Abstract
The eukaryotic initiation factor eIF4F recruits ribosomes to capped mRNAs while eIF2 mediates start codon recognition to initiate protein synthesis. Increasing interest in targeting translation to suppress tumor growth has led to the development of new classes of inhibitors, including 4EGi-1, which disrupts eIF4F complexes. However, the full effects of this inhibitor and its potential uses in the treatment of other disease states remain unclear. Here, we show that overall rates of protein synthesis in primary human cells were affected only modestly by eIF4F disruption using the mTOR inhibitor Torin1, yet were highly sensitive to 4EGi-1. Translational suppression occurred even at concentrations of 4EGi-1 that were below those required to significantly alter eIF4F levels but were instead found to increase the association of ribosomal complexes containing inactive eIF2α. Although highly stable in culture, the effects of 4EGi-1 on both cellular protein synthesis and ribosome association were readily reversible upon inhibitor removal. In addition, despite potently inhibiting translation, prolonged exposure to 4EGi-1 had only modest effects on cell morphology and protein abundance without affecting viability or stress tolerance to any significant degree, although differential effects on heat shock protein (hsp) expression highlighted distinct 4EGi-1-sensitive modes of hsp induction. In contrast, 4EGi-1 potently suppressed poxvirus replication as well as both reactivation and lytic phases of herpesvirus infection. These findings identify a novel way in which 4EGi-1 affects the host cell's protein synthesis machinery and demonstrate its potential as a noncytotoxic inhibitor of diverse forms of viral infection.
The vast majority of eukaryotic mRNAs are capped by a 7-methyl-GTP at their 5′ end. Ribosome recruitment and initiation of protein synthesis involves the activity of numerous eukaryotic translation initiation factors (eIFs). The cap-binding eIF4F complex consists of three subunits assembled through the interaction of eIF4G, a large scaffolding protein; eIF4E, a cap-binding protein; and eIF4A, an RNA helicase that unwinds the mRNA secondary structure to facilitate scanning (Fig. 1 A) (41). The ability of eIF4G to interact with eIF4E and form an active eIF4F complex is regulated by a family of small eIF4E-binding proteins (4E-BPs) (12). By binding the same site on eIF4E as eIF4G, 4E-BPs act as competitive inhibitors of eIF4F formation. In their hypophosphorylated state, 4E-BPs bind to eIF4E, but stimuli that activate the mammalian target of rapamycin (mTOR) result in their phosphorylation, releasing eIF4E to interact with eIF4G. In addition, eIF4G associates with poly(A) binding protein (PABP), which binds the poly(A) tail at the 3′ end of the mRNA. These interactions mediate 5′-to-3′-end communication thought to function in both quality control and translational stimulation of fully processed, mature mRNAs (Fig. 1A) (17, 19, 49). Finally, eIF4G interacts with eIF3 to bridge the 43S preinitiation complex (PIC) to the mRNA (Fig. 1A) (15).
FIG. 1.
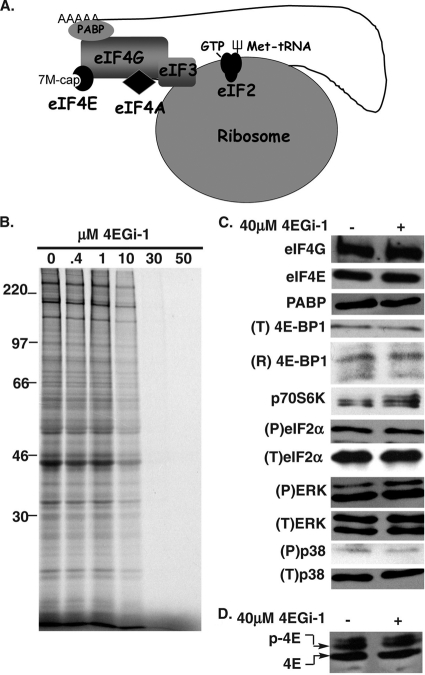
Inhibition of translation in primary human fibroblasts by 4EGi-1. (A) Regulation of ribosome recruitment by cellular translation initiation factors. The core eIF4F complex consists of eIF4E, which binds the 7-methyl-GTP (7M-cap) present at the 5′ end of the mRNA; eIF4G, the central scaffolding protein; and eIF4A, an RNA helicase. eIF4G also associates with the multifactor complex eIF3, which bridges the cap-binding complex and the 43S ribosome to form the 48S initiation complex. The 43S ribosome is formed through the interaction of the 40S ribosome and a ternary complex consisting of the multisubunit initiation factor, eIF2, together with GTP and the initiator Met tRNA (fork symbol). Finally, additional interactions between eIF4G, PABP, and the poly(A) tail are thought to mediate circularization of the mRNA. (B) Effects of 4EGi-1 on rates of translation in primary cells. NHDFs were treated for 3 h with increasing micromolar concentrations of 4EGi-1 and then metabolically labeled with [35S]Met/Cys for 1 h. Whole-cell extracts were resolved by SDS-PAGE, and then fixed, dried gels were exposed to X-ray film. Migration of molecular weight (MW) standards is indicated to the left of the autoradiogram. (C) NHDFs were treated for 4 h with DMSO or 40 μM 4EGi-1, and then whole-cell extracts were analyzed by Western blotting with the indicated antibodies. Total levels of 4E-BP1 (T) were examined using nonresolving 7.5% gels, while phosphorylated forms of 4E-BP1 were resolved (R) in 17.5% gels. Phosphorylation of p70S6K is evident as retarded mobility in 12.5% gels. (D) Samples described for panel C were fractionated by isoelectric focusing, and membranes were probed with anti-eIF4E antibody. Migration of the phosphorylated (p-4E) and hypophosphorylated (4E) forms of eIF4E are indicated to the left.
The 43S PIC is formed through the association of the 40S ribosome with a ternary complex consisting of eIF2, GTP, and the initiator methionine tRNA (Fig. 1A). eIF2 is composed of three subunits that play various roles in mediating the initiation of polypeptide synthesis (32). eIF2β and eIF2γ bind GTP, mRNA, and the initiator tRNA. GTP hydrolysis to promote translation initiation is mediated by eIF5, which binds eIF2β. Guanine nucleotide exchange to replenish GTP on eIF2 for a new round of initiation is mediated by eIF2B, which also binds eIF2β. The limiting amounts of eIF2B present in the cell are rapidly sequestered in an inactive complex with eIF2 itself through phosphorylation of Ser51 on eIF2α, resulting in global inhibition of translation initiation. As such, eIF2α is considered the regulatory subunit of the eIF2 complex, which is inactivated by a number of eIF2α kinases that respond to a variety of environmental cues to suppress translation (32).
Given their importance in regulating protein synthesis, it is not surprising that these regulatory factors also contribute to the genesis of various disease states. In particular, aberrant expression or function of a number of initiation factors has been found to play a central role in cellular transformation (39). Increased availability of initiation complexes enhances the translational efficiency of otherwise poorly translated low-abundance or structurally complex mRNAs, which have a high requirement for the cap-binding and helicase activities of eIF4F. Such mRNAs generally encode growth-promoting and anti-apoptotic proteins, and as such, deregulation of eIF4F activity results in robust synthesis of a broad spectrum of factors that drive transformation. Overexpression of either eIF4E or eIF4G results in aberrant cell growth and transformation in vitro (5, 11, 40), and elevated initiation factor levels are found in numerous cancers, serving as a poor prognostic marker (13, 31). The dependence of transformed cells on high-level production of growth and anti-apoptotic factors has spurred interest in the development of therapeutic strategies targeting translation initiation. Indeed, antisense and small interfering RNA (siRNA) approaches to reduce eIF4E expression decrease the growth of tumor cells (14, 35). Recently, high-throughput screening identified a small molecule inhibitor, 4EGi-1, that blocks the interaction between eIF4E and eIF4G (26). 4EGi-1 decreases the abundance of growth-related and anti-apoptotic proteins and either slows the growth of, or induces apoptosis in, a number of cancer cell lines, highlighting potential new avenues for anticancer drug development (26).
