Abstract
Neisseria gonorrhoeae produces no known siderophores but can employ host-derived, iron-binding proteins, including transferrin and lactoferrin, as iron sources. Given the propensity of this pathogen to hijack rather than synthesize iron-sequestering molecules, we hypothesized that the ability to use siderophores produced by other bacteria, or xenosiderophores, may also play a role in the survival of the gonococcus. Among a panel of diverse siderophores, only the catecholate xenosiderophores enterobactin and salmochelin promoted growth of gonococcal strain FA19. Surprisingly, the internalization pathway was independent of TonB or any of the TonB-dependent transporters. Xenosiderophore-mediated growth was similarly independent of the pilin-extruding secretin formed by PilQ and of the hydrophobic-agent efflux system composed of MtrCDE. The fbpABC operon encodes a periplasmic-binding-protein-dependent ABC transport system that enables the gonococcus to transport iron into the cell subsequent to outer membrane translocation. We hypothesized that the FbpABC proteins, required for ferric iron transport from transferrin and lactoferrin, might also contribute to the utilization of xenosiderophores as iron sources. We created mutants that conditionally expressed FbpABC from an IPTG-inducible promoter. We determined that expression of FbpABC was required for growth of gonococcal strain FA19 in the presence of enterobactin and salmochelin. The monomeric component of enterobactin, dihydroxybenzoylserine (DHBS), and the S2 form of salmochelin specifically promoted FbpABC-dependent growth of FA19. This study demonstrated that the gonococcal FbpABC transport system is required for utilization of some xenosiderophores as iron sources and that growth promotion by these ferric siderophores can occur in the absence of TonB or individual TonB-dependent transporters.
Neisseria gonorrhoeae is a Gram-negative obligate human pathogen and the causative agent of the sexually transmitted disease gonorrhea. Gonorrhea is the second most commonly reported infectious disease in the United States, with 336,742 cases reported in 2008 (12). Infection is initiated on the urogenital or anorectal mucosa after intimate sexual contact. Uncomplicated infection in men presents as an acute urethritis manifesting symptoms including dysuria and a mucopurulent discharge. Infection is usually uncomplicated in men but if undetected or left untreated, can ascend, leading to epididymitis or prostatitis. Symptomatic gonococcal infection in women presents within 10 days of infection and manifests as urethritis or endocervicitis. However, up to 80% of infections in women are asymptomatic (20, 63), leading to more serious complications such as pelvic inflammatory disease (PID). PID occurs in 10 to 20% of infected women (19) and can result in chronic pelvic pain, ectopic pregnancy, and infertility. Ascension in both men and women can precede disseminated gonococcal infection (DGI), which is characterized by septic arthritis and dermatological manifestations. Current treatment options for gonococcal infections are limited to the administration of expanded-spectrum cephalosporins due to the increase in resistance to other antibiotics (12). Because of the increasingly limited treatment options, it has become imperative to understand the mechanisms employed by N. gonorrhoeae to establish infection in order to identify new therapies.
Most microorganisms require iron to establish infection; however, the strict iron-withholding mechanisms employed by the human host result in a free serum iron concentration of approximately 10−24 M (2), which is too low for the survival of microbial pathogens. Free iron concentrations are similarly limited on mucosal surfaces and in tissues by host iron binding proteins. Therefore, the ability to obtain iron from the mammalian host is recognized as a virulence factor, and like many bacteria, gonococci have developed efficient mechanisms to acquire iron from such a limiting environment. Iron acquisition systems have been characterized that allow gonococci to obtain iron from the host iron binding proteins transferrin (16), lactoferrin (8), and hemoglobin (13). Transport of iron derived from these host proteins across the bacterial outer membrane requires energy that is transduced from the proton motive force by the inner membrane Ton complex composed of TonB, ExbB, and ExbD (for a review, see reference 9). Ton-derived energy is employed by specific TonB-dependent transporters (Tdts) to import iron through the outer membrane. The pathogenic neisseriae employ the TonB-dependent transporter TbpA to acquire iron from human transferrin (16). TbpB, a surface-exposed lipoprotein (3), in association with TbpA, comprises the transferrin-iron uptake system. Both proteins are capable of binding transferrin; however, TbpB is able to discriminate between holo and apo forms of transferrin (18). All gonococcal isolates are able to acquire iron from transferrin through this system, and expression of TbpA and TbpB was shown to be necessary to allow gonococcal strain FA1090 to establish infection in human male volunteers (17), which demonstrates a link between iron acquisition systems and initiation of infection.
Siderophores are another potential source of iron available to N. gonorrhoeae. Siderophores are high-affinity iron binding compounds that are secreted by microorganisms to chelate ferric iron from the environment. N. gonorrhoeae produces no known siderophores but may be capable of utilizing xenosiderophores, or siderophores produced by other microorganisms that share the same ecological niche. N. gonorrhoeae colonizes the mucosal surfaces of the urogenital tract and the oropharynx, niches which may be coinhabited by other siderophore-producing bacterial and fungal pathogens (for a review, see reference 35). Biosynthesis of siderophores has been described for a number of bacteria, including Escherichia, Salmonella, Yersinia, Bacillus, Vibrio, and Mycobacterium species, as well as a number of fungi, including Aspergillus and Rhizopus species (for a review, see reference 51). In Gram-negative bacteria, iron uptake from siderophores is receptor mediated, a process in which the entire siderophore is internalized. The sequences of more than 30 outer membrane siderophore receptor proteins have been determined (67). Solving the crystal structures of some of these proteins revealed that these transporters share a similar architecture, being composed of a 22-stranded β-barrel and an amino-terminal plug domain (for a review, see reference 50). Gonococcal strain FA1090 employs the Tdt FetA to acquire iron from the catecholate xenosiderophore enterobactin (10). Gonococci may be able to utilize additional ferric chelates, since four putative Tdts (31, 66) with similarity to siderophore transporters have been identified.
Although most characterized iron internalization systems in N. gonorrhoeae are TonB dependent, TonB-independent acquisition of iron in Gram-negative bacteria has been described. The utilization of ferric citrate and heme does not require TonB in N. gonorrhoeae (7). Yersinia pestis, the causative agent of bubonic and pneumonic plague, utilizes the ABC transport system, YfuABC, to transport chelated iron (28). Iron transport assays demonstrated that TonB was not required in this process (52), suggesting that energy was not required for the transport of the iron across the outer membrane. Wyckoff et al. (70) identified an ABC transport system in Vibrio cholerae that is sufficient for ferric iron internalization in the absence of TonB. To date, outer membrane components that contribute to these uptake systems have yet to be identified.
The FbpABC iron acquisition system is the neisserial homologue to the YfuABC transport system. FbpABC functions as a component of the transferrin and lactoferrin iron uptake systems. The three genes encoding this system are arranged in an operon that is regulated by iron availability. The fbpA gene encodes a periplasmic iron binding protein; fbpB encodes a cytoplasmic permease, while fbpC encodes a nucleotide-binding protein, which provides the energy to transport iron bound by FbpA across the cytoplasmic membrane. fbpABC null mutants are incapable of growth when nonheme iron sources, including transferrin, lactoferrin, ferric nitrate, ferric citrate, and ferric chloride, are provided as a sole iron source (40). Providing such mutants with heme or hemoglobin as an iron source allows them to grow at rates similar to that of the wild-type organism (65).
Since the ability to use xenosiderophores may play a role in the survival of N. gonorrhoeae in vivo, we evaluated a panel of iron sources for gonococcal growth stimulation. We determined that some catecholate xenosiderophores promoted the growth of gonococcal strain FA19 but that growth in the presence of these iron sources occurred independent of a functional Ton system. This suggested that internalization of these iron sources through the outer membrane occurred in an energy-independent manner and did not require a Tdt for entry. Moreover, unlike strain FA1090, enterobactin growth promotion of FA19 did not require the Tdt FetA. To elucidate the mechanism by which xenosiderophore-bound iron stimulated gonococcal growth in a Ton-independent process, we explored systems required for the transport of ferric iron into the cell subsequent to outer membrane translocation. Since the FbpABC iron transport system is necessary for periplasmic iron transport in neisseriae, we created conditional strains that expressed the fbpABC operon from an IPTG-dependent promoter. We report that the expression of FbpABC is required for xenosiderophore growth promotion of strain FA19, indicating that this ABC transport system can be employed for Ton-independent iron acquisition from xenosiderophores.
