Abstract
Germination of Clostridium difficile spores is the first required step in establishing C. difficile-associated disease (CDAD). Taurocholate (a bile salt) and glycine (an amino acid) have been shown to be important germinants of C. difficile spores. In the present study, we tested a series of glycine and taurocholate analogs for the ability to induce or inhibit C. difficile spore germination. Testing of glycine analogs revealed that both the carboxy and amino groups are important epitopes for recognition and that the glycine binding site can accommodate compounds with more widely separated termini. The C. difficile germination machinery also recognizes other hydrophobic amino acids. In general, linear alkyl side chains are better activators of spore germination than their branched analogs. However, l-phenylalanine and l-arginine are also good germinants and are probably recognized by distinct binding sites. Testing of taurocholate analogs revealed that the 12-hydroxyl group of taurocholate is necessary, but not sufficient, to activate spore germination. In contrast, the 6- and 7-hydroxyl groups are required for inhibition of C. difficile spore germination. Similarly, C. difficile spores are able to detect taurocholate analogs with shorter, but not longer, alkyl amino sulfonic acid side chains. Furthermore, the sulfonic acid group can be partially substituted with other acidic groups. Finally, a taurocholate analog with an m-aminobenzenesulfonic acid side chain is a strong inhibitor of C. difficile spore germination. In conclusion, C. difficile spores recognize both amino acids and taurocholate through multiple interactions that are required to bind the germinants and/or activate the germination machinery.
Clostridium difficile is a Gram-positive, rod-shaped, obligate anaerobic bacterium. As a survival mechanism, C. difficile forms metabolically inactive spores during nutrient deprivation (23). Like those of Bacillus and Clostridium, C. difficile spores are highly resistant to many physical and chemical insults. Spores return to vegetative growth through a process called germination (12, 20). Germination is initiated as the spore encounters nutrient-rich environments, allowing the spores to revert to replicating cells (21, 32).
When C. difficile spores germinate in the human intestine, toxins are produced and host cells are damaged (24, 36). This disease is known as Clostridium difficile-associated disease (CDAD) and is responsible for approximately 25% of all antibiotic-associated diarrhea (37). Contamination of hospital environments with C. difficile spores is a key factor associated with infection spread (5). CDAD is primarily a nosocomial infection, and mortality and morbidity are estimated to cost the U.S. health care system over $3 billion per year (17, 22).
In a healthy individual, indigenous intestinal bacteria resist C. difficile colonization (30). Immunocompromised patients, such as cancer patients with severe neutropenia (18) and patients in the postoperative period following organ transplantation (2), are typically administered antibiotics prophylactically. Following aggressive antimicrobial treatments, the normal gut microflora is disrupted, allowing the germination of C. difficile spores. The resulting toxin-producing vegetative cells fill empty niches in the depleted microbial community, leading to the onset of CDAD (5). Hence, antibiotic treatments favor colonization by C. difficile and the establishment of CDAD in already weakened individuals (39). C. difficile is also the most common cause of diarrhea in HIV-infected patients (4). CDAD is difficult to treat and is commonly recurrent, resulting in an increase in morbidity and mortality rates (6).
Spore germination mechanisms have been studied mainly in Bacillus. In most cases, the germination process is triggered by the detection of low-molecular-weight germinants by a sensitive biosensor (21, 32). This sensor consists of a proteinaceous germination (Ger) receptor encoded, in general, by a tricistronic operon. Ger receptors are activated by a variety of small biomolecules, including amino acids, sugars, and nucleosides (11, 14). Proteins involved in germination are remarkably conserved in both Bacillus and Clostridium. Basic Local Alignment Search Tool (BLAST) searches of spore-specific proteins revealed analogs in all sequenced sporulating bacteria. Interestingly, C. difficile encodes analogs for all spore-specific proteins except Ger receptors and spore coat proteins (31). Possibly, C. difficile Ger receptors are too divergent from those of other sporulating bacteria. Alternatively, C. difficile may use a different set of proteins as germination receptors.
A recent article revealed that C. difficile spores recognize glycine (an amino acid) and taurocholate (a bile salt) as germinants (35). Furthermore, chenodeoxycholate, another bile salt, has been shown to inhibit C. difficile spore germination (34). Neither glycine nor taurocholate has been previously described as a germinant for spores of Bacillus or Clostridium species, suggesting a novel mode of germinant recognition in C. difficile spores. We have recently published kinetic analyses of C. difficile spore germination in the presence of taurocholate and glycine. We showed that C. difficile spores bind taurocholate and glycine by a complex cooperative mechanism where the affinity of the spore for one germinant is affected by binding of the other (26). We also showed that chenodeoxycholate is a competitive inhibitor of taurocholate (26). We have seen similar cooperative behavior in the germination of Bacillus cereus and Clostridium sordellii spores (1, 27). Thus, C. difficile spores probably encode unknown receptor proteins to bind these germinants (26).
Due to the scarcity of genetic tools, many of the metabolic capabilities encoded by C. difficile remain poorly understood (19). This has precluded the use of molecular microbiology to identify putative germination receptors encoded by C. difficile. As an alternative to genetic manipulation, we have used molecular probes to study the mechanism of Bacillus and Clostridium spore germination (1, 8, 26). This approach provides mechanistic information, even when the identity of the germination receptors is unknown (27). In the present study, we synthesized and tested a series of glycine and taurocholate analogs for the ability to induce or inhibit C. difficile spore germination. Structure-activity relationship analysis allowed us to determine which taurocholate and amino acid functional groups are necessary and sufficient to bind to and/or activate C. difficile spores. Our data suggest either the presence of multiple amino acid germination receptors or that the putative glycine receptor recognizes structurally diverse amino acids. Furthermore, the putative taurocholate germination receptor recognizes its cognate germinant through multiple molecular interactions. In fact, we have discovered one interesting molecule that is four times more active than the natural inhibitor, chenodeoxycholate, at inhibiting C. difficile spore germination.
MATERIALS AND METHODS
Materials.
Taurocholate and amino acid analogs were purchased from Sigma-Aldrich Corporation (St. Louis, MO) or Steraloids (Newport, RI) or were synthesized in the Abel-Santos laboratory. Reagents for synthesis were purchased from Sigma-Aldrich Corporation (St. Louis, MO) or Alpha Aesar (Ward Hill, MA). Thin-layer chromatography silica gel 60 F254 was purchased from EMD Chemicals (Gibbstown, NJ). Silica gel for column chromatography was purchased from Fisher Scientific (Pittsburg, PA).