However, the full effects of this new inhibitor as well as the role of many translation factors in the biology of normal human cells and other disease states, and thus their potential as therapeutic targets, remain poorly defined. Here, we investigated the effects of inhibiting translation initiation on cell viability and sensitivity to both stress and viral infection in primary normal human dermal fibroblasts (NHDFs). While the mTOR inhibitor Torin1 demonstrated that overall rates of protein synthesis were only modestly dependent upon eIF4F, relatively low concentrations of 4EGi-1 potently suppressed translation without significantly altering eIF4F levels and instead increased the association of ribosomal complexes containing phosphorylated eIF2α. Despite this, 4EGi-1 did not affect long-term cell viability or tolerance of distinct stresses, but potently inhibited infection by either herpes simplex virus or vaccinia virus (VacV). These findings demonstrate the potential of targeting the cellular protein synthesis machinery as a noncytotoxic, broad-spectrum approach to inhibiting viral infection.
MATERIALS AND METHODS
Cells and virus.
Primary NHDF (Clonetics), HeLa, BSC40, and Vero cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum (FBS). Propagation and infection with herpes simplex virus type 1 (HSV-1) strain KOS and vaccinia virus strain Western Reserve were performed as described previously (44, 46). Determination of infectious virus in experimental samples was performed by freeze-thawing of cultures, followed by titration on permissive Vero or BSC40 cells. Quiescent infection and reactivation was performed as described previously (25).
Antibodies and inhibitors.
Antibodies toward 4E-BP1, p38, phosphorylated p38, extracellular signal-regulated kinase (ERK), phosphorylated ERK, PABP, p70S6K, hsp27, hsp70, poly(ADP-ribose) polymerase 1 (PARP-1), cleaved-PARP-1, caspase-3, caspase-7, eIF2α, eIF3A, and ribosomal protein 3 were obtained from Cell Signaling Technologies. Commercial sources of other antibodies were as follows: eIF4E (BD Bioscience), Ser51-phosphorylated eIF2α (Research Genetics), ICP0, ICP4, and ICP5 (Abcam), HSV-1 (Maine Technologies), and vaccinia virus (Virostat). Antisera toward eIF4G, Us11, and ICP22 were gifts of Ian Mohr (NYU School of Medicine), Richard Roller (University of Iowa), and John Blaho (Mount Sinai School of Medicine), respectively. 4EGi-1 and MG132 were purchased from Calbiochem; 4EGi-1 was also purchased from Santa Cruz Biotechnology. Torin1 was a gift of Nathanael Gray (Dana-Farber Cancer Institute) and David Sabatini (Whitehead Institute for Biomedical Research). All experiments were performed such that the final volume of dimethyl sulfoxide (DMSO) was equal in all samples. DMSO and trypan blue were obtained from Sigma-Aldrich.
Metabolic labeling and TCA precipitation.
For metabolic labeling, 1 h before sample preparation cultures were incubated with 77 μCi [35S]methionine/cysteine ([35S]Met/Cys) (PerkinElmer) as described previously (46). Rates and patterns of protein synthesis were determined by resolution of whole-cell extracts on SDS-PAGE gels, followed by autoradiography. To quantify global rates of protein synthesis, whole-cell extracts were incubated in a solution of 1 M NaOH-2% H2O2 and then precipitated with trichloroacetic acid (TCA) in the presence of cold competing l-Met/l-Cys and bovine serum albumin (BSA). Samples were dried onto Whatman GF/C microfiber filters and counted using a Beckman liquid scintillation counter.
Western blotting, isoelectric focusing, and microscopy.
Western blotting and isoelectric focusing were performed as described previously (46). For microscopy, cells were washed in phosphate-buffered saline (PBS) and then fixed for 20 min in 3.7% formaldehyde. Samples were then washed extensively in PBS, permeabilized with 0.1% Triton, and washed in PBS again. Fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich) was added to samples for 40 min to stain actin networks and then washed in PBS-0.1% Tween before being incubated with Hoechst stain for 5 min. After washing samples in PBS-0.1% Tween, phase contrast and fluorescent images were obtained using a Leica DFC 500 microscope.
7-Methyl-GTP chromatography.
Cells were seeded on 10-cm2 tissue culture dishes and treated with DMSO or 4EGi-1. Cultures were washed in PBS containing DMSO or 4EGi-1 and then scraped into lysis buffer (50 mM HEPES [pH 7.4], 100 mM NaCl, 1.5 mM MgCl2, 2 mM Na3VO4, 2 mM EDTA, 25 mM glycerophosphate, and complete mini protease inhibitor cocktail [Roche]) containing DMSO or 4EGi-1. Samples were snap-frozen and thawed three times and then clarified by centrifugation at 10,000 × g for 10 min at 4°C. Soluble fractions were treated with RNase A (50 μg) and precleared with Sepharose 4B for 1 h, then incubated with 7-methyl-GTP-Sepharose 4B for 1 h. After binding, beads were recovered by centrifugation and incubated in lysis buffer containing DMSO or 4EGi-1 along with competing GTP (2 mM) for 1 h. Beads were washed 3 times in 600 μl lysis buffer containing DMSO or 4EGi-1 and then boiled in Laemmli buffer before being analyzed by Western blotting. For inhibitor reversal experiments, 4EGi-1 was replaced with DMSO during the wash steps prior to boiling in Laemmli buffer. The procedure for detergent-based lysis and 7-methyl-GTP chromatography was as described previously (47).
RESULTS
Inhibition of translation in human cells by 4EGi-1.
To explore the consequences of suppressing ongoing protein synthesis in normal human cells, we first examined the effects of 4EGi-1 in primary NHDFs. Confluent cultures were treated with increasing concentrations of 4EGi-1 for 3 h, and then rates of translation were measured by metabolic labeling with [35S]Met/Cys for 1 h. Whole-cell extracts were prepared and resolved by SDS-PAGE, and then fixed, dried gels were exposed to X-ray film. As illustrated in Fig. 1B, rates of translation were notably reduced at a concentration of 10 μM and were potently suppressed at concentrations of 30 μM and above. Toxicity of 4EGi-1 became evident in cultures only at concentrations above 60 μM (data not shown), demonstrating that significant translational suppression was tolerated for at least short periods in these cells. While the effects of 4EGi-1 on eIF4F complex formation have been characterized (26), little is known about the broader effects of this new inhibitor. Therefore, we examined the impact of 4EGi-1 on levels of translation factors and activities of key cellular signaling pathways involved in translational control in NHDFs. Western blotting of samples from confluent NHDFs treated with DMSO or 40 μM 4EGi-1 for 4 h revealed that 4EGi-1 did not affect the steady-state levels of eIF4G, eIF4E, PABP, or 4E-BP1, nor did it affect the abundance or phosphorylation status of the mitogen-activated protein kinase (MAPK) substrate, ERK, or stress-activated targets p38 and eIF2α (Fig. 1C). 4EGi-1 did have modest stimulatory effects on the phosphorylation of mTOR substrates p70S6K and 4E-BP1, evident as partially reduced antigen mobility in inhibitor-treated samples (Fig. 1C). Although consistently observed, this effect was normally modest and transient, which may reflect an initial cellular response to translational suppression (20, 21).
Notably, when these 4-h samples were examined by isoelectric focusing, 40 μM 4EGi-1 had no effect on eIF4E phosphorylation (Fig. 1D), which is mediated by an eIF4G-associated kinase as part of the eIF4F complex (33, 48), suggesting that eIF4F was not disrupted at this concentration. We therefore examined the effects of this inhibitor on the composition of initiation complexes in NHDFs. Confluent cultures were treated with DMSO or 40 μM 4EGi-1 for 4 h. As binding of 4EGi-1 to eIF4E has been shown to be reversible, cell extracts were prepared by freeze-thawing in the absence of detergent, as previously performed in characterizing the inhibitor (26), and 4EGi-1 was added to all buffers used during the assay. Extracts were precleared, and then cap-binding eIF4E and associated proteins were recovered on 7-methyl-GTP-Sepharose 4B and analyzed by Western blotting. Input samples demonstrated that 4EGi-1 did not affect the abundance of numerous initiation factors (Fig. 2 A). When eIF4E-associated proteins were examined, 4EGi-1 was found to modestly stabilize the interaction of 4E-BP1 but did not affect the binding of eIF4G to any significant degree. Linearity of blots was confirmed by sample titration, and the same results were observed when extracts were prepared using NP-40-based detergent lysis (not shown). In line with this observation, numerous studies have shown that modest changes in 4E-BP1 binding to eIF4E are not sufficient to elicit changes in eIF4F levels in many cell types, including NHDFs (46, 52), and likely reflect differences in the relative abundances of each of these proteins. These findings were in agreement with the high 4EGi-1 concentrations previously reported necessary to achieve notable eIF4F disruption (26). Indeed, disruption of eIF4F was evident in NHDFs at higher 4EGi-1 concentrations but was coincident with the onset of cytotoxicity (not shown).