MATERIALS AND METHODS
Bacterial growth conditions.
Gonococcal strains were maintained on GC medium base (GCB; Difco) containing Kellogg's supplement 1 (37) and 12 μM ferric nitrate. The medium was supplemented with 1 μg/ml erythromycin, 100 μg/ml streptomycin, or 50 μg/ml kanamycin for selection of mutant strains. For iron depletion conditions, liquid cultures of gonococcal strains were grown in a chemically defined medium (CDM) (69) which was rendered iron free by treatment with Chelex 100 (Bio-Rad). When necessary, gonococcal strains were grown in CDM with 12 μM ferric nitrate for iron-replete conditions. For induction of the fbpABC operon, strains were grown in the presence of 2 mM IPTG. Cultures were grown in a shaking incubator at 37°C in an atmosphere supplemented with 5% CO2. Escherichia coli strains were cultured using Luria-Bertani (LB) broth (Difco) containing 100 μg/ml ampicillin, 50 μg/ml kanamycin, or 250 μg/ml erythromycin.
Bacterial strains and mutant construction.
All strains and plasmids used in this study are listed in Table 1. Gonococcal strain FA19 is a serum-resistant isolate from a disseminated infection, as has been described previously (45). MCV650, the tonB::Ω mutant strain, was constructed by transformation of FA19 with pVCU693 as described previously (31). pVCU693 was also used to transform strains KH12 and KH14 in order to create the mtrC tonB and mtrD tonB double mutants, MCV920 and MCV921, respectively. Successful inactivation of the tonB gene in the chromosome of these mutants was confirmed by PCR and by the inability to grow on human transferrin as a sole iron source. Plasmids pVCU704, pVCU705, pVCU706, and pVCU707 were described previously (31) and used to create the putative Tdt mutant strains MCV925, MCV926, MCV927, and MCV928, respectively, in strain FA19. Insertion of the Ω cassette into the chromosome was verified by PCR analysis as described previously (31). pVCU915 was constructed by inserting the aphA3 cassette from pUC18K (43) into the AgeI site of pVCU690 (39), thus disrupting the characterized tonB gene with a nonpolar kanamycin resistance gene. pVCU915 was linearized and used to transform strain FA6807 (5), resulting in the fetA tonB double mutant strain MCV917. Insertion of the disrupted tonB gene in the chromosome was confirmed by PCR and by the inability to grow on human transferrin as a sole iron source.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant description | Source or reference |
|---|---|---|
| Strains | ||
| FA19 | Wild type | 45 |
| KH12 | FA19 mtrC::Kan | 33 |
| KH14 | FA19 mtrD::Kan | 32 |
| FA6807 | FA19 fetA::Ω (Strr Spcr) | 5 |
| MCV650 | FA19 tonB::Ω (Strr Spcr) | 30 |
| MCV841 | FA19 pilQ::Ω (Strr Spcr) | 21 |
| MCV904 | FA19 transformed by pVCU912 (Ermr) | This study |
| MCV906 | FA19 transformed by pVCU913 (Ermr) | This study |
| MCV917 | FA19 fetA::Ω tonB::Kan | This study |
| MCV920 | FA19 mtrC::Kan tonB::Ω | This study |
| MCV921 | FA19 mtrD::Kan tonB::Ω | This study |
| MCV925 | FA19 tdfF::Ω | This study |
| MCV926 | FA19 tdfG::Ω | This study |
| MCV927 | FA19 tdfH::Ω | This study |
| MCV928 | FA19 tdfJ::Ω | This study |
| Plasmids | ||
| pCR2.1 | Kanr Ampr | Invitrogen |
| pHSS6GCU | Plasmid containing the gonococcal uptake sequence Kanr | 24 |
| pHSX-ermC-lacIOP | Kanr Ermr | 61 |
| pUC18K | Vector containing the aphA3 cassette (Kanr) | 43 |
| pUNCH290 | PCR2.1 containing the Ω cassette in the HincII site of pilQ1 | 14 |
| pVCU690 | pCR2.1 containing tonBΔ160-194 allele | 39 |
| pVCU693 | pCR2.1 containing the Ω cassette in the AgeI site of tonB | 31 |
| pVCU704 | pCR2.1 containing the Ω cassette in the PflMI site of tdfF | 31 |
| pVCU705 | pCR2.1 containing the Ω cassette in the StuI site of tdfG | 31 |
| pVCU706 | pCR2.1 containing the Ω cassette in the HincII site of tdfH | 31 |
| pVCU707 | pCR2.1 containing the Ω cassette in the HincII site of tdfJ | 31 |
| pVCU907 | pHSS6GCU containing the fbpA allele from pVCU911 | This study |
| pVCU908 | pHSS6GCU fbpA upstream and coding | This study |
| pVCU909 | pCR2.1 fbpA upstream and coding region | This study |
| pVCU910 | pHSS6GCU containing fbpA upstream and coding region from pVCU909 | This study |
| pVCU911 | pET22b(+) containing FA19 fbpA allele | 53 |
| pVCU912 | pHSS6GCU fbpA ermC lacIP (−RBS) | This study |
| pVCU913 | pHSS6GCU fbpA ermC lacIP | This study |
| pVCU915 | MCV690 containing the aphA3 cassette in the AgeI site of tonB | This study |
To create the conditional fbpA mutant, MCV906, the coding region of the fbpA gene from gonococcal strain FA19, including 674 bp upstream of the promoter, was PCR amplified using gene splicing by overlap extension (SOEing) (36). The first round of PCR employed two sets of primers, oVCU293 (TGGATCCCCCCCAAATGCCGTCTGAAGAAATG) and oVCU324 (GATATCCCATGAGTAGGATCCGGTTATTGTTTGTTCTGAC), to amplify the upstream region and oVCU325 (CTACTCATGGATATCCCCTCATTCATTTAGGAGAAA) and oVCU326 (GGCGATGGGTTCCAAATTGAAGGTGGATAC) to amplify the coding region. Primers oVCU324 and oVCU325 overlap to create a novel EcoRV site. Primary PCR products were combined and subjected to a second round of PCR using oVCU293 and oVCU326. In the final amplicon, the upstream and coding regions of fbpA were fused, thus removing all iron-responsive elements in the promoter region and replacing them with a novel EcoRV site. The final PCR product was cloned into the plasmid pCR2.1 (Invitrogen) resulting in plasmid pVCU909. TOP10 E. coli cells were transformed with pVCU909, and transformants were selected on LB plates containing 100 μg/ml ampicillin. The fbpA fragment was subcloned into the vector pHSS6GCU (24), which contains a gonococcal DNA uptake sequence. The resulting plasmid, pVCU910, was used to transform Nova Blue E. coli (Novagen), and transformants were selected on LB plates containing 50 μg/ml kanamycin. The plasmid was then linearized by digestion with EcoRV, and the ermC lacIP fragment from pHSX-ermC-lacIOP (61) (Table 1) was ligated into the EcoRV site, creating plasmid pVCU913. Wild-type strain FA19 was then transformed with pVCU913, creating mutant strain MCV906, in which the fbpA gene is regulated by the IPTG-inducible tac promoter. Successful integration of the inducible promoter was verified by PCR. MCV906 exhibited wild-type levels of growth in the presence of 2 mM IPTG when human transferrin was provided as the sole iron source; however, growth on human transferrin was also observed in the absence of IPTG due to the leakiness of the tac promoter.
A second mutant strain was created in order to circumvent the leakiness of the lac promoter. Plasmid pVCU911 contains the coding region of the fbpA gene inserted into the NdeI-XhoI sites of the polylinker of the plasmid pET22b(+) (Novagen). The fbpA gene was excised from pVCU911 by digestion with BglII and XhoI. The resultant BglII/XhoI fragment was treated with Klenow (Invitrogen) and ligated into the SmaI site of pHSS6GCU thus creating pVCU907. Primers oVCU293 and oVCU294 (TTCTAGAATCGATTTTCTCCTAAATGAATGAGGGTGTATACCTTG) were used to amplify 800 bp of the upstream of the fbpA gene. The 5′ end of oVCU293 encodes a BamHI site and the 5′ end of oVCU294 encodes an XbaI site, resulting in a PCR product that could be positionally cloned into pVCU907. The 800-bp upstream fragment of the fbpA gene was digested with BamHI and XbaI and cloned into pVCU907, creating plasmid pVCU908. The ermC lacIP fragment was ligated into an NdeI site downstream of the ribosome binding site, creating plasmid pVCU912. pVCU912 was used to transform gonococcal strain FA19, and erythromycin-resistant colonies were selected in the presence of 2 mM IPTG. Homologous recombination yielded strain MCV904, in which the IPTG-inducible lac promoter had been inserted downstream of the ribosome binding site of the native promoter of the fbpA gene. The location of the promoter was verified in the chromosome by PCR, and MCV904 was incapable of growth when transferrin was provided as a sole iron source in the absence of IPTG.
