Abstract
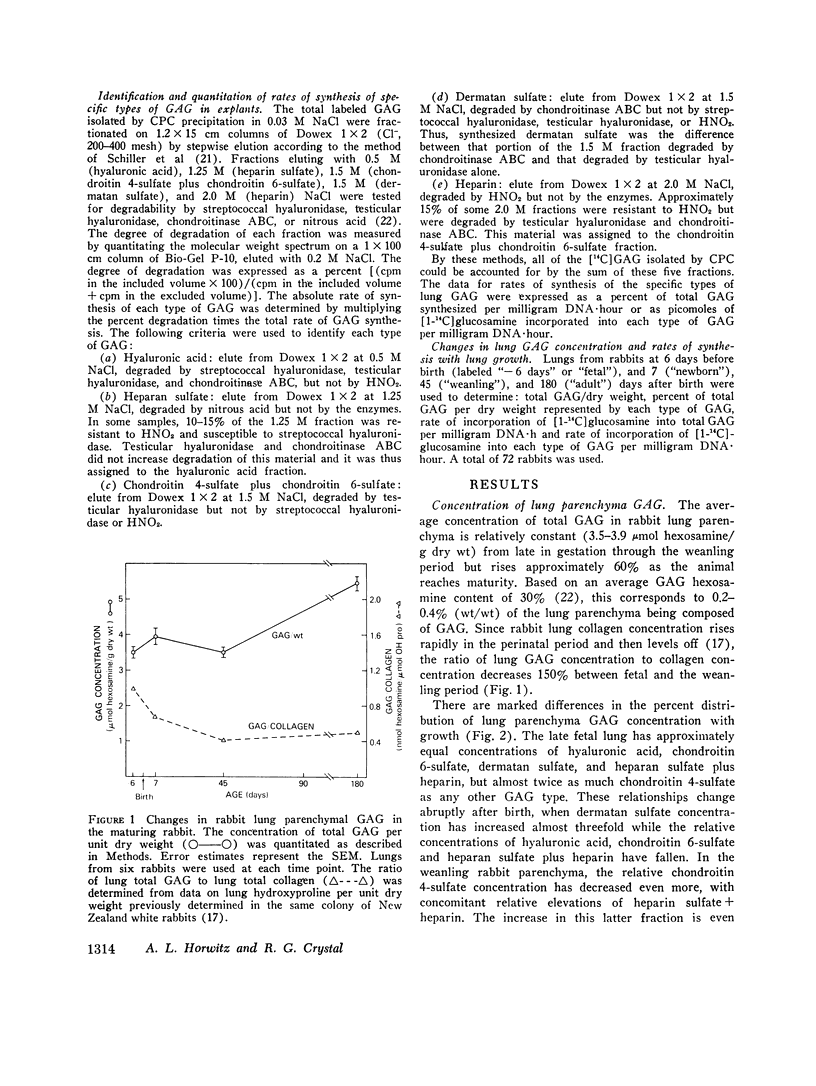
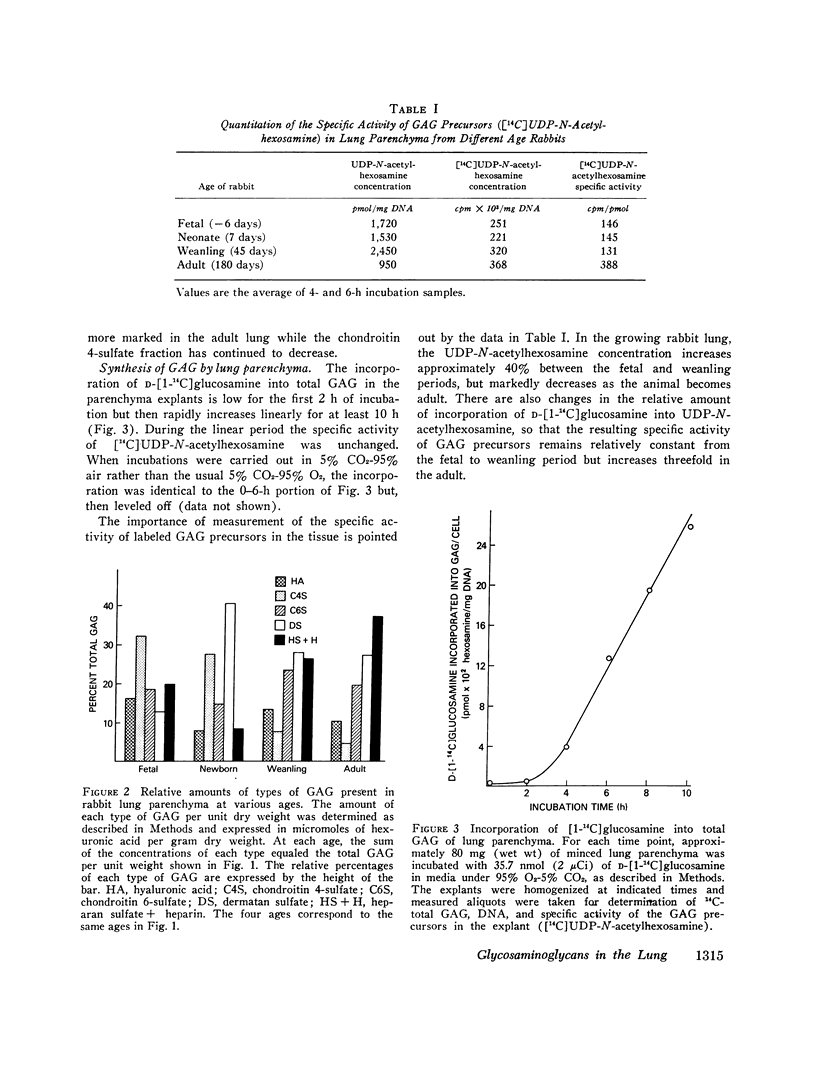
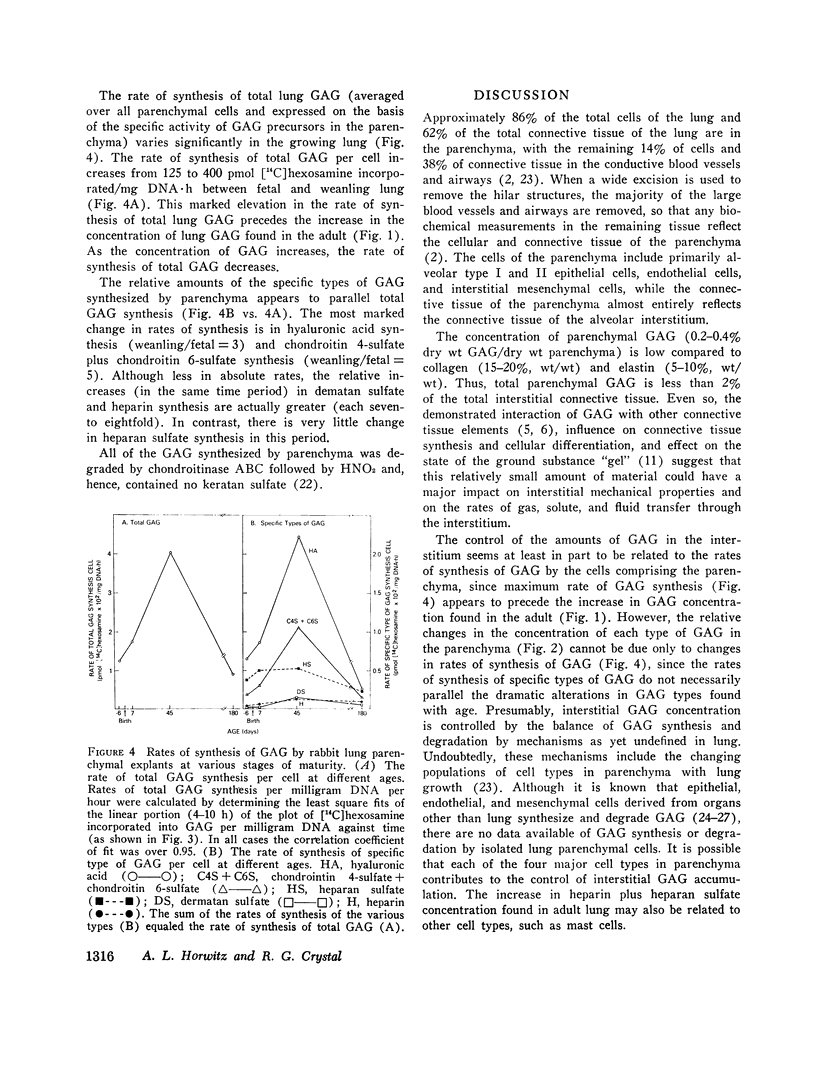
The function of lung is fundamentally linked to the connective tissue composition of the alveolar interstitium. The composition and synthesis of one class of interstitial connective tissue components, the glycosaminoglycans (GAG), was determined in lung parenchyma of rabbits at different stages of development. Parenchymal GAG content ranged between 0.2 and 0.4% (wt/wt) of dry weight, with highest concentration in adult lung. There were significant changes in types of GAG present at different ages. Fetal lungs contained a relatively high proportion of chondroitin 4-sulfate while the GAG in lung parenchyma of older animals was predominantly dermatan sulfate, heparan sulfate, and heparin. Methods were developed for the study of rates of synthesis of GAG by incorporation of [1-14C]glucosamine into lung explants. The rate of synthesis of total GAG per cell increased with development to a maximum in lung from weanling rabbits and fell to low rates of synthesis in mature rabbits. Fetal rabbit lung parenchyma synthesized mostly hyaluronic acid and heparan sulfate, while in weanling rabbit parenchyma hyaluronic acid and chondroitin 4/6-sulfate synthesis was greatest. In mature animals, the rates of synthesis of all types of GAG were relatively low but there was a relatively greater emphasis on synthesis of dermatan sulfate and heparin. These results may have significance in changes in lung function during development and in effects on other connective tissue components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K. H., McConnell S. D., Crystal R. G. Lung collagen composition and synthesis. Characterization and changes with age. J Biol Chem. 1974 May 10;249(9):2674–2683. [PubMed] [Google Scholar]
