Abstract
Human chorionic gonadotropin (hCG) is a glycoprotein hormone essential to pregnancy. hCG is heterodimeric and functionally defined by its β subunit. hCGβ evolved from the β subunit of luteinizing hormone in two phases. In the first phase, type I genes (hCGβ3, -5, -7, and -8) acquired changes affecting gene expression and extending the proteins' C terminus. In the second phase, type II genes (hCGβ1 and -2) were formed by the insertion of a DNA element into the type I 5′ end. The insertion includes the small noncoding RNA gene snaR-G and has been predicted to drastically change the protein products encoded. We trace the insertion to the common ancestor of the African great apes and show that it contains transcription signals, including snaR-G. Type II transcripts are predominantly expressed in testis. Contrary to predictions, the product of the major mRNA splice form is hCGβ. A novel peptide is encoded by alternatively spliced transcripts. These findings support the view that type II genes evolved in African great apes to function in the male reproductive system.
Chorionic gonadotropin (CG) is essential for reproduction in primates. CG is a heterodimer composed of a unique β subunit that defines its function and an α subunit that is shared with luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH). CG, LH, and FSH are gonadotropins critical to human fertility and reproduction. LH and FSH are expressed in the anterior pituitary gland and act on the gonads via the hypothalamic-pituitary-gonadal axis. In the ovaries, FSH stimulates steroid hormone synthesis and initiates maturation of follicles, and LH stimulates ovulation and conversion of the follicle to the corpus luteum (reviewed in references 1 and 10). CG is secreted at the onset of pregnancy by cytotrophoblast cells of the embryo (7) and stimulates the corpus luteum to maintain progesterone production and sustain pregnancy (29). In the testis, FSH stimulates Sertoli cells, LH stimulates Leydig cells to secrete testosterone, and both support spermatogenesis (for a recent review, see reference 53). Accumulating evidence also points to a testicular role for human CG (hCG). CG stimulates fetal Leydig cells prior to LH stimulation (20), and the hCGβ subunit has been detected in Leydig cells (3).
The CGβ genes evolved from LHβ with changes that affect their transcription as well as their protein products. Humans have six CGβ genes and a single LHβ gene arranged in an ∼40-kb gene cluster on chromosome 19 (Fig. 1A) (49). The CGβ genes can be separated into two classes. Four genes (hCGβ3, -5, -7, and -8) closely resemble the CGβ genes found in all other primates: we propose the term type I CGβ for this set. Two genes (hCGβ1 and -2), designated type II, have a large substitution at their 5′ ends. These genes, which appear to be restricted to the African great apes (humans, chimpanzees, and gorillas), display altered tissue expression and splicing patterns (6, 51). The type II genes have been predicted to specify wholly distinct proteins as a result of a reading frameshift (6, 13, 22). These putative protein products have not been detected, however, and the role of the type II genes remains unclear.
FIG. 1.
Structure and evolution of type II CGβ genes. (A) Diagram of the LH/CGβ gene cluster on human chromosome 19q13.33. Straight arrows indicate direction of transcription, the large curved arrow denotes inverted segmental duplication of the original CGβ1 gene, and arrowheads depict snaR-G genes. (B) Schematic of LHβ and type I and II CGβ gene structures, illustrating the replacement in type II CGβ genes of a segment common to type I CGβ genes with an insert containing snaR-G (dotted lines). Untranslated regions (UTRs) are depicted as solid boxes; open reading frames (ORFs) are shown as open boxes; introns and promoter regions are shown by solid lines; directions of transcription are shown by bent arrows. The CGβ1/2 first exon is defined here by the first donor splice site (6), and its UTRs (gray boxes) are defined by the first potential ORF. (Adapted from reference 25a with permission of the publisher.)
Type I CGβ is thought to have arisen by duplication of LHβ (16) in the common ancestor of anthropoid primates (41), followed by relatively minor mutations that caused an upstream shift of the transcription start site and extended the C terminus beyond the original LHβ translation stop codon (Fig. 1B). Subsequently, the type I CGβ gene copy number expanded in the primate lineage, such that there are at least two CGβ genes in Old World monkeys and apes and more in the great apes (41).
In the evolution of type II CGβ genes, an ∼0.7-kb sequence (22) containing the snaR-G gene (44, 46) replaced ancestral type I sequence encompassing its proximal promoter and nearly all of its 5′ untranslated region (UTR) (Fig. 1B). This substitution gave rise to the ancestor of the hCGβ1 gene, which was subsequently duplicated together with the adjacent hCGβ5 gene, to give hCGβ2 and hCGβ3 (Fig. 1A) (23). snaR-G1 and -G2 are located 86 bp upstream of the transcriptional start sites of hCGβ1 and -β2, respectively, in the opposite orientation to the type II hCGβ gene (44, 46). The snaR genes are a family of ∼30 genes in humans that are transcribed by polymerase III (Pol III) into small noncoding RNA (44). Type II mRNAs are found in testis, where they are present at levels comparable to those of type I genes, in contrast to placenta, where type I transcripts vastly predominate (4, 51). Type II mRNAs also differ from type I mRNAs in their splicing patterns and predicted translation initiation sites, thereby altering their protein products (4, 6, 13, 51, 52).
Altogether, these observations suggested that the substitution event introduced a novel presumptive promoter element into type II genes, modified their tissue expression pattern and splicing, and drastically changed the proteins encoded. We therefore considered the possibility that the type II hCGβ genes generate product(s) that serves specialized roles in the testis and perhaps in other tissues of humans and closely related species. To examine this hypothesis, we traced the evolution of the type II genes and characterized their expression at the RNA and protein levels. We show that the type II inserted DNA is an assemblage of several chromosomal sequences that originated in the African great apes. The promoter activity of the insert is influenced by its embedded snaR-G gene and by upstream sequences. Alternatively spliced hCGβ1 and hCGβ2 mRNAs are detected in testis and, to a lesser extent, in other tissues, including adrenal gland and fetal brain. Translation of the shorter mRNAs gives rise to hCGβ protein, rather than a hypothetical novel polypeptide, while the longer mRNAs encode a small peptide with predicted neuropeptide/hormone cleavage sites. We conclude that the evolution of type II CGβ genes has allowed differential tissue expression of hCGβ hormone and of a novel peptide, supporting a critical role in the male reproductive system.
MATERIALS AND METHODS
Bioinformatics.
Type II inserts were searched against the human genome (hg18) using BLAT search (31) in the UCSC genome browser (32). Genomic PCR clones were aligned in Clustal-W with chimpanzee CGβ1 and CGβ1B (EU000308) and gorilla CGβ1L (DQ238548) and CGβ2 (DQ238550). GgV PCR product was searched against Western gorilla whole-genome shotgun sequences from the NCBI Trace Archive using MegaBLAST (61). In silico translation was performed by Translate from the Swiss Institute of Bioinformatics (18). Predicted proteins were examined for signal peptide sequence on the SignalP 3.0 server (2).
Genomic PCR.
Bonobo (PR00251), chimpanzee (PR00643), lowland gorilla (PR00573), and Sumatran orangutan (PR00253) genomic DNA was purchased (IPBIR, Camden, NJ). Genomic DNA from human 293 cells was prepared as described previously (55). Primers used to amplify sequence encompassing snaR-G were as follows: (i) forward hCGβ1/2 promoter (5′-GCCATTCTGTTTACCACAGG-3′), (ii) forward hCGβ1 promoter (5′-GAGCAAGGCTCGGTATCC-3′), (iii) reverse hCGβ1/2 exon 1 (5′-CATGTCTCTCTCTTAGCGG-3′), (iv) reverse CGβ1/2 intron 1 (5′-CCTCCTTCCACAGCTCACA-3′), and (v) reverse chimpanzee CGβ1 intron 1 (5′-CTCCTTCCACCGCTCACG-3′). PCR product was ligated into pCRII-TOPO vector (Invitrogen), cloned, and sequenced in both directions.
Tissue expression.
Adrenal gland (Adr) and fetal brain (FBra) human tissue total RNAs were purchased from Clontech. Adipose (Adi), bladder (Bla), brain (Bra), cervix (Cer), colon (Col), esophagus (Eso), heart (Hrt), kidney (Kid), liver (Liv), lung (Lun), postmenopausal ovary (Ova), term placenta (Pla), prostate (Prs), skeletal muscle (SM), small intestine (SInt), spleen (Spl), testis (Tes), thymus (Thm), thyroid (Thr), and trachea (Tra) human tissue total RNAs (composites of 3 individuals) were from Ambion. RNA (5 μg) was incubated with DNase I (amplification grade; Invitrogen) and then reverse transcribed with a “lock-dock” oligo(dT)18 primer (8) and Superscript Reverse Transcriptase II in accordance with instructions (Invitrogen). cDNA was amplified with HotStar Taq DNA polymerase (Qiagen) using hCGβ3/5/7/8-specific primers F3/5/7/8 (5′-GACCCCACCATAGGCAGAG-3′) and R3/5/7/8 (5′-GGTAGTTGCACACCACCTGA-3′) or hCGβ1/2-specific primers F1/2 (5′-CGGAGCTAGCTGTATCTGAGAGAGAGCAG-3′; NheI site underlined) and R1/2 (5′-CGGACTTAAGGATTGAGAAGCCTTTATTGTG-3′; AflII site underlined). Fresh PCR product was sequenced directly or ligated into pCR2.1 plasmid using TOPO TA cloning (Invitrogen).
