Abstract
Cyclin A is known to promote S-phase entry in mammals, but its critical targets in this process have not been defined. We derived a novel human cyclin A mutant (CycA-C1), which can activate cyclin-dependent kinase but cannot promote S-phase entry, and isolated replication licensing factor Mcm7 as a factor that interacts with the wild-type cyclin A but not with the mutant. We demonstrated that human cyclin A and Mcm7 interact in the chromatin fraction. To address the physiological significance of the cyclin A-Mcm7 interaction, we isolated an Mcm7 mutant (Mcm7-3) that is capable of association with CycA-C1 and found that it can also suppress the deficiency of CycA-C1 in promoting S-phase entry. Finally, RNA interference experiments showed that the CycA-C1 mutant is defective for the endogenous cyclin A function in S-phase entry and that this defect can be suppressed by the Mcm7-3 mutant. Our findings demonstrate that interaction with Mcm7 is essential for the function of cyclin A in promoting S-phase entry.
Key transitions within the cell cycles of eukaryotes are controlled by cyclin-dependent kinases (CDKs). The kinase activity of CDKs depends on their association with cyclins. Cyclins are differentially expressed during the cell cycle, and specific cyclin-CDK complexes are assembled and activated at different cell cycle stages. Each of these cyclin-CDK complexes executes a unique cell cycle function that is essential for cell cycle progression (23, 30).
One of the two major functions of cyclin is to promote the activation of CDK (31). This is ascribed to a “cyclin box,” a conserved domain within all cyclins that is necessary for binding to CDK. The other major function of cyclin is to target CDK to its specific substrates (22, 28). Presumably, cyclin-specific domains other than the cyclin box are responsible for this function. However, there is only limited information on the exact cyclin domains that determine substrate specificity. Systematic analyses of the domains specific for each cyclin would be very important for clarifying the cyclin-specific substrates, which represent a major gap in our current knowledge of cell cycle control.
Cyclin A is important in the vertebrate cell cycle for the onset of both DNA replication and mitosis. Elimination of cyclin A function by microinjecting antibodies against cyclin A or by expressing antisense cyclin A RNA has been shown to inhibit DNA synthesis and mitosis (10, 25). Also, precautious expression of cyclin A in G1 cells accelerated their S-phase entry, indicating that cyclin A is rate limiting for the G1-to-S (G1/S) transition (27). Deletion analyses of Xenopus laevis cyclin A (19) have shown that it can be separated into three domains: the N-terminal domain, which is essential for periodic degradation of the cyclin A protein; the central cyclin box domain; and the C-terminal domain, whose function is largely unknown. X-ray crystallography of the human cyclin A-CDK2 complex has revealed that cyclin A interacts with CDK via the cyclin box domain and that the C-terminal domain is basically free of the CDK interaction (16). In addition, a conserved substrate-docking site in the cyclin box domain has been identified (29). This site is implicated in binding to various cyclin-CDK substrates through their cyclin-binding motifs (RXL motifs) but is not likely to contribute to substrate specificity, since the docking site and the RXL motifs are widely conserved among different cyclins and substrates (22, 28). In contrast, the C-terminal domain contains several clusters of charged amino acid residues on the protein surface and could therefore be involved in the interaction with a specific target protein(s). This prompted us to systematically mutagenize these clusters in the C-terminal domain of human cyclin A2 (a somatic subtype of cyclin A, hereafter referred to as cyclin A or CycA), and we isolated a mutant that is capable of activating CDK but is defective for promoting S-phase entry. This mutant, which we call a “targeting mutant,” is supposedly defective for targeting a specific substrate(s) required for promoting S-phase entry.
Here we report that we identified replication licensing factor Mcm7 as a specific target of cyclin A for promoting S-phase entry, using the cyclin A “targeting mutant” as a screening tool. We showed that cyclin A interacts with Mcm7 in human cells and that the interaction is essential for the promotion of S-phase entry.
MATERIALS AND METHODS
Cells and plasmids.
All cells used were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C with 5% CO2. pCS2MT (34)-based expression vectors carrying myc-tagged human cyclin A2 (pCSMTcycA) (4) were used for constructing all the alanine-scanning cyclin A mutants by site-directed mutagenesis (20). Other plasmids used were provided as described in the supplemental material.
Transfection, flow cytometry, and immunoprecipitation.
Five micrograms (or other amounts, as indicated) of each of the cyclin A- and Mcm7-expressing vectors was cotransfected with 1 μg of a green fluorescent protein (GFP)-expressing vector, pEGFP-C1 (Clontech Laboratories, Inc., Mountain View, CA), using the modified Ca-phosphate method (5). Sixteen hours after the transfection, cells were refed and were then cultured for an additional 24 h before being harvested. Flow cytometry was performed as described previously (4), and data for the GFP-positive cells were gated. Immunoprecipitation was performed as described previously (4), and histone H1 kinase assays were done by standard methods (5). The sources of the antibodies used are described in the supplemental material.
Yeast two-hybrid (Y2H) screening.