FIG. 2.
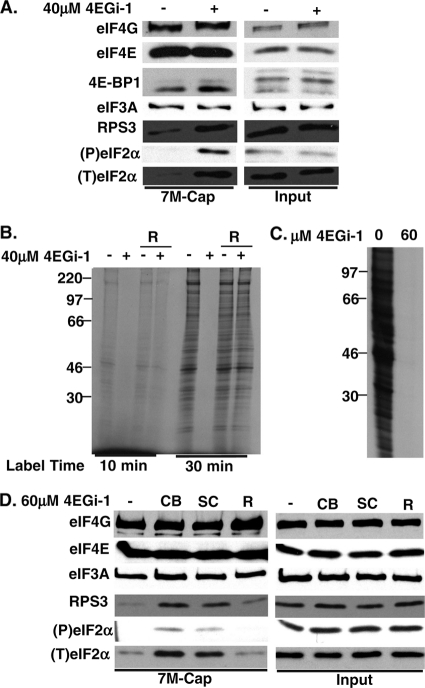
Reversible effects of 4EGi-1 on both the composition of translation initiation complexes and rates of translation. (A) NHDFs were treated for 4 h with DMSO or 40 μM 4EGi-1, and then soluble cell extracts were subjected to 7-methyl-GTP chromatography. Cap-bound and input samples were analyzed by Western blotting with the indicated antibodies. (P), phosphorylated; (T), total. 4E-BP1 was examined using 17.5% gels to resolve phosphorylated species. (B) NHDFs were treated with DMSO (−) or 40 μM 4EGi-1 (+) for 4 h. Cells were then rinsed in growth medium containing DMSO or 4EGi-1 and metabolically labeled for 10 min or 30 min in the continued presence of inhibitor (lanes 1 to 2 and 5 to 6) or rinsed in medium containing DMSO to remove (R) inhibitor and labeled in the presence of DMSO alone (lanes 3 to 4 and 7 to 8). Whole-cell extracts were resolved by SDS-PAGE and then fixed, dried gels were exposed to X-ray film. Migration of molecular weight (MW) standards is indicated to the left of the autoradiogram. (C) HeLa cells were treated with DMSO solvent control (0) or 60 μM 4EGi-1 for 3 h and then labeled with [35S]Met/Cys for 1 h. Whole-cell extracts were prepared and resolved by SDS-PAGE and fixed, dried gels were exposed to X-ray film. Migration of MW markers is indicated to the left. (D) HeLa cells were treated with DMSO (−) or 60 μM 4EGi-1 for 4 h. Independent sources of 4EGi-1 from Calbiochem (CB) and Santa Cruz Biotechnology (SC) were compared. An additional sample was prepared from cells treated with 60 μM 4EGi-1, but the inhibitor was removed (R) by omitting it from wash buffers at the end of the assay to examine reversibility. Samples were resolved by SDS-PAGE and analyzed by Western blotting with the indicated antibodies.
This demonstrated that 4EGi-1 suppressed translation at concentrations below those necessary to disrupt eIF4F. We therefore examined the levels of other components of the translation initiation complex that forms on eIF4F. The 7-methyl-GTP-bound samples described above were analyzed by Western blotting with antibodies toward eIF3A, a component of the functional core of the eIF3 complex (24), and the 40S ribosomal protein rps3. While 4EGi-1 had no effect on the abundance or association of eIF3A, it did increase the binding of rps3 (Fig. 2A). Given its central role in regulating global protein synthesis, we then examined whether 4EGi-1 had any effect on eIF2α. Similar to whole-cell extracts (Fig. 1C), input samples demonstrated that 4EGi-1 did not induce eIF2α phosphorylation (Fig. 2A). However, large amounts of phosphorylated eIF2α were found in complexes isolated from 4EGi-1-treated cultures. Overall, these findings suggest that this compound increased the association of ribosomal complexes containing phosphorylated, inactive eIF2 with eIF4F.
It has been reported that extensive dialysis of eIF4E bound to 4EGi-1 reverses inhibitor binding, but the reversibility of its effects on translation have not been examined (26). We therefore examined the kinetics with which translation was restored in NHDFs upon removal of 4EGi-1. Confluent NHDFs were first treated with either DMSO or 40 μM 4EGi-1 for 4 h. Cultures were then rinsed in growth medium and metabolically labeled in the presence of DMSO alone, while a second set of samples was metabolically labeled in the continuous presence of either DMSO or 4EGi-1. Cultures were metabolically labeled for either 10 min or 30 min, and then whole-cell extracts were prepared and resolved by SDS-PAGE. As illustrated in Fig. 2B, the amount of [35S]Met/Cys incorporated into proteins increased with increasing labeling times and was potently reduced in the continuous presence of 4EGi-1. However, in cultures where the inhibitor had been washed out, only a very modest reduction in [35S]Met/Cys incorporation was observed after 10-min labeling, while no significant differences were observed after 30-min labeling. This demonstrated that the effects of 4EGi-1 were very rapidly reversed in cultured cells.
Next, we examined whether the effect of 4EGi-1 on the composition of initiation complexes was unique to primary NHDFs and whether this was a reversible modification. A recent report demonstrated that 100 μM 4EGi-1 inhibited translation in HeLa cells with only modest induction of cellular stress over an 8-h period, although the levels of eIF4F were not examined (28). We therefore treated HeLa cells with 60 μM 4EGi-1 and first confirmed that this concentration also suppressed translation. HeLa cells were treated with DMSO or 4EGi-1 for 3 h and were then metabolically labeled for 1 h. Whole-cell extracts were resolved by SDS-PAGE, and fixed, dried gels were exposed to X-ray film. As illustrated in Fig. 2C, 60 μM 4EGi-1 potently suppressed global rates of protein synthesis in HeLa cells. We then assayed the composition of initiation complexes in HeLa cells treated with DMSO or 60 μM 4EGi-1 for 4 h by 7-methyl-GTP chromatography. Similar to findings in NHDFs, the levels of translation initiation factors and rps3, as well as the phosphorylation of eIF2α in input samples, remained unaffected in 4EGi-1-treated samples (Fig. 2D). Analysis of 7-methyl-GTP-bound complexes demonstrated that 4EGi-1 had no significant effect on the association of eIF4E with eIF4G or with eIF3A but did increase the association of rps3 and phosphorylated eIF2α, suggesting that this effect was not cell-type specific. The same effect was also observed in samples treated with 4EGi-1 from an independent source (Fig. 2D). Finally, given the reversibility of the effects of 4EGi-1 on translation (Fig. 2B), we examined whether its effects on rps3 and eIF2α association were also reversible in the same experiment. In prior experiments, we had noted that when 4EGi-1 was washed out of cultures for 10 min prior to sample preparation the increased association of ribosomal complexes and eIF2α was lost (not shown). However, the increased association of rps3 and phosphorylated eIF2 was also reversed even if the inhibitor was simply omitted from buffers during the final wash stages of the assay after recovery of cap-bound initiation complexes (Fig. 2D). Overall, these findings demonstrate that the effects of 4EGi-1 on both cellular translation and the composition of initiation complexes were rapidly reversible.
To determine its contribution to rates of protein synthesis, we then used the catalytic site-specific mTOR inhibitor Torin1 to disrupt eIF4F (43). To confirm its efficacy, confluent NHDFs were treated with DMSO or 100 nM Torin1 for 24 h, and then soluble cell extracts were prepared and initiation complexes analyzed as described above. As illustrated in Fig. 3 A, Torin1 treatment caused a robust dephosphorylation of 4E-BP1 in input samples, which correlated with a large increase in the association of 4E-BP1 with eIF4E and a concomitant loss of eIF4G binding. We then compared the effects of both inhibitors on the rates of translation in HeLa cells and NHDFs. Cells were treated with DMSO, 4EGi-1, or Torin1 for 24 h and then metabolically labeled for 1 h. Whole-cell lysates were resolved by SDS-PAGE, and fixed, dried gels were exposed to X-ray film. While 4EGi-1 potently suppressed translation in both HeLa and NHDF cells, Torin1 had only modest effects on total rates of protein synthesis in both cases (Fig. 3B). This demonstrated that robust translation of many mRNAs within the cell did not require high levels of eIF4F and that potentially small, undetected effects of 4EGi-1 on eIF4G binding to eIF4E at the concentrations used would contribute very little to the overall suppression of global translation by this inhibitor. We also noted that 4EGi-1-treated HeLa cells were visibly stressed by 24 h, while NHDFs were indistinguishable from DMSO controls (not shown), and Western blotting of HeLa cell extracts demonstrated that significant caspase-3 processing, indicative of apoptosis, was detectable in 4EGi-1-treated cultures at 1 day (Fig. 3C).