Northern blots.
293 cell RNA was 3′ radiolabeled (47), resolved alongside transfected cell RNA in 5% acrylamide-7 M urea gels, and blotted as described previously (44). Antisense oligonucleotide probes were 5′-CAGTCCCTCACACCCTCGCCTAGCCTTGAG-3′ for snaR-G1 and 5′-GTACCCTCGAACCCTCCCCTCCCTGAGG-3′ for snaR-G2.
Promoter analysis.
The proximal promoter and 5′ UTR of hCGβ2 were amplified from 293 cell DNA (∼200 ng) over 30 PCR cycles with primers F1 (5′-GACAGCTAGCCCTCTTCCCTGTG-3′; NheI site underlined) and R1 (5′-CTGTAAGCTTGGAAAGTAAATTGAGTCTCCG-3′; HindIII site underlined). The hCGβ1 promoter/5′ UTR was amplified with primers F1 and R1.1 (5′-CGGAAATGTGGATCTACCC-3′), a reverse primer unique to hCGβ1, followed by reamplification with primers F1 and R1. To generate β1L and β2L constructs, R1/F1 PCR products were double digested with NheI and HindIII and ligated into similarly digested pGL3-Enhancer vector (Promega). To generate snaR-G deletion constructs β1ΔL and β2ΔL, the R1/F1 PCR product was reamplified with primers R1 and F2.1 (5′-GACAAAATTTGTTGTCGGGCCCATCCTT-3′; ApoI site underlined), unique to hCGβ1, or F2.2 (5′-GACAAAATTTGGTGTCGGGGATCTCCTT-3′), unique to hCGβ2. F2.1/R1 and F2.2/R1 PCR products were sequentially digested with HindIII and ApoI, combined with F1/R1 PCR products digested with NheI and ApoI, and ligated into pGL3-Enhancer plasmid. To generate β1/2S and β1/2ΔS constructs, β1/2L and β1/2ΔL constructs were PCR amplified with primers F3 (5′-GACAGCTAGCGAGGAGGTTCCACCT-3′; NheI site underlined) and R1; after NheI and HindIII digestion, PCR products were ligated into similarly digested pGL3-Enhancer plasmid. HeLa S3 cells were transfected in duplicate over 48 h with reading frame constructs, and cell extracts were analyzed by dual luciferase reporter assay (Promega).
Reading frame analysis.
To generate β1a-F1/2/3-FFL and β2b-F1/2/3-FFL constructs, pCR2.1-hCGβ1a and -hCGβ2b were PCR amplified with primers F1/2 and either RF1 (5′-GTCCAAGCTTGGGGCAGTAGCCGGCA-3′), RF2 (5′-GTCCAAGCTTGGCAGTAGCCGGCACA-3′), or RF3 (5′-GTCCAAGCTTGGGCAGTAGCCGGCAC-3′) (HindIII sites are underlined; variable numbers of G residues are in bold; the 3′ ends of the primers are complementary to the 3′ end of the hCGβ clone). PCR products were digested with HindIII and NheI and ligated into similarly digested pGL3-Basic vector (Promega). The resultant constructs lacked a promoter, so the hCGβ1/2b sequence and the downstream luciferase gene were excised from pGL3 by digestion with NheI and XbaI and then subcloned into similarly digested pcDNA3.1(+) vector (Invitrogen). To generate the β2b′-FFL construct, pCR2.1-hCGβ2b was PCR amplified with primers F1/2 and RF2′ (5′-GTCCAAGCTTCCCTTCTCATGCCAGTGATG-3′; HindIII site is underlined). PCR product was digested, ligated into pGL3 vector, and subcloned into pcDNA3.1(+) vector as described above. HeLa S3 cells were transfected in triplicate over 24 h with reading frame constructs, and extracts were analyzed by dual luciferase reporter assay and by immunoblotting with antiluciferase antibody (Zymed).
In vitro translation assay.
pCR2.1-hCGβ1a, -hCGβ2a, and -hCGβ2b constructs were digested with AflII and NheI and subcloned into pcDNA3.1(+) vector. Constructs were linearized by AflII or SanDI digestion, and protein products were labeled with [35S]methionine in rabbit reticulocyte lysate TNT or wheat germ extract systems (Promega). Labeled protein was resolved in 0.75-mm, 10 to 22% SDS-polyacrylamide gels and dried onto a 0.2-μm polyvinylidene difluoride (PVDF) membrane (Bio-Rad) at 80°C for 2 h. Labeled protein was visualized by autoradiography.
In vivo protein synthesis analysis.
HeLa S3 cells were transfected in duplicate over 48 h with pcDNA3.1(+)-hCGβ1a, -hCGβ2a, and -hCGβ2b constructs. Cells from one plate were harvested in growth medium, washed in phosphate-buffered saline (PBS), and lysed in 50 μl Triton buffer (50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 0.5 mM dithiothreitol [DTT]) for 5 min on ice. Nuclei were pelleted at 4,000 rpm for 5 min. Supernatant (15 μl) was incubated with or without 1,000 U peptide:N-glycosidase F (PNGase F; New England Biolabs) at 37°C for 1 h, in accordance with instructions. Samples were resolved in 15% SDS-polyacrylamide gels. Protein was transferred to a 0.2-μm PVDF membrane and immunoblotted with anti-hCGβ antibody (clone SB6). Cells from a second plate were lysed in 500 μl Trizol, and total RNA was extracted (Invitrogen). cDNA was synthesized as described above and PCR amplified with primers F1/2 and R1/2. To generate the β2b′-FLAG construct, pCR2.1-hCGβ2b was PCR amplified with primers F1/2 and RFLAG (5′-CTCGAAGCTTTCACTTGTCATCGTCATCCTTGTAATCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCCACTGGTCTGCCCCTTCT-3′; HindIII site is underlined; 3×FLAG nucleotides are in bold). PCR product was digested with HindIII and NheI and ligated into similarly digested pcDNA3.1(+) vector. Mutations A175C, A265C, and A271C were introduced into the β2b′-FLAG construct using the QuikChange site-directed mutagenesis kit (Stratagene). β2b′-FLAG constructs were transfected into HeLa cells over 24 h. Protein samples were resolved in 0.75-mm, 10 to 22% SDS-polyacrylamide gels, transferred to an 0.2-μm PVDF membrane, and probed with anti-FLAG antibody (clone M2; Sigma). Total RNA was extracted from a parallel experiment; cDNA was synthesized as described above and PCR amplified with primers F1/2 and RFLAG.
Nucleotide sequence accession numbers.
The PCR products sequenced in this work were submitted to GenBank under accession numbers GQ259379.1 to GQ259415.1.
RESULTS
Origin of the type II CGβ insertion.
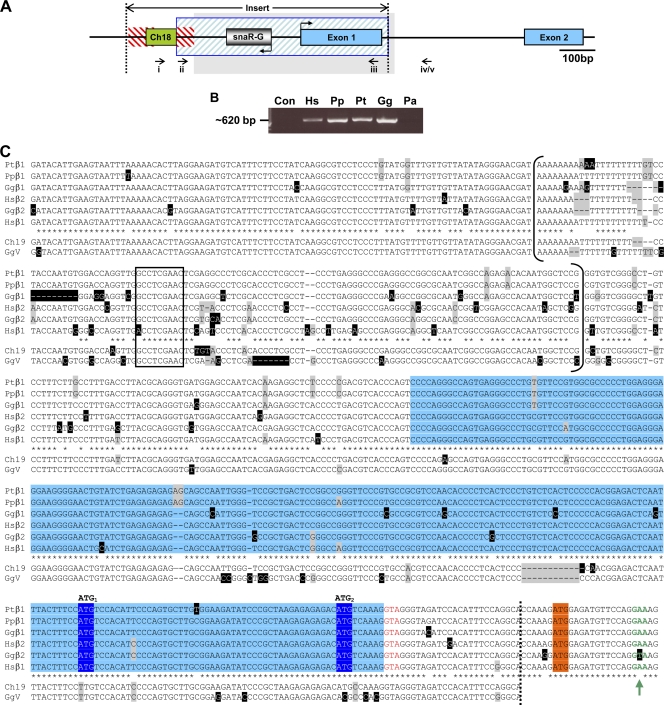
Bioinformatic searches of the human genome revealed that the type II insert (736 bp in hCGβ1 and 724 bp in hCGβ2) is a composite of four genomic sequences (Fig. 2A; also see Data Set S1 in the supplemental material). Most of it (∼0.6 kb), including snaR-G and the unique first exon of hCGβ1/2, is orthologous to a chromosome 19 region located ∼1.5 Mb downstream of the LH/CGβ cluster. This region contains a homologous snaR gene, snaR-F, in the same position and orientation as in the insert (45, 46). Abutting the 5′ end of this region is a short sequence (80 bp) homologous to chromosome 18, embedded in Alu sequences. At the extreme 5′ end of the insert is an Alu-Y SINE sequence (∼73 bp) that overlaps the 5′ end of the chromosome 18 homology region, and the 5′ end of the chromosome 19 homology segment also consists of Alu sequence (Fig. 2A). The positions of the Alu sequences suggest that this SINE family had a role in the assembly of the composite insert and possibly in its substitution into an ancestral type I gene.