The C-terminal domains (amino acids 297 to 432) of wild-type and mutant (C1) cyclin A were subcloned into a pGBDu-C3 vector (15) and used as baits for screening a pACT2 (Clontech Laboratories, Inc.)-based human B cell cDNA library according to mating methods (15). First, the yeast (Saccharomyces cerevisiae) cells carrying the wild-type cyclin A bait plasmid were mated with those carrying the human cDNA library (2 × 106 clones), and the diploids were selected for the histidine-prototrophic (His+) and adenine-prototrophic (Ade+) phenotypes. After the bait plasmid from these clones (ca. 4,900 clones) was cured by the 5-fluoroorotic acid selection method (2) and the bait-independent false positives were excluded, the remaining 191 true-positive clones were transformed with the cyclin A-C1 (CycA-C1) mutant bait plasmid and selected for the His− and Ade− phenotypes.
Chromatin fractionation.
Cell extracts from U2-OS and HT1080 cells were prepared and fractionated according to the method described by Fujita et al. (7, 8), with some modifications. Briefly, cells were lysed on ice for 20 min in 1 ml of modified CSK buffer (10 mM HEPES-KOH [pH 7.5], 100 mM NaCl, 300 mM sucrose, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonate, 10 μg/ml each of leupeptin, pepstatin, and aprotinin, 50 mM NaF, and 0.1 mM sodium vanadate) containing 0.1% Triton X-100 and 0.1 mM ATP, followed by centrifugation at 3,000 rpm for 3 min. The supernatant was cleared of debris by centrifugation at 15,000 rpm for 5 min (soluble fraction). The precipitate was suspended in 1 ml of CSK buffer containing 0.1% Triton X-100, 1 mM ATP, and 1,000 U/ml of DNase I (Roche Diagnostics, Indianapolis, IN), treated at 25°C for 30 min, and cleared by centrifugation at 3,000 rpm for 3 min (chromatin-bound fraction).
Isolation of Mcm7 suppressor mutants by Y2H screening.
The C-terminal domain of Mcm7 (amino acids 447 to 719) was mutagenized by PCR with 45 cycles of 30 s at 94°C, 30 s at 61°C, and 1 min at 72°C, in the presence of 8 mM MgCl2 and was subcloned into pACT2 to construct a mutant Mcm7 library (2.6 × 104 clones). The yeast cells carrying the mutant Mcm7 library were mated with those carrying the CycA-C1 mutant bait, and a total of 6 × 105 diploids were screened for the His+ and Ade+ phenotype. The mutant Mcm7 C-terminal domains recovered from 11 clones, which exhibited the strongest CycA-C1-interactive phenotype among 830 positive clones, were sequenced, and four of them were shown to carry single-amino-acid substitutions (Mcm7-3, Mcm7-8, Mcm7-9, and Mcm7-11). These mutations were subcloned into the pCS2HA (34)-based vector carrying full-length Mcm7 cDNA by recombinant PCR.
RNA interference experiments.
Small interfering RNAs (siRNAs) for silencing mouse cyclin A2 (siRNA 1 [si1], 5′-ACUGUAAGGUUGAAAGCUUAGCAAU-3′, and siRNA 237 [si237], 5′-UCCUUCAUGGAAAGCAGUCAGUAAA-3′) were designed and provided by iGene Therapeutics (Tsukuba, Japan). For evaluation of their silencing efficiency, they were cotransfected with a hemagglutinin (HA)-tagged mouse cyclin A expression vector into 293 cells, using X-tremeGene transfection reagent (Roche Diagnostics).
NIH 3T3 cell lines carrying doxycycline-inducible wild-type or the C1 mutant cyclin A were established using the retroviral RevTet-On system (Clontech Laboratories, Inc.), and single-cell-derived clones were isolated and selected for the equivalent expression levels of wild-type and the C1 mutant cyclin A's. They were grown with 10 μg/ml of doxycycline for 24 h and then transfected with the si1 RNA (compensated with a scrambled control double-stranded RNA [dsRNA] to a total of 4 nM), HA-tagged Mcm7-3 expression vector (0.2 μg), and pEGFP-C1 (0.4 μg), using X-tremeGene reagent. Twenty-four hours after the transfection, the cells were fixed and subjected to flow cytometry.
RESULTS
Isolation of a human cyclin A “targeting mutant.”
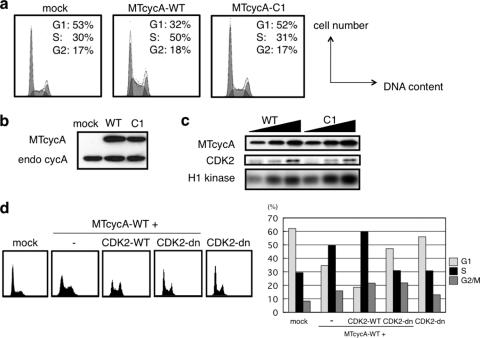
When mouse NIH 3T3 cells were transfected with the vector expressing wild-type human cyclin A2 (cycA-WT), the transfected cells exhibited a characteristic cell cycle profile, with a decrease in the G1-phase population and an increase in the S-phase population (Fig. 1 a). Similar phenotypes have been observed for tetracycline-inducible cyclin A2 (27) and cyclin E1 (18, 24); i.e., the overexpression of these cyclins leads to an acceleration of the G1/S transition. We also observed that coexpression of the dominant negative CDK2 mutant (CDK2-dn) but not wild-type CDK2 (CDK2-WT) suppressed the G1/S acceleration caused by the overexpression of wild-type cyclin A (Fig. 1d), suggesting that the G1/S acceleration by cyclin A depends on CDK activity. Note that with the amount of CDK2-dn expression vector we used, and even with a larger amount (data not shown), CDK2-dn alone did not cause any dominant negative effect. This might reflect a different level of sensitivity of the cell type to a dominant negative effect of CDK2-dn on the cell cycle.