FIG. 3.
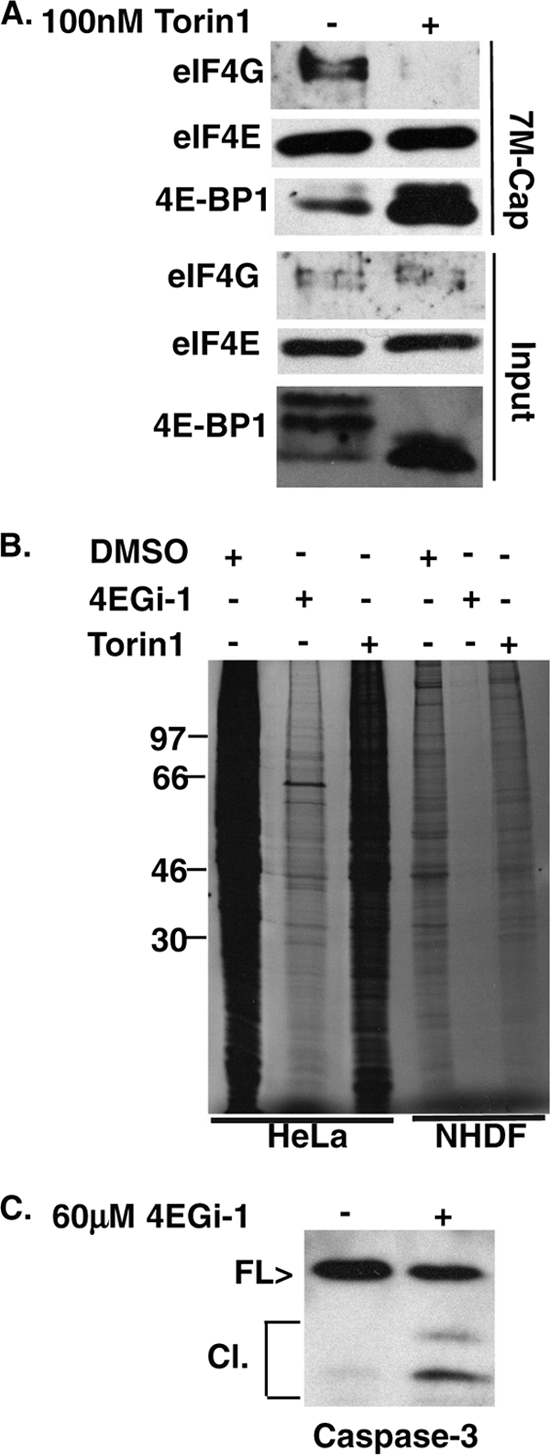
4EGi-1 and the mTORC inhibitor Torin1 have distinct effects on rates of translation. (A) NHDFs were treated with DMSO (−) or 100 nM Torin1 (+) for 1 day, and then eIF4E and associated proteins were recovered from soluble cell extracts on 7-methyl-GTP-Sepharose and analyzed by Western blotting with the indicated antibodies. 4E-BP1 was resolved in 17.5% SDS-PAGE gels to separate phosphorylated species. (B) NHDFs or HeLa cells were treated with DMSO, 40 μM (NHDFs) or 60 μM (HeLa cells) 4EGi-1, or 100 nM Torin1 for 1 day. Cultures were metabolically labeled for 1 h, and then whole-cell extracts were resolved by SDS-PAGE and fixed, dried gels were exposed to X-ray film. MW standards are indicated to the left. (C) HeLa cells were treated with DMSO or 60 μM 4EGi-1 for 1 day, and then whole-cell extracts were analyzed by Western blotting against caspase-3. Migration of full-length (FL) and cleaved (Cl) forms is indicated to the left.
Primary cells remain viable and stress tolerant despite prolonged exposure to 4EGi-1.
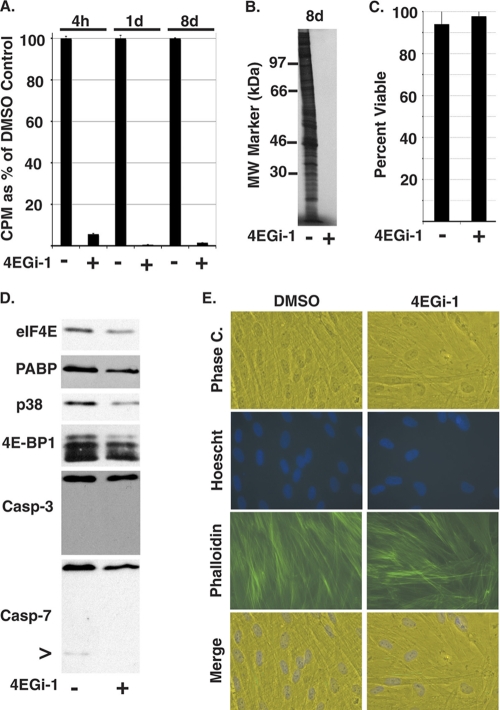
As 4EGi-1 had no obvious effects on NHDF morphology after 1 day, we then examined more prolonged exposure to this inhibitor. Previous studies demonstrated that 4EGi-1 inhibited cell growth or induced apoptosis in different cell types over periods of 3 to 7 days (26, 42), suggesting that this inhibitor is relatively stable in culture. In agreement with results from these reports, when added to low-density cultures of NHDFs, 40 μM 4EGi-1 completely inhibited cell growth for more than 3 days (not shown). As such, to test its longer-term effects, confluent cultures of NHDFs were treated with 40 μM 4EGi-1, replenishing fresh drug-containing medium every 3 days. Samples were metabolically labeled for 1 h prior to harvesting, and whole-cell extracts were prepared after 4 h, 1 day, and 8 days. TCA precipitation demonstrated that rates of translation were reduced to 5% of control samples by 3 to 4 h posttreatment while continued exposure to 4EGi-1 further reduced rates to ∼1% of control samples at later points (Fig. 4 A). The degree and global nature of translational suppression at 8 days posttreatment was evident on autoradiographs of samples resolved by SDS-PAGE (Fig. 4B). Despite this, cell viability, as determined by trypan blue exclusion, remained unaffected after an 8-day treatment (Fig. 4C), although the abundance of a number of cellular antigens and phosphorylation of the mTOR substrate 4E-BP1 were reduced (Fig. 4D). When the same samples were resolved by SDS-PAGE and total protein was visualized by silver staining, overall protein levels appeared very similar in treated and untreated cultures (not shown). Thus, the antigens examined by Western blotting (Fig. 4D) did not appear to be exceptionally stable and suggested that primary cells may adapt to 4EGi-1-mediated inhibition of translation, perhaps through concomitant suppression of protein turnover or entry into an arrested metabolic state. Levels of caspase-3 or -7 were also reduced by 4EGi-1 treatment, but this appeared to reflect the global decrease in protein abundance in these cultures rather than indicate apoptosis, as cleaved forms of these proteins were generally undetectable, or, when detected, their abundance also decreased (Fig. 4D). Morphologically, 4EGi-1- and DMSO-treated cultures remained similar after 8 days, and although the intensity of staining in inhibitor-treated cells was modestly decreased, the structural integrity of the actin cytoskeleton remained intact (Fig. 4E). More-frequent drug replenishment did not cause cytotoxicity (not shown), in line with the stability of 4EGi-1. In addition, removal of 4EGi-1 from cultures treated for 8 days resulted in a substantial restoration of translation rates within just 30 min, further demonstrating that cells remained viable and that the effects of this inhibitor remained reversible (not shown). As such, these findings suggested that the low levels of ongoing translation observed in 4EGi-1-treated primary human cells were sufficient to sustain cellular homeostasis.
FIG. 4.