FIG. 2.
Sequence of the type II insert in African great apes. (A) Schematic of the type II CGβ insert (between dotted lines) in the context of CGβ1/2 gene structure (exons in blue). Homology to chromosome 18 (green box), Alu (red hatching), and upstream chromosome 19 sequence (blue hatching) is shown. Bent arrows denote snaR-G and CGβ1/2 transcription directions. PCR forward primers targeting genomic sequence downstream of snaR-G1 or -G2 (i) or snaR-G1 (ii) were coupled with reverse primers targeting hCGβ1/2 exon 1 (iii) and intron 1 (iv) or chimpanzee CGβ1 intron 1 (v). The region shaded gray is aligned in panel C. (B) Ethidium bromide-stained agarose gel of PCR amplification products obtained with primers i and iii, specific for hCGβ1/2 promoter and exon 1, and 293 cell (Hs), bonobo (Pp), chimpanzee (Pt), gorilla (Gg), or orangutan (Pa) genomic DNA or no template (Con). (C) Sequence alignment of African great ape genomic DNA from the CGβ1 and -β2 proximal promoter and first exon (blue shading). snaR-G genes are bracketed, and their B-box promoter is boxed. Upstream chromosome 19 (Ch19) and GgV clone sequences are also aligned, and the limit of their homology is demarcated by the dotted line. Nucleotide heterogeneity is highlighted in gray, and unique bases are highlighted in black. Asterisks denote identity among all sequences except Ch19 and GgV. Putative CGβ1/2 start codons (ATG1 and ATG2) are shaded dark blue, and the start codon of the CGβ antecedent is shaded orange. The new splice donor site (red text) and the consensus splice donor site (green text and arrow) are shown.
Conservation of type II CGβ genes.
To examine the phylogeny of the type II genes, we initially analyzed the genomic DNA of five great ape species by PCR amplification using primers directed against the insert sequence (primers i and iii [Fig. 2A]). Products were generated from human, bonobo, chimpanzee, and gorilla but not from orangutan (Fig. 2B), supporting the earlier suggestion that CGβ1/2 evolved in the African great apes (22). We then employed combinations of PCR primers, located both within and outside the insert sequence (primers i to v [Fig. 2A]), to define the type II CGβ genes present in African great ape species and to compare their sequences.
Analysis of the resulting clones revealed that all African great apes have an snaR-G gene proximal to CGβ1/2 and that this gene displays the greatest degree of sequence heterogeneity in the type II insertion (Fig. 2C; see also Data Set S2 in the supplemental material). The structure of the LH/CGβ cluster has not yet been defined in gorilla, but this species appears to have distinct CGβ1 and CGβ2 genes (22). Correspondingly, gorilla has both snaR-G1 and snaR-G2, which have diverged considerably from each other, as in humans (45, 46). Within the Pan genus the snaR-G genes are almost identical to one another and are more closely related to snaR-G1 than to snaR-G2 of humans (Fig. 2C). Furthermore, there was no evidence for snaR-G2 or CGβ2 in either Pan species, consistent with the existence of two tandem copies of CGβ1 but no CGβ2 in chimpanzee (23) or bonobo. We conclude that African great apes have two type II CGβ genes, although both are CGβ1 in Pan, and that they carry divergent snaR-G genes.
The first exon region displays good sequence conservation across all species (Fig. 2C). Of particular note, a known hCGβ1/2 splice donor site (6) is conserved, as are two potential translational start codons. The first of these (ATG1 [Fig. 2C]) is represented by TTG in the ancestral human chromosome 19 region and in an additional copy of the type II insert found in gorilla that also does not adjoin a CGβ gene (GgV; see Data Set S2 in the supplemental material). The second potential start codon (ATG2) is also absent from GgV, which has ACG in this position. These observations suggest that the first two AUG codons of the CGβ1/2 genes serve a translational role that evolved after the substitution event creating the type II genes.
Expression of alternatively spliced mRNAs.
Unlike type I genes, alternately spliced hCGβ1/2 transcripts have been detected in human tissues (4, 6, 13, 51). To define the protein products of the type II genes, we first generated cDNA libraries by reverse transcription-PCR (RT-PCR) amplification. We used an oligo(dT)-containing primer for the RT step and PCR primers located close to the 5′ end of exon 1 and the 3′ end of exon 3, such that the products would be nearly full-length cDNAs containing every potential open reading frame (ORF). A comparable library was constructed for type I transcripts.
Examination of a large set of human tissues yielded the expected 561-bp type I product from term placenta after 25 PCR cycles (Fig. 3A, top). Further amplification (35 PCR cycles) revealed expression in prostate and testis, as well as in skeletal muscle, thymus, and trachea (Fig. 3A, middle). There was no evidence of alternatively spliced type I mRNAs. For type II transcripts, at least 35 PCR cycles were required to detect products, consistent with the relatively low expression of these genes in placenta (51). The highest type II expression was observed in testis, in which two major amplification products (671 and 837 bp) were apparent (Fig. 3A, bottom). These results were obtained with testis RNA pooled from three individuals. Identical band patterns were obtained from two additional individual testis samples (data not shown). After a further 3 cycles of PCR amplification, the short transcript was also detected in adrenal gland and fetal brain, but not in placenta, and several additional bands appeared in testis (Fig. 3B). Products were also weakly detected in cervix and bladder (data not shown). Hence, the type II substitution appears to greatly affect hCGβ tissue expression and to enable alternative splicing.
FIG. 3.
Tissue expression of hCGβ mRNA. (A) Tissue expression of type I mRNA (top panel, 25 cycles; middle panel, 35 cycles) and of type II mRNA (bottom panel, 38 cycles) as determined by RT-PCR. Tissue name abbreviations are defined in Materials and Methods. (B) Comparison of hCGβ1/2 PCR products from positive tissues in panel A. (C) Alternatively spliced hCGβ1/2 mRNAs. The number of clones isolated from each tissue is given. (D) Schematic of the alternative splicing of hCGβ1 and hCGβ2 clones. Nucleotide numbers indicate splice donor (black) or acceptor (gray) sites relative to the transcription start site.
Cloning and sequencing of the type II PCR products revealed alternatively spliced mRNAs from both hCGβ1 and hCGβ2, which were differentially expressed in tissues (Fig. 3C and D). Multiple splice forms were detected in testis, where hCGβ2 transcripts appeared to predominate over those originating from hCGβ1, whereas adrenal gland and fetal brain clones were derived exclusively from hCGβ1 (Fig. 3C; see also Data Set S3 in the supplemental material). The variation in mRNA structures results from differential splicing to the consensus acceptor site of exon 2 (Fig. 3D). As noted earlier (6), alternative splicing of hCGβ1/2 mRNAs appears to result from the mutation of the type I donor site from GT to GA and the introduction of a noncanonical donor site in the ∼0.7-kb insert that is 47 nucleotides (nt) upstream of the former site (position +232 [Fig. 3D]). Short transcripts (β1a and β2a [Fig. 3D]), corresponding to the predominant short RT-PCR products (Fig. 3B), result from splicing at the introduced donor site. These mRNAs are generated from both type II genes, but splice site selection in longer mRNAs appears to be dictated by the gene of origin. The longer species (β1b and β2b [Fig. 3D]), corresponding to the predominant long RT-PCR products (Fig. 3B), are derived by utilization of constitutive donor sites ∼400 nt downstream of the transcription start site. Both of these alternative splice forms were previously observed in term placenta (6), testis (4), and pituitary gland (13), although the gene of origin was not identified as hCGβ1 or hCGβ2.
We also detected a novel rare hCGβ1 splice form in testis, β1c, which contains an additional exon (exon 1B [Fig. 3D]). It results from splicing of the exon 1 donor site of β1a mRNAs to a constitutive acceptor site 90 nt downstream at +322 and of the β1b donor site to the consensus acceptor site of exon 2. The PCR product of β1c (789 bp) is visible between the two major testis bands (Fig. 3B, white arrowhead). Further work will be required to establish the abundance of this and other hCG mRNAs in individual tissue samples.