FIG. 1.
Loss of the S-phase entry-promoting function in a human cyclin A mutant (CycA-C1). (a, b, and c) NIH 3T3 cells were cotransfected with 1 μg of GFP-expressing vector and 5 μg each of mock, myc-tagged (MT) wild-type (WT) or mutant (C1) cyclin A (cycA)-expressing vector and subjected to FACS analysis of the GFP-positive cells (a) and anti-cyclin A Western blot analysis (b); 1, 2, and 4 μg of the cyclin A-expressing vectors were used for anti-myc tag immunoprecipitation/histone H1 kinase assays (c). (d) NIH 3T3 cells were cotransfected with 1 μg of GFP-expressing vector and 5 μg of either mock, MTcycA-WT-expressing, or MTcycA-WT vector plus 2 μg each of wild-type or dominant negative CDK2-expressing vector and subjected to FACS analysis of the GFP-positive cells. The transfection control contained the CDK2-dn vector without cyclin A.
We employed this G1/S acceleration assay to isolate the “targeting mutant” of cyclin A, a mutant deficient specifically for targeting CDK to the factor(s) involved in promoting the G1/S transition. To exclude any loss-of-function mutations that impair CDK binding and activation, we introduced alanine substitution mutations into ca. 20 charged amino acid clusters on the surface of the cyclin A protein (deduced from the cyclin A-CDK2 crystal structure) except for those in the cyclin box domain. These mutants were myc tagged and transiently overexpressed in the NIH 3T3 cells and assayed for their G1/S acceleration activity and associated CDK activity. Among them, one mutant (CycA-C1) did not accelerate the G1/S transition (Fig. 1a), while its protein expression level, association with CDK, and associated CDK activity were equivalent to those of the wild-type (Fig. 1b and c), and it was therefore considered to be a “targeting mutant.” Its mutation sites (D345 and K349; see Fig. S1 in the supplemental material) reside in the C-terminal, cyclin A-specific domain and face the solvent in the tertiary structure model of the cyclin A-CDK2-substrate peptide complex.
The cyclin A targeting mutant is deficient for targeting a factor(s) other than the Rb protein.
Retinoblastoma (Rb) protein is well known as a key regulatory factor that is hyperphosphorylated by multiple CDK complexes during the G1/S transition. Along with cyclin D and cyclin E, cyclin A has been shown to overcome the Rb-derived G1 arrest by targeting Rb (13). We therefore determined whether the CycA-C1 mutant can target Rb by examining its ability to overcome Rb-derived G1 arrest. Overexpressing Rb in Rb-deficient SaOS-2 cells resulted in a marked G1 arrest, and coexpression of wild-type cyclin A overcame the arrest (Fig. 2 a). Coexpression of the CycA-C1 mutant also overcame the arrest, concomitant with the phosphorylation of Rb (Fig. 2b), demonstrating that the CycA-C1 mutant can target Rb normally; i.e., it is defective for targeting a factor(s) other than Rb.
FIG. 2.
The cyclin A-C1 mutant can promote phosphorylation and inactivation of the retinoblastoma protein (Rb). Rb-deficient SaOS-2 cells were cotransfected with 1 μg of GFP-expressing vector and 5 μg of mock or Rb-expressing vector with or without 5 μg of wild-type (WT) and mutant (C1) cyclin A-expressing vectors and subjected to FACS analysis of the GFP-positive cells (a) and anti-Rb Western blot analysis (b). Rb-p, the phosphorylated form of Rb.
Screening for the specific target factor(s) for cyclin A in G1/S acceleration.
Given that the CycA-C1 mutant is defective for targeting an essential factor(s) in the G1/S transition, we reasoned that such a factor(s) can bind to the wild-type cyclin A but not to the mutant. We therefore employed a Y2H system to screen for such a specific factor(s). The C-terminal domains of the wild-type and C1 mutant cyclin A's, each fused to the GAL4 DNA-binding domain, were used as baits for screening a human B cell cDNA library fused to the GAL4 activation domain. We performed the primary Y2H screening with the wild-type cyclin A bait and isolated 191 bait-dependent positive clones, which were subjected to secondary screening with the CycA-C1 mutant bait. We isolated 19 clones that did not interact with the mutant bait and identified 18 of them as derived from 14 different proteins, including nuclear, cytosolic, and mitochondrial proteins (the remaining one was derived from an expressed sequence tag [EST] clone).
We focused on nuclear proteins, since the key regulatory mechanism for G1/S transition takes place in the nucleus. Among them was Mcm7, a subunit of the heterohexameric MCM complex that is an essential replication licensing factor and a DNA helicase (35). The Y2H clone contained the C-terminal domain (amino acids 447 to 719) of Mcm7, adjacent to the ATP-binding domain (see Fig. 4a). Our data suggest that the C1 mutation specifically affects the interaction between cyclin A and Mcm7, because in transient expression experiments CycA-C1 can target Rb normally (Fig. 2). These experiments suggest that Mcm7, as a component of the core replication machinery, is one of the important targets of cyclin A for promoting the G1/S transition.