The effects of extended exposure to 4EGi-1 in NHDFs. (A) NHDFs were treated with DMSO (−) or 40 μM 4EGi-1 (+) and then 1 h prior to the indicated time points cultures were metabolically labeled and whole-cell extracts prepared. 35S incorporation was quantified by TCA precipitation and counts per minute (CPM) are presented as a percentage of DMSO controls, arbitrarily set at 100%. (B) An autoradiograph of samples from cultures treated with DMSO (−) or 40 μM 4EGi-1 (+) for 8 days. MW standards are indicated to the left. (C) NHDFs were treated with DMSO (−) or 40 μM 4EGi-1 (+) for 8 days, and then cells were trypsinized and incubated with trypan blue. Percentage viability represents the number of dye-excluding cells as a percentage of the total cell number. (D) Whole-cell extracts from NHDFs treated for 8 days with DMSO or 40 μM 4EGi-1 were analyzed by Western blotting with the indicated antibodies. Phosphorylated species of 4E-BP1 were resolved in 17.5% gels. > indicates a low-level caspase-7 cleavage product evident in the DMSO-treated sample. (E) NHDFs were treated with DMSO or 40 μM 4EGi-1 for 8 days and then washed in PBS and fixed in formaldehyde. Samples were permeabilized and stained with FITC-conjugated phalloidin (actin; green) and Hoechst (DNA; blue). Phase contrast and fluorescent images were captured on a Leica DFC 500 microscope.
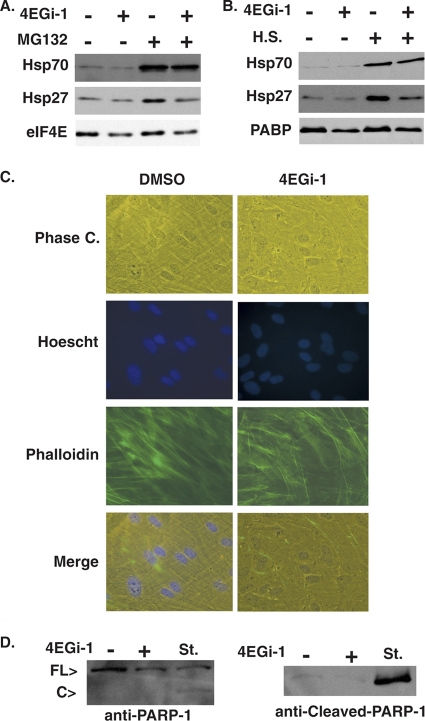
While NHDFs remained viable over extended periods of repeated exposure to 4EGi-1, it was possible that they were in a fragile, stress-sensitive state. To test this we exposed NHDFs that had been treated with 4EGi-1 to two distinct forms of stress. First, cultures were exposed to MG132, a broad-spectrum chemical inhibitor of proteasome and lysosome function. NHDFs were first treated with DMSO or 40 μM 4EGi-1 for 7 days, replenishing the inhibitor midway. Cultures were then treated with fresh DMSO or 4EGi-1 combined with either DMSO solvent control or 10 μM MG132. Twenty-four hours posttreatment whole-cell extracts were prepared and levels of hsp27 and hsp70, induced during cellular stress responses, along with eIF4E as a control antigen were examined by Western blotting. While patterns of eIF4E expression remained unaltered upon exposure to MG132, high levels of hsp70 expression were induced in both DMSO- and 4EGi-1-treated cultures, demonstrating that inhibitor-treated NHDFs were not only viable but remained stress responsive (Fig. 5 A). However, 4EGi-1 did reduce the accumulation of hsp27, demonstrating distinct mechanisms by which these small and large hsps were induced and suggesting that robust accumulation of hsp27, when other hsps are induced, is not essential for tolerance of chemical stress.
FIG. 5.
4EGi-1-treated NHDFs remain tolerant of and responsive to distinct stresses. (A) NHDFs were treated with DMSO (−) or 40 μM 4EGi-1 (+) for 7 days and then treated with fresh DMSO (−) or 40 μM 4EGi-1 (+) combined with either DMSO (−) or 10 μM MG132 (+) for an additional 24 h. Whole-cell extracts were prepared and analyzed by Western blotting with the indicated antibodies. (B) NHDFs were treated for 7 days with DMSO (−) or 40 μM 4EGi-1 (+), followed by either continued incubation at 37°C (−) or heat shock (H.S.) at 41°C (+) for 24 h. Whole-cell extracts were analyzed by Western blotting with the indicated antibodies. (C) NHDFs were treated for 7 days with DMSO or 40 μM 4EGi-1 and then heat shocked at 41°C for a further 24 h. Cultures were washed in PBS, fixed in formaldehyde, and incubated with FITC-conjugated phalloidin (actin; green) and Hoechst (DNA; blue). Images were captured on a Leica DFC 500 microscope. (D) Whole-cell extracts from cultures treated with DMSO (−) or 40 μM 4EGi-1 (+) for 7 days and then cultured at 41°C for 24 h were analyzed by Western blotting with antibodies against full-length or cleaved forms of PARP-1. Whole-cell extracts from NHDFs treated for 4 h with 1 μM staurosporine (St.) were used as a control for apoptosis. Full-length (FL) and cleaved (C) forms of the protein detected using anti-PARP-1 antibody are indicated to the left.
To ensure that this was representative of a general capacity to tolerate and respond to distinct cellular stresses, NHDFs were treated with DMSO or 4EGi-1 for 7 days as described above and then either maintained at 37°C or transferred to an incubatorat 41°C for 24 h. Similar to findings with MG132, a representative cellular antigen (PABP) remained unaltered while thermal stress caused a robust increase in hsp70 expression that was insensitive to 4EGi-1, and hsp27 accumulation was again reduced in the presence of this inhibitor (Fig. 5B). Thermally stressed DMSO- and 4EGi-1-treated cultures were also fixed in formaldehyde and incubated with phalloidin and Hoechst to stain the actin cytoskeleton and nucleus, respectively. Phase-contrast and fluorescent imaging demonstrated that the morphology of cells together with the integrity of the actin cytoskeleton and nucleus remained intact under heat-shock conditions in both control and inhibitor-treated cultures (Fig. 5C). Finally, we determined whether apoptosis might be occurring in 4EGi-1-treated cultures after thermal stress using the apoptotic indicator PARP-1. While total levels of PARP-1 were reduced in 4EGi-1-treated cultures, a corresponding cleavage product indicative of apoptosis was not detected (Fig. 5D). In contrast, total PARP-1 levels were reduced, and a PARP-1 cleavage product was evident in NHDFs treated for 4 h with the apoptosis-inducing agent staurosporine (1 μM) (Fig. 5D). An antibody that specifically detects the cleaved form of PARP-1 showed more clearly that only low levels of cleaved PARP-1 were present in DMSO- or 4EGi-1-treated cultures, while large amounts of cleaved PARP-1 accumulated in staurosporine-treated cultures (Fig. 5D). Overall, these findings demonstrated that extensive exposure to 4EGi-1 did not adversely affect the cells' overall capacity to tolerate diverse stress conditions but did alter the accumulation of specific hsps.
4EGi-1 potently inhibits both reactivation and lytic replication of HSV-1.
Having found that primary human cells were highly tolerant of 4EGi-1-mediated translational suppression, we then examined whether this inhibitor could be used to reduce their sensitivity to infection by DNA viruses, which have recently been found to stimulate the formation of translation initiation complexes during infection (1, 3, 22, 44-47). Among these, herpes simplex virus type 1 (HSV-1) is a widespread human pathogen that exists in two distinct states, termed latent and lytic (36). During latent infection the viral genome is largely silent, but reactivation results in the production of new viral progeny that then spread and replicate through a lytic mode in newly infected permissive cell types, resulting in recurrent disease symptoms. Both reactivation and lytic replication produce new viruses but are distinct processes. To determine whether 4EGi-1 could suppress either or both modes of viral infection, we first tested its effects on virus reactivation.
