Abstract
While methyl viologen had only a small effect on P700+ rereduction kinetics after far-red pulses in KCN-treated wild-type Synechocystis sp. strain PCC6803 and an NdhF3/NdhF4 (NdhF3/F4)-defective mutant, it involved a rather slow P700+ rereduction in an NdhF1-defective mutant. This strongly indicates that (i) active electron flow from metabolites to plastoquinone is suppressed upon deletion of ndhF1 and (ii) photosystem 1-mediated cyclic electron transport is dependent on NdhF3/F4-type NDH-1 complexes.
Cyanobacteria possess multiple type 1 NADPH dehydrogenase (NDH-1) complexes with functionally distinct profiles (1, 8). In the well-characterized mesophilic cyanobacterium Synechocystis sp. strain PCC6803, two types of NDH-1 complexes have been identified; one type, containing NdhD1/NdhF1 (NdhD1/F1) (NDH-1L) or NdhD2/F1 (NDH-1L′), has been considered to be a component of the major photosystem 1 (PS1)-dependent cyclic electron transport pathway, while the other type, containing NdhD3/F3 (NDH-1MS) or NdhD4/F4 (NDH-1MS′), has been shown to be essential for CO2 uptake (10, 11, 15). Surprisingly, although CO2 uptake by cyanobacterial cells is driven by PS1-mediated cyclic electron transport (9), inactivation of the ndhD3 and ndhD4 (or ndhF3 and ndhF4) genes had only a moderate effect on the PS1-mediated electron flow (10). It has also been suggested that NDH-1 participates in the respiratory electron flow (6) with its NDH-1L complexes (10, 19). However, Cooley et al. reported a strong influence of succinate dehydrogenase (SDH) on the plastoquinone (PQ) redox poise and claimed SDH as the major respiratory electron donor of the PQ pool instead of the NDH-1 complex (3). They suggested that the absence of PS1-dependent cyclic electron flow in the ndhB-defective M55 mutant lacking all types of active NDH-1 complexes results from a suppression of succinate formation due to a high NADPH/NADP ratio (4). As the cyanobacterial electron transport network is rather complex, there are several open questions. These include (i) are NdhD3/D4-type NDH-1 complexes involved in PS1-mediated cyclic electron transport processes, (ii) which is the major respiratory pathway(s) (to PQ), and (iii) how do the pathways possibly interact? These questions led us to examine quantitatively the respiratory and cyclic electron transport rates in various ndhD- and ndhF-defective mutants by the analysis of P700+ oxidation-reduction kinetics. Here we show that NdhF3/F4-type NDH-1 complexes are involved in a PS1-mediated cyclic electron transport pathway distinct from the pathway that is dependent on NdhF1-type NDH-1 complexes and that electron transport from metabolites via NDH-1 complexes apparently is highly regulated by the redox state of the PQ pool.
The construction of various ndh deletion mutants has been reported in detail previously (7, 10, 11) and deposited on the website CyanoMutants (http://www.kazusa.or.jp/cyano/mutants/). Cells were grown at 30°C in BG11 medium (12), buffered with 20 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES)-KOH (pH 8.0) and bubbled with 3% (vol/vol) CO2-enriched air under continuous illumination (50 microeinsteins/m2·s) using white fluorescence lamps. The cultures were bubbled with ambient air overnight, harvested by centrifugation, and resuspended