC TCA AGT GCC CT-3′) and antisense (5′-CCC TCG AGT TAC CCT GCA GCC CCG GCA AA-3′) primers were designed with inclusion of NdeI and XhoI restriction sites, respectively. Thirty cycles of PCR were performed as follows: denaturation at 94°C for 15 s (120 s in cycle 1), annealing at 55°C for 30 s, and polymerization at 72°C for 60 s (180 s in cycle 30). The amplified DNA fragment was digested by NdeI and XhoI and ligated with pET19b vector (Novagen, Madison, WI). The plasmid containing the correct sequence was introduced into Escherichia coli BL21 Star (DE3)/pLysS cells (Invitrogen, Carlsbad, CA). The bacteria were grown in Luria broth containing 100 μg of ampicillin/ml and 34 μg of chloramphenicol/ml. When the optical density at 600 nm reached 0.6, the expression of recombinant SAG1 with a histidine tag was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 30°C for 4 h. Recombinant SAG1 proteins accumulated as inclusion bodies in E. coli and were solubilized by using a protein refolding kit (Novagen) and purified by affinity chromatography using His-Bind resin (Novagen) according to the manufacturer's recommendations.
Construction of an immunoglobulin gene library.
Peripheral blood (15 ml) was obtained from an immunocompetent adult man with acute acquired toxoplasmosis. Lymphocytes were separated from the blood by density-gradient centrifugation using Ficoll-Paque (Pharmacia, Uppsala, Sweden). Construction of a combinatorial immunoglobulin gene library from the lymphocytes was performed as previously described (32). Briefly, total RNA was purified from lymphocytes and used to synthesize cDNA. Genes encoding κ and λ light chains and the Fd regions of γ and μ heavy chains were amplified by 30 cycles of PCR. The light-chain genes were first ligated with an expression vector, pFab1-His2, and then introduced into E. coli JM109 cells. The vector with inserts was then ligated with the Fd heavy-chain genes and introduced into E. coli cells.
Screening of clones producing anti-T. gondii SAG1 antibodies.
Screening of positive clones producing anti-T. gondii SAG1 antibodies was performed by colony blotting as previously described (9). Approximately 5 × 103 colonies per 90-mm plate were grown on Luria broth agar containing 50 μg of ampicillin/ml at 37°C. Colonies were transferred to nitrocellulose filters and then incubated on the surface of fresh plates containing 1 mM IPTG at 30°C for 6 h. The filters were treated with chloroform vapor and then incubated with lysis buffer (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM MgCl2, 1.5% bovine serum albumin, 1 μg of DNase/ml, and 40 μg of lysozyme/ml) overnight. After being washed with PBS containing 0.05% Tween 20 (PBST), the filters were subjected to blocking with PBS containing 3% skim milk for 1 h. Each filter was incubated with 400 μg of recombinant SAG1 and then with plasma from the patient. Positive signals on the filters were detected with a horseradish peroxidase (HRP)-conjugated goat antibody to human whole IgG (ICN Pharmaceuticals, Aurora, OH) and a Konica HRP-1000 immunostaining kit (Konica Co., Tokyo, Japan). Positive clones were identified in the original plates and then cultured in 1.5 ml of super broth medium (30-g tryptone, 20-g yeast extract, and 10-g 4-morpholinepropanesulfonic acid per liter [pH 7.0]) containing 50 μg of ampicillin/ml. After treatment with 0.1 mM IPTG in a 30°C shaker for 12 h, the bacteria were pelleted by centrifugation, resuspended in 100 μl of PBS containing 1 mM phenylmethylsulfonyl fluoride, and then sonicated. Lysates were centrifuged at 12,000 × g for 10 min, and the supernatants were subjected to a second screening by an enzyme-linked immunosorbent assay (ELISA). The light-chain gene from a positive clone confirmed by ELISA and subsequent immunofluorescence analysis was replaced with light-chain genes from the library. The light-chain-shuffled plasmid with the cloned Fd heavy chain was introduced into the bacteria and then screened again.
ELISA.