- Buonassisi V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp Cell Res. 1973 Feb;76(2):363–368. doi: 10.1016/0014-4827(73)90388-1. [DOI] [PubMed] [Google Scholar]
- CLAUSEN B. Influence of age on connective tissue. Uronic acid and uronic acid-hydroxyproline ratio in human aorta, myocardium, and skin. Lab Invest. 1962 Dec;11:1340–1345. [PubMed] [Google Scholar]
- Crystal R. G. Lung collagen: definition, diversity and development. Fed Proc. 1974 Nov;33(11):2248–2255. [PubMed] [Google Scholar]
- De Luca L., Wolf G. Effect of vitamin A on the mucopolysaccharides of lung tissue. Arch Biochem Biophys. 1968 Jan;123(1):1–8. doi: 10.1016/0003-9861(68)90097-0. [DOI] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman S. L., Burri P. H., Weibel E. R. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974 Sep;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Ginsburg V. The metabolism of glucosamine by tissue culture cells. Exp Cell Res. 1966 Mar;41(3):592–600. doi: 10.1016/s0014-4827(66)80109-x. [DOI] [PubMed] [Google Scholar]
- Laros C. D., Kuyper C. M., Janssen H. M. The chemical composition of fresh human lung parenchyma. An approach to the pathogenesis of lung emphysema. Respiration. 1972;29(5):458–467. doi: 10.1159/000192915. [DOI] [PubMed] [Google Scholar]
- Laros C. D. The pathogenesis of lung emphysema. A hypothesis. Respiration. 1972;29(5):442–457. doi: 10.1159/000192914. [DOI] [PubMed] [Google Scholar]
- Margolis R. U., Margolis R. K., Chang L. B., Preti C. Glycosaminoglycans of brain during development. Biochemistry. 1975 Jan 14;14(1):85–88. doi: 10.1021/bi00672a014. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Wusteman F. S. The glycosaminoglycans of human tracheobronchial cartilage. Biochem J. 1970 Dec;120(4):777–785. doi: 10.1042/bj1200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Acid mucopolysaccharides in cultured human fibroblasts. Lancet. 