Transcription signals in type II inserts.
The substitution that gave rise to type II CGβ genes deleted the proximal 52-bp sequence of the type I promoter which is critical for transcription (19) and replaced it with sequence that in its previous genomic location is not known to function as a promoter. To examine the ability of this inserted sequence to direct transcription, we fused upstream and 5′ untranslated regions of hCGβ1 or hCGβ2 to the firefly luciferase (FFL) reporter gene (Fig. 4A). Longer constructs (β1L and β2L) contain a fragment of the hCGβ1 or hCGβ2 5′ UTR (174 bp; up to ATG1), its type II-specific sequence (−481/469 bp), and the distal region (−481/469 to −861/849 bp) common to both type I and type II genes. Shorter constructs (β1S and β2S) lack the common upstream region. Versions of each construct, designated Δ, from which the snaR-G genes were deleted were produced to determine whether these sequences can modulate type II CGβ expression.
FIG. 4.
Type II hCGβ promoter analysis. (A) Schematic of hCGβ1/2 promoter, 5′ UTR, and first exon. The type II insert is demarcated by the dotted line. The extent of hCGβ1/2 sequence included in reporter constructs β1/2L (white bar), β1/2LΔ (hatched bar), β1/2S (black bar), and β1/2SΔ (gray bar) is shown below. (B) Relative luciferase activity in HeLa S3 cells transfected with the β1/2L and β1/2S constructs and their corresponding snaR-G deletion constructs diagrammed in panel A. Activity is normalized to β1L construct, and standard deviations (n = 4 duplicates) are shown. Two-tailed Student's t test: *, P < 0.002, and **, P < 0.001. (C) Northern blots of total RNA from transfected JAr cells. (Top) Cells were transfected with β1L (0, 0.5, 1, or 2 μg) or β1LΔ (2 μg) as indicated, and blots were probed for snaR-G1. (Bottom) As in top panel except that cells were transfected with β2L or β2LΔ and blots were probed for snaR-G2. (D) As in panel C, except that HeLa S3 cells were transfected with 0, 0.3, 0.75, 1.5, or 3 μg of β1L or β2L or with 3 μg of β1LΔ or β2LΔ.
Initially, several cell lines were screened for expression of FFL from β1L, using Renilla luciferase expression from cotransfected cytomegalovirus (CMV)-Renilla plasmid as internal control. Strong activity was obtained in HeLa cells (derived from cervical adenocarcinoma), with lesser activity in JAr cells (placental choriocarcinoma) and low or very low activity in CHME-5 (fetal microglia), 293 (transformed fetal kidney), and 293T and MCF-7 (mammary adenocarcinoma) cells (data not shown). This wide variation is in keeping with the restricted tissue expression of hCGβ1/2. In HeLa cells, β1L and β2L displayed equal activities (Fig. 4B). The β1S and β2S constructs were as active as the corresponding long forms, indicating that the type II-specific sequence is sufficient for transcription from these genes (Fig. 4B).
Deletion of the snaR-G gene reduced expression from the shorter promoter constructs by 40 to 45% (compare β1SΔ to β1S and β2SΔ to β2S [Fig. 4B]). The effects of the deletion were attenuated in the context of the longer promoter constructs, where promoter activity was reduced by ∼20% for β2LΔ and no reduction was observed in the case of β1LΔ (Fig. 4B). Thus, the snaR-G sequence can serve as a transcriptional activator of hCG1β and hCG2β, and the promoter-distal region can compensate at least in part for snaR-G deletion. These results indicate a functional interplay between these two parts of the type II promoter as well as the existence of transcriptional differences between the two type II genes. The expression of snaR-G1 and -G2 from β1L and β2L, respectively, was confirmed in JAr cells (Fig. 4C), showing that these snaR genes are transcriptionally competent. HeLa cells expressed snaR-G1, as expected, but snaR-G2 expression was barely detectable (Fig. 4D), suggesting a requirement for cell-specific transcription factors or the rapid turnover of snaR-G2 in these cells.
Coding capacity of hCGβ1 and -β2 genes.
Sequence substitution introduced two new upstream potential start codons (ATG1 and ATG2 [Fig. 2C]) into type II CGβ. These codons are evolutionarily conserved in the African great apes and would initiate translation in reading frames that differ from one another (Fig. 5A). Since the type I initiation site is missing from type II β1a/2a mRNAs due to splicing, it has been assumed that translation begins at ATG1, yielding a novel 132-amino-acid (aa) protein product. This hypothetical product, which has not been detected, is unrelated in sequence to hCGβ and has no recognized functional motifs. On the other hand, initiation at ATG2 would yield a product that is similar to hCGβ along most of its length. Initiation at the third or fourth AUG would yield truncated versions of the same products, while initiation at the distant fifth AUG would result in the synthesis of a short ∼3-kDa product from the third reading frame (not illustrated).
FIG. 5.
Type II hCGβ reading frame usage. (A) Schematic of potential hCGβ1a, -2a, and -2b cDNA reading frames. Predicted translation products initiated at ATG1 (black bars) or ATG2 (gray bars) are depicted, and their sizes in amino acids (kDa) are indicated. Exons are depicted as open boxes, and the SanDI restriction site is shown by an arrowhead. (B) Autoradiography of protein products from hCGβ1a, -2a, or -2b cDNA labeled in rabbit reticulocyte (top) or wheat germ (bottom) cell-free transcription/translation systems. Short peptide bands are denoted by the white bracket. (C) Schematic of potential hCGβ1a, -2a, and -2b reading frames. Exons are depicted as open boxes. Reading frames are numbered 1 to 3 with subscript numbers denoting the order of ATG codons relative to the cDNA 5′ end. The first stop codon of each reading frame is numbered in gray. Linkage of the FFL gene to the 5′ part of hCGβ cDNAs is denoted by a dotted line for all F1 to F3 constructs and by a dashed line for the F1′ β2b construct. (D) Immunoblot of extract of HeLa cells transfected with reading frame constructs indicated. Blots were probed with antibodies (Ab) specific for firefly luciferase (FFL; top panel, short exposure; middle panel, longer exposure) and α-tubulin (Tub; bottom panel). (E) Relative luciferase activity in the same extracts as in panel D (n = 4 with duplicate samples).
To characterize the translation products of hCGβ1 and hCGβ2, we first programmed the rabbit reticulocyte cell-free coupled transcription-translation system with constructs specifying the three most abundant type II testis mRNAs, namely, β2a, β2b, and β1a (Fig. 3C). The constructs were linearized by digestion with AflII, which cuts outside the hCG sequence, or with SanDI, which cuts within the second ORF (Fig. 5A, arrowhead). Translation of AflII-linearized β1a and β2a clones yielded two radiolabeled products, an intense upper band and a weaker lower band (Fig. 5B, top). The upper bands had apparent molecular masses of ∼16 kDa and ∼17 kDa, respectively, while the lower bands comigrated at ∼15 kDa. These correspond to predicted products of 16.7 and 14.0 kDa from β1a and 17.4 and 14.0 kDa from β2a when translation is assumed to begin at ATG2 and ATG1, respectively (Fig. 5A; see also Data Set S4 in the supplemental material). Analysis of the products obtained with SanDI-linearized templates confirmed this interpretation. SanDI digests within the reading frame beginning with ATG2 to give a predicted product of ∼12.9 kDa but outside the ORF beginning with ATG1 (Fig. 5A; see also Data Set S4). The upper bands from both β1a and β2a were abolished after SanDI digestion and replaced by a diffuse smear migrating in the lower region of the gel (Fig. 5B, top), where resolution is compromised by the large amount of globin present in the reticulocyte lysate.
We repeated the transcription/translation experiment using the wheat germ-coupled system to obtain better resolution of short proteins (Fig. 5B, bottom). The AflII-digested β1a and β2a templates again yielded two products, although the upper (∼16-kDa or ∼17-kDa) and lower (∼15-kDa) bands generated were approximately equal in intensity, possibly reflecting the different initiation site selection preferences of plants and animals (39). As expected, SanDI-digested β1a and β2a yielded bands of ∼15 kDa and ∼12 kDa in the wheat germ system. We conclude that the upper bands obtained in vitro with the AflII-digested template represent protein initiated at the second AUG. These findings raise the possibility that hCG-related proteins are the principal products of type II genes.
Start codon preference in vivo.