in fresh BG11 medium. Kinetics of P700 oxidation-reduction was determined in a Dual-PAM-100 system (Walz, Effeltrich, Germany) using standard 1-cm cuvettes as described previously (2, 13, 14). P700 was specifically excited by 5-s far-red (FR) pulses at 730 nm with maximal intensity (75 W/m2). This light intensity was high enough to oxidize P700 substantially at low electron inflow but was subsaturating at high electron inflow. Both the maximal P700 oxidation level during the pulse and the subsequent P700+ rereduction kinetics in the dark provide information on the electron transport through the PQ pool and the cytochrome (cyt) b6f complex (2, 13, 14, 17, 18), which are shared components of the photosynthetic and respiratory electron transport chains. Also, as FR light selectively excites PS1, electron transport originating from water-splitting PS2, which may complicate the interpretation of the data, could be neglected. To separate the three remaining major routes (i.e., cyclic, PQ-reducing, and PQ-oxidizing respiratory electron flows) (Fig. 1, routes A, B, and C, respectively), site-specific chemical agents were applied as described previously (18) and the rates were determined as follows. Without any addition, the rate of P700+ rereduction, k1, should reflect the difference between electron transport rates into and out of the PQ pool: k1 = kA + kB − kC, where kA, kB, and kC represent the electron transport rates in routes A, B and C, respectively. In the presence of KCN, electron transport to molecular oxygen via terminal oxidases is blocked; therefore, the P700+ rereduction kinetics, k2, should show the sum of the electron flows via routes A and B: k2 = kA + kB. Hence, the difference between k1 and k2 (k1 < k2) corresponds to electron transport rates in route C. Electron donation to PQ via cyclic electron transport can be eliminated by adding the PS1 electron acceptor methyl viologen (MV). Combined application of KCN and MV inactivates both routes A and C; therefore, while P700+ rereduction rates under these conditions (k3) directly reflect the electron transport rates from respiration via route B (k3 = kB), the difference between k2 and k3 (k2 > k3) gives the cyclic electron transport rate (k2 − k3 = kA + kB − kB = kA). The P700+ rereduction kinetics was fitted by single exponential decays, and the resulting rate constants (k1, k2, and k3) were used to calculate the electron transfer rates in different pathways as described above.
FIG. 1.
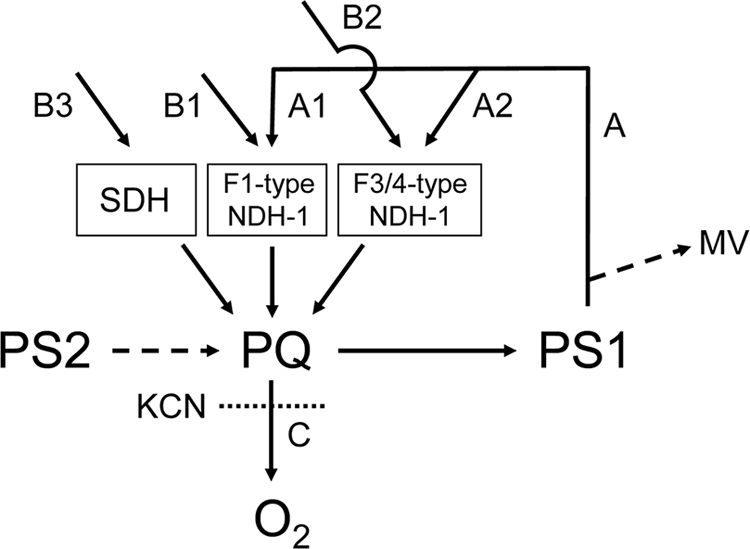
Major electron transport pathways around P700 in cyanobacteria. A, NDH-1 complex-dependent PS1-mediated cyclic routes, subdivided into F1-type NDH-1-dependent (A1) and F3/F4-type NDH-1-dependent (A2) routes; B, electron flow from metabolites to PQ via respiratory enzymes such as F1-type NDH-1 (B1), F3/F4-type NDH-1 (B2), and SDH (B3); C, electron flow from PQ to oxygen mediated by terminal oxidases. The inhibitory site of KCN and electron leakage due to MV are also indicated.
Figure 1 shows the electron transport scheme with all potential major electron transfer pathways around PQ being modified according to the NDH-1 heterogeneity. Both routes A and B are subdivided into electron flows mediated by F1-type (A1 and B1) and F3/F4-type (A2 and B2) NDH-1 complexes and SDH (B3).