Each well of ELISA plates was sensitized with recombinant SAG1 (0.5 μg/well) or recombinant C-Igl (30) of Entamoeba histolytica (0.5 μg/well) as a control protein overnight at 4°C. The plates were washed with PBST and then blocked with PBS containing 3% skim milk for 1 h. Supernatant (100 μl) containing Fab fragments or the purified Fab fragments were added to the wells and incubated for 1 h at room temperature. After being washed with PBST, the wells were incubated with 100 μl of HRP-conjugated sheep antibody to human IgG Fab (ICN Pharmaceuticals, Aurora, OH) for 1 h at room temperature and then reacted with 200 μl of a substrate solution (0.4 mg of o-phenylenediamine/ml in citric acid-phosphate buffer [pH 5.0] containing 0.001% hydrogen peroxide) for 30 min. The reaction was stopped by the addition of 50 μl of 2.5 N H2SO4, and the optical density at 490 nm was determined with a model 550 microplate reader (Bio-Rad, Hercules, CA). The plasma of the patient diluted 1:400 with PBS containing 3% skim milk was used as a positive control.
DNA sequencing.
Cloned light-chain genes and Fd heavy-chain genes were recloned into sequencing vectors. Cycle sequencing in both directions was performed with a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) using M13 primers. The sequences were obtained using a model ABI Prism 3100 genetic analyzer (Applied Biosystems).
Purification of Fab fragments.
Positive clones were cultured in one liter of super broth medium. Portions (20 ml) of each resultant supernatant, prepared as described above, were filtrated through 0.22-μm-pore-size filters. Purification of Fabs was performed by affinity chromatography using His-Bind Resin (Novagen, Madison, WI) according to the manufacturer's instructions.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting.
Purified Fab fragments were analyzed on 12.5% acrylamide gels containing sodium dodecyl sulfate under reducing conditions, transferred to a nitrocellulose filter, and then incubated with an HRP-conjugated goat antibody to the human κ chain, an HRP-conjugated goat antibody to the human Fab fragment, and an HRP-conjugated mouse antibody to His-tagged protein (His-probe [H-3]; Santa Cruz Biotechnology, Santa Cruz, CA) (9). Development was performed with a Konica immunostaining kit.
Immunofluorescence analysis.
Indirect immunofluorescent staining was performed essentially as previously described (33). Tachyzoites obtained from mice were fixed with 4% paraformaldehyde in PBS. Fluorescein isothiocyanate-conjugated goat IgG to human IgG Fab (ICN Pharmaceuticals, Aurora, OH) was used as secondary antibody.
Confocal microscopy.
HeLa cells infected with T. gondii tachyzoites were incubated on multispot glass slides and fixed with 4% paraformaldehyde in PBS for 30 min. After being washed with PBS, the glass slides were incubated with 10% sucrose in PBS for 1 h and then stored at −80°C until use. The slides were treated with 0.05% Triton X-100 in PBS for 5 min. After being washed with PBS, the samples were blocked with 3% bovine serum albumin in PBS for 30 min and then incubated with Fab fragments for 1 h at room temperature. After being washed again with PBS, the cells were incubated with Alexa Fluor 488-labeled goat anti-human IgG antibody (Molecular Probes, Eugene, OR) for 1 h. After final washing, the stained trophozoites were mounted using glycerol containing 1.25 μg of DAPI/ml, 1.25 mg of 1,4-diazabicyclo(2,2,2)octane/ml, and 10% PBS. Samples were observed by using a Zeiss LSM700 confocal laser scanning microscope. Tachyzoites isolated from mice were also fixed with 4% paraformaldehyde and used as antigen.
Determination of affinity constant.
Measurement of the affinity of purified Fabs by surface plasmon resonance was performed by using a BIAcore 3000 instrument (Biacore AB, Uppsala, Sweden) according to the general procedure outlined by the manufacturer. The recombinant SAG1 was immobilized onto a CM5 chip (Biacore) at a low density. Association and dissociation constants were determined using the software (BIAevaluation 3.1) provided by the manufacturer.
Attachment assay.