1969 Oct 18;2(7625):838–841. doi: 10.1016/s0140-6736(69)92289-2. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Glagov S. Acid mucopolysaccharide patterns in aging human cartilage. J Clin Invest. 1966 Jul;45(7):1103–1111. doi: 10.1172/JCI105416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochem J. 1965 Sep;96(3):710–716. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Stimulation of extracellular matrix synthesis in the developing cornea by glycosaminoglycans. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2310–2313. doi: 10.1073/pnas.71.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B. The influence of glycosaminoglycans on the formation of fibers from monomeric tropocollagen in vitro. Eur J Biochem. 1973 Apr 2;34(1):129–137. doi: 10.1111/j.1432-1033.1973.tb02739.x. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., DORFMAN A. Effect of age on the heparin content of rat skin. Nature. 1960 Jan 9;185:111–112. doi: 10.1038/185111a0. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. Dermatan sulfate-protein: isolation from and interaction with collagen. Arch Biochem Biophys. 1968 Dec;128(3):567–578. doi: 10.1016/0003-9861(68)90064-7. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusteman F. S. Glycosaminoglycans of bovine lung parenchyma and pleura. Experientia. 1972 Aug 15;28(8):887–888. doi: 10.1007/BF01924923. [DOI] [PubMed] [Google Scholar]
- Wusteman F. S., Gold C., Wagner J. C. Glycosaminoglycans and calcification in the lesions of progressive massive fibrosis and in pleural plaques. Am Rev Respir Dis. 1972 Jul;106(1):116–118. doi: 10.1164/arrd.1972.106.1.116. [DOI] [PubMed] [Google Scholar]
- Wusteman F. S., Johnson D. B., Dodgson K. S. The use of 'normal' rats in studies on the acid mucopolysaccharides of lung. Life Sci. 1968 Dec 15;7(24):1281–1287. doi: 10.1016/0024-3205(68)90257-9. [DOI] [PubMed] [Google Scholar]