Preparation of iron-containing compounds.
The ferric siderophores used in this study were purchased from EMC Microcollections (Tubingen, Germany) except for acinetobactin and alcaligin, which were kindly provided by Luis Actis and Sandra Armstrong, respectively. Each siderophore was resuspended to a final concentration of 1 mg/ml in sterile deionized water, except for ferric enterobactin, which was dissolved in methanol prior to dilution in water. Human transferrin (hTf) (Sigma) was obtained in the apo form and dissolved in ferration buffer (500 mM Tris, 150 mM NaCl, 100 mM NaHCO3, pH 8.6) at a final concentration of 10 mg/ml. Iron solution (100 mM sodium citrate, 100 mM NaHCO3, 5 mM FeCl3) was added to apo-hTf in order to attain 30% iron saturation. The mixture was incubated at room temperature for 2 h and then dialyzed overnight at 4°C against dialysis buffer (40 mM Tris, 150 mM NaCl, 20 mM NaHCO3, pH 7.4) and filter sterilized prior to use. Bovine transferrin (bTf) (Sigma) was obtained in the apo form, dissolved in CDM at a final concentration of 10 mg/ml, and filter sterilized prior to use.
Xenosiderophore utilization assays.
Plate bioassays to evaluate xenosiderophore utilization were performed using CDM plates supplemented with 2.5 μM apo bTf to chelate contaminating iron. Mutant or control strains were inoculated onto the plates using a sterile Dacron swab (Puritan). A sterile pipette was used to inoculate the agar with 10 μl of a 1-mg/ml solution of the indicated xenosiderophore. Plates were incubated at 37°C in a 5% CO2 atmosphere for 24 h and then evaluated for gonococcal growth in the presence of each iron source.
Generation of iron-dependent growth curves.
Growth curves were generated using the following protocol, modified from one described previously (54). Gonococcal strains were plated from freezer stocks onto GCB plates containing Kellogg's supplement 1 and 12 μM ferric nitrate. Single nonpiliated, transparent colonies were passaged onto GCB plates containing Kellogg's supplement 1 and 12 μM ferric nitrate. Mutant strains MCV904 and MCV906 were maintained on GCB plates containing 2 mM IPTG. Plates were incubated approximately 18 h at 37°C in the presence of 5% CO2. A sterile Dacron swab was used to resuspend gonococcal strains in iron-free CDM. Strains were then grown for 1 h prior to assay and then diluted to an OD600 of 0.02. After dilution, approximately 8.7 × 106 bacteria were added to each well of a sterile 96-well flat-bottomed microtiter plate. Human transferrin (30% saturated) was added to a final concentration of 7.5 μM and served as a control for each assay. In separate wells, each xenosiderophore was added, resulting in a final concentration of 10 μM. CDM containing apo bovine transferrin, added as an iron chelator, was added to each well to bring the final volume to 100 μl. The final concentration of apo bovine transferrin in each well was 2.5 μM. The initial optical density at 600 nm (OD600) value was determined, and the plate was incubated at 37°C and 5% CO2 with shaking at 225 rpm. OD600 readings were taken every 2 h thereafter. Each strain and iron source was tested in triplicate, and each assay was conducted a minimum of three times.
Purification of recombinant FbpA and generation of rabbit polyclonal antibody.
Recombinant FbpA was purified in order to generate polyclonal antibodies. pVCU911 (53) contains the fbpA coding region from gonococcal strain FA19 cloned into the expression vector pET-22b(+) (Novagen), thus generating full-length FbpA with a C-terminal His tag. This vector was used to transform E. coli expression strain BL21 (Novagen). Transformants were isolated, grown to mid-log phase, and induced with 1 mM β-d-thiogalactopyranoside (IPTG) for 3 h to express recombinant FbpA. After induction, cells were pelleted by centrifugation and resuspended in lysis buffer (10 mM imidazole and 1 mg/ml lysozyme; Sigma). The samples were incubated on ice for 30 min and then subjected to three 20-s bursts of sonication using a Sonifer 450 sonicator (Branson). Samples were subjected to centrifugation to remove the insoluble fraction. The soluble fraction was applied to a nickel affinity column (Qiagen), and purified rFbpA was isolated. Elution fractions were pooled and dialyzed overnight against phosphate-buffered saline (PBS). Purified rFbpA was sent to Covance Research Products, Inc. (Denver, PA), where it was used to immunize two female Elite New Zealand White (NZW) rabbits to generate polyclonal antibodies as described previously (42).
Western analysis.
Gonococcal cultures were grown in CDM under iron-depleted or iron-replete conditions. After 4 h of growth, cultures were standardized to cell density and pelleted. Cell pellets consisting of approximately 1 × 1012 CFU were resuspended in 100 μl of 2× Laemmli solubilizing buffer (41) containing 5% β-mercaptoethanol (Sigma). Samples were heated at 95°C for 5 min, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (41) followed by transfer to nitrocellulose (Schleicher and Schuell) (64). Blots were blocked for 1 h in 5% skim milk in 1× Tris-buffered saline (TBS) and subsequently probed with anti-rFbpA, anti-FetA (gift from Fred Sparling), or anti-TonB antibodies (38). Secondary antibodies were either goat anti-mouse or goat anti-rabbit alkaline phosphate conjugates (Bio-Rad). Blots were developed using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma).
Statistical analysis.
Statistical significance was determined using a two-tailed, unpaired Student's t test in which a P value of ≤0.05 was considered significant.
RESULTS
Xenosiderophores promote growth of gonococcal strain FA19.
Neisseria gonorrhoeae produces no known siderophores but may be capable of utilizing siderophores produced by other microorganisms that may share the same ecological niche. We tested a variety of xenosiderophores of both catecholate and hydroxamate classes for their ability to promote growth of gonococcal strain FA19 (Table 2). Gonococci were inoculated onto a CDM plate containing apo bovine transferrin to sequester free iron; 10 μg of each xenosiderophore was then applied to the plate. FA19 grew in the presence of a hydroxamate siderophore aerobactin and three catecholates: dihydroxybenzoylserine (DHBS), enterobactin, and mixed salmochelins. The same results were obtained when Desferal (deferroxamine mesylate; final concentration of 1, 5, or 10 μM) was added to the plate as an iron chelator (data not shown). To confirm these results, we performed liquid growth assays in order to assess growth promotion of FA19 in the presence of 10 μM each xenosiderophore (Fig. 1). FA19 grew in this format when provided DHBS, enterobactin, or salmochelin but not when aerobactin was provided as a sole iron source. In some experiments, siderophores were pretreated with Amberlite XAD to remove contaminating free iron. The results of these growth experiments (data not shown) were identical to those shown in Fig. 1.
TABLE 2.
Growth promotion of FA19 by xenosiderophores
| Xenosiderophore | Growtha |
|---|---|
| Acinetobactin | − |
| Aerobactin | + |
| Alcaligin | − |
| Arthrobactin | − |
| Coprogen | − |
| DHBS | + |
| Enterobactin | + |
| Ferrichrome | − |
| Ferrichrysin | − |
| Ferricrocin | − |
| Ferrihodin | − |
| Ferrirubin | − |
| Fusigen | − |
| Neocoprogen | − |
| Ornibactins (C4, C6, C8) | − |
| Rhizoferrin | − |
| Salmochelins (S2/S4) | + |
| Schizokinen | − |
| Vibriobactin | − |
| Yersiniabactin | − |
+, growth; −, no growth.
FIG. 1.