The attachment assay was performed essentially as described previously by Mineo and Kasper (23). MDBK cells were cultured in MEM supplemented with 10% fetal bovine serum at 37°C in 5% CO2. For the assay, the MDBK cells were plated on a 96-well cell culture plate at a concentration of 2.3 × 106 cells/ml and incubated until a subconfluent monolayer was formed. The medium was removed, and the cells were washed with PBS. The cells were fixed with 2.5% glutaraldehyde in PBS for 30 min at 37°C and then washed with PBS. Tachyzoites (5 × 105 cells) of the T. gondii RH strain were incubated with various concentrations of Fab in MEM without fetal bovine serum for 90 min at 37°C, washed with ice-cold PBS, and then suspended in MEM with 10% fetal bovine serum. CP33, a human monoclonal antibody Fab specific for Entamoeba histolytica lectin (34), was used as a control Fab. The fixed MDBK cells were incubated with the treated tachyzoites for 30 min at 37°C in 5% CO2 and washed several times in warm medium to remove unbound parasites. The preparation of tachyzoites and MDBK cells was treated with 3.7% formaldehyde in PBS for 30 min at 4°C. Bound parasites were detected by immunofluorescence with a rabbit polyclonal antibody (ViroStat, Portland, OR) to T. gondii tachyzoites as the primary antibody and fluorescein isothiocyanate-conjugated goat IgG to rabbit IgG as the secondary antibody (ICN Pharmaceuticals, Aurora, OH). The number of parasites per 100 host cells was determined in three experiments.
Passive immunization and challenge.
Three groups of 6-week-old female ICR mice (eight mice per group) received an i.p. injection of 0.5 ml of PBS containing 0.1, 1.0, or 10 mg of the human Fab to T. gondii at 24 h before challenge. Control mice received an i.p. injection of 10 mg of CP33 in PBS. All mice were challenged i.p. with 100 viable tachyzoites of the T. gondii RH strain. Each group received injections of the same amount of human Fab at 1 and 72 h after challenge. Mice were fed and housed under normal conditions and were observed until 60 days after challenge.
Statistical analysis.
Statistical analyses of differences in inhibition of tachyzoite attachment by Fab fragments in vitro were performed by using the Student t test. Kaplan-Meier analysis was used to assess survival, and a log-rank (Mantel-Cox) test was used for comparison between two groups. Differences were considered to be significant at P < 0.05. Analyses were performed using SPSS v13.0.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in the present study have been deposited in the DDBJ, EMBL, and GenBank databases under accession numbers AB585999 to AB586001.
RESULTS
Cloning of human Fab clones.
The combinatorial immunoglobulin gene library constructed from peripheral lymphocytes of a patient with toxoplasmosis contained approximately 3 × 106 clones. We screened 6 × 105 clones by colony blotting with recombinant SAG1. Secondary screening by ELISA gave 30 positive clones (0.005%) to SAG1. When the reactivities of these Fabs to T. gondii tachyzoites were examined by indirect immunofluorescence microscopy, only one clone, designated Tox11, showed positive staining on the cell surface. However, sequence analysis of the light-chain gene showed deletion of the second framework and complementarity-determining region (CDR) 2 in Tox11. Therefore, the heavy-chain gene of Tox11 was recombined with light-chain genes from the library, and then a shuffled library consisting of 105 clones was constructed. We screened 2 × 103 clones from the light-chain shuffled library by ELISA and indirect immunofluorescence microscopy. Finally, two clones, designated Tox203 and Tox1403, were obtained. These Fab fragments showed remarkably strong positive staining patterns on the surface of tachyzoites in indirect immunofluorescence microscopy.
Reactivity of human Fabs.