Synthesis of T09 and T10.
Methoxylated taurocholate analogs (3-methoxy-7,12-dihydroxytaurocholate [T09] and 3,7-dimethoxy-12-hydroxytaurocholate [T10]) were prepared following published procedures (3). Briefly, to a solution of taurocholate (1 equivalent) in dry 1,4-dioxane, methyl iodide (50 equivalents) and sodium hydride (4 equivalents) were added under nitrogen. The reaction mixture was heated to 40°C for 48 h with stirring. After the initial 48 h, sodium hydride (4 equivalents) was added daily to the reaction mixture for four additional days. The reaction mixture was then diluted with dichloromethane and washed with 1 M HCl and twice with water. The organic layer was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The resulting residue was purified by silica gel column chromatography eluted with a step gradient from 100% dichloromethane to 60% dichloromethane/acetone. Two different compounds were obtained. 1H nuclear magnetic resonance (NMR) and mass spectrometry showed that one compound had a single methoxy group and the second compound had two methoxy groups. The compounds were tentatively identified as T09 and T10, as determined by published OH reactivity (13, 15).
Synthesis of T11 to T16 and T18 to T21.
The taurocholate analogs CAAMSA (T11), CAAPSA (T12), CApSA (T13), CAoSA (T14), CAmSA (T15), hypotaurocholate (T16), CAAPA (T18), CA2APA (T19), CAABA (T20), and CA2ABA (T21) were prepared following published procedures (7, 38). Briefly, cholic acid (1 equivalent) was activated with 1.4 equivalents of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) and 1.3 equivalents of N-methylmorpholine (NMO) in dimethylformamide (DMF). After the mixture was stirred for 5 min, 1.2 equivalents of the appropriate amino sulfonic acid or amino acid was added. The reaction mixture was heated to 90°C for 40 min and then cooled to room temperature. The solution was poured into 100 ml of ice-cold diethyl ether, resulting in a precipitate. The ether suspension was kept at 4°C overnight. The ether layer was decanted, and the resinous residue was dissolved in 25 ml 0.2 N NaOH-MeOH and poured into 100 ml cold diethyl ether. The ether solution was kept at 4°C for at least 2 h, and the resulting precipitate was filtered and washed with diethyl ether. If necessary, the product was recrystallized by dissolving it in hot ethanol to saturation, followed by the addition of ethyl acetate until a precipitate appeared. The solution was kept at −20°C for 2 h to allow complete precipitation and then filtered to retrieve the product. The precipitated residue was further purified by silica gel column chromatography eluted with a step gradient from 100% dichloromethane to 100% ethanol. CA2APA (T19) and CA2ABA (T21) were obtained as side products of the synthesis of CAAPA (T18), and CAABA (T20), respectively. Compound structures were verified by 1H-NMR, Fourier transform infrared spectroscopy (FTIR), and mass spectrometry.
Synthesis of CAHESA (T22).
Conjugation of cholate to the sulfonic acid alkyl linker by an ester was prepared following established protocols for Fischer esterification (10, 16, 33). Briefly, to a solution of cholate (1 equivalent) and hydroxy ethane sulfonic acid (4 equivalents), concentrated sulfuric acid was added dropwise and refluxed for 1 h. The reaction mixture was poured into cold diethyl ether, and a precipitate formed immediately. The diethyl ether suspension was left overnight at 4°C. The precipitate was filtered, dissolved in 0.2 N NaOH-MeOH, precipitated a second time in diethyl ether, and kept at 4°C for at least 2 h. The crude precipitate was filtered and purified by silica gel column chromatography eluted by step gradient from 100% dichloromethane to 30% dichloromethane-ethyl alcohol (EtOH). The compound structure was verified by 1H-NMR, FTIR, and mass spectrometry.
Bacterial strains and spore preparation.
C. difficile strain 630 (ATCC BAA-1382) was obtained from the American Type Culture Collection (ATCC). C. difficile cells were plated in BHIS (brain heart infusion broth supplemented with 5 mg/ml yeast extract and 0.1% l-cysteine) agar supplemented with 1% yeast extract, 0.1% l-cysteine-HCl, and 0.05% sodium taurocholate to yield single-cell clones. Single C. difficile colonies were grown in BHIS broth and spread plated onto agar to obtain bacterial lawns. The plates were incubated for 5 days at 37°C in an anaerobic environment (5% CO2, 10% H2, and 80% N2). The resulting bacterial lawns were collected by flooding them with ice-cold deionized water. Spores were pelleted by centrifugation and resuspended in fresh deionized water. After two washing steps, the spores were separated from vegetative and partially sporulated forms by centrifugation through a 20% to 50% HistoDenz gradient. The spore pellet was washed five times with water, resuspended in sodium thioglycolate (0.5 g/liter), and stored at 4°C.
Activation of C. difficile spore germination by amino acids and taurocholate analogs.
Spores were diluted in germination buffer (100 mM sodium phosphate buffer [pH 6.0] containing 5 mg/ml sodium bicarbonate) to an optical density at 580 nm (OD580) of 1.0. To test for taurocholate agonists of spore germination, the spore suspensions were individually supplemented with 6 mM corresponding taurocholate analogs and 12 mM glycine. To test for amino acid agonists of spore germination, spore suspensions were supplemented with 12 mM corresponding amino acid analogs and 6 mM taurocholate. Spore germination was evaluated based on the decrease in the OD580 at 30°C each minute for 90 min. Germination rates were set to 100% for C. difficile spores germinated with 6 mM sodium taurocholate and 12 mM glycine. The percent germination for all analogs was calculated as the fraction of the germination rate compared to these conditions.
Inhibition of C. difficile spore germination by amino acids and taurocholate analogs.
To test for antagonists of spore germination, spore aliquots were individually supplemented with various concentrations of taurocholate analogs or amino acid analogs. Spore suspensions were incubated for 15 min at room temperature while the OD580 was monitored. If no germination was detected, taurocholate and glycine were added to 6 and 12 mM final concentrations, respectively. Relative OD580 values were obtained every minute for 90 min after germinant addition and were plotted against the logarithm of inhibitor concentrations. As expected, germination decreased in the presence of active germination inhibitors. The resulting data were fitted using the four-parameter logistic function of SigmaPlot v.9 to obtain 50% inhibitory concentration (IC50) values (28, 29).