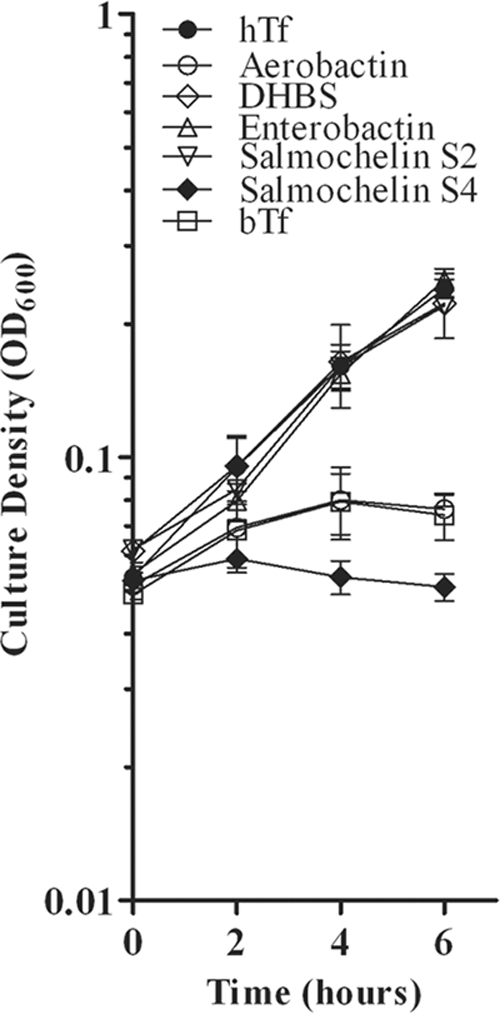
Xenosiderophore-stimulated growth of wild-type gonococcal strain FA19. Strain FA19 was grown in CDM containing 2.5 μM bovine transferrin alone (negative control) or in CDM with bovine transferrin and supplemented with either 7.5 μM human transferrin (positive control) or 10 μM the tested xenosiderophores. The density (OD600) of each culture was monitored for 6 h. Each time point represents the mean and standard deviation of results from three independent experiments.
To further characterize salmochelin-mediated growth, we sought to determine if specific salmochelins promoted growth of FA19. Salmochelin S4 is the cyclical, C-glucosylated derivative of enterobactin, while salmochelin S2 is the linearized hydrolysis product of S4 (6). We tested each salmochelin individually in a liquid growth assay (Fig. 1). FA19 was able to grow only when the S2 form of salmochelin was provided as an iron source. This suggests that gonococci are capable only of using the linear and not the cyclic molecule as an iron source.
Xenosiderophore-promoted growth is not affected by individual loss of TdfF, TdfG, TdfH, or TdfJ.
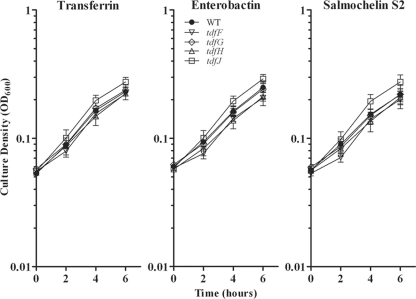
Four putative Tdts have been identified by sequence similarity to siderophore and heme transporters expressed by other Gram-negative bacteria (31, 66). To determine if these incompletely characterized systems play a role in xenosiderophore iron acquisition, we repeated the liquid growth assay with strains containing individual mutations in TdfF, TdfG, TdfH, or TdfJ (Fig. 2). The growth patterns observed were identical to that of the parental strain, demonstrating that none of these putative Tdts are individually involved in iron acquisition from the xenosiderophores tested.
FIG. 2.
Xenosiderophore-stimulated growth of wild-type (WT) gonococcal strain FA19 compared to growth of isogenic mutants lacking putative TonB-dependent transporters. Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with either 7.5 μM human transferrin (positive control), 10 μM enterobactin, or 10 μM salmochelin S2. Each time point represents the mean and standard deviation of results from three independent experiments. The strains used in this assay were FA19 (wild type), MCV925 (tdfF), MCV926 (tdfG), MCV927 (tdfH), and MCV928 (tdfJ).
Xenosiderophore-promoted growth is TonB and FetA independent.
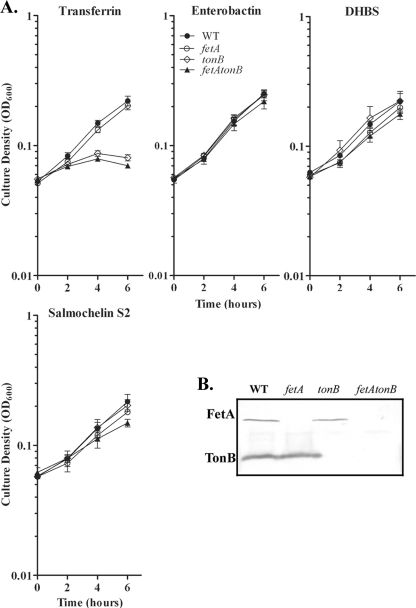
Siderophore uptake in other Gram-negative bacteria is characterized as TonB dependent (25); therefore we investigated the potential role of TonB in xenosiderophore-mediated growth promotion of strain FA19. We created an isogenic tonB::Ω mutant of strain FA19 and performed liquid growth assays to evaluate the ability of this strain to utilize xenosiderophores. Xenosiderophore-promoted growth was independent of the characterized Ton system, as the polar tonB mutant exhibited growth patterns identical to those of the wild-type strain, FA19 (Fig. 3 A).
FIG. 3.
Xenosiderophore-stimulated growth and protein expression by FA19 and isogenic mutants. (A) Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with either 7.5 μM human transferrin, 10 μM enterobactin, 10 μM DHBS, or 10 μM salmochelin S2. Each time point represents the mean and standard deviation of results from three independent experiments. WT, wild type. (B) TonB and FetA expression by FA19 (wild type), FA6807 (fetA), MCV650 (tonB), and MCV917 (fetA tonB). Strains were grown in CDM under iron-depleted conditions for 4 h. Gonococci were lysed, subjected to SDS-PAGE, and, following transfer of proteins to nitrocellulose, probed with an anti-FetA monoclonal antibody or anti-TonB polyclonal antibody.
FetA is a TonB-dependent outer membrane transporter that has been shown to be involved in utilization of enterobactin by gonococcal strain FA1090 (10). Our data suggested that strain FA19 was able to utilize enterobactin in the absence of a functional TonB. Therefore, we conducted liquid growth assays with the isogenic fetA::Ω mutant, FA6807, to characterize the role of FetA in growth promotion by various xenosiderophores. Utilization of DHBS, enterobactin, and salmochelin S2 was FetA independent (Fig. 3A). We also created an isogenic tonB fetA double mutant to explore the possibility that FetA was energized for enterobactin import by a mechanism independent of TonB. The tonB fetA double mutant exhibited growth patterns similar to those of the wild-type strain; therefore, growth promotion of FA19 by DHBS, enterobactin, and salmochelin S2 did not require functional TonB or FetA proteins. The phenotypes of the strains used in this assay were confirmed by Western analysis, with which we verified that FA6807 was incapable of expressing FetA and that MCV650 was incapable of expressing TonB (Fig. 3B). In contrast to strain FA1090, FA19 does not require TonB or FetA to utilize enterobactin. The fetA promoter is phase variable due to a slip strand in a poly(C) tract between the −35 and −10 promoter elements (11). The optimal promoter has 12 C residues in this repeat region and results in high levels of FetA expression (11), whereas our laboratory variant of strain FA19 has only 10 C residues and consequently expresses low levels of FetA. Therefore, variances in promoter strength and levels of FetA expression may explain the differences in enterobactin utilization by these two strains.
Xenosiderophore-promoted growth does not require the Mtr efflux system.