To assess the start codon usage of type II mRNAs in vivo, we fused 5′ fragments of β1a upstream of the FFL reporter (Fig. 5C). The fragments encompass the start codons of all three reading frames. Versions were created containing a 1-nucleotide deletion or insertion at the β1a/FFL junction so that translation initiation could be evaluated in each reading frame (see Data Set S5 in the supplemental material). Constructs in which FFL is in frame with reading frame 1 of β1a mRNA, using ATG1 or ATG4, are designated F1 (Fig. 5C and Table 1). Similarly, constructs in which FFL is in frame with reading frame 2 of β1a mRNA, using ATG2 or ATG3, are designated F2. Finally, constructs in which FFL is in frame with reading frame 3 of β1a mRNA, using ATG5, are designated F3.
TABLE 1.
Type II mRNA start codon usage in vivo
| Construct | AUG order | Reading frame | Observed banda | Molecular mass (kDa) |
AUG context | |
|---|---|---|---|---|---|---|
| Predicted | Observedb | |||||
| FFL | ++++ | 60.7 | 60.5 | ACCaugG | ||
| β1a/2a | 1 | 1 | − | 69.8 | TCCaugT | |
| 2 | 2 | +++ | 68.0 | 68.6 | GACaugT | |
| 3 | 2 | ++ | 66.6 | 66.1 | AGCaugG | |
| 4 | 1 | + | 66.3 | 66.4 | GACaugG | |
| 5 | 3 | − | TCAaugC | |||
| β2b′ | 1 | 1 | − | 68.4 | TCCaugT | |
| 3 | 1 | + | 65.0 | 65.2 | AAGaugG | |
Expression levels based on anti-FFL antibody immunoblot analysis as in Fig. 5D. −, no detectable expression; + to ++++, low to high expression.
Apparent molecular masses were calculated using GeneTools software (v4.01; SynGene) calibrating against unmodified FFL protein and molecular mass markers (Precision Plus; Bio-Rad).
Constructs were transfected into HeLa cells, and FFL expression was assessed by immunoblotting with antiluciferase antibody (Fig. 5D; Table 1) The predominant products generated from construct F2 migrated as a close doublet, and the mobility of the slower, dominant band indicated probable initiation at the second AUG of β1a (Fig. 5D; Table 1). These findings are consistent with those obtained in vitro. Lesser amounts of product were generated from F1; its mobility suggested initiation at the fourth AUG rather than the first. Little or no product was detected from F3. These results were supported by luciferase assays (Fig. 5E), although the activity produced by F1 was somewhat greater than expected relative to F2. Possibly the N-terminal extension in the fusion product generated by initiation at ATG2 in F2 interferes with FFL enzymatic action to a greater extent than the shorter extension resulting from initiation at ATG4 in F1. As seen in Table 1, the observed selection of β1a mRNA start codons is compatible with the position and context rules for cap-dependent initiation (33, 34). Similar results would be expected for β2a mRNA, which has an almost identical 5′ region (Fig. 2C; see also Data Set S5 in the supplemental material), establishing that type II genes can potentially generate hCG-related proteins in vivo.
hCGβ2 encodes hCGβ protein.
β2a mRNA is the most abundant type II transcript detected in testis, and initiation at its second AUG is predicted to result in synthesis of hCGβ. After cleavage of its N-terminal signal peptide, hCGβ derived from hCGβ2 would differ from type I hCGβ protein by a solitary amino acid substitution (Ala for Asp 117 [Fig. 6A]), a variation that corresponds to a common polymorphism and is present in hCGβ7. The corresponding hCGβ1 product would have Asp 117 but a truncated C terminus, missing the last eight residues. To determine whether type II hCGβ mRNAs can give rise to hCGβ in vivo, we transfected clones obtained from testis into HeLa cells and probed the resulting cell extracts by immunoblotting with anti-hCGβ antibody.
FIG. 6.
Type II hCGβ protein products. (A) Sequence alignment of hCGβ. Key residues are numbered relative to the signal peptide cleavage site (solid line). The Phe residue in the signal peptide sequence (underlined) is replaced by Leu in hCGβ5. Known N- and O-linked glycosylation sites are denoted by gray and black shading, respectively. (B) (Top) Immunoblot of extract of HeLa cells transfected with construct expressing β1a, β2a, or β2b mRNA or empty vector and probed with anti-hCGβ antibody. Extract was incubated in the presence or absence of PNGase F (PNGF) before electrophoresis. (Bottom) Longer exposure of the same immunoblot. An apparent hCGβ1-derived band is denoted by the white arrow. (C) Total RNA extracted from cells in panel B was DNase I treated, reverse transcribed in the absence or presence of reverse transcriptase (RT), and PCR amplified for 20 or 25 cycles. PCR products were visualized in an ethidium bromide-stained agarose gel. (D) Immunoblot of extract from HeLa cells transfected with C-terminal FLAG-tagged peptide β2b′ construct, ATG mutants, or empty vector. Blot was probed with anti-FLAG antibody. (E) Amino acid sequence of putative peptides translated from β2b or β1c mRNAs. Predicted subtilisin-like proprotein convertase (1) and N-Arg dibasic convertase (2) cleavage sites, as well as a peptide amidation site (3), are denoted by arrowheads. Positions of adenine mutations in ATG codons at nucleotides A175, A265, and A271 relative to the transcription start site are marked. (F) PCR product of RNA isolated from cell extract in panel D, amplified over 20 or 25 cycles.
A prominent immunoreactive band of ∼23 kDa was produced in cells transfected with β2a (Fig. 6B). The mobility of this band was lower than that expected for unmodified hCGβ (17.4 kDa), possibly because hCGβ is extensively modified by N- and O-linked glycosylation after entering the endoplasmic reticulum and Golgi apparatus (reviewed in reference 12). When the cell extract was treated with peptide:N-glycosidase F (PNGase F) to remove N-glycan chains (40), the prominent β2a-specific band shifted to a mobility corresponding to ∼16 kDa, in good agreement with the expected size of unmodified hCGβ (Fig. 6B). These results demonstrate that the β2a mRNA of hCGβ2 is translated into an hCG precursor protein from conserved ATG2, that its distinct signal peptide is sufficient for transport into the endoplasmic reticulum, and that the protein is processed by cleavage and N glycosylation like type I hCGβ.
No prominent band was seen in untreated extracts of cells transfected with the clone specifying β1a, but long exposure revealed a faint immunoreactive band after PNGase F treatment (Fig. 6B), indicating that this gene encodes truncated hCGβ, albeit more weakly. The low level of hCGβ expression in vivo from hCGβ1 relative to that from hCGβ2 contrasted markedly with their equivalent expression in vitro (Fig. 5B), and the discrepancy could not be attributed to low levels of β1a mRNA as shown by RT-PCR analysis (Fig. 6C). The 5′ ends of the mRNAs are almost identical, and so differences in translation initiation efficiency are unlikely. Consistent with the hCGβ1 C-terminal truncation, the β1a product had a slightly higher mobility (∼15 kDa) than that of β2a (∼16 kDa). This truncation affects the C terminus, which is thought to be involved in hCGβ folding (43), and eliminates Ser 138 (Fig. 6A), one of four sites of O glycosylation (5). We conclude that hCGβ1 is also capable of generating a form of hCGβ: the truncated product accumulates to lower levels than the full-length protein generated by hCGβ2, possibly because of lower stability.
Novel peptide encoded by β2b mRNA.
The second most abundant type II CGβ transcript detected in testis was β2b (Fig. 3C). In the reticulocyte coupled transcription/translation system, the AflII-digested β2b template appeared to yield a small peptide band (Fig. 5B, top) although its molecular weight could not be estimated; peptides with apparent molecular masses of ∼8 and ∼5 kDa were produced in the wheat germ system (Fig. 5B, bottom, white bracket). These products are consistent with translation of frame 1 by initiation at ATG1 and ATG3 (Fig. 5A; see also Data Set S4 in the supplemental material), yielding hypothetical peptides of 60 aa (6.9 kDa) and 30 aa (3.5 kDa), respectively. No such peptides are predicted to result from initiation at any of the other AUG codons in this mRNA (Fig. 5A; see also Data Set S4). Thus, the β2b mRNA is able to generate short peptides in vitro by initiation at its first and third AUGs.
In transfection experiments analogous to those conducted with β1a fusion constructs, the 5′ fragment of β2b cDNA fused to FFL produced no immunoreactive bands (Fig. 5D) and elicited only background luciferase activity (Fig. 5E) in any of the three frames, F1 to F3. The failure to synthesize FFL is consistent with the presence of stop codons in the first two frames and the distant location of the AUG of the third frame (ATG6 [Fig. 5C; see also Data Set S5 in the supplemental material). These negative findings rule out several unorthodox translational mechanisms as explanations for the origin of the short peptides. To confirm the coding potential of β2b frame 1, we generated a shorter reporter construct in which FFL was fused to a fragment lacking the frame 1 stop codon (β2bF1′ [Fig. 5C; see also Data Set S5]). This construct gave rise to FFL fusion protein (Fig. 5D) and luciferase activity (Fig. 5E), demonstrating that β2b can give rise to translation products in vivo as well as in vitro.