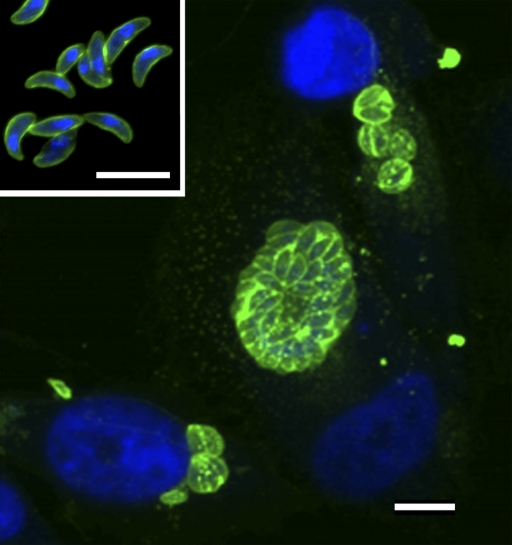
Tox203 and Tox1403 were cultured in large scale, and recombinant Fab fragments were purified by affinity chromatography. The purified Fab fragments showed two bands on SDS-PAGE with molecular masses of 24 and 26 kDa, respectively, under reducing conditions. These bands were identified as light and heavy chains by Western immunoblot analysis. The reactivities of Tox203 and Tox1403 with recombinant SAG1 were compared by ELISA. The optical density of Tox203 (1.90) was higher than that of Tox1403 (0.99) at 2 μg/ml. Confocal microscopy was used to localize the antigen recognized by Tox203 and Tox1403 on tachyzoites. The entire surfaces of free tachyzoites and multiplying tachyzoites in host cells were stained (Fig. 1). The host cells were not stained with these Fabs.
FIG. 1.
Confocal laser scanning microscopy of the T. gondii antigen recognized by human Fab fragment Tox203. Free tachyzoites (inset) of T. gondii RH strain and HeLa cells infected with tachyzoites were fixed. These cells were incubated with purified Tox203 and then with Alexa Fluor 488-labeled goat antibody to human IgG (green). Nuclei were counterstained with DAPI (blue). Scale bar, 10 μm.
Primary structure and gene usage of human Fabs.
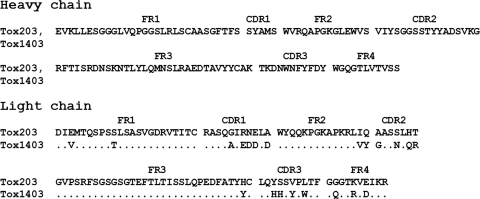
The heavy- and light-chain genes of Tox203 and Tox1403 were sequenced. The deduced amino acid sequences are shown in Fig. 2. The CDR sequences in the light chains differed somewhat between Tox203 and Tox1403, with changes in 5 of 10 amino acids in CDR1, 4 of 7 in CDR2, and 4 of 10 in CDR3. The germ lines of the heavy- and light-chain genes were analyzed by sequence homology using IgBLAST (NCBI; http://www.ncbi.nlm.nih.gov/igblast/) (Table 1). The closest germ line for the V segment in the heavy-chain gene was VH3-23, and those for the D and J segments were D1-7 and JH4, respectively. The closest V germ line in the light chains of both clones was Vκ1-17. For the J segments, Jκ4 was used in Tox203 and Jκ1 in Tox1403.
FIG. 2.
Deduced amino acid sequences of genes coding for heavy- and light-chain variable regions in human Fabs to T. gondii SAG1. FR, framework region; CDR, complementarity-determining region. Dots indicate identical residues.
TABLE 1.
Comparison of gene usage for heavy (H)- and light (L)-chain variable regions of human anti-T. gondii SAG1 Fab fragments
| Clone(s) | V segment |
D segment |
J segment |
|||
|---|---|---|---|---|---|---|
| Closest germ line | % Identity | Closest germ line | % Identity | Closest germ line | % Identity | |
| Tox203-H, Tox1403-H | VH3-23 | 98.3 | D1-7 | 100 | JH4 | 100 |
| Tox203-L | Vκ1-17 | 93.7 | NAa | NA | Jκ4 | 100 |
| Tox1403-L | Vκ1-17 | 89.8 | NA | NA | Jκ1 | 94.7 |
NA, not applicable.
Affinity of human Fab fragments.
The affinities of Tox203 and Tox1403 for recombinant SAG1 were measured by surface plasmon resonance. The dissociation constants were 3.09 × 10−9 M and 2.01 × 10−8 M, respectively (Table 2). Since the affinity of Tox203 was approximately 7 times higher than that of Tox1403, we chose to use Tox203 in further experiments.
TABLE 2.
Association and dissociation constants for the binding of recombinant human Fabs to T. gondii SAG1, measured by surface plasmon resonancea
| Fab | Ka (1/M) | Kd (M) |
|---|---|---|
| Tox203 | 1.9 × 108 | 3.09 × 10−9 |
| Tox1403 | 9.0 × 107 | 2.01 × 10−8 |
Ka, association constant; Kd, dissociation constant.