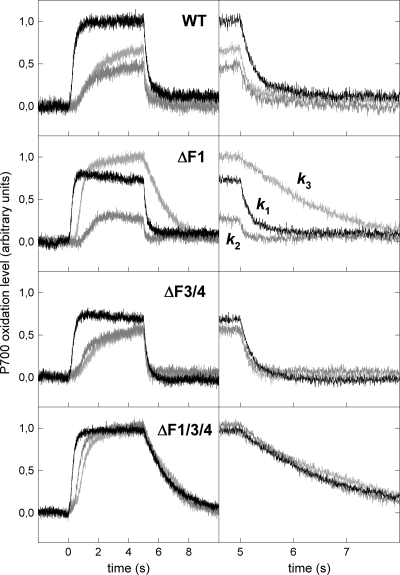
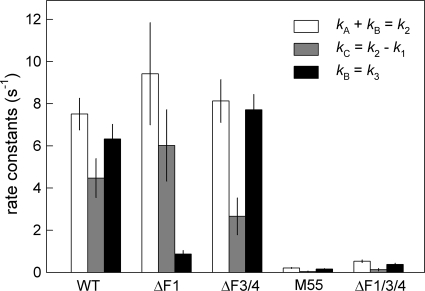
Figure 2 shows oxidation (rise) and reduction (decay) kinetics of P700 upon switching FR pulses on and off, respectively, measured in the absence or presence of inhibitors. While rereduction of P700+ in the dark depends only on the electron transport through PQ and cyt b6f, the rate and extent of P700 oxidation depend on both P700+-reducing and P700-oxidizing processes, i.e., electron in- and outflow, respectively, to and from PS1. In the absence of any inhibitor (Fig. 2, black traces) the rise (oxidation) kinetics were similar (half-life [t1/2] ≅ 300 to 330 ms) in wild-type (WT), ΔF3/F4, and ΔF1/F3/F4 cells but faster in the ΔF1 mutant (t1/2 ≅ 220 ms), indicating a highly efficient P700 oxidation in the last strain. In contrast, the decay kinetics (k1) are similar (t1/2 ≅ 210 to 260 ms) in wild-type (WT) and ΔF1 cells but much faster in the ΔF3/F4 mutant (t1/2 ≅ 130 ms) and very slow (≅1.6 s) in the triple knockout mutant and M55 (data not shown). While the former observation indicates a larger difference between the electron in- and outflow at PQ/cyt b6f (compare the white and gray bars in Fig. 3) in the ΔF3/F4 mutant, the latter shows a largely impaired respiratory electron flow to PQ in all cases of NDH-1 inactivation, in agreement with previous observations (4, 10). Addition of KCN (Fig. 2, dark gray traces) lowered the P700 oxidation levels significantly and produced slower rise and faster decay kinetics, respectively, in the WT, ΔF1, and ΔF3/F4 strains, indicating a more reduced PQ pool in the presence of KCN. This, in turn, shows a significant electron transport to oxygen via terminal oxidases in these strains. In contrast, the triple-deletion and M55 strains were unaffected by KCN, showing a very low rate of respiratory electron transport (4, 10) (Fig. 3). Addition of MV to the KCN-treated samples (Fig. 2, light gray traces) hardly affected the P700+ oxidation-reduction kinetics in WT, ΔF3/F4, and ΔF1/F3/F4 strains (Fig. 2, light versus dark gray traces), resulting in nearly identical k3 and k2 values for these strains (Fig. 3, white versus black bars). The high k3 values for WT and ΔF3/F4 strains indicate a high capacity for electron donation from metabolites to PQ, which confirms earlier observations (3, 4, 6). In contrast, MV strongly increases and decreases the rate of P700+ rise and decay, respectively, in the ΔF1 mutant (Fig. 2 and 3). This indicates a strong suppression of electron flow from respiratory routes in this mutant (Fig. 1, route B) and highly active cyclic electron transport via route A2 (via F3/F4-type NDH-1), in agreement with the fast P700 oxidation-reduction kinetics in the nontreated samples. As cyclic routes make only a minor contribution to P700+ reduction in WT and ΔF3/F4 strains, the contribution of the PQ-reducing pathways (similar to the plastoquinol [PQH2]-oxidizing pathways [5]) appears to be highly flexible and possibly dependent on other pathways. Electron flow in one route may suppress another route(s), and elimination of one path may activate another one. For this reason it is not possible to determine the individual contributions of A1, A2, and B1 to B3 when more than two paths are active. Nevertheless, the higher kA value in the ΔF1 mutant relative to WT (Fig. 3, white versus black bars) may indicate that elimination of the F1-dependent (A1 and B1) paths results in an upregulation of the A2 flow. Quantification of the electron transport rates to terminal oxidases (route C) (Fig. 3, gray bars) showed a higher rate in the ΔF1 mutant than in WT, while the rate was much lower in the ΔF3/F4 mutant and negligible in the triple mutant. Thus, high-yield electron flow in route A2 in the ΔF1 strain is accompanied by a high electron flow to terminal oxidases.