To visualize the predicted novel peptide, we generated a construct in which the β2b ORF is linked directly to sequence encoding a C-terminal epitope tag (2bF1′-3×FLAG [see also Data Set S5 in the supplemental material]). When this construct was transfected into HeLa cells, a band at ∼12 kDa was detected by immunoblotting with anti-FLAG antibody (Fig. 6D). Single point mutations were made in the three in-frame AUG codons (ATG1, ATG3, and ATG4), substituting cytosine for adenine at nucleotide positions 175, 265, and 271 (Fig. 6E, top). Equivalent expression of mRNA was confirmed by RT-PCR (Fig. 6F). Uniquely, the A175C mutant, which inactivates the first AUG, eliminated the expression of tagged product, and no shorter products were observed (Fig. 6D). We conclude that hCGβ2 encodes a novel peptide by translation of β2b mRNA from conserved ATG1.
DISCUSSION
The CGβ genes have undergone rapid expansion and evolution in primates. Two members of this gene family, CGβ1 and CGβ2, referred to here as type II CGβ genes, are restricted to the African great apes. This study indicates that the type II genes are responsible for tissue-specific expression of hCGβ and generate a novel peptide with potential biological activity (Fig. 7A).
FIG. 7.
CGβ gene expression and evolution. (A) Schematic of CGβ gene products and their predominant tissue expression. (B) Alternative phylogenetic tree models for the duplication and loss of CGβ1/2 in the African great apes. Branching highlighted in gray was proposed by Laan and colleagues (23).
Origin and evolution of type II CGβ genes.
The region of chromosome 19 containing CGβ genes has undergone extensive duplication of an ancestral LHβ gene in primates (41) and represents a hot spot of human genomic diversity (21). Type II CGβ genes originated through replacement of a portion of an ancestral type I CGβ gene with a composite of chromosome 19 and 18 and Alu sequences, replacing the type I proximal promoter and 5′ UTR sequence with a novel promoter containing a small noncoding RNA gene. Although substitution events can lead to gene inactivation (pseudonization) (60), many lines of evidence indicate that type II CGβ genes are transcriptionally and translationally active. First, type II genes display a higher degree of exon sequence conservation between humans and chimpanzees than does LHβ (22). Second, several studies (4, 6, 13), as well as the present one (Fig. 3), have isolated hCGβ1/2-derived cDNAs from human tissue. Third, hCGβ1/2 mRNA has been detected in polysomes (6). Finally, as reported here, two translational start codons in the sequence unique to CGβ1/2 are phylogenetically conserved (Fig. 2C) and functional (Fig. 5 and 6).
The chimpanzee LH/CGβ cluster contains five CGβ genes, rather than six as in humans, including two copies of CGβ1 and no CGβ2 (23). Gorilla, on the other hand, appears to have distinct CGβ1 and CGβ2 genes (22). Correspondingly, we found distinctive CGβ1 and CGβ2 promoter regions containing distinct snaR-G1 and snaR-G2 in gorilla, but only CGβ1 and snaR-G1 in chimpanzees (see Data Set S2 in the supplemental material). Two models can be envisioned to account for the species-specific duplication of these genes (Fig. 7B). Both suppose a chimpanzee-specific duplication of CGβ1, but they differ in the evolutionary history of CGβ2. Model 1 proposes that CGβ2 arose independently in human and gorilla but not in chimpanzee, while model 2 proposes that the CGβ duplication leading to CGβ1 and CGβ2 took place in the common ancestor of African great apes and that CGβ2 was subsequently lost in chimpanzees. Although model 1 was considered more parsimonious on the basis of chimpanzee-human comparisons (23), it appears less attractive when all three species are considered, requiring the postulate of parallel gene duplication in human and gorilla. We therefore favor model 2, which is further supported by the almost perfect identity of chimpanzee and bonobo snaR-G, consistent with a recent duplication of CGβ1 in Pan.
Type II hCGβ transcription.
Type II genes differ from type I at the RNA level in three important respects. They have distinct 5′ end sequences (Fig. 1 and 2), type II mRNAs are variable as a result of differential splicing of the first exon to the orthodox exon 2 acceptor site (Fig. 3D), and type II mRNA is predominantly expressed in testis and weakly expressed in placenta, where type I hCGβ mRNA is abundant (Fig. 3A) (6, 51). These type II characteristics are either directly attributable to, or strongly associated with, their defining insertion and the concomitant deletion of type I sequence. Two experimentally determined Ets-2 sites are present in the proximal 52 bp of the type I promoter that were lost during type II CGβ genesis (19). Ets-2 is an enhancer of several genes essential to placental development (14, 54, 57, 59), so the absence of its binding sites from type II genes may account for their relatively low expression in human placenta.
Although the chimeric insert is composed of intergenic sequences, in type II CGβ genes it functions as a promoter sufficient for transcription in HeLa cells (Fig. 4). It is predicted to be rich in factor binding sites (22), including potential AP-2 sites in snaR-G. Deletion of snaR-G reduced type II promoter activity (Fig. 4B), indicating that this sequence or its active transcription plays a part in regulating CGβ transcription. Transcription of noncoding RNA by Pol III can influence local chromatin structure and thereby allow transcription of an adjacent gene by Pol II (38). Correspondingly, a recent study established the binding of Pol III to snaR-G1 and -G2, together with the adjacent binding of Pol II (50). In addition, we note that the most divergent snaR-G genes are gorilla GgV, which is not tethered to a CGβ gene, and a sequence upstream of gorilla CGβ1, which is a suspected pseudogene (see below). Both of these divergent snaR-G genes contain internal deletions (Fig. 2C). These data support the involvement of snaR-G in the functionality of the neighboring CGβ gene.
Translation products of type II hCGβ and functional implications.
We demonstrate for the first time that proteins are generated from hCGβ1 and especially hCGβ2. Phylogenetic comparisons implied that the first two AUGs of type II mRNAs evolved to allow protein synthesis from these genes. We show that hCGβ is translated from the second start codon (ATG2) of β2a mRNA and that a novel short peptide is translated from the first start codon (ATG1) of β2b mRNA (Fig. 5 and 6). Expression of these mRNAs in testis suggests that the type II genes were exapted to serve a neoteric function in this organ.
hCGβ protein, derived from β2a mRNA, undergoes intracellular maturation, including transport to the endoplasmic reticulum via a distinct signal peptide leader sequence, cleavage of this sequence, and N glycosylation (Fig. 6B). The β2a mRNA product is an authentic form of hCGβ, differing from type I-derived hCGβ by a solitary substitution at residue 117 (Fig. 6A). The A-to-C replacement, resulting in Asp117/Ala replacement (see Data Set S4 in the supplemental material), is a known human single nucleotide polymorphism (SNP) with an ∼50% occurrence in all type I CGβ genes. The β2a mRNA was especially abundant in the testis sample tested (Fig. 3C), suggesting that this transcript is a source of intratesticular hCGβ. Previous studies have documented the involvement of hCG in the male reproductive system. Testicular Leydig cells are responsive to hCG, resulting in testosterone secretion and the support of spermiogenesis (24, 37, 56). hCGβ has been detected in the interstitium of adult testis in the absence of hCGα (3), leading to speculation that free hCGβ subserves a novel function possibly through its cystine knot topology, which is a characteristic of the transcription growth factor β (TGF-β) family (35). In addition, hCG is a primary stimulant of fetal Leydig cells during testicular development, allowing testosterone secretion and masculinization of the male genital tract (11, 20, 26, 36, 42). The source of fetal testicular hCG has been questioned (27). Our findings warrant further study of the expression of hCG genes, and in particular of type II CGβ, in fetal testis, as well as examination of their potential roles in the paracrine/autocrine stimulation of fetal Leydig cells.
β2b mRNA, the second most abundant human type II mRNA detected, encodes a novel 60-aa polypeptide that contains predicted cleavage sites for two peptidases, subtilisin-like proprotein convertase and nardilysin endopeptidase (Fig. 6E, top). The former is expressed extensively in mammalian neural and endocrine cells and has a major role in processing neuropeptide and peptide hormone precursors (30), while the latter cleaves propeptides (48) and is expressed in human testis and mouse neural tissues during early development (17). The expression of this peptide in tissues awaits confirmation, but the relatively high expression of its mRNA, the conservation of its start codon, and the presence of specific propeptide cleavage sites all suggest a functional role. Although not detected here, a novel peptide of 42 aa is predicted to be synthesized from the relatively rare β1c mRNA (Fig. 6E, bottom). This peptide would contain the same propeptide cleavage sites and a peptide amidation site common to many secretory peptide hormones. Peptide hormones have numerous and varied roles in spermatogenesis (9, 15, 28). Thus, our findings raise the possibility that type II CGβ genes generate other peptides, in addition to the known hCGβ, that function in the male reproductive tract.