Inhibition of tachyzoite attachment by Fab fragments in vitro.
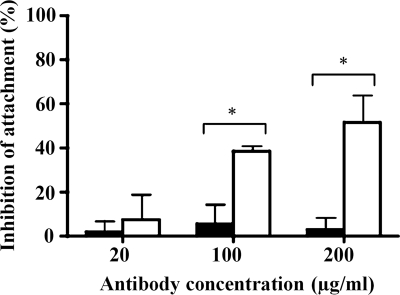
To examine the effect of Tox203 on parasite-host cell attachment, a quantitative attachment assay was performed in vitro. Tox203 significantly blocked tachyzoite attachment to MDBK cells in comparison with a control Fab, CP33, which is specific for E. histolytica (P < 0.05). The blocking effects of Tox203 were concentration dependent, ranging from 7% at 20 μg/ml to 52% at 200 μg/ml (Fig. 3).
FIG. 3.
Inhibition of attachment of T. gondii tachyzoites by human Fab Tox203. Tachyzoites were pretreated with various concentrations of Tox203 and then incubated with fixed MDBK cells. Controls were pretreated with CP33 or PBS. The number of attached tachyzoites per 100 host cells was counted. The inhibition rate (%) of attachment in Tox203-treated (white columns) and CP33-treated (black columns) groups was determined as follows: [1 − number in experimental group/number in PBS-treated control] × 100. The results are presented as means ± the standard deviations of data from three experiments. *, P < 0.05 versus CP33.
Protection of mice from lethal toxoplasmosis by Fab fragments.
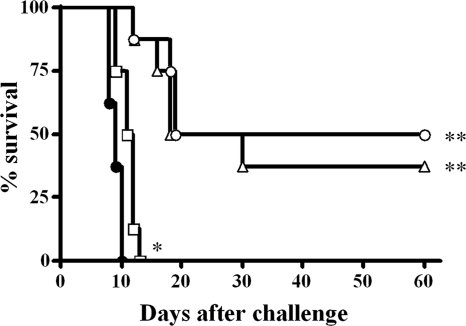
An in vivo experiment was performed to evaluate whether Tox203 protected mice from lethal T. gondii infection. Fab fragments were injected in mice 24 h prior and 1 and 72 h after challenge. In the control group, all mice died within 10 days after challenge. Mice that received injections of 1 mg of Tox203 had a median survival period of 11.5 days, which was significantly longer than that of 9.0 days in the control group (P = 0.01), although all mice died within 13 days after challenge (Fig. 4). In contrast, survival was prolonged significantly in mice treated with 5 or 10 mg of Tox203, in comparison to the control group (both P = 0.001). By 60 days after challenge, 38 and 50% of mice were alive in the groups treated with 5 and 10 mg of Tox203, respectively.
FIG. 4.
Survival profiles of ICR mice treated with human Fab Tox203 against lethal toxoplasmosis. Mice received i.p. injection of 1 mg (□), 5 mg (▵), or 10 mg (○) of the Fab at 24 h before i.p. challenge with 100 tachyzoites of the T. gondii RH strain. Mice received two i.p. injections with the same amount of the Fab at 1 and 72 h, respectively, after challenge. Control mice received i.p. injections of 10 mg of CP33 (•). *, P = 0.01; **, P = 0.001 versus CP33.
DISCUSSION
In the present study, two human monoclonal Fab fragments to T. gondii SAG1 were generated from a combinatorial immunoglobulin gene library derived from a patient with toxoplasmosis. To our knowledge, this is the first report of bacterial expression of human Fab fragments to SAG1 of T. gondii. Phage display systems have been used for screening of Fab fragments from immunoglobulin gene libraries (3, 35), while we have used colony blotting for screening and prepared human Fabs to major surface molecules of several pathogens (8, 9, 20, 34). Since fewer clones are screened in colony blotting, antibodies to major antigens must be abundant in the libraries. Indeed, SAG1 is the most abundant surface molecule of T. gondii (17), and serum antibodies to this molecule have been detected in patients with toxoplasmosis (6, 12, 19, 28). Therefore, the results of the present study show that screening by colony blotting is effective for libraries constructed from immune patients.