FIG. 2.
Oxidation-reduction kinetics of P700 triggered by FR illumination in WT and ΔF1, ΔF3/F4, and ΔF1/F3/F4 mutants. P700+ rereduction is also shown in a shorter time window (right). Black traces, no addition; dark gray traces, 1 mM KCN; light gray traces, 1 mM KCN plus 100 μM MV. k1, k2, and k3 are the corresponding rate constants of P700+ rereduction, respectively. The levels of 0.0 and 1.0 on the y axis correspond to completely reduced and oxidized P700 populations, respectively.
FIG. 3.
Respiratory and cyclic electron flows (see Fig. 1) as determined from P700+ rereduction decays according to Fig. 2. White bars, sum of electron donation via routes A and B (k2 = kA + kB); gray bars, electron flow in route C (kC = k2 − k1); black bars, electron transport from metabolism (route B, kB = k3).
Experimental data show a high-yield electron flow from metabolites to PQ (routes B) (3, 4, 6; this study). Whether these routes also include SDH (3, 4) or only NDH-1 complexes still remains to be shown. However, our results provide strong evidence for the participation of the NDH-1 complex in these processes. Metabolic flux analysis also shows the predominance of the oxidative pentose phosphate pathway in the carbon metabolism of Synechocystis (16), which questions any major contribution of citric acid cycle intermediates in respiration. As PQ is a common component of all respiratory routes, the efficiency of electron donation to or from PQ should mainly depend on the properties of different complexes involved in routes A1, A2, B1 to B3, and C. Possibly, electron donation to or from each complex depends on the PQ redox level. Assuming that P700 is fully reduced in the dark and fully oxidized by FR light in nontreated WT samples, the steady-state PQ oxidation levels during FR illumination in the presence of KCN can be estimated to be 0.46, 0.24, 0.55, and 1.00 for WT, ΔF1, ΔF3/F4, and ΔF1/F3/F4 strains, respectively (Fig. 2). This implies that electron donation from metabolites to PQ favors electron donation from PQ to oxidases at a low oxidation level of PQ. Probably the F3/F4-type NDH-1 complexes are able to efficiently donate electrons to PQ even at a low PQ oxidation level. As already shown, CO2 uptake of cyanobacterial cells is energized by PS1 (9) and depends on NdhF3/F4-type NDH-1 complexes (10, 11). Although the mechanism of these processes is not yet completely understood, our present study clearly indicates a PS1-mediated cyclic electron flow which is dependent on NdhF3/F4-type NDH-1 complexes.
Acknowledgments
We thank Achim Trebst for helpful discussion, and Claudia König and Erdmut Thomas for excellent technical assistance.
Support by the Federal Ministry of Education and Research (BMBF, project Bio-H2; G.B. and M.R.), the EU/NEST project Solar-H2 (M.R.), and the German Research Foundation (DFG, project C1 in SFB 480; M.R.) is gratefully acknowledged.
Footnotes
Published ahead of print on 29 August 2010.