Cyclin A specifically interacts with Mcm7 in mammalian cells.
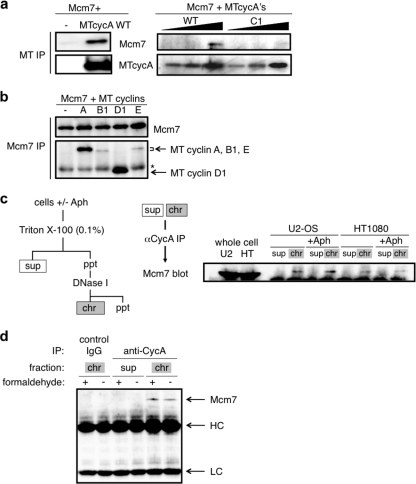
In order to verify that cyclin A and Mcm7 interact in mammalian cells, we first examined the coimmunoprecipitation of the exogenous cyclin A (myc-tagged) and Mcm7 (HA-tagged) proteins, which are transiently expressed in human 293 cells. Figure 3 a shows that anti-myc tag antibodies coimmunoprecipitated Mcm7 with the wild-type cyclin A but much less efficiently with the CycA-C1 mutant. Thus, the allele-specific interaction of cyclin A with Mcm7 in the Y2H system was also confirmed in the mammalian cells. We also compared other cyclins for their abilities to associate with Mcm7. Anti-Mcm7 antibodies coimmunoprecipitated cyclins B1, D1, and E, with much lower affinities for cyclin B1 and cyclin E but with a higher affinity for cyclin D1 than for cyclin A (Fig. 3b).
FIG. 3.
Interaction of cyclin A and Mcm7 in human cells. (a) 293 cells were cotransfected with 5 μg of Mcm7- and MT cyclin A (5 μg in the left panel and 1, 2, and 4 μg in the right panel for the wild type; 1, 2, and 4 μg in the right panel for C1)-expressing vectors, and the cell extracts were subjected to anti-myc tag immunoprecipitation (IP), followed by anti-Mcm7 and anti-MT Western blot analysis. (b) 293 cells were cotransfected with 5 μg each of Mcm7- and myc-tagged-cyclin (cyclin A, cyclin B1, cyclin D1, and cyclin E)-expressing vectors, and the cell extracts were subjected to anti-Mcm7 immunoprecipitation, followed by anti-Mcm7 and anti-myc tag Western blot analysis. The asterisk indicates the IgG heavy chain. (c) Cell extracts from asynchronous or aphidicolin (Aph)-blocked U2-OS and HT1080 cells were fractionated into soluble (sup) and chromatin-bound (chr) fractions and subjected to anti-cyclin A (αCycA) immunoprecipitation, followed by anti-Mcm7 Western blot analysis. (d) Cell extracts from asynchronous U2-OS cells treated with (+) or without (-) 0.2% formaldehyde were fractionated into soluble and chromatin-bound fractions as for panel c and subjected to anti-cyclin A (rabbit polyclonal) immunoprecipitation, followed by anti-Mcm7 (rabbit polyclonal) Western blot analysis. The chromatin-bound fractions were also immunoprecipitated with control rabbit IgG. Arrows indicate Mcm7, the IgG heavy chain (HC), and the IgG light chain (LC). ppt, precipitates.
Next, we asked if the endogenous cyclin A and Mcm7 proteins interact in human cells, especially in the chromatin fraction. The human cell lines U2-OS and HT1080 were cultured with or without the DNA polymerase α inhibitor aphidicolin, and the cell extracts were fractionated into soluble and chromatin-bound fractions. As shown in Fig. 3c, human endogenous Mcm7 was coimmunoprecipitated with endogenous cyclin A specifically in the chromatin-bound fraction. Mcm7 was not immunoprecipitated with a control antibody in the chromatin-bound fractions (Fig. 3d). We also observed coimmunoprecipitation of cyclin A and Mcm7 in the chromatin-bound fraction with a formaldehyde treatment of the cells to cross-link adjacent proteins in vivo (Fig. 3d), suggesting that the coimmunoprecipitation is not derived from a postfractionation interaction of the proteins independently bound to chromatin. These results demonstrate that human cyclin A specifically interacts with Mcm7 in the chromatin fraction in vivo. The addition of aphidicolin had no effect on the yield of Mcm7 coimmunoprecipitated with cyclin A, suggesting that cyclin A interacts with Mcm7 before the loading of DNA polymerase α onto the replication preinitiation complex (preIC).
Interaction of cyclin A and Mcm7 is essential for promoting S-phase entry.