C. difficile spore germination in BHIS medium.
To test for germination in complex media, spores were resuspended in BHIS alone and with combinations of taurocholate, chenodeoxycholate, glycocholate, glycine, l-arginine, and l-phenylalanine. Bile salts were added to a final concentration of 6 mM, and amino acids were added at a final concentration of 12 mM. Relative OD580 values were obtained every minute for 90 min after germinant addition.
RESULTS AND DISCUSSION
C. difficile has been shown to germinate in the presence of glycine and taurocholate (35). The question that has remained unanswered is how glycine and taurocholate interact with the putative binding sites. In the current work, we tested 30 amino acid analogs and 22 taurocholate analogs as activators (Fig. 1 A) or inhibitors (Fig. 1B) of C. difficile spore germination. Structure-activity relationship analysis of germinant analogs allows a better understanding of the microenvironment of the C. difficile germination machinery. Activators of the germination pathway identify which functional groups are essential for binding and activation of the C. difficile germination machinery. On the other hand, inhibiting agents provide structural details about functional groups that allow only binding. Inhibition assays serve as an indirect method to map physiochemical configurations in receptor binding sites. Competitive inhibitors most likely bind to the same site as the cognate germinant. Strong competitive inhibitors complement the germinant binding site shape, size, hydrophobicity, and hydrogen bonding pattern. Inactive compounds yield information on functional groups that interfere with germinant binding (8). As expected, changing glycine and taurocholate functional groups affected the germination of C. difficile spores.
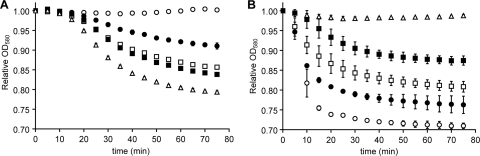
FIG. 1.
Germination kinetic graphs showing agonistic and antagonistic behavior of molecules with C. difficile spores. (A) Activation of germination. C. difficile spores were treated with a fixed concentration of taurocholate (6 mM), and glycine was added at 0 mM (○), 8 mM (•), 10 mM (□), 12 mM (▪), and 14 mM (▵) final concentrations. For clarity, the data are shown at 5-min intervals and for only five glycine concentrations. (B) Inhibition of germination. C. difficile spores were incubated with 0 mM (○), 0.0005 mM (•), 0.001 mM (□), 0.075 mM (▪), and 7.5 mM (▵) concentrations of CAmSA (T15) and supplemented with taurocholate (6 mM) and glycine (12 mM). For clarity, the data are shown at 5-min intervals and for only five CAmSA (T15) concentrations. Although data were collected for 90 min, only 75 min are shown in both graphs for clarity. The error bars indicate standard deviations.
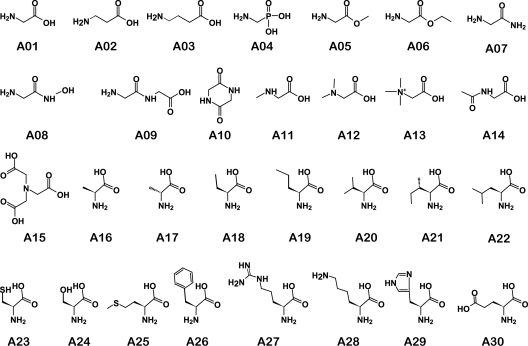
Glycine (A01) has a methylene bridge that separates the carboxylic and amine groups and is the simplest of the 20 common amino acids. To find determinants required for glycine recognition, we individually supplemented taurocholate-treated spores with 30 different glycine analogs (Fig. 2). Each of the glycine analogs differs from the parent compound by a single modification in either the length of the alkyl chain, substitutions to the amino group, or changes in the carboxylate group. β-Alanine (A02) and γ-aminobutyric acid (A03) have an ethylene and a propylene bridge between the amino and carboxylate group, respectively. These changes progressively increase the distance between the amino and carboxylate groups. β-Alanine and γ-aminobutyric acid are as effective as glycine as cogerminants of C. difficile spores (Fig. 3). Thus, lengthening the chain between the amino and carboxylate functional groups does not interfere with recognition by the putative glycine germination receptor.
FIG. 2.
Amino acids assessed for activation or inhibition of glycine-mediated germination in C. difficile spores. A01, glycine; A02, β-alanine; A03, γ-aminobutyric acid; A04, aminomethylphosphonic acid; A05, glycine methyl ester; A06, glycine ethyl ester; A07, glycinamide; A08, glycine hydroxamate; A09, diglycine; A10, glycine anhydride; A11, sarcosine; A12, N,N-dimethylglycine; A13, betaine; A14, N-acetylglycine; A15, nitrilotriacetic acid; A16, l-alanine; A17, d-alanine; A18, l-2-aminobutyric acid; A19, l-norvaline; A20, l-valine; A21, l-isoleucine; A22, l-leucine; A23, l-cysteine; A24, l-serine A25, l-methionine; A26, l-phenylalanine; A27, l-arginine; A28, l-lysine; A29, l-histidine; A30, l-aspartic acid.
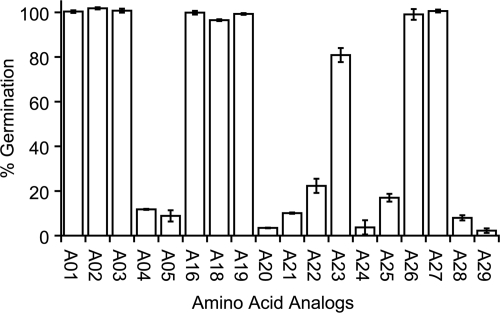
FIG. 3.
Comparison of amino acids as agonists of C. difficile spore germination. Spores were treated with taurocholate (6 mM) and amino acid analogs at 12 mM. Germination was determined by the decrease in the OD580 for 90 min at 30°C. The percent germination for each analog was calculated based on glycine/taurocholate germination set as 100%. The error bars indicate standard deviations.