REFERENCES
- 1.Battchikova, N., and E. M. Aro. 2007. Cyanobacterial NDH-1 complexes: multiplicity in function and subunit composition. Physiol. Plant. 131:22-32. [DOI] [PubMed] [Google Scholar]
- 2.Bernát, G., N. Waschewski, and M. Rögner. 2009. Towards efficient hydrogen production: the impact of antenna size and external factors on electron transport dynamics in Synechocystis PCC 6803. Photosynth. Res. 99:205-216. [DOI] [PubMed] [Google Scholar]
- 3.Cooley, J. W., C. A. Howitt, and W. F. J. Vermaas. 2000. Succinate:quinol oxidoreductases in the cyanobacterium Synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport. J. Bacteriol. 182:714-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooley, J. W., and W. F. J. Vermaas. 2001. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J. Bacteriol. 183:4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutthann, F., M. Egert, A. Marques, and J. Appel. 2007. Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentration in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1767:161-169. [DOI] [PubMed] [Google Scholar]
- 6.Mi, H., T. Endo, U. Schreiber, T. Ogawa, and K. Asada. 1992. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 33:1233-1237. [Google Scholar]
- 7.Ogawa, T. 1991. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803. Proc. Natl. Acad. Sci. U. S. A. 88:4275-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa, T., and H. Mi. 2007. Cyanobacterial NADPH dehydrogenase complexes. Photosynth. Res. 93:69-77. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa, T., A. Miyano, and Y. Inoue. 1985. Photosystem-I-driven inorganic carbon transport in the cyanobacterium, Anacystis nidulans. Biochim. Biophys. Acta 808:77-84. [Google Scholar]
- 10.Ohkawa, H., H. B. Pakrasi, and T. Ogawa. 2000. Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC 6803. J. Biol. Chem. 275:31630-31634. [DOI] [PubMed] [Google Scholar]
- 11.Shibata, M., H. Ohkawa, T. Kaneko, H. Fukuzawa, S. Tabata, A. Kaplan, and T. Ogawa. 2001. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. U. S. A. 98:11789-11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanier, R. Y., R. Kunisawa, M. Mandel, and G. Cohen-Bazire. 1971. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35:171-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsunoyama, Y., G. Bernát, N. G. Dyczmons, D. Schneider, and M. Rögner. 2009. Multiple Rieske proteins enable short- and long-term light adaptation of Synechocystis sp. PCC 6803. J. Biol. Chem. 284:27875-27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkmer, T., D. Schneider, G. Bernát, H. Kirchhoff, S. O. Wenk, and M. Rögner. 2007. Ssr2998 of Synechocystis sp. PCC 6803 is involved in regulation of cyanobacterial electron transport and associated with the cytochrome b6f complex. J. Biol. Chem. 282:3730-3737. [DOI] [PubMed] [Google Scholar]
- 15.Xu, M., G. Bernát, A. Singh, H. Mi, M. Rögner, H. B. Pakrasi, and T. Ogawa. 2008. Properties of mutants of Synechocystis sp. strain PCC 6803 lacking inorganic carbon sequestration systems. Plant Cell Physiol. 49:1672-1677. [DOI] [PubMed] [Google Scholar]
- 16.Yang, C., Q. Hua, and K. Shimizu. 2002. Metabolic flux analysis in Synechocystis using isotope distribution from 13C-labeled glucose. Metab. Eng. 4:202-216. [DOI] [PubMed] [Google Scholar]
- 17.Yeremenko, N., R. Jeanjean, P. Prommeenate, V. Krasikov, P. J. Nixon, W. F. J. Vermaas, M. Havaux, and H. C. P. Matthijs. 2005. Open reading frame ssr2016 is required for antimycin A-sensitive photosystem I-driven cyclic electron flow in the cyanobacterium Synechocystis sp PCC 6803. Plant Cell Physiol. 46:1433-1436. [DOI] [PubMed] [Google Scholar]
- 18.Yu, L., J. D. Zhao, U. Mühlenhoff, D. A. Bryant, and J. H. Golbeck. 1993. Psae is required for in-vivo cyclic electron flow around photosystem-I in the cyanobacterium Synechococcus sp-Pcc-7002. Plant Physiol. 103:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, P., N. Battchikova, T. Jansen, J. Appel, T. Ogawa, and E. M. Aro. 2004. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis PCC 6803. Plant Cell 16:3326-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]