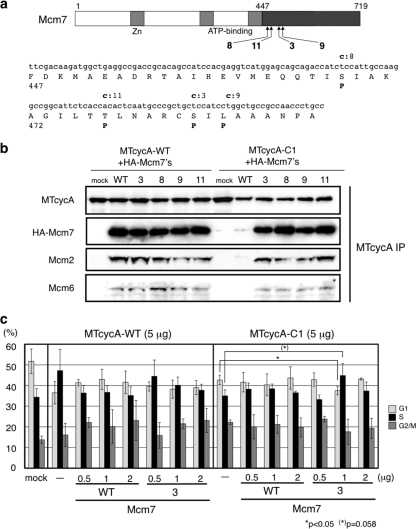
To determine if the interaction with Mcm7 is essential for cyclin A function to promote S-phase entry, we attempted to isolate Mcm7 “suppressor” mutants that restore the interaction with the CycA-C1 mutant. We mutagenized the C-terminal domain of Mcm7 and constructed a mutant Mcm7 Y2H library, which was screened with the CycA-C1 bait. Four strongly interactive clones were found to carry single mutations, which were clustered around the N-terminal region in the mutagenized domain (Fig. 4 a). We subcloned these mutations into full-length Mcm7 and examined these mutant proteins for coimmunoprecipitation with the wild-type and mutant cyclin A's. As shown in Fig. 4b, all the Mcm7 mutants (designated Mcm7-3, Mcm7-8, Mcm7-9, and Mcm7-11) showed increased affinity for the CycA-C1 mutant. We also detected the coimmunoprecipitation of endogenous Mcm2 and Mcm6, other subunits of the MCM complex, with cyclin A only when the Mcm7 proteins were coexpressed, suggesting that these Mcm7 proteins are assembled into the MCM complex and associated with cyclin A. In fact, we observed that all the wild-type and mutant Mcm7 proteins were recovered in both soluble and chromatin-bound fractions and that they coimmunoprecipitated almost equally with Mcm2 and Mcm6 in both fractions (data not shown), suggesting that the mutations do not affect Mcm7 binding to other MCM proteins and loading onto chromatin.
FIG. 4.
Isolation of Mcm7 mutant proteins that restore the interaction with CycA-C1 and suppression of the deficiency of CycA-C1 in promoting S-phase entry by the Mcm7-3 mutant. (a) Mutation sites in the Mcm7-C domain that interacted with CycA-C1 bait in the yeast two-hybrid screening. Small and capital letters in bold indicate the substituted nucleotides and amino acid residues in the corresponding mutants (3, 8, 9, and 11), respectively. (b) The four single mutations in Mcm7-C were subcloned into full-length Mcm7 cDNA. 293 cells were cotransfected with 5 μg of myc-tagged-cyclin A (wild-type or C1)-expressing vector and 2 μg of wild-type or mutant Mcm7 (Mcm7-3, Mcm7-8, Mcm7-9, and Mcm7-1)-expressing vector, and the cell extracts were subjected to anti-myc tag immunoprecipitation, followed by Western blot analysis using antibodies against the myc tag, the HA tag, Mcm2, and Mcm6. (c) NIH 3T3 cells were cotransfected with GFP-expressing vector and myc-tagged wild-type- or the C1 mutant cyclin A-expressing vector, together with wild-type Mcm7 vector or the Mcm7-3 mutant-expressing vector and subjected to FACS analysis of the GFP-positive cells. The significances of the differences between the mock- and Mcm7-transfected cells were analyzed by the Student t test (see Table S1 in the supplemental material).
Then we asked if these Mcm7 mutants can suppress the defect of the CycA-C1 mutant. We carried out a G1/S acceleration assay for cyclin A combined with the Mcm7-3 mutant (Fig. 4c; see Fig. S2 in the supplemental material for representative original fluorescence-activated cell sorter [FACS] profiles). As shown in Fig. 1, overexpression of wild-type cyclin A caused G1/S acceleration, while the CycA-C1 mutant did not. However, when the CycA-C1 mutant was coexpressed with a relatively small amount of the Mcm7-3 mutant (1 μg of the Mcm7-3 expression vector versus 5 μg of the CycA-C1 expression vector), the overexpression of the CycA-C1 mutant resulted in a reduction of the G1 phase and an increase of the S phase (statistical significances of the differences, P = 0.043 for the G1 phase and 0.058 for the S phase), indicating that the Mcm7-3 mutant can suppress the defect of the CycA-C1 mutant in G1/S acceleration. With a larger amount (2 μg) of the Mcm7-3 mutant, coexpression of the Mcm7-3 mutant did not suppress the defect of the CycA-C1 mutant, which might be derived from sequestration of the cyclin A protein by excess Mcm7 protein. Consistent with this finding, we also observed a decrease in the S-phase when we cotransfected wild-type CycA with either wild-type Mcm7, the Mcm7-3 mutant, or only the Mcm7 C-terminal (CycA-interactive) domain (Fig. 4c and data not shown). The wild-type Mcm7 in any amount did not suppress the defect of CycA-C1, suggesting that the increased affinity for the Mcm7-3 mutant is necessary and sufficient for the CycA-C1 mutant to promote S-phase entry. Cotransfection of other Mcm7 mutants that are also able to bind to CycA-C1 (Mcm7-8, Mcm7-9, and Mcm7-11) together with CycA-C1 resulted in a significant increase in the S-phase population and/or a decrease in the G1-phase population compared with that of cells transfected with CycA-C1 alone (see Fig. S3 and Table S2 in the supplemental material).