One interesting observation in the study was the partial deletion of the light chain in the Tox11 clone. However, by shuffling of light-chain genes in combination with the cloned heavy chain, we were able to obtain two clones, Tox203 and Tox1403. The affinity of Tox203 was 7 times higher than that of Tox1403. Therefore, shuffling of light-chain genes was effective in finding a better combination of heavy and light chains from the combinatorial library (34).
Mouse and rabbit antibodies to SAG1 are able to block adherence and/or invasion (23, 24, 27), although only a subset of anti-SAG1 antibodies exert these blocking effects (11). In the present study, neutralization of adherence to MDBK cells was detected with Tox203. The surface of T. gondii tachyzoites is covered with SRS proteins, and these proteins constitute a superfamily of at least 160 members, some of which are developmentally regulated (16). The central peptide sequence of SAG1 seems to be conserved in all members of the SRS family (37). It is unclear whether Tox203 is reactive with all proteins in the SRS family; however, confocal microscopy in the present study demonstrated that the antigen recognized by Tox203 was located across the entire surface of both intra- and extracellular tachyzoites (18).
The closest germ lines of the cloned heavy-chain gene were VH3-23, D1-7, and JH4 for the V, D, and J segments, respectively. A search of nucleotide databases located three human anti-SAG1 heavy-chain genes (AY506559, AY789123, and AY789125). These genes also had VH3-23 and JH4 in the V and J segments, but the D segments were D3-16 and D3-9. No biological data are available for these immunoglobulin molecules, but it is likely that the VH3 antibody is the major immunoglobulin to SAG1 of T. gondii. This appears to agree well with the proposal that VH3 antibodies are important for defense against a variety of viruses (2, 15) and bacteria (1, 29), as well as a parasite (31, 34). The closest V germ line in the light chains was Vκ1-17, and the J segments were Jκ4 in Tox203 and Jκ1 in Tox1403. Anti-SAG1 light-chain gene sequences (AY506560, AY789124, and AY789126) were also found in database searches, and the germ lines in all of these genes were Vκ1D-39 and Jκ1.
An effective vaccine against human T. gondii infection is desirable, and SAG1 is one of the most promising vaccine candidates (4, 10, 26). However, toxoplasmosis is also problematic in an immunocompromised host, and passive immunization may be useful in such a host due to reduced cellular immunity. Passive immunization of mice with anti-SAG1 antibody following challenge with a lethal dose of tachyzoites significantly increased survival compared to mice treated with control ascites (7). T. gondii tachyzoites treated with mouse monoclonal antibodies to SAG1 are reported to be gathered together, destroyed, deformed, and swollen, with the formation of holes and gaps on the surface (21).
Passive immunization of mice with Tox203 in our study also significantly reduced mortality in mice challenged with T. gondii. However, a whole IgG molecule is required to elicit complement activation and antibody-dependent cellular cytotoxicity. Recent advances enable production of whole IgG molecules using cloned genes for variable regions, and we plan to evaluate the in vivo effect of IgG molecules including Tox203 in further studies.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (to H.T.) and the National Science Foundation of China (Grant No. 30170851) (to X.-J.C.).