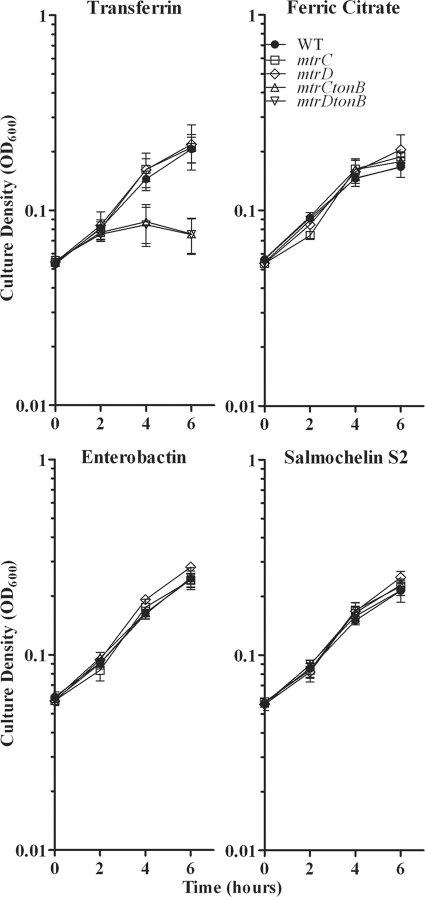
Since the energy provided by TonB was not necessary for xenosiderophore growth promotion of FA19, we sought to identify an alternative source of energy that might be involved in transport of these iron sources into the gonococcal cell. The MtrC-MtrD-MtrE efflux pump in N. gonorrhoeae harnesses the proton motive force to constitutively export hydrophobic antimicrobial agents from the bacterial cell (33). Moreover, it has been shown that TonB is necessary for high levels of induced efflux of hydrophobic agents (58), demonstrating a link between TonB- and Mtr-mediated efflux. We hypothesized that the energy harnessed from the proton motive force by the Mtr system may provide the energy necessary for transport of xenosiderophores across the gonococcal outer membrane in the absence of TonB. We performed liquid growth assays with mutants KH12 and KH14, in which mtrC and mtrD, respectively, were insertionally inactivated (32, 33). mtrC encodes the periplasmic membrane fusion protein and mtrD encodes the cytoplasmic membrane transporter of the Mtr-mediated efflux system (62). Each of these gene products is individually essential for efflux activity (62). The liquid growth assays demonstrated that both mutants exhibited wild-type levels of growth in the presence of enterobactin and salmochelin S2 (Fig. 4). We subsequently created isogenic mtrC tonB (MCV920) and mtrD tonB (MCV921) double mutants to determine whether the two systems have redundant functions. MCV920 and MCV921 were evaluated in the liquid growth assay, where growth promotion by 10 μM ferric citrate was used as a positive control since ferric citrate utilization is TonB independent (7) (Fig. 4). Again, all mutants exhibited growth patterns similar to the wild-type strain in the presence of enterobactin and salmochelin S2, thus eliminating the possibility that the Mtr efflux system provides an alternative source of energy for xenosiderophore transport into the gonococcal cell.
FIG. 4.
Xenosiderophore-stimulated growth of FA19 and isogenic mtr and mtr tonB mutant strains. Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with 10 μM ferric citrate (positive control), 7.5 μM human transferrin, 10 μM enterobactin, or 10 μM salmochelin S2. Each time point represents the mean and standard deviation of results from three independent experiments. The strains used in this study were FA19 (wild type [WT]), KH12 (mtrC), KH14 (mtrD), MCV920 (mtrC tonB), and MCV921 (mtrD tonB).
Xenosiderophore iron acquisition is PilQ independent.
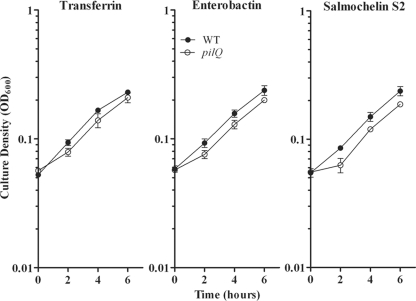
To identify the mechanism by which enterobactin and salmochelin S2 entered the periplasm in the absence of a TonB-dependent transporter, we investigated the role of a potential candidate, PilQ. PilQ is an integral outer membrane secretin protein (59) that forms a 6.5-nm gated pore and is necessary for biogenesis of the gonococcal type IV pilus (23). A study by Chen et al. (14) demonstrated that PilQ was responsible for nonspecific leakage of heme and protoporphyrins, allowing utilization of these iron sources in the absence of TonB and the Tdt specific for hemoglobin utilization. We therefore tested an isogenic pilQ::Ω mutant, MCV841, in xenosiderophore-dependent liquid growth assays (Fig. 5). The growth levels of MCV841 were decreased at each time point for all iron sources tested, including the positive control, transferrin, compared to results for the wild type. However, the rates of growth and final culture densities for MCV841 when incubated with transferrin, enterobactin, or salmochelin S2 were not significantly different from each other. Therefore, the pore created by PilQ is not the portal of entry into the gonococcal cell for these xenosiderophore iron sources under these experimental conditions.
FIG. 5.
Xenosiderophore-stimulated growth of FA19 and isogenic pilQ mutant. Strains FA19 (wild type [WT]) and MCV841 (pilQ) were grown in CDM containing 2.5 μM bovine transferrin, supplemented with either 7.5 μM human transferrin (positive control), 10 μM enterobactin, or 10 μM salmochelin S2. Each time point represents the mean and standard deviation of results from three independent experiments.
Growth promotion by DHBS, enterobactin, and salmochelin S2 is FbpABC dependent.
We next focused on transport of iron from these xenosiderophore iron sources across the periplasm and cytoplasmic membrane. In N. gonorrhoeae the gene products encoded by the fbpABC operon are required for transport of ferric iron into the cell subsequent to translocation through the outer membrane. FbpABC is homologous to the YfuABC system present in pathogenic Yersinia species, which is involved in TonB-independent iron acquisition (60). Therefore, we hypothesized that the FbpABC iron transport system required for iron transport from transferrin might also contribute to the utilization of xenosiderophores as iron sources.
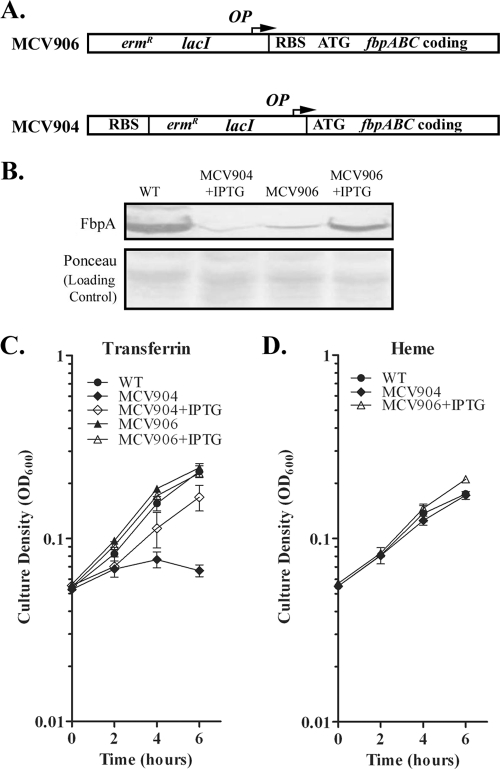
To test this hypothesis we employed an inducible promoter system to allow us to evaluate the potential role of FbpABC in xenosiderophore iron acquisition. Two conditional FbpABC mutants were constructed: MCV904 and MCV906. In mutant MCV906 the promoter of the fbpABC operon was replaced by the IPTG-inducible promoter of plasmid pHSX-ermC-lacIOP (61). This promoter allows for some transcription in the absence of IPTG (47); consequently, a second mutant was created. MCV904 contains the same promoter; however, it has been inserted downstream of the ribosome binding site, thus preventing expression of FbpA at both the transcriptional and translational levels (Fig. 6 A).
FIG. 6.
Characterization of conditional FbpABC expression strains. (A) Genetic organization of the fbpABC locus in mutant strains MCV904 and MCV906. In strain MCV906, the native promoter has been replaced by an inducible promoter (OP). In MCV904 the inducible promoter (OP) was inserted downstream of the native ribosome binding site (RBS). The arrow indicates the direction of transcription. WT, wild type. (B) FbpA expression in FA19 and the mutants that conditionally express FbpA. Strains were grown in CDM under iron-stressed conditions. Some cultures were supplemented with 2 mM IPTG (+IPTG). Gonococci were lysed, subjected to SDS-PAGE, and, following transfer of proteins to nitrocellulose, probed with a polyclonal anti-rFbpA antibody. Recombinant FbpA (rFbpA) was used as a positive control. (C) Growth with hTf by mutants that conditionally express FbpA. Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with 7.5 μM human transferrin. MCV904 and MCV906 were grown in the presence (+IPTG) or absence of 2 mM IPTG. (D) Growth with heme by mutants that conditionally express FbpA. Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with 4 μM heme. Each time point represents the mean and standard deviation of results from three independent experiments.