Aminomethylphosphonic acid (A04) is a glycine analog in which the carboxylate has been changed to a phosphonate. This substitution exchanges a carbon atom for phosphorus while retaining the negative charge. A04 significantly decreased C. difficile spore germination (Fig. 3). Furthermore, methylation of the carboxylate in glycine methyl ester (A05) resulted in germination rates of less than 10% compared to glycine-triggered germination (26). Any other modification of the carboxylate (glycine ethyl ester [A06[, glycinamide [A07], and glycine hydroxamate [A08]) resulted in compounds that were unable to activate or inhibit C. difficile spore germination (data not shown). The sum of these data suggests that there is a specific requirement for a carboxylate functional group for recognition by the glycine germination receptor to activate germination.
The distance between the amino and carboxylate of diglycine (A09) is similar to that of γ-aminobutyric acid (A03). However, whereas γ-aminobutyric acid is a good agonist for C. difficile spore germination, diglycine has no effect (data not shown). Thus, the addition of an internal amide must interfere with compound binding. Possibly there is a requirement for a more hydrophobic linker between the two functional-group ends. Glycine anhydride (A10) is the result of the dehydration of diglycine, forming a cyclic diamide. Without a free amine or carboxylate, this compound is unable to activate or inhibit spore germination (data not shown). The rigidity and bulkiness of the analog may prevent interaction with the glycine binding site.
Alkylation (sarcosine [A11], N,N-dimethylglycine [A12], and betaine [A13]), acetylation (N-acetylglycine [A14]), or other modification (nitrilotriacetic acid [A15]) to the amino group of glycine resulted in compounds that can neither activate nor inhibit C. difficile spore germination (data not shown). This suggests that activation of C. difficile spores by glycine has a requirement for a free primary amino group regardless of the presence of an unmodified carboxylate group.
To test the effect of side chain substitution in amino acid recognition by the C. difficile germination machinery, we exposed taurocholate-treated C. difficile spores to other amino acid analogs (Fig. 2). l-Alanine (A16) has been shown to act as a germinant and/or cogerminant in other sporulating bacteria (1, 14). The stereoisomer, d-alanine (A17), has been shown to inhibit alanine-mediated germination in Bacillus (25). In C. difficile, l-alanine was as efficient at triggering germination as glycine (A01) (Fig. 3). Interestingly, d-alanine was unable to inhibit the germination of spores treated with l-alanine and taurocholate (data not shown). d-Alanine was also inactive as an agonist for C. difficile spore germination (data not shown). This implies that stereochemistry is important for recognition and binding of amino acids.
To determine whether amino acid analogs with longer linear alkyl side chains were able to activate C. difficile spore germination, we exposed taurocholate-treated spores to l-2-aminobutyric acid (A18) and l-norvaline (A19). Both of these amino acid analogs were able to activate germination to levels similar to those with glycine (A01). l-valine (A20) is a branched isomer of l-norvaline and has similar chemical and physical properties. However, this slight difference in structure reduced C. difficile spore germination by more than 90% compared to that of l-norvaline (Fig. 3). Similarly, l-isoleucine (A21) and l-leucine (A22) are poor germinants of C. difficile spores compared to l-norvaline. The data suggest that branched alkyl side chains are unable to be recognized by the putative amino acid germination receptor in C. difficile.
l-Cysteine (A23) is an amino acid with a methanethiol side chain. l-Serine (A24), on the other hand, is an l-cysteine analog in which the thiol group is substituted for a hydroxyl group. Interestingly, whereas l-cysteine is a good germinant of C. difficile spores (Fig. 3), the more polar l-serine is almost inactive. The germination activity of l-cysteine is not due solely to hydrophobicity, since the more hydrophobic l-methionine (A25) is a poor germinant compared to l-cysteine. This suggests that C. difficile spores recognize the thiol group specifically as a determinant for germination.
Surprisingly, l-phenylalanine (A26), with a bulky aromatic side chain, is as effective as glycine as a germinant of C. difficile spores (Fig. 3). We would expect that since branched amino acids are inactive, the bulkier side chain of l-phenylalanine would also be restricted from the binding site. We cannot completely rule out the possibility that the putative glycine receptor is able to accommodate phenyl but not branched alkyl side chains. However, we find that possibility unlikely due to their sizes relative to glycine. Hence, we postulate that aromatic amino acids are recognized by a separate binding site in the C. difficile spore. Indeed, other Bacillus and Clostridium strains are able to recognize structurally different germinants by encoding multiple receptors (8, 12, 21, 27).
l-Arginine (A27) is also a strong cogerminant for C. difficile spores (Fig. 3). Although l-arginine has a linear alkyl chain, it also contains a positively charged, branched guanidinium group. Similarly, l-lysine (A28) is linear with a positively charged amino side chain, yet l-lysine is a weak germinant compared to l-arginine. l-Histidine (A29) contains an aromatic side chain, like l-phenylalanine (A26), but it is positively charged, like l-arginine's side chain. However, unlike A26 or l-arginine, l-histidine could not efficiently activate C. difficile spore germination. l-Aspartic acid (A30) has a short acidic side chain and was similarly unable to affect C. difficile spore germination (data not shown). Thus, l-arginine was the only polar and/or charged amino acid tested that was able to induce C. difficile spore germination. Since l-arginine has physicochemical properties that are very different from those of the other amino acids that are able to activate C. difficile spore germination, it suggests there is a specific recognition site for l-arginine binding.
Earlier studies (35) reported that only glycine was able to trigger C. difficile spore germination in the presence of taurocholate. This study reportedly used three combinations of defined media to narrow down active germination stimulators. Although glycine was supplemented with only 7 amino acids, phenylalanine and arginine were in a mixture supplemented with 15 other amino acids. The presence of multiple weak germinants in the defined media containing phenylalanine and arginine could mask their ability to stimulate germination. Furthermore, in those experiments, l-phenylalanine and l-arginine were supplemented at concentrations (1.21 mM and 1.15 mM, respectively) lower than those used for the current experiments (12 mM).
Glycine, l-arginine, and l-phenylalanine individually or in pairs do not trigger C. difficile spore germination in the absence of taurocholate (data not shown). However, a cocktail of l-phenylalanine, l-arginine, and glycine (all at 12 mM) was able to effectively trigger C. difficile spore germination in the absence of taurocholate (Fig. 4). Chenodeoxycholate (6 mM) is not able to inhibit the germination of C. difficile spores treated with amino acids only. Other clostridia have been shown to use amino acids alone as germination signals (27).