Finally, we asked if the interaction with Mcm7 is essential for the endogenous cyclin A function in S-phase entry. To address this, we attempted to knock down the endogenous cyclin A in mouse cells and express exogenous human cyclin A with or without the Mcm7-3 mutant. We designed siRNAs specific for the mouse cyclin A, checked their knockdown efficiency, and employed the siRNA designated si1 (Fig. 5 a, left panel). First, the NIH 3T3 cells stably expressing the exogenous human cyclin A, either the wild-type or C1 mutant, were cotransfected with the si1 RNA and GFP, gated for GFP-positive and -negative cells by FACS, and analyzed for their cell cycle distribution. Endogenous cyclin A expression was silenced to a nondetectable level by si1 RNA transfection, as shown in Fig. 5a (right panel). Figure 5b shows the ratio of each cell cycle population in the transfected and nontransfected cells; i.e., values above 1.0 represent an increase and those beneath 1.0 a reduction in the population of each phase (see Fig. S6 in the supplemental material for direct plots of these populations in the GFP-positive and -negative cells and Fig. S4 for representative original FACS profiles). When the control cells not expressing the exogenous cyclin A were transfected with the si1 RNA, the G1 population was increased while the S and G2/M populations were reduced, demonstrating that S-phase entry was inhibited, although not completely, by knocking down the endogenous cyclin A (about 10% of the si1-transfected cells were still in S phase; see Table S3 and Fig. S6 in the supplemental material). S-phase entry was not inhibited by the si1 RNA in the wild-type-cyclin A-expressing cells but was inhibited in the CycA-C1 mutant-expressing cells, as in the control cells. This suggests that the CycA-C1 mutant is defective for the endogenous cyclin A function in S-phase entry. Next, we cotransfected the Mcm7-3 mutant with the si1 RNA (Fig. 5c; see Fig. S6 in the supplemental materials for direct plots of the cell cycle populations and Fig. S5 for representative original FACS profiles). In the wild-type-cyclin A-expressing cells, the Mcm7-3 mutant had little effect on the S-phase population. In contrast, in the CycA-C1 mutant-expressing cells, coexpression of the Mcm7-3 mutant resulted in a decrease of the G1 phase and an increase of the S- and G2/M phases, indicating that inhibition of S-phase entry by the siRNA was suppressed. These results suggest that the interaction with Mcm7 is essential for the cyclin A function in promoting S-phase entry.
FIG. 5.
Cyclin A-Mcm7 interaction is essential for S-phase entry. (a) Left panel: 293 cells were cotransfected with the vector expressing HA-tagged mouse cyclin A (HA-mCycA) and the indicated concentrations of siRNAs, and the cell lysates were subjected to anti-HA Western blot analysis. Right panel: NIH 3T3 cells stably expressing no exogenous cyclin (mock) or myc-tagged human wild-type (WT) or mutant (C1) cyclin A were transfected with the indicated amounts of si1 RNA, and the cell lysates were subjected to anti-cyclin A Western blot analysis. MTCycA, myc-tagged human cyclin A; endo CycA, endogenous mouse cyclin A. (b and c) The NIH 3T3 cell lines described for panel a were cotransfected with si1 RNA (0, 2, and 4 nM) and a GFP-expressing vector, in the absence (b) and presence (c) of the Mcm7-3 mutant-expressing vector. For panel c, pCS2HA was used for the control transfection without Mcm7-3. Twenty-eight hours after transfection, the cell cycle distributions of both GFP+ (transfected) and GFP− (nontransfected) cells were analyzed by FACS. Values are ratios of the percentages of G1-, S-, and G2/M-phase cells in GFP+ cells relative to those for GFP− cells. The significances of the differences between cyclin A-expressing cells and mock-expressing cells (b) and between Mcm7-3-transfected cells and mock-transfected cells (c) were analyzed by the Student t test (see Tables S3 and S4 in the supplemental material).
DISCUSSION
Previous studies have suggested that cyclin A is rate limiting for the G1/S transition (27). It has also been shown that cyclin A-CDK2 is colocalized at subnuclear sites of DNA replication in mammalian cells (3). A number of evidences have suggested that cyclin A-CDK2 is involved in the phosphorylation of many components of the DNA replication initiation complex, thereby promoting the initiation of DNA replication (see below). However, there has been little information on a specific target(s) essential for the cyclin A function in promoting G1/S transition. Thus, the cyclin A targeting mutant (CycA-C1) we isolated is particularly interesting for addressing this point, since it is the first cyclin A mutant reported that is capable of activating CDK but defective for promoting the G1/S transition in vivo. Its mutation sites are located outside the cyclin box domain in which a conserved substrate docking site has been identified (29). The docking site is implicated in binding to various cyclin-CDK substrates but is not likely to contribute to substrate specificity. In contrast, the mutation sites in our targeting mutant (see Fig. S1, red areas, in the supplemental material) reside in the C-terminal, cyclin A-specific domain, suggesting that these sites are involved in recognizing a specific substrate(s).