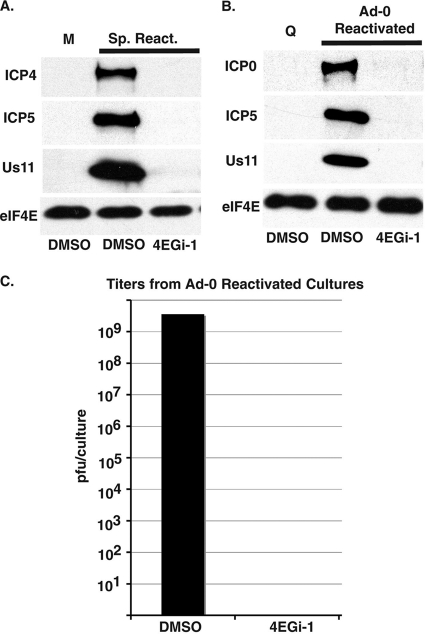
Reactivation of HSV-1 in vivo is complex and poorly understood but thought to occur either through inefficient spontaneous events or in a more controlled manner in response to stress stimuli that trigger production of the virus-encoded reactivator protein ICP0 (7). Both spontaneous and controlled reactivation can be studied in vitro using models whereby HSV-1 can be made to enter a latent-like state, termed quiescence, in cultured human cells by infecting cells at elevated temperatures (25). Low-level spontaneous reactivation and spread occurs over a 5-day period when quiescently infected cultures are returned to 37°C, while efficient controlled reactivation is achieved by transducing cultures with adenoviral vectors encoding HSV-1 ICP0 (25). To test the effect of 4EGi-1 on virus reactivation, mock or quiescent infection was established in NHDFs and this nonproductive state was maintained for 6 days. Cultures were then returned to 37°C and incubated in the presence of either DMSO or 40 μM 4EGi-1 for 5 days, after which time whole-cell extracts were prepared and analyzed by Western blotting. While viral antigens from both early (ICP4), mid (ICP5), and late (Us11) stages of the viral life cycle were readily detectable in samples from DMSO-treated cultures undergoing spontaneous reactivation, they remained completely undetectable in 4EGi-1-treated samples (Fig. 6 A). As spontaneous reactivation is a relatively inefficient process, we also tested the ability of 4EGi-1 to inhibit reactivation mediated by ICP0. Quiescently infected cultures were returned to 37°C and then mock transduced or transduced with an adenovirus encoding ICP0 in the presence of DMSO or 40 μM 4EGi-1. Forty-eight hours posttransduction, whole-cell extracts were prepared and analyzed by Western blotting. While no viral antigens were detectable in cultures that were mock transduced, high levels of all viral antigens assayed were detected in ICP0-transduced, DMSO-treated cultures (Fig. 6B). In contrast, no viral antigens were detectable in 4EGi-1-treated samples. This included ICP0 itself, suggesting that 4EGi-1 was capable of inhibiting de novo production of critical triggers of virus reactivation. The inability to detect viral proteins in inhibitor-treated samples correlated with a failure to detect infectious virus in cultures treated with 4EGi-1, contrasting with the high levels present in DMSO-treated cultures (Fig. 6C). As such, 4EGi-1 was found to be a highly potent inhibitor of both spontaneous and controlled reactivation of HSV-1.
FIG. 6.
4EGi-1 inhibits reactivation of HSV-1. (A) NHDFs were mock infected (M) or infected with HSV-1 at an elevated temperature to establish quiescent infection, which was maintained for 6 days. Cultures were then returned to 37°C and quiescent virus was allowed to spontaneously reactivate (Sp. React.) in the presence of DMSO or 40 μM 4EGi-1 for 5 days. Whole-cell extracts were prepared and analyzed by Western blotting with the indicated antibodies. (B) NHDFs were quiescently infected with HSV-1 for 6 days and then returned to 37°C. Cultures were transduced with medium, maintaining quiescence (Q), or an adenovirus encoding HSV-1 ICP0 (Ad-0 Reactivated) in the presence of DMSO or 40 μM 4EGi-1 for 2 days. Whole-cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. (C) NHDFs were quiescently infected for 6 days and then reactivated by transduction with an adenovirus encoding HSV-1 ICP0 in the presence of DMSO or 40 μM 4EGi-1 for 2 days. Infectious virus in cultures was determined by titration on permissive Vero cells and presented as PFU/culture.
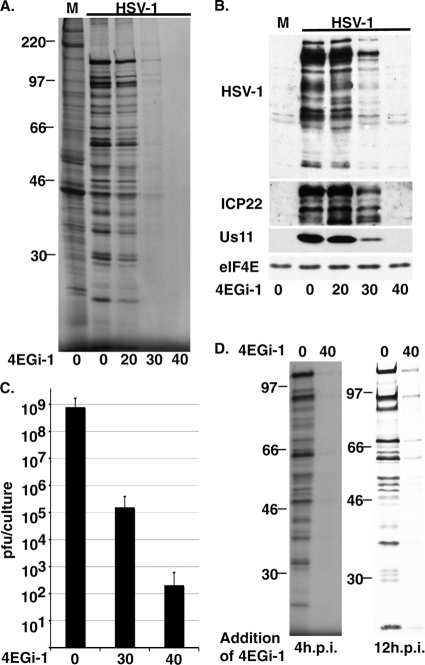
In contrast to the process of virus reactivation from a silent state within the cell, incoming viral particles that establish a productive lytic infection carry with them a wide range of viral proteins whose various functions ensure the rapid onset of efficient virus replication (36). We therefore tested the efficacy of 4EGi-1 against the lytic phase of HSV-1 infection. NHDFs were pretreated with DMSO or increasing concentrations of 4EGi-1 and then mock infected or infected at high multiplicity of infection (MOI, 5) for 11 h. Prior to sampling, cultures were metabolically labeled for 1 h (10 to 11 hours postinfection [hpi]), and then whole-cell extracts were resolved by SDS-PAGE and exposed to X-ray film. In DMSO-treated cultures, HSV-1 infection resulted in a characteristic pattern of host shutoff and robust synthesis of viral proteins (Fig. 7 A). While 20 μM 4EGi-1 had only modest effects, 30 μM and 40 μM concentrations caused an increasingly potent inhibition of viral protein synthesis. Quantification of the effects of 4EGi-1 by TCA precipitation demonstrated that 40 μM 4EGi-1 reduced translation rates to 1.4% of that of control samples (not shown). When the abundance of viral antigens was measured in the same samples, the accumulation of HSV-1 proteins was reduced in a dose-dependent manner, reaching undetectable levels at 40 μM 4EGi-1 (Fig. 7B). Blots presented in Fig. 7B were intentionally saturated to demonstrate the severity of the defect in antigen accumulation at higher concentrations of inhibitor. The effects on antigen accumulation were reflected in the levels of virus production in inhibitor-treated cultures infected at an MOI of 5 for 11 h, with those in cultures treated with 40 μM 4EGi-1 declining to just 400 infectious particles per culture, more than 106-fold lower than that of DMSO controls (Fig. 7C). This was not due to cytotoxic effects of the combination of inhibitor and viral infection, and 40 μM-treated virus-infected cultures were morphologically indistinguishable from mock-infected cultures (not shown). We also examined the effects of adding 4EGi-1 to cultures after infection was already established. Initially, 40 μM 4EGi-1 was added to cultures at 4 h after infection for a further 4 h, and then cultures were metabolically labeled for 1 h (8 to 9 hpi) prior to sample lysis. Autoradiographs demonstrated that 4EGi-1 potently suppressed ongoing translation at this point (Fig. 7D), demonstrating that the effects of the inhibitor were not simply due to a block in viral entry. To determine its effects when added very late in infection, 40 μM 4EGi-1 was added to cultures 12 h after infection for a period of 4 h, and then cultures were metabolically labeled for 1 h. Although 4EGi-1 again potently suppressed rates of protein synthesis, some viral protein synthesis was evident (Fig. 7D). HSV-1 encodes numerous proteins that interact with eIF4G, eIF4A, PABP, and ribosomal proteins (6, 9, 10, 23, 37, 45), in addition to encoding proteins capable of modulating both the activity of eIF2 kinases and directly influencing phosphate turnover on eIF2 (27). It remains to be determined whether any of these proteins might partially counteract the effects of 4EGi-1 at very late stages of infection.
FIG. 7.
4EGi-1 potently suppresses lytic HSV-1 replication. (A) NHDFs were pretreated for 4 h and then mock infected (M) or infected with HSV-1 at an MOI of 5 in the presence of DMSO (0) or increasing micromolar concentrations of 4EGi-1. Ten hours postinfection, cultures were metabolically labeled for 1 h and whole-cell extracts were resolved by SDS-PAGE. Fixed, dried gels were exposed to X-ray film. MW standards are indicated to the left. (B) Samples as described for panel A were analyzed by Western blotting using the indicated antibodies. M, mock infection. (C) NHDFs were infected with HSV-1 at an MOI of 5 in the presence of increasing micromolar concentrations of 4EGi-1 for 11 h. Infectious virus production was determined by titration on permissive Vero cells and represented as PFU/culture. (D) NHDFs were infected with HSV-1 at an MOI of 5 for 4 h (left) or 12 h (right), and then DMSO or 40 μM 4EGi-1 was added for 4 h, followed by metabolic labeling for 1 h. Whole-cell extracts were resolved by SDS-PAGE and fixed, dried gels were exposed to X-ray film. Migration of MW markers is indicated to the left.