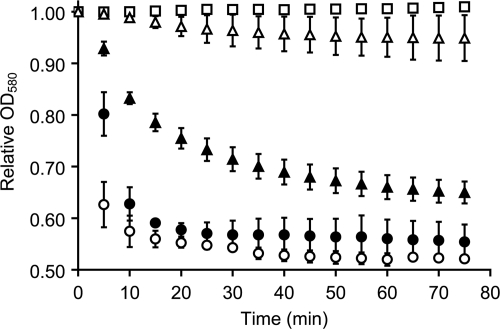
FIG. 4.
Germination kinetic graph showing behavior of C. difficile spores and germinants in buffer and complex media. C. difficile spores were resuspended in germination buffer and treated with l-phenylalanine, l-arginine, and glycine (each at 12 mM) (○) or l-phenylalanine, l-arginine, glycine (each at 12 mM), and chenodeoxycholate (6 mM) (•). Purified spores were also suspended in BHIS medium (□), BHIS supplemented with 12 mM taurocholate (▴), or BHIS supplemented with 12 mM taurocholate and 12 mM chenodeoxycholate (▵). For clarity, data are shown at 5-min intervals and only for 75 min. The error bars indicate standard deviations.
When C. difficile spores are resuspended in BHIS medium, germination is very slow, even though BHIS contains a complex amino acid mixture that includes glycine, l-arginine, and l-phenylalanine (Fig. 4). It is possible that BHIS contains amino acids that are weak germinants and that compete with glycine, l-phenylalanine, and l-arginine for binding. Binding of these alternative substrates causes a fraction of the spore population to germinate at a lower rate. We have seen similar behavior in the germination of C. sordellii spores (27). Interestingly, supplementing BHIS with taurocholate (Fig. 5, T01) augmented C. difficile spore germination (Fig. 4). The taurocholate-enhanced spore germination in BHIS was inhibited by chenodeoxycholate. Thus, it seems that amino-acid-only C. difficile spore germination occurs only when a limited number of amino acids are present and is disfavored with complex amino acid mixtures.
FIG. 5.
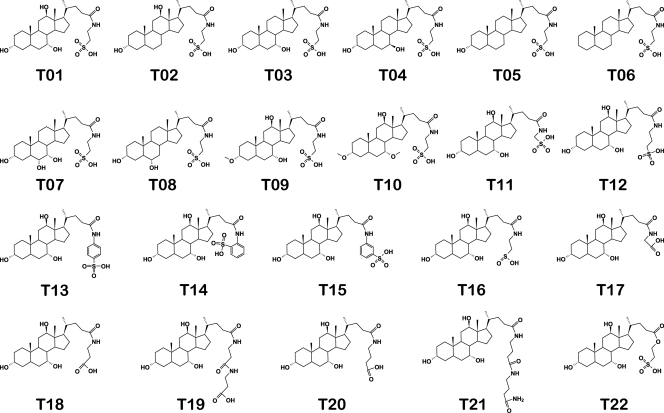
Taurocholate analogs assessed for activation or inhibition of taurocholate-mediated germination in C. difficile spores. T01, taurocholate; T02, taurodeoxycholate; T03, taurochenodeoxycholate; T04, tauroursodeoxycholate; T05, taurolithocholate; T06, taurocholanate; T07, taurohycholate; T08, taurohyodeoxycholate; T09, 3-methoxy-7,12-dihydroxytaurocholate; T10, 3,7-dimethoxy-12-hydroxytaurocholate; T11, CAAMSA; T12, CAAPSA; T13, CApSA; T14, CAoSA; T15, CAmSA; T16, hypotaurocholate; T17, glycocholate; T18, CAAPA; T19, CA2APA; T20, CAABA; T21, CA2ABA; T22, CAHESA.
All glycine analogs and amino acids were further tested for the ability to inhibit C. difficile spore germination. Spores were treated with each analog or amino acid in the presence of taurocholate and glycine. However, no individual amino acid analog inhibited C. difficile spore germination (data not shown).
Taurocholate (T01) is a natural bile salt that has hydroxyl groups at positions 3, 7, and 12 of the cholate skeleton. All three hydroxyl groups are in the alpha configuration. Taurocholate also has a side chain consisting of taurine attached to the cholate skeleton by an amide bond. Taurocholate activates C. difficile spore germination with a 50% effective concentration (EC50) of 15.9 mM. Taurocholate analogs were tested for the ability to activate germination in the presence of glycine and for the ability to inhibit germination in the presence of taurocholate and glycine.
To understand the importance of hydroxyl groups on the cholate backbone of taurocholate, analogs T02 to T08 (Fig. 5) were tested as agonists and antagonists of C. difficile spore germination. These analogs differ from taurocholate (T01) in the number, placement, or stereochemistry of the hydroxyl groups. Taurodeoxycholate (T02) lacks only the hydroxyl group at the 7 position. This change was sufficient to reduce germination by more than 70% (Table 1). Meanwhile, taurochenodeoxycholate (T03) is missing only the hydroxyl at position 12 and was able to induce germination only to 10% of the level of taurocholate. Tauroursodeoxycholate (T04) is an isomer of taurochenodeoxycholate in which the 7-hydroxyl is in the beta configuration. Alteration of the stereochemistry of this one hydroxyl group further decreased germination activity from 10% for taurochenodeoxycholate to 3% for tauroursodeoxycholate (Table 1).
TABLE 1.
Effect of taurocholate hydroxyl groups on C. difficile spore germination
 |
a C. difficile spores were individually treated with 6 mM glycine and 12 mM taurocholate analogs T01 to T10. The percent germination was calculated based on taurocholate/glycine germination set as 100%. Standard deviations are shown in parentheses.
b C. difficile spores were incubated with various concentrations of taurocholate analogs for 15 min prior to the addition of 6 mM taurocholate and 12 mM glycine. The IC50 was calculated by plotting the extent of germination versus the logarithm of analog T02 to T10 concentrations. Standard deviations are shown in parentheses.
c NA, no activity under the conditions tested.