FbpA expression by MCV904 and MCV906 was evaluated by Western analysis probing with an anti-rFbpA polyclonal serum (Fig. 6B). Proteins were transferred to nitrocellulose and probed with rFbpA-specific antiserum. In strain FA19, expression of the FbpABC system is regulated by iron availability (22), where maximal FbpA protein detection occurs under iron-depleted conditions. Strain MCV906 was able to grow with or without IPTG, but maximal FbpA protein expression was detected under IPTG-positive (+IPTG) conditions. MCV904 grew under +IPTG conditions, resulting in very low levels of FbpA expression. As expected, MCV904 grew in the absence of IPTG when heme was provided as the sole iron source (Fig. 6D), since heme utilization by gonococci does not require FbpABC (65). To further characterize these strains, we performed a liquid growth assay in which MCV904 and MCV906 were grown in the presence or absence of 2 mM IPTG and hTf was provided as the sole iron source (Fig. 6C). MCV906 was able to grow on transferrin-derived iron in either the presence or absence of IPTG. MCV904 was unable to grow when hTf was provided as the sole iron source in the absence of IPTG; however, addition of IPTG resulted in growth stimulation in the presence of human transferrin.
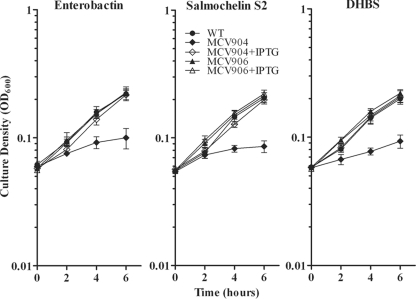
The Fbp dependence of gonococcal utilization of iron from xenosiderophores was evaluated using the liquid growth assay. Strains FA19, MCV904, and MCV906 were grown in the presence of enterobactin, DHBS, or salmochelin S2 (Fig. 7). MCV904 was unable to grow in the absence of IPTG when DHBS, enterobactin, and salmochelin S2 were provided as sole iron sources, indicating that FbpABC is necessary for growth promotion by these xenosiderophores. As seen with transferrin-iron utilization, MCV906 in the presence and absence of IPTG was capable of growth on the ferric siderophores. Likewise, MCV904 (+IPTG) grew in the presence of the ferric siderophores; however, in the absence of inducer, MCV904 was incapable of growth on the ferric siderophores. These observations directly link expression of the fbpABC operon to use of these xenosiderophore preparations as a sole iron source.
FIG. 7.
Xenosiderophore-stimulated growth of FA19 and conditional FbpABC expression strains. Strains were grown in CDM containing 2.5 μM bovine transferrin, supplemented with 10 μM enterobactin, 10 μM DHBS, or 10 μM salmochelin S2. MCV 904 and MCV906 were grown in the presence (+IPTG) or absence of 2 mM IPTG. Each time point represents the mean and standard deviation of results from three independent experiments. Differences between MCV904 (without IPTG) and FA19 in xenosiderophore-supported growth yields were statistically significant at 2-, 4-, and 6-h time points. WT, wild type.
DISCUSSION
While the pathogenic neisseriae do not synthesize siderophores as a means of iron acquisition, the best-characterized systems for internalization of this necessary nutrient involve hijacking iron from the host's iron binding proteins. Three two-component systems have been characterized in N. gonorrhoeae, which enable the use of human transferrin, human lactoferrin, and hemoglobin as iron sources (for a review, see reference 56). One component of each system is a TonB-dependent transporter while the other component is a lipid-modified protein that participates in ligand binding and, in the case of transferrin iron utilization, makes the process of iron internalization more efficient (3). Encoded elsewhere on the chromosome, the FbpABC gene products are necessary for mobilization of ferric iron, removed from transferrin and lactoferrin, through the periplasm and across the cytoplasmic membrane (15, 46). Genes encoding four other TonB-dependent transporters (TdfF, TdfG, TdfH and TdfJ) have been identified in the genome sequences of N. gonorrhoeae strains (31, 66). These predicted transporters share similarity with siderophore or heme transporters and are not encoded near predicted lipoproteins, in contrast to the characterized transport systems. The genes that encode TdfF and TdfG are proximate to genes that could encode periplasmic binding proteins with iron-related functions. However, none of the predicted transporters are encoded within loci that include predicted ABC transport systems.
The fet locus is present in the genomes of all Neisseria species. Unlike the examples of TonB-dependent transporters listed above, the fet locus encompasses the only example of a complete periplasmic-binding-protein-dependent, ABC transport system found in the neisserial chromosome. The FetA transporter was demonstrated by Carson et al. (10) to enable gonococcal strain FA1090 to utilize the heterologous siderophore enterobactin, derived from supernatants from enterobactin-overproducing E. coli. While the linked fetB gene was implicated in use of the xenosiderophore preparations, the rest of the putative uptake system has not yet been characterized. In a follow-up study, Carson et al. (11) demonstrated that FetA was subject to phase variation by virtue of a C-string in the promoter region. Changes in the number of C residues resulted in altered FetA expression levels presumably due to modification of promoter strength. Due to its ubiquitous presence and relatively high sequence conservation among neisserial strains, FetA has been considered a viable vaccine candidate (4).
In the current study, we screened a broad panel of xenosiderophores for their abilities to support in vitro growth of gonococcal strain FA19. While both catecholate- and hydroxamate-type siderophores were tested, only the catecholate siderophores enterobactin and salmochelin supported growth in liquid-phase assays conducted over a short time course. While our initial screening approach, utilizing plate bioassays, demonstrated that the hydroxamate-type siderophore aerobactin also supported growth, this was not confirmed by the liquid growth assay. Since growth on plates requires significantly more time to develop, we hypothesize that aerobactin use by N. gonorrhoeae is less efficient than is the use of the catechols.
Salmochelin and enterobactin were utilized efficiently by FA19, resulting in high-density growth yields in less than 6 h. To assess the specificity of these utilization systems, we tested the breakdown products of enterobactin and salmochelin for their growth promotion abilities. Interestingly, DHBS and salmochelin S2 supported growth, while salmochelin S4 did not. These results suggest that gonococcal strain FA19 may preferentially employ the small, linear forms of these catecholate siderophores. The observation that gonococci can utilize salmochelin and enterobactin as sole iron sources is interesting given the recent recognition that the innate immune system interacts differentially with these siderophore molecules. Lipocalin, a protein produced by epithelial cells and in the granules of polymorphonuclear leukocytes, binds to enterobactin and prevents its use as an iron source by enteric bacteria (27). However, salmochelin, which is produced by Salmonella species (55), uropathogenic E. coli (34), and some Klebsiella strains (49), is the glucosylated form of enterobactin and is not bound by the innate immunity protein lipocalin (26). Thus, the ability to modify enterobactin, by the action of the iro locus, leads to escape from the sequestering capabilities of lipocalin. Iro-positive E. coli strains are, as a result, more virulent (26). Within this context, it is interesting that N. gonorrhoeae, which might very well coinhabit the same ecological niche with E. coli and in particular with uropathogenic E. coli, has evolved the capacity to internalize both the unmodified and the glucosylated forms of enterobactin.
While the original goal of this study was to identify siderophores that could be utilized by N. gonorrhoeae and then to link growth promotion with expression of one or more of the predicted TdTs, we were surprised to find that gonococcal strain FA19 employed enterobactin and salmochelin in a mechanism that did not depend upon expression of individual TdTs or TonB. These findings contrasted with those of Carson et al. (10), who found that enterobactin utilization was FetA and TonB dependent in strain FA1090. The reason for these contrasting findings is not completely clear but may be related to the level of expression of FetA in strain FA19. Maximal expression levels of FetA were achieved by Carson et al. (11) with 12 C residues in the promoter region; however, the variant of FA19 used in this study had only 10 C residues in the fetA promoter, making the distance between −35 and −10 elements only 15 residues, which is suboptimal. Other differences between these strains exist, including expression levels of other regulatory factors (57), which may have the effect of suppressing expression of FetA in the FA19 background. FA19 and FA1090 are both serum-resistant clinical isolates. The genomes of both strains have been sequenced; however, the extent to which these strains differ at the phenotypic level has not yet been determined. We propose that Ton-independent ferric-siderophore acquisition is a trait of both goncoccal strains; however, increased FetA expression in FA1090 allows for the identification of Ton-dependent uptake over the background of Ton-independent internalization.