Using this mutant as a screening tool, we found that the replication licensing factor Mcm7 interacts with wild-type cyclin A but not with the CycA-C1 mutant. The cyclin A-interacting domain in Mcm7 is supposedly the C-terminal domain adjacent to the Walker B motif of its ATP-binding domain, and this domain has been reported to be involved in binding to the Rb protein (32). There are three putative RXL motifs in the Mcm7 C-terminal domain, but apparently they are not involved in the interaction with the cyclin A C-terminal domain. Rather, since all four “suppressor” mutations in Mcm7, which restore the interaction with the CycA-C1 mutant, are proline substitutions and are clustered in a helical region (Glu446 to Ala486; deduced by the NewJoint method of the PAPIA system, Computational Biology Research Center, Tokyo, Japan; http://mbs.cbrc.jp/papia-cgi/ssp_menu.pl) relatively proximal to the Walker B motif, this helical region appears to contribute to the structural moiety of the Mcm7 C-terminal domain for interacting with cyclin A. We therefore speculate that the proline substitutions might contribute to a relaxed conformation of the C-terminal domain so that it can interact broadly with wild-type and mutant cyclin A's.
The physiological significance of the cyclin A-Mcm7 interaction was verified in a G1/S acceleration assay and an endogenous cyclin A-knockdown/-complementation assay. The Mcm7-3 “suppressor” mutant could suppress the defect of the CycA-C1 mutant for accelerating the G1/S transition and complementing the endogenous cyclin A function in promoting S-phase entry. Recently, cyclin E has been shown to facilitate the loading of the MCM complex onto chromatin in cells exiting quiescence, in a CDK-independent fashion (9). In contrast, our results suggest that cyclin A accelerates the G1/S transition in a CDK-dependent manner (Fig. 1d). It has been presumed that cyclin A-CDK activity contributes to the G1/S transition in cycling cells lacking cyclin E (9). Direct phosphorylation of Mcm7 by CDK, however, has not been observed (14, 26), suggesting that cyclin A-CDK phosphorylates another protein(s) involved in promotion of S-phase entry. The interaction of endogenous cyclin A and Mcm7 proteins in the chromatin fraction was not affected by aphidicolin (Fig. 3c), suggesting that the cyclin A-Mcm7 interaction promotes the G1/S transition by facilitating the prereplicative complex (preRC) and/or preIC formation. Since phosphorylation by CDK of preRC components, including Cdc6, Cdt1, and some Mcm proteins, has been known to inhibit preRC formation (1, 6), we propose that cyclin A-CDK facilitates preIC formation via a cyclin A-Mcm7 interaction, utilizing Mcm7 as an anchor/docking component. Recent studies with budding yeast have demonstrated that S-phase CDK activity is required for both preloading complex (Sld2/Dpb11/GINS/Polɛ) formation and the loading of this complex onto the chromosomal replication origin complex, which includes Sld3, Cdc45, and Mcm proteins (21, 33). Sld2 binds to Dpb11 and Sld3 binds to Cdc45 in a CDK phosphorylation-dependent manner. In an analogous manner, cyclin A-CDK2 might regulate these steps in mammalian cells.
To our surprise, knocking down the endogenous cyclin A by siRNA did not completely arrest the cell cycle. It has been reported that even high-efficiency siRNAs against cyclin A (11) or short hairpin RNAs (shRNAs) against cyclin A in cyclin E1−/− E2−/− cells (12) did not completely arrest the cell cycle, and moreover, that fibroblasts lacking both cyclins A1 and A2 grow normally (17). These observations imply that although involved in the promotion of S-phase entry, cyclin A is not essential for cell cycle progression. It has been speculated that in the metazoan somatic cell cycle, A-, D-, and E-type cyclins contribute redundantly to G1- and S-phase progression and that A- and B-type cyclins contribute redundantly to G2- and M-phase progression. Further genetic analyses are necessary to address their specific roles under certain conditions, especially in some developmental stages and cell lineages.
Supplementary Material
Acknowledgments
We thank R. Eisenman, M. Fujita, B. Clurman, B. Kelly, and M. Otsubo for discussions and materials; P. James, P. Noirot, K. Haga, and A. Sakai for materials and suggestions on yeast two-hybrid systems; C. Sherr, D. Morgan, S. Van den Heuvel, and M. Carrington for materials; and I. Yano, K. Watanabe, K. Tateishi, H. Nakayama, and A. Ito for technical assistance.
This work was supported by grants from the NIH and a Grant-in-Aid for Scientific Research on Priority Area (C) (Cancer Biology) from the Ministry of Education, Culture, Science and Technology of Japan.
We declare no conflict of interest.