Antiviral properties of 4EGi-1 are largely eIF4F independent.
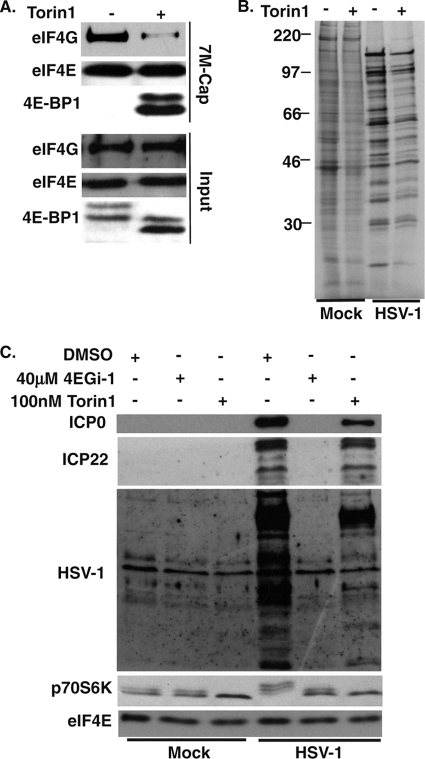
To determine the contribution of eIF4F to HSV-1 protein synthesis and accumulation, we examined the effects of Torin1 during infection. To verify that Torin1 did indeed disrupt eIF4F in infected cells, NHDFs were infected at an MOI of 5 for 11 h in the presence of DMSO or 100 nM Torin1, and cap-binding eIF4E and associated proteins were recovered from soluble cell extracts on 7-methyl-GTP-Sepharose and analyzed by Western blotting. Compared with DMSO control samples, Torin1 treatment caused a robust dephosphorylation of 4E-BP1, evident in both faster antigen mobility in input samples and a large increase in the association of 4E-BP1 with eIF4E in cap-bound samples (Fig. 8 A). This was accompanied by a robust decrease in eIF4G binding to eIF4E compared to the DMSO-treated control (Fig. 8A). We then compared the effects of Torin1 on rates of host and viral protein synthesis. NHDFs were treated with DMSO or 100 nM Torin1 for 4 h and then mock infected or infected at an MOI of 5 for 11 h. Cultures were metabolically labeled for 1 h (10 to 11 hpi) and then whole-cell extracts were resolved by SDS-PAGE and fixed, dried gels were exposed to X-ray film. As illustrated in Fig. 8B, Torin1 reduced rates of translation in both mock and infected samples to comparable degrees, suggesting that host and viral mRNAs have similar requirements for eIF4F activity. However, the extent of repression was not as dramatic as that observed for 4EGi-1 (Fig. 7A). To directly compare the effects of both inhibitors on the production of viral proteins, NHDFs were pretreated for 4 h with DMSO, 40 μM 4EGi-1, or 100 nM Torin1 and then mock infected or infected at an MOI of 5 for 11 h. Whole-cell extracts were then analyzed by Western blotting. While Torin1 caused notable reductions in the accumulation of viral structural proteins detected with anti-HSV-1 antibody, together with specific viral proteins such as ICP0 and ICP22, each of these viral antigens was undetectable in 4EGi-1-treated samples (Fig. 8C). HSV-1 infection resulted in mTOR activation and phosphorylation of p70S6K, evident as a reduction in antigen mobility, which was not only inhibited but reduced to below basal mock-infected levels in the presence of Torin1 (Fig. 8C). In contrast, p70S6K phosphorylation in cells infected with HSV-1 in the presence of 4EGi-1 remained the same as that in DMSO- or 4EGi-1-treated mock-infected samples, most likely due to the failure of the virus to establish infection and produce the protein(s) required for mTOR activation.
FIG. 8.
The mTORC inhibitor Torin1 disrupts eIF4F but does not suppress HSV-1 protein production as potently as 4EGi-1. (A) NHDFs were pretreated for 4 h with DMSO (−) or 100 nM Torin1 (+) and then infected with HSV-1 at an MOI of 5 for 11 h. eIF4E and associated proteins were recovered from soluble cell extracts on 7-methyl-GTP-Sepharose and analyzed by Western blotting with the indicated antibodies. Phosphorylated forms of 4E-BP1 were resolved using 17.5% gels. (B) NHDFs were pretreated for 4 h with DMSO (−) or 100 nM Torin1 (+) and then mock infected (mock) or infected (HSV-1) at an MOI of 5 for 11 h. Cultures were metabolically labeled from 10 to 11 hpi and then whole-cell extracts were resolved by SDS-PAGE and fixed, dried gels exposed to X-ray film. Migration of MW markers is indicated to the left of autoradiograms. (C) NHDFs were pretreated for 4 h with DMSO, 40 μM 4EGi-1, or 100 nM Torin1 as indicated in the panels above, then mock infected or infected with HSV-1 at an MOI of 5 for 11 h. Whole-cell extracts were analyzed by Western blotting with the indicated antibodies.
Suppression of poxvirus replication by 4EGi-1.
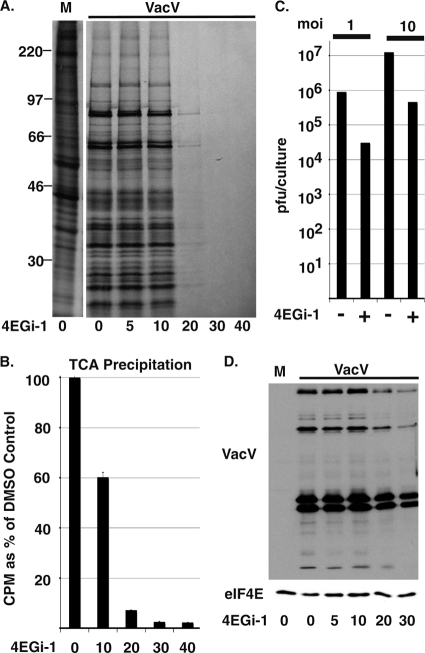
Finally, to determine whether 4EGi-1 was capable of inhibiting the replication of viruses other than HSV-1 we then tested its effects on vaccinia virus (VacV), the laboratory prototype for poxvirus infection (30). Poxviruses are unique among human DNA viruses as they replicate exclusively in the cytoplasm of infected cells. We therefore treated NHDFs with increasing concentrations of 4EGi-1 for 4 h and then infected cultures with VacV at an MOI of 10. Fifteen hours postinfection, cultures were metabolically labeled for 1 h and then whole-cell extracts were resolved by SDS-PAGE and fixed, dried gels were exposed to X-ray film. Poxvirus infection of DMSO-treated NHDFs resulted in the characteristic shutoff of host translation and robust synthesis of poxvirus polypeptides (Fig. 9 A). While lower concentrations of 4EGi-1 had no significant effect on the pattern or rates of viral protein synthesis, modest inhibition of translation was evident at 10 μM, while concentrations above 20 μM resulted in dramatic suppression of translation. TCA precipitation and quantification showed that 30 μM and 40 μM 4EGi-1 reduced translation rates in infected cultures to 2.4% and 2.3%, respectively, of those in DMSO-treated cultures (Fig. 9B). Notably, when 4EGi-1 was added to cultures at 12 hpi it retained the capacity to inhibit ongoing viral protein synthesis when cultures were metabolically labeled from 16 to 17 hpi (not shown), demonstrating that its effects were not due to inhibition of viral entry into the cell.
FIG. 9.
4EGi-1 suppresses poxvirus protein synthesis and replication. (A) NHDFs were pretreated for 4 h with DMSO (0) or increasing micromolar concentrations of 4EGi-1 and then mock infected (M) or infected with VacV at an MOI of 10 for 15 h, followed by metabolic labeling for 1 h. Whole-cell extracts were resolved by SDS-PAGE and then gels were fixed, dried, and exposed to X-ray film. Migration of MW standards is indicated to the left. (B) 35S-labeled protein in VacV samples described in panel A was quantified by TCA precipitation, and counts per minute (CPM) are presented as a percentage of DMSO controls, arbitrarily set at 100%. (C) NHDFs were pretreated with DMSO (−) or 30 μm 4EGi-1 (+) and infected with VacV at an MOI of 1 or 10 for 16 h. Infectious virus was quantified by titration on permissive BSC40 cells and presented as PFU/culture. (D) NHDFs were treated with DMSO (0) or increasing micromolar concentrations of 4EGi-1 and then mock infected (M) or infected with VacV at an MOI of 10 for 16 h. Whole-cell extracts were analyzed by Western blotting using antibodies against VacV or eIF4E as a loading control.