Utilization of enterobactin and salmochelin in our study was independent of both TonB and individual TdT expression. We also tested the hypothesis that the outer membrane PilQ complex participated in iron acquisition, which has already been documented for heme entry when high-affinity transport was disabled (14). However, in the FA19 background the pilQ mutant was not impaired in its ability to employ xenosiderophores as sole iron sources. We additionally entertained the hypothesis that the TonB independence of xenosiderophore-promoted growth was due to cross talk between TonB-dependent uptake and hydrophobic agent efflux systems. MtrC and MtrD form the cytoplasmic membrane complex that is responsible for energizing efflux through the outer membrane component MtrE (33). Since efflux of hydrophobic compounds depends upon the proton motive force and the MtrC and MtrD proteins (68), it was formally possible that MtrC or MtrD might energize xenosiderophore iron utilization in the absence of a functional Ton system. However, single and double mutants lacking TonB and/or the MtrCD components retained the ability to internalize enterobactin and salmochelin, thus eliminating this possible mechanism of TonB bypass.
The use of chelated iron by recombinant E. coli strains that express heterologous iron transport genes has been demonstrated in previous studies (28, 70). In these cases, growth on limiting inorganic iron was achieved by overexpression of proteins homologous to the gonococcal FbpABC proteins. When the FbpABC homologs were produced by E. coli, the requirement for TonB and Tdts for iron internalization was eliminated. These findings prompted us to test whether expression of the fbpABC operon in N. gonorrhoeae was necessary for utilization of salmochelin- or enterobactin-derived iron. Since all ferric iron internalization characterized to date in Neisseriae requires the expression of FbpABC (1, 15), we engineered two strains, MCV904 and MCV906, that conditionally expressed these gene products as a function of IPTG addition. With these strains, we determined that the iron from salmochelin and enterobactin was indeed internalized by N. gonorrhoeae in an FbpABC-dependent fashion. These results are consistent with studies of V. cholerae FbpABC (44) and Y. pestis YfuABC (28) which demonstrated that this periplasmic-binding protein dependent ABC transport system was sufficient to internalize iron in the absence of TonB or Tdts. However, the current study is the first to demonstrate that siderophore-derived iron can similarly be acquired by the neisserial FbpABC system.
The mechanism by which the siderophore-derived iron crosses the gonococcal outer membrane has not yet been resolved. Due to the specificities, defined in the current study, for DHBS and S2 salmochelin, it is possible that the FbpABC-dependent uptake system relies upon a low iron binding affinity. The binding affinities of S2 and DHBS for iron are lower than those of the intact, cyclic forms of enterobactin or salmochelin (49); thus our observation that these derivatives are specifically internalized by N. gonorrhoeae in an FbpABC-dependent mechanism suggests that the ability of FbpABC to acquire the iron may depend upon its loose association with the xenosiderophore. While intact enterobactin is also employed by N. gonorrhoeae in our assays, it is difficult to ensure that any preparation of enterobactin is free of the smaller breakdown products, including DHBS. Gonococcal metabolism, when provided glucose as a carbon source, is also associated with release of organic acids and a resultant decrease in the pH of the medium (48). This decreased pH could also hasten the release of iron from the xenosiderophores and facilitate its solubilization under aerobic conditions.
One possible portal through the outer membrane for iron, either bound to the siderophore or released from it extracellularly, is porin. Linearized siderophores or soluble, released iron could passively diffuse through channels in the outer membrane made by porins. The observation that growth promotion with these xenosiderophores was achieved only at relatively high concentrations suggests that concentration-dependent diffusion could play a role in entry. While porin-deficient mutants are not viable (29), we tried to block entry with large sugars such as stachyose (data not shown). Growth promotion with any iron source tested was inhibited by the addition of this sugar, suggesting that porin-mediated diffusion was indeed blocked, but the specificity for siderophore-derived iron could not be ascertained. Thus, our favored model at present involves the extracellular removal of iron from xenosiderophores and passage of the ferric iron through porins into the periplasm where FbpA can bind and shuttle the ferric ion to the cytoplasmic permease and ATPase components FbpB and FbpC.
In summary, we demonstrated that gonococcal strain FA19 is capable of utilizing the xenosiderophores enterobactin and salmochelin. Internalization of iron from these heterologous siderophores was independent of TonB and TonB-dependent transporters but was dependent upon expression of the FbpABC system. The means by which the iron crosses the gonococcal outer membrane remains to be defined, but it may involve nonspecific diffusion through ubiquitous, constitutively expressed porins. Solubilization and liberation of iron from the siderophores may be facilitated by acidic conditions produced by growth on glucose and by low-affinity interactions between siderophore derivatives and iron. The ability of the gonococcus to utilize siderophores that are produced by colocalized bacteria adds to the repertoire of iron-scavenging mechanisms deployed by this stealth pathogen.
Acknowledgments
Funding for this work was provided by U.S. Public Health Service grant numbers AI065555, AI047141 and AI084400 from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
We gratefully acknowledge Luis Actis and Sandra Armstrong for providing the xenosiderophores acinetobactin and alcaligin, respectively. We also thank the following collaborators for their contributions: Fred Sparling for providing the FetA-specific antiserum and fetA mutants; Bill Shafer for providing the mtrC, mtrD, and mtrE mutants; and Rob Nicholas for providing the pilQ mutant.
Editor: S. M. Payne
Footnotes
Published ahead of print on 1 November 2010.
REFERENCES
- 1.Adhikari, P., S. A. Berish, A. J. Nowalk, K. L. Veraldi, S. A. Morse, and T. A. Mietzner. 1996. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J. Bacteriol. 178:2145-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisen, P., A. Leibman, and J. Zweier. 1978. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 253:1930-1937. [PubMed] [Google Scholar]
- 3.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, J. S., E. A. Thompson, P. Kriz, K. A. Jolley, and M. C. Maiden. 2009. A common gene pool for the Neisseria FetA antigen. Int. J. Med. Microbiol. 299:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beucher, M., and P. F. Sparling. 1995. Cloning, sequencing, and characterization of the gene encoding FrpB, a major iron-regulated, outer membrane protein of Neisseria gonorrhoeae. J. Bacteriol. 177:2041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bister, B., D. Bischoff, G. J. Nicholson, M. Valdebenito, K. Schneider, G. Winkelmann, K. Hantke, and R. D. Sussmuth. 2004. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals 17:471-481. [DOI] [PubMed] [Google Scholar]
- 7.Biswas, G. D., J. E. Anderson, and P. F. Sparling. 1997. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol. Microbiol. 24:169-179. [DOI] [PubMed] [Google Scholar]
- 8.Biswas, G. D., and P. F. Sparling. 1995. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect. Immun. 63:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun, V. 1995. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16:295-307. [DOI] [PubMed] [Google Scholar]
- 10.Carson, S. D., P. E. Klebba, S. M. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson, S. D., B. Stone, M. Beucher, J. Fu, and P. F. Sparling. 2000. Phase variation of the gonococcal siderophore receptor FetA. Mol. Microbiol. 36:585-593. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2009. Sexually transmitted disease surveillance, 2008. Centers for Disease Control and Prevention, Atlanta, GA.
- 13.Chen, C. J., P. F. Sparling, L. A. Lewis, D. W. Dyer, and C. Elkins. 1996. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect. Immun. 64:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C. J., D. M. Tobiason, C. E. Thomas, W. M. Shafer, H. S. Seifert, and P. F. Sparling. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, C. Y., S. A. Berish, S. A. Morse, and T. A. Mietzner. 1993. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol. Microbiol. 10:311-318. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 18.Cornelissen, C. N., and P. F. Sparling. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 178:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crossman, S. H. 2006. The challenge of pelvic inflammatory disease. Am. Fam. Physician 73:859-864. [PubMed] [Google Scholar]
- 20.Densen, P. 1989. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin. Microbiol. Rev. 2(Suppl):S11-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeRocco, A. J. 2007. Molecular analysis of transferrin binding protein B in Neisseria gonorrhoeae. Ph.D. thesis. Virginia Commonwealth University, Richmond, VA.