Footnotes
Published ahead of print on 15 November 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Blow, J. J., and A. Dutta. 2005. Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell Biol. 6:476-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso, M. C., H. Leonhardt, and B. Nadal-Ginard. 1993. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell 74:979-992. [DOI] [PubMed] [Google Scholar]
- 4.Chibazakura, T., S. G. McGrew, J. A. Cooper, H. Yoshikawa, and J. M. Roberts. 2004. Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107. Proc. Natl. Acad. Sci. U. S. A. 101:4465-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clurman, B. E., R. J. Sheaff, K. Thress, M. Groudine, and J. M. Roberts. 1996. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 10:1979-1990. [DOI] [PubMed] [Google Scholar]
- 6.Diffley, J. F. 2004. Regulation of early events in chromosome replication. Curr. Biol. 14:R778-R786. [DOI] [PubMed] [Google Scholar]
- 7.Fujita, M., T. Kiyono, Y. Hayashi, and M. Ishibashi. 1997. In vivo interaction of human MCM heterohexameric complexes with chromatin. Possible involvement of ATP. J. Biol. Chem. 272:10928-10935. [DOI] [PubMed] [Google Scholar]
- 8.Fujita, M., C. Yamada, T. Tsurumi, F. Hanaoka, K. Matsuzawa, and M. Inagaki. 1998. Cell cycle- and chromatin binding state-dependent phosphorylation of human MCM heterohexameric complexes. A role for cdc2 kinase. J. Biol. Chem. 273:17095-17101. [DOI] [PubMed] [Google Scholar]
- 9.Geng, Y., Y. M. Lee, M. Welcker, J. Swanger, A. Zagozdzon, J. D. Winer, J. M. Roberts, P. Kaldis, B. E. Clurman, and P. Sicinski. 2007. Kinase-independent function of cyclin E. Mol. Cell 25:127-139. [DOI] [PubMed] [Google Scholar]
- 10.Girard, F., U. Strausfeld, A. Fernandez, and N. J. Lamb. 1991. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67:1169-1179. [DOI] [PubMed] [Google Scholar]
- 11.Gong, D., J. R. Pomerening, J. W. Myers, C. Gustavsson, J. T. Jones, A. T. Hahn, T. Meyer, and J. E. Ferrell, Jr. 2007. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr. Biol. 17:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanashiro, K., M. Kanai, Y. Geng, P. Sicinski, and K. Fukasawa. 2008. Roles of cyclins A and E in induction of centrosome amplification in p53-compromised cells. Oncogene 27:5288-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinds, P. W., S. Mittnacht, V. Dulic, A. Arnold, S. I. Reed, and R. A. Weinberg. 1992. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70:993-1006. [DOI] [PubMed] [Google Scholar]
- 14.Ishimi, Y., Y. Komamura-Kohno, Z. You, A. Omori, and M. Kitagawa. 2000. Inhibition of Mcm4,6,7 helicase activity by phosphorylation with cyclin A/Cdk2. J. Biol. Chem. 275:16235-16241. [DOI] [PubMed] [Google Scholar]
- 15.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffrey, P. D., A. A. Russo, K. Polyak, E. Gibbs, J. Hurwitz, J. Massague, and N. P. Pavletich. 1995. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376:313-320. [DOI] [PubMed] [Google Scholar]
- 17.Kalaszczynska, I., Y. Geng, T. Iino, S. Mizuno, Y. Choi, I. Kondratiuk, D. P. Silver, D. J. Wolgemuth, K. Akashi, and P. Sicinski. 2009. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell 138:352-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly, B. L., K. G. Wolfe, and J. M. Roberts. 1998. Identification of a substrate-targeting domain in cyclin E necessary for phosphorylation of the retinoblastoma protein. Proc. Natl. Acad. Sci. U. S. A. 95:2535-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi, H., E. Stewart, R. Poon, J. P. Adamczewski, J. Gannon, and T. Hunt. 1992. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell 3:1279-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkel, T. A., J. D. Roberts, and R. A. Zakour. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154:367-382. [DOI] [PubMed] [Google Scholar]
- 21.Masumoto, H., S. Muramatsu, Y. Kamimura, and H. Araki. 2002. S-Cdk-dependent phosphorylation of Sld2 essential for chromosomal DNA replication in budding yeast. Nature 415:651-655. [DOI] [PubMed] [Google Scholar]
- 22.Miller, M. E., and F. R. Cross. 2001. Cyclin specificity: how many wheels do you need on a unicycle? J. Cell Sci. 114:1811-1820. [DOI] [PubMed] [Google Scholar]
- 23.Morgan, D. O. 1995. Principles of CDK regulation. Nature 374:131-134. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsubo, M., and J. M. Roberts. 1993. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 259:1908-1912. [DOI] [PubMed] [Google Scholar]
- 25.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereverzeva, I., E. Whitmire, B. Khan, and M. Coue. 2000. Distinct phosphoisoforms of the Xenopus Mcm4 protein regulate the function of the Mcm complex. Mol. Cell. Biol. 20:3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnitzky, D., L. Hengst, and S. I. Reed. 1995. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol. Cell. Biol. 15:4347-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts, J. M. 1999. Evolving ideas about cyclins. Cell 98:129-132. [DOI] [PubMed] [Google Scholar]
- 29.Schulman, B. A., D. L. Lindstrom, and E. Harlow. 1998. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. U. S. A. 95:10453-10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 31.Sherr, C. J. 1993. Mammalian G1 cyclins. Cell 73:1059-1065. [DOI] [PubMed] [Google Scholar]
- 32.Sterner, J. M., S. Dew-Knight, C. Musahl, S. Kornbluth, and J. M. Horowitz. 1998. Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7. Mol. Cell. Biol. 18:2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka, S., T. Umemori, K. Hirai, S. Muramatsu, Y. Kamimura, and H. Araki. 2007. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445:328-332. [DOI] [PubMed] [Google Scholar]
- 34.Turner, D. L., and H. Weintraub. 1994. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8:1434-1447. [DOI] [PubMed] [Google Scholar]
- 35.Tye, B. K. 1999. MCM proteins in DNA replication. Annu. Rev. Biochem. 68:649-686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.