As expected, taurolithocholate (T05) and taurocholanate (T06), which lack hydroxyls at positions 7 and 12 and 3, 7, and 12, respectively, are unable to activate germination. Taurohycholate (T07) and taurohyodeoxycholate (T08) are isomers of taurocholate (T01) and taurodeoxycholate (T02), respectively, in which the 12-hydroxyl groups were moved to the 6 position. Neither of these compounds is able to significantly activate germination of C. difficile spores. The sum of these data suggests that both the 7 and 12 α-hydroxyls of taurocholate are important determinants for binding and activation of C. difficile spores.
The 3-hydroxyl position of the cholate molecule is more nucleophilic than the other two hydroxyls. Similarly, the 7-hydoxyl is more reactive than the 12-hydroxyl (13, 15). Thus, methylation of taurocholate yielded two compounds that we putatively identified as 3-methoxy-7,12-dihydroxytaurocholate (T09) and 3,7-dimethoxy-12-hydroxytaurocholate (T10). Interestingly, 3-methoxy-7,12-dihydroxytaurocholate neither induces nor inhibits C. difficile spore germination. As expected, 3,7-dimethoxy-12-hydroxytaurocholate was also inactive. This suggests that the ability to donate a 3-hydroxyl hydrogen bond is essential for recognition of taurocholate as a germinant for C. difficile spores.
To determine if analogs differing in the number, location, or stereochemistry of the hydroxyl groups can inhibit taurocholate-mediated germination, C. difficile spores were treated with taurocholate (T01), glycine (A01), and compounds T02 to T10 (Table 1). Only taurochenodeoxycholate (T03), tauroursodeoxycholate (T04), and taurohyodeoxycholate (T08) showed germination-inhibitory properties. All three inhibitors have the common feature of lacking the 12-hydroxyl group. Since the 12-hydroxyl group was necessary for triggering spore germination, these results suggest that this hydroxyl is necessary for activation of germination but not for binding of taurocholate to the putative C. difficile germination receptor. The inhibitory compounds also have hydroxyl groups at either the 6 or 7 position (but not both), indicating that having one (but not two) hydroxyls in the B ring is important for inhibition of taurocholate-mediated germination of C. difficile spores.
To determine the taurine side chain functional groups responsible for recognition by the C. difficile germination machinery, analogs T11 to T22 were tested for the ability to induce spore germination in the presence of glycine (Fig. 5). All of these compounds differ from taurocholate in the structure of the side chain and retain the cholate skeleton with hydroxyl groups at the 3, 7, and 12 positions.
CAAMSA (T11) is a taurocholate analog in which the alkyl linker between the sulfonate and the amide was shortened by one methylene. CAAPSA (T12), on the other hand, is a taurocholate analog in which the linker was lengthened by one methylene (Fig. 5). The results show that spores treated with CAAMSA germinate to levels comparable with those of spores treated with taurocholate (T01) (Table 2 ). In contrast, the longer alkyl chain, CAAPSA, does not activate germination. To further analyze the necessity for the alkyl linker in germination, we synthesized three analogs containing a benzene ring in place of the ethylene linker of taurine (Fig. 5). These analogs differed in the position of the sulfonate, para (CApSA [T13]), ortho (CAoSA [T14]), or meta (CAmSA [T15]), with respect to the amino group in the benzene ring. These three taurocholate analogs were unable to activate C. difficile spore germination. This suggests that C. difficile spores are activated by taurocholate analogs with shorter, but not longer or bulkier, linkers.
TABLE 2.
Effect of the taurocholate side chain (R) on C. difficile spore germination
a C. difficile spores were individually treated with 6 mM glycine and 12 mM taurocholate analogs T11 to T22. The percent germination was calculated based on taurocholate/glycine germination set as 100%. Standard deviations are shown in parentheses.
b C. difficile spores were incubated with various concentrations of taurocholate analogs for 15 min prior to the addition of 6 mM taurocholate and 12 mM glycine. The IC50 was calculated by plotting the extent of germination versus the logarithm of analog T11 to T22 concentrations. Standard deviations are shown in parentheses.
c N/A, no activity under the conditions tested.
Hypotaurocholate (T16) differs from taurocholate by a substitution of a sulfonate for a sulfinate (Fig. 5). This analog was unable to activate C. difficile spore germination. Interestingly, glycocholate (T17) is a CAAMSA (T11) analog in which a sulfonate has been substituted for a carboxylate. In our hands, glycocholate, like CAAMSA, is able to significantly activate C. difficile spore germination in buffer (Table 2). A previous report had determined that glycocholate is not a germinant for C. difficile spores in BHIS medium (35). Indeed, when we tested glycocholate in BHIS medium, the C. difficile spore germination rate dropped almost 10-fold. However, addition of glycine to BHIS medium partially restored glycocholate-mediated germination (data not shown). Hence, compound mixtures in BHIS medium seem to intrinsically inhibit C. difficile spore germination.
CAAPA (T18) is a carboxylated analog of taurocholate (T01). Interestingly, whereas taurocholate, CAAMSA (T11), and glycocholate (T17) are able to activate C. difficile spore germination, CAAPA is inactive. Similarly, CA2APA (T19), CAABA (T20), and CA2ABA (T21), with longer side chains, are also inactive. These results suggest that although a carboxylate is able to partially substitute for sulfonate, it is not optimal for activation of C. difficile spore germination.
CAHESA (T22) is a taurocholate analog in which an amide group is substituted for an ester. CAHESA was unable to trigger germination in C. difficile spores (Table 2). This suggests that the hydrogen bond ability of the amide group is necessary for C. difficile spore germination.
To determine whether taurocholate analogs with modified side chains (T11 to T22) (Fig. 5) can inhibit C. difficile spore germination, we analyzed the effects of these analogs on C. difficile spores treated with taurocholate (T01) and glycine (A01). Taurocholate analogs with one less carbon (T11) or one more carbon (T12) in the taurine side chain were unable to inhibit germination (Table 2). Interestingly, while the addition of a benzene ring in the linker with the sulfonate in the para (CApSA [T13]) or the ortho (CAoSA [T14]) position does not inhibit spore germination, having the sulfonate in the meta position (CAmSA [T15]) results in strong inhibition of C. difficile spore germination. CAmSA (T15) has an IC50 of 58.3 μM and is the strongest inhibitor reported so far. The conversion of the sulfonate functional group to a sulfinate (T16) resulted in a compound with slight inhibitory activity at an IC50 of 640 μM. Interestingly, compounds with longer alkyl linkers followed by a carboxylate (T20 and T21) are able to inhibit C. difficile spore germination, whereas shorter carboxylate end groups (T17 to T19) are inactive. CAABA (T20) has an IC50 of 762 μM, whereas the longer side chain, CA2ABA (T21), containing two amide groups has an IC50 of approximately 3,000 μM. Replacement of the amide group of taurocholate with an ester (T22) results in a compound with slight inhibitory activity (IC50, 1,322 μM).