CGβ function in African great apes.
Human and chimpanzee type II CGβ genes display a degree of exon sequence conservation similar to that of LHβ, implying functional conservation (22). In chimpanzee, CGβ1B has only one nonconservative amino acid change in its potential CGβ sequence whereas CGβ1 has five nonconservative substitutions, principally in the C-terminal region (Table 2; also see Data Set S6 in the supplemental material). Chimpanzee CGβ1 may therefore be less likely to encode an active form of the protein. The functionality of both gorilla type II CGβ genes has been called into question (22). A single nucleotide insertion in exon 2 of gorilla CGβ1 would lead to a frameshift preventing CGβ protein synthesis (Table 2; see also Data Set S6). In gorilla CGβ2, a 12-nucleotide deletion in exon 2 would result in a deletion of four leucine residues from the CGβ signal peptide and its likely inactivation as a transport signal (see Data Set S7). However, gorilla CGβ2 contains a reversion mutation in the former type I donor splice site (Fig. 2C, green arrow) that is known to restore splicing at this site in humans (6). The resultant mRNA would contain the type I translation start site, predicted to generate CGβ protein with the N-terminal extension MEMLQ (see Data Set S6) compatible with recognition as a signal peptide (see Data Set S7). In short, the splice site reversion could serve as a compensatory mutation, allowing gorilla CGβ2 to generate CGβ protein despite the absence of four leucines from the signal sequence.
TABLE 2.
Conservation of type II CGβ genes in African great apes
| Species | Gene | S/NSa | Indeld | Comment |
|---|---|---|---|---|
| Human | CGβ1 | 4/3 | Premature stop: 8-aa C-terminal truncation | |
| CGβ2 | 1/0 | |||
| Chimpanzee | CGβ1 | 0/5b | ||
| CGβ1B | 2/1 | |||
| Gorilla | CGβ1 | 1/3c | 1 In | Single nucleotide insertion: frameshift |
| CGβ2 | 2/3b | 12 Del | Deletion of 12 nucleotides: loss of 4 Leu residues |
Synonymous (S) and nonsynonymous (NS) nucleotide changes in the CGβ reading frame (frame 2) of CGβ1 and CGβ2 genes, as determined by their departure from the consensus in the type II sequence alignment (see data set S6 in the supplemental material).
All nonsynonymous changes result in neutral or nonconservative amino acid changes based on the Blosum 62-amino-acid substitution matrix (25), except for a Lys-to-Arg change in gorilla CGβ2.
Many nonconservative amino acid changes due to frameshift after residue 31.
Nucleotide insertions (In) and deletions (Del).
In humans, CGβ2 encodes hCGβ whereas the hCGβ1 product is truncated and has several nonconservative substitutions (Fig. 6 and Table 2). Thus, each of the African great apes has at least one type II gene that is a potential source of CGβ protein and short active peptides. The differences among their active CGβ genes would be consistent with a role in the rapid evolution of male reproductive genes, as is evident in primates and particularly notable in chimpanzees and humans (58).
Supplementary Material
Acknowledgments
We thank S. Christakos, L. Goldsmith, T. Pe'ery, and M. Rogers for suggestions and comments on the manuscript. Polyclonal anti-hCGβ antibody (clone SB6) was kindly provided by A. F. Parlow of the National Hormone and Peptide Program.
This study was initiated with funding from the National Institutes of Health (R01 AI034552).
Footnotes
Published ahead of print on 15 November 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adashi, E. Y. 1994. Endocrinology of the ovary. Hum. Reprod. 9:815-827. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Berger, P., M. Gruschwitz, G. Spoettl, S. Dirnhofer, S. Madersbacher, R. Gerth, W. E. Merz, E. Plas, and N. Sampson. 2007. Human chorionic gonadotropin (hCG) in the male reproductive tract. Mol. Cell. Endocrinol. 260-262:190-196. [DOI] [PubMed] [Google Scholar]
- 4.Berger, P., W. Kranewitter, S. Madersbacher, R. Gerth, S. Geley, and S. Dirnhofer. 1994. Eutopic production of human chorionic gonadotropin beta (hCG beta) and luteinizing hormone beta (hLH beta) in the human testis. FEBS Lett. 343:229-233. [DOI] [PubMed] [Google Scholar]
- 5.Birken, S., and R. E. Canfield. 1977. Isolation and amino acid sequence of COOH-terminal fragments from the beta subunit of human choriogonadotropin. J. Biol. Chem. 252:5386-5392. [PubMed] [Google Scholar]
- 6.Bo, M., and I. Boime. 1992. Identification of the transcriptionally active genes of the chorionic gonadotropin beta gene cluster in vivo. J. Biol. Chem. 267:3179-3184. [PubMed] [Google Scholar]
- 7.Bonduelle, M. L., R. Dodd, I. Liebaers, A. Van Steirteghem, R. Williamson, and R. Akhurst. 1988. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum. Reprod. 3:909-914. [DOI] [PubMed] [Google Scholar]
- 8.Borson, N. D., W. L. Salo, and L. R. Drewes. 1992. A lock-docking oligo(dT) primer for 5′ and 3′ RACE PCR. PCR Methods Appl. 2:144-148. [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar, V., and A. Bartke. 1992. The influence of beta-endorphin on testicular endocrine function in adult rats. Biol. Reprod. 47:1-5. [DOI] [PubMed] [Google Scholar]
- 10.Channing, C. P., T. Hillensjo, and F. W. Schaerf. 1978. Hormonal control of oocyte meiosis, ovulation and luteinization in mammals. Clin. Endocrinol. Metab. 7:601-624. [DOI] [PubMed] [Google Scholar]
- 11.Clements, J. A., F. I. Reyes, J. S. Winter, and C. Faiman. 1976. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J. Clin. Endocrinol. Metab. 42:9-19. [DOI] [PubMed] [Google Scholar]
- 12.de Medeiros, S. F., and R. J. Norman. 2009. Human choriogonadotrophin protein core and sugar branches heterogeneity: basic and clinical insights. Hum. Reprod. Update 15:69-95. [DOI] [PubMed] [Google Scholar]
- 13.Dirnhofer, S., M. Hermann, A. Hittmair, R. Hoermann, K. Kapelari, and P. Berger. 1996. Expression of the human chorionic gonadotropin-beta gene cluster in human pituitaries and alternate use of exon 1. J. Clin. Endocrinol. Metab. 81:4212-4217. [DOI] [PubMed] [Google Scholar]
- 14.Ezashi, T., A. D. Ealy, M. C. Ostrowski, and R. M. Roberts. 1998. Control of interferon-tau gene expression by Ets-2. Proc. Natl. Acad. Sci. U. S. A. 95:7882-7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabbri, A., G. Knox, E. Buczko, and M. L. Dufau. 1988. Beta-endorphin production by the fetal Leydig cell: regulation and implications for paracrine control of Sertoli cell function. Endocrinology 122:749-755. [DOI] [PubMed] [Google Scholar]
- 16.Fiddes, J. C., and H. M. Goodman. 1980. The cDNA for the beta-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3′-untranslated region. Nature 286:684-687. [DOI] [PubMed] [Google Scholar]
- 17.Fumagalli, P., M. Accarino, A. Egeo, P. Scartezzini, G. Rappazzo, A. Pizzuti, V. Avvantaggiato, A. Simeone, G. Arrigo, O. Zuffardi, et al. 1998. Human NRD convertase: a highly conserved metalloendopeptidase expressed at specific sites during development and in adult tissues. Genomics 47:238-245. [DOI] [PubMed] [Google Scholar]
- 18.Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R. D. Appel, and A. Bairoch. 2003. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh, D., T. Ezashi, M. C. Ostrowski, and R. M. Roberts. 2003. A central role for Ets-2 in the transcriptional regulation and cyclic adenosine 5′-monophosphate responsiveness of the human chorionic gonadotropin-beta subunit gene. Mol. Endocrinol. 17:11-26. [DOI] [PubMed] [Google Scholar]
- 20.Habert, R., H. Lejeune, and J. M. Saez. 2001. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol. Cell. Endocrinol. 179:47-74. [DOI] [PubMed] [Google Scholar]
- 21.Hallast, P., L. Nagirnaja, T. Margus, and M. Laan. 2005. Segmental duplications and gene conversion: human luteinizing hormone/chorionic gonadotropin beta gene cluster. Genome Res. 15:1535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallast, P., K. Rull, and M. Laan. 2007. The evolution and genomic landscape of CGB1 and CGB2 genes. Mol. Cell. Endocrinol. 260-262:2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallast, P., J. Saarela, A. Palotie, and M. Laan. 2008. High divergence in primate-specific duplicated regions: human and chimpanzee chorionic gonadotropin beta genes. BMC Evol. Biol. 8:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson, V., O. Djoseland, O. Torgersen, E. M. Ritzen, F. S. French, and S. N. Nayfeh. 1976. Hormones and hormonal target cells in the testis. Andrologia 8:195-202. [DOI] [PubMed] [Google Scholar]