Editor: J. H. Adams
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Abadi, J., J. Friedman, R. A. Mageed, R. Jefferis, M. C. Rodriguez-Barradas, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of V(H)3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed] [Google Scholar]
- 2.Andris, J. S., P. H. Ehrlich, L. Ostberg, and J. D. Capra. 1992. Probing the human antibody repertoire to exogenous antigens. Characterization of the H and L chain V region gene segments from anti-hepatitis B virus antibodies. J. Immunol. 149:4053-4059. [PubMed] [Google Scholar]
- 3.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. U. S. A. 89:9339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biemans, R., D. Gregoire, M. Haumont, A. Bosseloir, L. Garcia, A. Jacquet, C. Dubeaux, and A. Bollen. 1998. The conformation of purified Toxoplasma gondii SAG1 antigen, secreted from engineered Pichia pastoris, is adequate for serorecognition and cell proliferation. J. Biotechnol. 66:137-146. [DOI] [PubMed] [Google Scholar]
- 5.Bird, R. E., K. D. Hardman, J. W. Jacobson, S. Johnson, B. M. Kaufman, S. M. Lee, T. Lee, S. H. Pope, G. S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science 242:423-426. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, F. R., D. A. Silva, J. P. Cunha-Junior, M. A. Souza, T. C. Oliveira, S. R. Bela, G. G. Faria, C. S. Lopes, and J. R. Mineo. 2008. Reverse enzyme-linked immunosorbent assay using monoclonal antibodies against SAG1-related sequence, SAG2A, and p97 antigens from Toxoplasma gondii to detect specific immunoglobulin G (IgG), IgM, and IgA antibodies in human sera. Clin. Vaccine Immunol. 15:1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha, D. Y., I. K. Song, G. S. Lee, O. S. Hwang, H. J. Noh, S. D. Yeo, D. W. Shin, and Y. H. Lee. 2001. Effects of specific monoclonal antibodies to dense granular proteins on the invasion of Toxoplasma gondii in vitro and in vivo. Korean J. Parasitol. 39:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, X. J., H. Hayasaka, K. Watanabe, Y. L. Tao, J. Y. Liu, H. Tsukamoto, T. Horii, K. Tanabe, and H. Tachibana. 2007. Production of high-affinity human monoclonal antibody Fab fragments to the 19-kilodalton C-terminal merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 75:3614-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, X. J., S. Ihara, M. Takekoshi, and H. Tachibana. 2000. Entamoeba histolytica: bacterial expression of a human monoclonal antibody which inhibits in vitro adherence of trophozoites. Exp. Parasitol. 96:52-56. [DOI] [PubMed] [Google Scholar]
- 10.Fang, R., H. Feng, H. Nie, L. Wang, P. Tu, Q. Song, Y. Zhou, and J. Zhao. 2010. Construction and immunogenicity of pseudotype baculovirus expressing Toxoplasma gondii SAG1 protein in BALB/c mice model. Vaccine 28:1803-1807. [DOI] [PubMed] [Google Scholar]
- 11.Grimwood, J., and J. E. Smith. 1996. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int. J. Parasitol. 26:169-173. [DOI] [PubMed] [Google Scholar]
- 12.Harning, D., J. Spenter, A. Metsis, J. Vuust, and E. Petersen. 1996. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, X. L., M. E. Grigg, J. C. Boothroyd, and K. C. Garcia. 2002. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat. Struct. Biol. 9:606-611. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, C. A., and J. S. Remington. 1994. Immunopathogenesis of toxoplasmic encephalitis. J. Infect. Dis. 170:1057-1067. [DOI] [PubMed] [Google Scholar]
- 15.Ikematsu, H., N. Harindranath, Y. Ueki, A. L. Notkins, and P. Casali. 1993. Clonal analysis of a human antibody response. II. Sequences of the VH genes of human IgM, IgG, and IgA to rabies virus reveal preferential utilization of VHIII segments and somatic hypermutation. J. Immunol. 150:1325-1337. [PMC free article] [PubMed] [Google Scholar]
- 16.Jung, C., C. Y. Lee, and M. E. Grigg. 2004. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 34:285-296. [DOI] [PubMed] [Google Scholar]
- 17.Kasper, L. H., J. H. Crabb, and E. R. Pfefferkorn. 1983. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J. Immunol. 130:2407-2412. [PubMed] [Google Scholar]