Conclusions.
In this study, we used taurocholate and glycine analogs to better understand how the C. difficile germination machinery recognizes its germinants. Chemical probes can reveal the chemical, physical, and spatial requirements of the germination receptor binding site. The present study has shown that the C. difficile germination machinery recognizes a number of amino acid side chains and that the putative glycine receptor requires both a free carboxylate and a free amino group to recognize glycine, but the binding site is flexible enough to accommodate longer separations between the two functional groups. Alkyl amino acid side chains seem to be recognized in a narrow hydrophobic groove that allows the binding of linear chains but excludes branched isomers. We have seen a similar branched-chain restriction in the recognition of inosine analogs by B. cereus spores (9). These size and polarity restrictions also suggest the existence of separate binding sites for l-phenylalanine, l-arginine, and possibly l-cysteine.
We could also speculate that the binding region for l-alanine in C. difficile is divergent enough from the l-alanine binding site in Bacillus to impede the binding and inhibition by d-alanine. Because none of the amino acid analogs was able to compete with glycine to inhibit C. difficile spore germination, the functional groups in the amino acid moieties are needed for both binding and activation of the putative amino acid germination receptor.
The putative taurocholate binding sites of C. difficile spores were less flexible in compounds allowed to bind and activate germination. Hydroxyl groups at positions 3 and 12 seem to be required for both binding and activation of C. difficile spore germination. In contrast, hydroxyl groups in the B ring appear to be important only for binding. Hence, the data imply that there is a requirement for hydrogen bonding with hydroxyls at specific locations and configurations in the C. difficile germination binding pocket.
Recognition of the taurine side chain seems to be even more restricted. Even small changes in the linker length and rigidity, amide bond, or oxidation state of the sulfonate group had a large effect on C. difficile spore germination. Although the sulfonate group is optimal for spore germination activation, it can be partially substituted with a carboxylate as long as the alkyl chain is short. These data suggest that the binding site for taurocholate recognizes the taurine side chain selectively.
In contrast to agonist specificity, the putative taurocholate binding receptor was more flexible in regard to antagonist binding. The meta-sulfonic benzene derivative CAmSA (T15) is active at concentrations approximately 275-fold lower than taurocholate and is almost 4 times more active than the natural inhibitor chenodeoxycholate (26, 34). The benzene ring is a rigid functional group with little free rotation. We speculate that the sulfonate in the meta position is able to fit tightly into the sulfonate binding pocket but the overall receptor does not recognize the benzene ring to trigger germination. This is further confirmed by the inactivity of the ortho and para isomers that would spatially place the sulfonate in different locations. The rigidity and positioning of the m-sulfonate probably provides an entropic advantage over alkyl sulfonates. It is possible that longer alkyl side chains are too flexible to allow the sulfonate moiety to bind efficiently to the putative taurocholate. The discovery of CAmSA and its strong inhibitory effect has revealed a new path to designing compounds for CDAD prophylaxis.
Interestingly, BHIS medium seems to have intrinsic inhibitory activity against amino acid (but not taurocholate) activation of C. difficile spore germination. Since biological medium is a better representation of potential metabolites present in the host, it indicates that taurocholate-mediated germination is the preferred pathway for human infection.
In conclusion, the putative taurocholate glycine receptors in C. difficile recognize multiple functional groups in their respective germinants. Hence, even subtle changes in the germinant structure can be detrimental to the binding ability of the germination machinery of C. difficile spores.
Footnotes
Published ahead of print on 22 October 2010.
REFERENCES
- 1.Abel-Santos, E., and T. Dodatko. 2007. Differential nucleoside recognition during Bacillus cereus 569 (ATCC 10876) spore germination. New J. Chem. 31:748-755. [Google Scholar]
- 2.Arslan, H., E. K. Inci, O. K. Azap, H. Karakayali, A. Torgay, and M. Haberal. 2007. Etiologic agents of diarrhea in solid organ recipients. Transplant Infect. Dis. 9:270-275. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay, P., V. Janout, L. H. Zhang, and S. L. Regen. 2001. Ion conductors derived from cholic acid and spermine: importance of facial hydrophilicity on Na+ transport and membrane selectivity. J. Am. Chem. Soc. 123:7691-7696. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, J. G. 2007. Clostridium difficile: old and new observations. J. Clin. Gastroenterol. 41:S24-S29. [Google Scholar]
- 5.Cloud, J., and C. P. Kelly. 2007. Update on Clostridium difficile associated disease. Curr. Opin. Gastroenterol. 23:4-9. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, L. F., E. Valiente, and B. W. Wren. 2009. Clostridium difficile: a continually evolving and problematic pathogen. Infect. Genet. Evol. 9:1410-1417. [DOI] [PubMed] [Google Scholar]
- 7.Dayal, B., K. R. Rapole, C. Patel, B. N. Pramanik, S. Shefer, G. S. Tint, and G. Salen. 1995. Microwave-induced rapid synthesis of sarcosine conjugated bile acids. Bioorg. Med. Chem. Lett. 5:1301-1306. [Google Scholar]