- 25.Henikoff, S., and J. G. Henikoff. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U. S. A. 89:10915-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Henke, A., and J. Gromoll. 2008. New insights into the evolution of chorionic gonadotropin. Mol. Cell. Endocrinol. 291:11-19. [DOI] [PubMed] [Google Scholar]
- 26.Huhtaniemi, I. T., C. C. Korenbrot, and R. B. Jaffe. 1977. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J. Clin. Endocrinol. Metab. 44:963-967. [DOI] [PubMed] [Google Scholar]
- 27.Huhtaniemi, I. T., C. C. Korenbrot, and R. B. Jaffe. 1978. Content of chorionic gonadotropin in human fetal tissues. J. Clin. Endocrinol. Metab. 46:994-997. [DOI] [PubMed] [Google Scholar]
- 28.Ivell, R., and R. Anand-Ivell. 2009. Biology of insulin-like factor 3 in human reproduction. Hum. Reprod. Update 15:463-476. [DOI] [PubMed] [Google Scholar]
- 29.Jameson, J. L., and A. N. Hollenberg. 1993. Regulation of chorionic gonadotropin gene expression. Endocr. Rev. 14:203-221. [DOI] [PubMed] [Google Scholar]
- 30.Jansen, E., T. A. Ayoubi, S. M. Meulemans, and W. J. Van de Ven. 1995. Neuroendocrine-specific expression of the human prohormone convertase 1 gene. Hormonal regulation of transcription through distinct cAMP response elements. J. Biol. Chem. 270:15391-15397. [DOI] [PubMed] [Google Scholar]
- 31.Kent, W. J. 2002. BLAT—the BLAST-like alignment tool. Genome Res. 12:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent, W. J., C. W. Sugnet, T. S. Furey, K. M. Roskin, T. H. Pringel, A. M. Zahler, and D. Haussler. 2002. The human genome browser at UCSC. Genome Res. 12:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak, M. 1981. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 9:5233-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozak, M. 1984. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 12:857-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapthorn, A. J., D. C. Harris, A. Littlejohn, J. W. Lustbader, R. E. Canfield, K. J. Machin, F. J. Morgan, and N. W. Isaacs. 1994. Crystal structure of human chorionic gonadotropin. Nature 369:455-461. [DOI] [PubMed] [Google Scholar]
- 36.Leinonen, P. J., and R. B. Jaffe. 1985. Leydig cell desensitization by human chorionic gonadotropin does not occur in the human fetal testis. J. Clin. Endocrinol. Metab. 61:234-238. [DOI] [PubMed] [Google Scholar]
- 37.Liu, L., J. L. Southers, S. M. Banks, D. L. Blithe, R. E. Wehmann, J. H. Brown, H. C. Chen, and B. C. Nisula. 1989. Stimulation of testosterone production in the cynomolgus monkey in vivo by deglycosylated and desialylated human choriogonadotropin. Endocrinology 124:175-180. [DOI] [PubMed] [Google Scholar]
- 38.Lunyak, V. V., G. G. Prefontaine, E. Nunez, T. Cramer, B. G. Ju, K. A. Ohgi, K. Hutt, R. Roy, A. Garcia-Diaz, X. Zhu, et al. 2007. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science 317:248-251. [DOI] [PubMed] [Google Scholar]
- 39.Lutcke, H. A., K. C. Chow, F. S. Mickel, K. A. Moss, H. F. Kern, and G. A. Scheele. 1987. Selection of AUG initiation codons differs in plants and animals. EMBO J. 6:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maley, F., R. B. Trimble, A. L. Tarentino, and T. H. Plummer, Jr. 1989. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 180:195-204. [DOI] [PubMed] [Google Scholar]
- 41.Maston, G. A., and M. Ruvolo. 2002. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol. Biol. Evol. 19:320-335. [DOI] [PubMed] [Google Scholar]
- 42.Molsberry, R. L., B. R. Carr, C. R. Mendelson, and E. R. Simpson. 1982. Human chorionic gonadotropin binding to human fetal testes as a function of gestational age. J. Clin. Endocrinol. Metab. 55:791-794. [DOI] [PubMed] [Google Scholar]
- 43.Muyan, M., and I. Boime. 1998. The carboxyl-terminal region is a determinant for the intracellular behavior of the chorionic gonadotropin beta subunit: effects on the processing of the Asn-linked oligosaccharides. Mol. Endocrinol. 12:766-772. [DOI] [PubMed] [Google Scholar]
- 44.Parrott, A. M., and M. B. Mathews. 2007. Novel rapidly evolving hominid RNAs bind nuclear factor 90 and display tissue-restricted distribution. Nucleic Acids Res. 35:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrott, A. M., and M. B. Mathews. 2009. snaR genes: recent descendants of Alu involved in the evolution of chorionic gonadotropins. Cold Spring Harbor Symp. Quant. Biol. 74:363-373. [DOI] [PubMed] [Google Scholar]
- 46.Parrott, A. M., M. Tsai, P. Batchu, K. Ryan, H. L. Ozer, B. Tian, and M. B. Mathews. 2010. The evolution and expression of the snaR family of small non-coding RNAs. Nucleic Acids Res. doi: 10.1093/nar/gkq1856. [DOI] [PMC free article] [PubMed]
- 47.Parrott, A. M., M. R. Walsh, and M. B. Mathews. 2007. Analysis of RNA:protein interactions in vivo: identification of RNA-binding partners of nuclear factor 90. Methods Enzymol. 429:243-260. [DOI] [PubMed] [Google Scholar]
- 48.Pierotti, A. R., A. Prat, V. Chesneau, F. Gaudoux, A. M. Leseney, T. Foulon, and P. Cohen. 1994. N-arginine dibasic convertase, a metalloendopeptidase as a prototype of a class of processing enzymes. Proc. Natl. Acad. Sci. U. S. A. 91:6078-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Policastro, P. F., S. Daniels-McQueen, G. Carle, and I. Boime. 1986. A map of the hCG beta-LH beta gene cluster. J. Biol. Chem. 261:5907-5916. [PubMed] [Google Scholar]
- 50.Raha, D., Z. Wang, Z. Moqtaderi, L. Wu, G. Zhong, M. Gerstein, K. Struhl, and M. Snyder. 2010. Close association of RNA polymerase II and many transcription factors with Pol III genes. Proc. Natl. Acad. Sci. U. S. A. 107:3639-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rull, K., P. Hallast, L. Uuskula, J. Jackson, M. Punab, A. Salumets, R. K. Campbell, and M. Laan. 2008. Fine-scale quantification of HCG beta gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol. Hum. Reprod. 14:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rull, K., and M. Laan. 2005. Expression of beta-subunit of HCG genes during normal and failed pregnancy. Hum. Reprod. 20:3360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sofikitis, N., N. Giotitsas, P. Tsounapi, D. Baltogiannis, D. Giannakis, and N. Pardalidis. 2008. Hormonal regulation of spermatogenesis and spermiogenesis. J. Steroid Biochem. Mol. Biol. 109:323-330. [DOI] [PubMed] [Google Scholar]
- 54.Staun-Ram, E., S. Goldman, and E. Shalev. 2009. Ets-2 and p53 mediate cAMP-induced MMP-2 expression, activity and trophoblast invasion. Reprod. Biol. Endocrinol. 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauss, W. M. 1998. Preparation of genomic DNA from mammalian tissue. p. 2.2.1-2.2.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. I. John Wiley & Sons, Inc., New York, NY.
- 56.Wang, H., D. L. Segaloff, and M. Ascoli. 1991. Lutropin/choriogonadotropin down-regulates its receptor by both receptor-mediated endocytosis and a cAMP-dependent reduction in receptor mRNA. J. Biol. Chem. 266:780-785. [PubMed] [Google Scholar]
- 57.Wen, F., J. A. Tynan, G. Cecena, R. Williams, J. Munera, G. Mavrothalassitis, and R. G. Oshima. 2007. Ets2 is required for trophoblast stem cell self-renewal. Dev. Biol. 312:284-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wyckoff, G. J., W. Wang, and C. I. Wu. 2000. Rapid evolution of male reproductive genes in the descent of man. Nature 403:304-309. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto, H., M. L. Flannery, S. Kupriyanov, J. Pearce, S. R. McKercher, G. W. Henkel, R. A. Maki, Z. Werb, and R. G. Oshima. 1998. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 12:1315-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, Z., P. M. Harrison, Y. Liu, and M. Gerstein. 2003. Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res. 13:2541-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Z., S. Schwartz, L. Wagner, and W. Miller. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203-214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.