- 18.Lee, B. Y., M. H. Ahn, H. C. Kim, and D. Y. Min. 2001. Toxoplasma gondii: ultrastructural localization of specific antigens and inhibition of intracellular multiplication by monoclonal antibodies. Korean J. Parasitol. 39:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letillois, M. F., V. Laigle, F. Santoro, M. Micoud, and B. F. Chumpitazi. 1995. Toxoplasma gondii surface antigen-1 in sera of HIV-infected patients as an indicator of reactivated toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 14:899-903. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., H. Shao, Y. Tao, B. Yang, L. Qian, X. Yang, B. Cao, G. Hu, H. Tachibana, and X. Cheng. 2006. Production of an anti-severe acute respiratory syndrome (SARS) coronavirus human monoclonal antibody Fab fragment by using a combinatorial immunoglobulin gene library derived from patients who recovered from SARS. Clin. Vaccine Immunol. 13:594-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, K. Y., D. B. Zhang, Q. K. Wei, J. Li, G. P. Li, and J. Z. Yu. 2006. Biological role of surface Toxoplasma gondii antigen in development of vaccine. World J. Gastroenterol. 12:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 23.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 79:11-20. [DOI] [PubMed] [Google Scholar]
- 24.Mineo, J. R., R. McLeod, D. Mack, J. Smith, I. A. Khan, K. H. Ely, and L. H. Kasper. 1993. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 150:3951-3964. [PubMed] [Google Scholar]
- 25.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen, H. V., S. L. Lauemoller, L. Christiansen, S. Buus, A. Fomsgaard, and E. Petersen. 1999. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect. Immun. 67:6358-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen, E., H. V. Nielsen, L. Christiansen, and J. Spenter. 1998. Immunization with Escherichia coli produced recombinant T. gondii SAG1 with alum as adjuvant protect mice against lethal infection with Toxoplasma gondii. Vaccine 16:1283-1289. [DOI] [PubMed] [Google Scholar]
- 28.Santoro, F., D. Afchain, R. Pierce, J. Y. Cesbron, G. Ovlaque, and A. Capron. 1985. Serodiagnosis of toxoplasma infection using a purified parasite protein (P30). Clin. Exp. Immunol. 62:262-269. [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman, G. J., and A. H. Lucas. 1991. Variable region diversity in human circulating antibodies specific for the capsular polysaccharide of Haemophilus influenzae type b. Preferential usage of two types of VH3 heavy chains. J. Clin. Invest. 88:911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachibana, H., X. J. Cheng, G. Masuda, N. Horiki, and T. Takeuchi. 2004. Evaluation of recombinant fragments of Entamoeba histolytica Gal/GalNAc lectin intermediate subunit for serodiagnosis of amebiasis. J. Clin. Microbiol. 42:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tachibana, H., X. J. Cheng, H. Tsukamoto, and J. Itoh. 2009. Characterization of Entamoeba histolytica intermediate subunit lectin-specific human monoclonal antibodies generated in transgenic mice expressing human immunoglobulin loci. Infect. Immun. 77:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachibana, H., X. J. Cheng, K. Watanabe, M. Takekoshi, F. Maeda, S. Aotsuka, Y. Kaneda, T. Takeuchi, and S. Ihara. 1999. Preparation of recombinant human monoclonal antibody Fab fragments specific for Entamoeba histolytica. Clin. Diagn. Lab. Immunol. 6:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tachibana, H., S. Kobayashi, Y. Kato, K. Nagakura, Y. Kaneda, and T. Takeuchi. 1990. Identification of a pathogenic isolate-specific 30,000-Mr antigen of Entamoeba histolytica by using a monoclonal antibody. Infect. Immun. 58:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tachibana, H., K. Watanabe, X. J. Cheng, H. Tsukamoto, Y. Kaneda, T. Takeuchi, S. Ihara, and W. A. Petri, Jr. 2003. VH3 gene usage in neutralizing human antibodies specific for the Entamoeba histolytica Gal/GalNAc lectin heavy subunit. Infect. Immun. 71:4313-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takekoshi, M., F. Maeda, H. Tachibana, H. Inoko, S. Kato, I. Takakura, T. Kenjyo, S. Hiraga, Y. Ogawa, T. Horiki, and S. Ihara. 1998. Human monoclonal anti-HCMV neutralizing antibody from phage display libraries. J. Virol. Methods 74:89-98. [DOI] [PubMed] [Google Scholar]
- 36.Tomizuka, K., H. Yoshida, H. Uejima, H. Kugoh, K. Sato, A. Ohguma, M. Hayasaka, K. Hanaoka, M. Oshimura, and I. Ishida. 1997. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat. Genet. 16:133-143. [DOI] [PubMed] [Google Scholar]
- 37.Velge-Roussel, F., I. Dimier-Poisson, D. Buzoni-Gatel, and D. Bout. 2001. Anti-SAG1 peptide antibodies inhibit the penetration of Toxoplasma gondii tachyzoites into enterocyte cell lines. Parasitology 123:225-233. [DOI] [PubMed] [Google Scholar]