- 8.Dodatko, T., M. Akoachere, N. Jimenez, Z. Alvarez, and E. Abel-Santos. 2010. Dissecting interactions between nucleosides and germination receptors in Bacillus cereus 569 spores. Microbiology 156:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodatko, T., M. Akoachere, S. M. Muehlbauer, F. Helfrich, A. Howerton, C. Ross, V. Wysocki, J. Brojatsch, and E. Abel-Santos. 2009. Bacillus cereus spores release alanine that synergizes with inosine to promote germination. PLoS One 4:e6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer, E. and A. Speier. 1895. Darstellung der Ester. Ber. Dtsch. Chem. Ges. 28:3252-3258. [Google Scholar]
- 11.Fisher, N., and P. Hanna. 2005. Characterization of Bacillus anthracis germinant receptors in vitro. J. Bacteriol. 187:8055-8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, S. J., and K. Johnstone. 1990. Pulling the trigger: the mechanism of bacterial spore germination. Mol. Microbiol. 4:137-141. [DOI] [PubMed] [Google Scholar]
- 13.Gargiulo, D., T. A. Blizzard, and K. Nakanishi. 1989. Synthesis of mosesin-4, a naturally occurring steroid saponin with shark repellent activity, and its analog 7-β-galactosyl ethyl cholate. Tetrahedron 45:5423-5432. [Google Scholar]
- 14.Hornstra, L. M., Y. P. De Vries, M. H. J. Wells-Bennik, W. M. De Vos, and T. Abee. 2006. Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 72:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida, T., and F. C. Chang. 1982. Potential bile acid metabolites. 6. Stereoisomeric 3,7-dihydroxy-5β-cholanic acids. J. Org. Chem. 47:2966-2972. [Google Scholar]
- 16.Indu, B., W. R. Ernst, and L. T. Gelbaum. 1993. Methanol-formic acid esterification equilibrium in sulfuric acid solutions: influence of sodium salts. Ind. Eng. Chem. Res. 32:981-985. [Google Scholar]
- 17.Kuijper, E. J., J. T. Van Dissel, and M. H. Wilcox. 2007. Clostridium difficile: changing epidemiology and new treatment options. Curr. Opin. Infect. Dis. 20:376-383. [DOI] [PubMed] [Google Scholar]
- 18.Maschmeyer, G., and A. Haas. 2008. The epidemiology and treatment of infections in cancer patients. Int. J. Antimicrob. Agents 31:193-197. [DOI] [PubMed] [Google Scholar]
- 19.Minton, N., G. Carter, M. Herbert, T. O'Keeffe, D. Purdy, M. Elmore, A. Ostrowski, O. Pennington, and I. Davis. 2004. The development of Clostridium difficile genetic systems. Anaerobe 10:75-84. [DOI] [PubMed] [Google Scholar]
- 20.Moir, A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526-530. [DOI] [PubMed] [Google Scholar]
- 21.Moir, A., B. M. Corfe, and J. Behravan. 2002. Spore germination. Cell. Mol. Life Sci. 59:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien, J. A., B. J. Lahue, J. J. Caro, and D. M. Davidson. 2007. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect. Control. Hosp. Epidemiol. 28:1219-1227. [DOI] [PubMed] [Google Scholar]
- 23.Paredes, C. J., K. V. Alsaker, and E. T. Papoutsakis. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 3:969-978. [DOI] [PubMed] [Google Scholar]
- 24.Poxton, I. R., J. McCoubrey, and G. Blair. 2001. The pathogenicity of Clostridium difficile. Clin. Microbiol. Infect. 7:421-427. [DOI] [PubMed] [Google Scholar]
- 25.Preston, R. A., and H. A. Douthit. 1988. Functional relationships between l- and d-alanine, inosine and NH4Cl during germination of spores of Bacillus cereus. J. Gen. Microbiol. 134:3001-3010. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez, N., M. Liggins, and E. Abel-Santos. 2010. Kinetic evidence for the presence of putative germination receptors in C. difficile spores. J. Bacteriol. 192:4215-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez, N., and E. Abel-Santos. 2010. Requirements for germination of Clostridium sordellii spores in vitro. J. Bacteriol. 192:418-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodbard, D., and Y. Feldman. 1978. Kinetics of two-site immunoradiometric (‘sandwich’) assays. I. Mathematical models for simulation, optimization, and curve fitting. Mol. Immunol. 15:71-76. [DOI] [PubMed] [Google Scholar]
- 29.Rodbard, D., and S. W. McClean. 1977. Automated computer analysis for enzyme multiplied immunological techniques. Clin. Chem. 23:112-115. [PubMed] [Google Scholar]
- 30.Rolfe, R. D., S. Helebian, and S. M. Finegold. 1981. Bacterial interference between Clostridium difficile and normal fecal flora. J. Infect. Dis. 143:470-475. [DOI] [PubMed] [Google Scholar]
- 31.Sebaihia, M., B. W. Wren, P. Mullany, N. F. Fairweather, N. Minton, R. Stabler, N. R. Thomson, A. P. Roberts, A. M. Cerdenõ-Tárraga, H. Wang, M. T. Holden, A. Wright, C. Churcher, M. A. Quail, S. Baker, N. Bason, K. Brooks, T. Chillingworth, A. Cronin, P. Davis, L. Dowd, A. Fraser, T. Feltwell, Z. Hance, S. Holroyd, K. Jagels, S. Moule, K. Mungall, C. Price, E. Rabbinowitsch, S. Sharp, M. Simmonds, K. Stevens, L. Unwin, S. Whithead, B. Dupuy, G. Dougan, B. Barrell, and J. Parkhill. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779-786. [DOI] [PubMed] [Google Scholar]
- 32.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 33.Shanmugam, S., B. Viswanathan, and T. K. Varadarajan. 2004. Esterification by solid acid catalysts: a comparison. J. Mol. Catal. A Chem. 223:143-147. [Google Scholar]
- 34.Sorg, J. A., and A. L. Sonenshein. 2009. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J. Bacteriol. 191:1115-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorg, J. A., and A. L. Sonenshein. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surowiec, D., A. G. Kuyumjian, M. A. Wynd, and C. E. Cicogna. 2006. Past, present, and future therapies for Clostridium difficile-associated disease. Ann. Pharmacother. 40:2155-2163. [DOI] [PubMed] [Google Scholar]
- 37.Tonna, I., and P. D. Welsby. 2005. Pathogenesis and treatment of Clostridium difficile infection. Postgrad. Med. J. 81:367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tserng, K. Y., D. L. Hachey, and P. D. Klein. 1977. An improved procedure for the synthesis of glycine and taurine conjugates of bile acids. J. Lipid Res. 18:404-407. [PubMed] [Google Scholar]
- 39.von Baum, H., A. Sigge, M. Bommer, W. V. Kern, R. Marre, H. Döhner, P. Kern, and S. Reuter. 2006. Moxifloxacin prophylaxis in neutropenic patients. J. Antimicrob. Chemother. 58:891-894. [DOI] [PubMed] [Google Scholar]