- 22.Desai, P. J., A. Angerer, and C. A. Genco. 1996. Analysis of Fur binding to operator sequences within the Neisseria gonorrhoeae fbpA promoter. J. Bacteriol. 178:5020-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake, S. L., and M. Koomey. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975-986. [DOI] [PubMed] [Google Scholar]
- 24.Elkins, C., C. E. Thomas, H. S. Seifert, and P. F. Sparling. 1991. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J. Bacteriol. 173:3911-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson, A. D., and J. Deisenhofer. 2002. TonB-dependent receptors—structural perspectives. Biochim. Biophys. Acta 1565:318-332. [DOI] [PubMed] [Google Scholar]
- 26.Fischbach, M. A., H. Lin, D. R. Liu, and C. T. Walsh. 2005. In vitro characterization of IroB, a pathogen-associated C-glycosyltransferase. Proc. Natl. Acad. Sci. U. S. A. 102:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flo, T. H., K. D. Smith, S. Sato, D. J. Rodriguez, M. A. Holmes, R. K. Strong, S. Akira, and A. Aderem. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917-921. [DOI] [PubMed] [Google Scholar]
- 28.Gong, S., S. W. Bearden, V. A. Geoffroy, J. D. Fetherston, and R. D. Perry. 2001. Characterization of the Yersinia pestis Yfu ABC inorganic iron transport system. Infect. Immun. 69:2829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotschlich, E. C., M. E. Seiff, M. S. Blake, and M. Koomey. 1987. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc. Natl. Acad. Sci. U. S. A. 84:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagen, T. A. 2006. Mechanisms of iron acquisition employed by Neisseria gonorrhoeae for survival within cervical epithelial cells. Ph.D. thesis. Virginia Commonwealth University, Richmond, VA.
- 31.Hagen, T. A., and C. N. Cornelissen. 2006. Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol. Microbiol. 62:1144-1157. [DOI] [PubMed] [Google Scholar]
- 32.Hagman, K. E., C. E. Lucas, J. T. Balthazar, L. Snyder, M. Nilles, R. C. Judd, and W. M. Shafer. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117-2125. [DOI] [PubMed] [Google Scholar]
- 33.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 34.Hantke, K., G. Nicholson, W. Rabsch, and G. Winkelmann. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. U. S. A. 100:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hider, R. C., and X. Kong. 2010. Chemistry and biology of siderophores. Nat. Prod. Rep. 27:637-657. [DOI] [PubMed] [Google Scholar]
- 36.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528-535. [PubMed] [Google Scholar]
- 37.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenney, C. D. 2002. Characterization of the transferrin-binding protein complex expressed by Neisseria gonorrhoeae. Ph.D. thesis. Virginia Commonwealth University, Richmond, VA.
- 39.Kenney, C. D., and C. N. Cornelissen. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J. Bacteriol. 184:6138-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khun, H. H., S. D. Kirby, and B. C. Lee. 1998. A Neisseria meningitidis fbpABC mutant is incapable of using nonheme iron for growth. Infect. Immun. 66:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 42.Masri, H. P., and C. N. Cornelissen. 2002. Specific ligand binding attributable to individual epitopes of gonococcal transferrin binding protein A. Infect. Immun. 70:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mey, A. R., E. E. Wyckoff, L. A. Hoover, C. R. Fisher, and S. M. Payne. 2008. Vibrio cholerae VciB promotes iron uptake via ferrous iron transporters. J. Bacteriol. 190:5953-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mietzner, T. A., G. Bolan, G. K. Schoolnik, and S. A. Morse. 1987. Purification and characterization of the major iron-regulated protein expressed by pathogenic Neisseriae. J. Exp. Med. 165:1041-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 48.Morse, S. A., S. Stein, and J. Hines. 1974. Glucose metabolism in Neisseria gonorrhoeae. J. Bacteriol. 120:702-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller, S. I., M. Valdebenito, and K. Hantke. 2009. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals 22:691-695. [DOI] [PubMed] [Google Scholar]
- 50.Noinaj, N., M. Guillier, T. J. Barnard, and S. K. Buchanan. 2010. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64:43-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oves-Costales, D., N. Kadi, and G. L. Challis. 2009. The long-overlooked enzymology of a nonribosomal peptide synthetase-independent pathway for virulence-conferring siderophore biosynthesis. Chem. Commun. (Camb.) 43:6530-6541. [DOI] [PubMed] [Google Scholar]
- 52.Perry, R. D., J. Shah, S. W. Bearden, J. M. Thompson, and J. D. Fetherston. 2003. Yersinia pestis TonB: role in iron, heme, and hemoprotein utilization. Infect. Immun. 71:4159-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price, G. A. 2005. Immunogenicity of the gonococcal transferrin binding proteins. Ph.D. thesis. Virginia Commonwealth University, Richmond, VA.
- 54.Price, G. A., H. P. Masri, A. M. Hollander, M. W. Russell, and C. N. Cornelissen. 2007. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 25:7247-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raffatellu, M., M. D. George, Y. Akiyama, M. J. Hornsby, S. P. Nuccio, T. A. Paixao, B. P. Butler, H. Chu, R. L. Santos, T. Berger, T. W. Mak, R. M. Tsolis, C. L. Bevins, J. V. Solnick, S. Dandekar, and A. J. Baumler. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5:476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohde, K. H., and D. W. Dyer. 2003. Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Front. Biosci. 8:1186-1218. [DOI] [PubMed] [Google Scholar]
- 57.Rouquette, C., J. B. Harmon, and W. M. Shafer. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 33:651-658. [DOI] [PubMed] [Google Scholar]
- 58.Rouquette-Loughlin, C., I. Stojiljkovic, T. Hrobowski, J. T. Balthazar, and W. M. Shafer. 2002. Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrC-MtrD-MtrE efflux pump requires TonB-ExbB-ExbD proteins. Antimicrob. Agents Chemother. 46:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russel, M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279:485-499. [DOI] [PubMed] [Google Scholar]
- 60.Saken, E., A. Rakin, and J. Heesemann. 2000. Molecular characterization of a novel siderophore-independent iron transport system in Yersinia. Int. J. Med. Microbiol. 290:51-60. [DOI] [PubMed] [Google Scholar]
- 61.Seifert, H. S. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215-220. [DOI] [PubMed] [Google Scholar]
- 62.Shafer, W. M., W. L. Veal, E. H. Lee, L. Zarantonelli, J. T. Balthazar, and C. Rouquette. 2001. Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis. J. Mol. Microbiol. Biotechnol. 3:219-224. [PubMed] [Google Scholar]
- 63.Sparling, P. F. 1990. Biology of Neisseria gonorrhoeae, p. 131-138. In K. K. Holmes, P.-A. Mardh, P. F. Sparling, and P. J. Weisner (ed.), Sexually transmitted diseases, 2nd ed. McGraw-Hill, New York, NY.
- 64.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner, P. C., C. E. Thomas, C. Elkins, S. Clary, and P. F. Sparling. 1998. Neisseria gonorrhoeae heme biosynthetic mutants utilize heme and hemoglobin as a heme source but fail to grow within epithelial cells. Infect. Immun. 66:5215-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner, P. C., C. E. Thomas, I. Stojiljkovic, C. Elkins, G. Kizel, D. A. Ala'Aldeen, and P. F. Sparling. 2001. Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiology 147:1277-1290. [DOI] [PubMed] [Google Scholar]
- 67.van der Helm, D., R. Chakraborty, A. D. Ferguson, B. S. Smith, L. Esser, and J. Deisenhofer. 2002. Bipartite gating in the outer membrane protein FecA. Biochem. Soc. Trans. 30:708-710. [DOI] [PubMed] [Google Scholar]
- 68.Veal, W. L., A. Yellen, J. T. Balthazar, W. Pan, B. G. Spratt, and W. M. Shafer. 1998. Loss-of-function mutations in the mtr efflux system of Neisseria gonorrhoeae. Microbiology 144(Pt. 3):621-627. [DOI] [PubMed] [Google Scholar]
- 69.West, S. E., and P. F. Sparling. 1985. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect. Immun. 47:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyckoff, E. E., A. R. Mey, A. Leimbach, C. F. Fisher, and S. M. Payne. 2006. Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J. Bacteriol. 188:6515-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]