To determine the effect of this translational suppression on poxvirus replication, NHDFs were treated with DMSO or 30 μM 4EGi-1 and infected at a multiplicity of infection of either 1 or 10 infectious particles per cell. At 16 h, samples were harvested by freeze-thawing and infectious virus was determined by titration on permissive BSC40 cells. In both instances 4EGi-1 reduced virus replication to approximately 3% that of control DMSO-treated cultures (Fig. 9C), closely mirroring the degree to which it suppressed rates of viral protein synthesis and demonstrating that 4EGi-1 inhibited virus replication regardless of the amount of incoming viral particles. However, although 4EGi-1 potently suppressed VacV replication, its effects on infectious virus production were much lower than that observed with HSV-1 (Fig. 7D). Indeed, in contrast to HSV-1 antigens (Fig. 7C), Western blotting against VacV antigens demonstrated that the accumulation of viral proteins was readily detectable in 4EGi-1-treated samples (Fig. 9D). As such, while 4EGi-1 affected translation rates in both cases it had distinct effects on the overall fate of poxvirus and herpesvirus infections.
DISCUSSION
Translation represents a fundamental step in the conversion of genetic code into functional protein, both for the host cell and invading viruses. As such, the degree to which, and means whereby, translation might be suppressed to create a suboptimal environment for virus replication without deleterious effects on the cell remains unclear. Here, we identify a previously unknown eIF4F-independent mode whereby the recently described small molecule 4EGi-1 affects the cellular translational machinery and show that this inhibitor potently suppresses ongoing protein synthesis in normal human cells without affecting long-term survival or the ability to tolerate stress, while at the same time inhibiting the replication of two very distinct human pathogens.
As our mechanistic understanding of how the complex process of protein synthesis regulation grows, it becomes clear that different mRNAs can have very distinct requirements for initiation factors to mediate their translation. Despite its cap-binding and helicase activities, growing evidence shows that high levels of eIF4F are largely required only for efficient translation of mRNAs with complex 5′ untranslated regions (UTRs), while structurally simpler and more abundant housekeeping messages are relatively insensitive to modulations in the activity of this complex (4, 34). Indeed, many interactions of eIF4F components are dispensable for maintaining significant rates of global protein synthesis, suggesting that this complex could play a regulatory rather than obligatory role in translation (16). In line with this, Torin1 significantly reduced eIF4F levels but had relatively modest effects on overall translation in either uninfected or HSV-1-infected cells, as well as on the accumulation of viral antigens. A very recent report demonstrated that Torin1 disrupted eIF4F to extents similar to those reported here and reduced HSV-1 replication by approximately 100-fold (29). However, it should be noted that these experiments were performed in mouse embryo fibroblast cells infected at an MOI of 0.05 for a period of 3 days, in which virus replication and spread amplifies viral growth defects. As such, our findings are in line with those of Moorman and colleagues and suggest that although eIF4F is important for maximal rates of translation, significant disruption of this complex does not adversely affect HSV-1 protein synthesis.
In contrast to Torin1, 4EGi-1 potently inhibited both host and viral protein synthesis. 4EGi-1 was recently identified in high-throughput in vitro screens for molecules that could bind to eIF4E and potentially disrupt its interaction with eIF4G (26). While 4EGi-1 disrupted eIF4F complexes in reticulocyte extracts and cultured cells, providing an important demonstration that such molecules could be developed, it did so only at relatively high concentrations that also induced apoptosis in many cell types (8, 26, 42). However, the possibility that 4EGi-1 might also affect other steps in the translation process has not been explored in detail, although a number of properties of this compound that were evident in its initial characterization suggest that this might indeed be the case (26). Notably, 4EGi-1 inhibited translation and reduced the growth of cell lines at concentrations lower than those required to disrupt eIF4F. In addition, 4EGi-1 affected translation driven from reporter constructs that employed the encephalomyocarditis virus (EMCV) or hepatitis C virus (HCV) internal ribosome entry site (IRES), both of which mediate eIF4F-independent translation (26). In contrast, 4EGi-1 did not affect translation when the cricket paralysis virus (CrPV) IRES was used. Notably, the CrPV IRES is distinct from both the EMCV and HCV IRES elements in that it drives ribosome recruitment and translation in a manner that is independent of initiation factors (38, 50). Indeed, Moerke et al. suggested that 4EGi-1 may have off-target effects and a number of recent reports support the notion of eIF4F-independent modes for 4EGi-1 (26). Although the composition of initiation complexes were not examined in their study, Mokas and colleagues recently reported that 4EGi-1 potently inhibited global translation in HeLa cells and resulted in the accumulation of 80S ribosomes, which was not observed when eIF4E levels were experimentally reduced (28). Another report demonstrated that 4EGi-1 downregulated c-FLIP protein levels to induce apoptosis in cancer cells, an effect that could not be mimicked by siRNA-mediated knockdown of eIF4E (8). Here we show that at concentrations lower than those required to significantly disrupt eIF4F, 4EGi-1 increased the association of ribosomal complexes containing phosphorylated, inactive eIF2α. This may reflect defects in the dissociation and recycling of initiation factors that normally occur during the dynamic process of ribosome assembly and translation initiation. While it remains to be determined how 4EGi-1 mediates this effect, which may be similar to how it stabilizes the eIF4E/4EBP-1 interaction, this finding offers an explanation for how 4EGi-1 might selectively affect EMCV and HCV IRES activities but not that of CrPV, as well as why this inhibitor affects global translation and cell growth at concentrations below those needed to disrupt eIF4F. As such, this is an important functional consideration when using this inhibitor as a tool to study translation in various biological processes and in developing the therapeutic potential of 4EGi-1 or related compounds.
The degree to which translation was inhibited in poxvirus-infected cells was directly correlated with the reduction in infectious virus produced in 4EGi-1-treated cultures, suppressing virus replication by 97%. However, although similar reductions in translation rates were observed in HSV-1-treated cultures, 4EGi-1 had a disproportionately large effect on viral antigen accumulation and production of infectious virus. While this might be due to fortuitous negative effects of 4EGi-1 on other processes during HSV-1 infection, another possible explanation may lie in differences between the herpesvirus and poxvirus life cycles. In contrast to poxviruses, the default fate of incoming herpesvirus genomes is repression mediated by cellular factors. These obstacles must be overcome by viral proteins, in particular ICP0 that is produced early in infection and essential for initiating a productive infection in cell types such as normal human cells (7, 25). Inefficient production of these proteins to below a critical threshold in 4EGi-1-treated cultures likely amplifies its inhibitory effects on the HSV-1 life cycle. Indeed, the residual rates of translation in infected cells at 40 μM 4EGi-1 detected by TCA precipitation may reflect low-level cellular, rather than viral, protein synthesis. Similarly, the highly potent inhibition of HSV-1 reactivation by 4EGi-1 is likely due in large part to its ability to suppress de novo production of ICP0, a critical initiator of the cascade of viral gene expression required for efficient reactivation. As such, this approach could be particularly potent in the case of infectious agents that depend upon efficient expression of viral proteins to overcome natural cellular restrictions.
The potent antiviral effects of 4EGi-1, particularly in the case of HSV-1, were all the more intriguing given the surprising tolerance primary human cells showed for this inhibitor. Not only did these cells remain viable for extended periods of exposure to 4EGi-1, they also retained the capacity to tolerate and respond to both chemical and thermal stress. It was notable that in both cases 4EGi-1 did impair the accumulation of hsp27 but had little or no effect on the accumulation of hsp70. Translation of both hsp27 and hsp70 mRNAs has been shown to be enhanced rather than repressed when eIF4F components are depleted in HeLa cells (18), further suggesting that 4EGi-1 affects hsp27 accumulation in an eIF4F-independent manner. On the other hand, selective enhancement of hsp70 synthesis during stress conditions has been shown to be facilitated by sequences specifically found in the 5′ UTR of the hsp70 mRNA (51), which may confer an advantage in competing for low levels of active initiation complexes and insensitivity to 4EGi-1. However, additional mechanisms of hsp70 induction may include changes in protein stability or large increases in the abundance of hsp70 mRNAs. Whatever the mechanism, the ability of 4EGi-1-treated cells to accumulate hsp70 is likely to play an important part in their ability to tolerate stress (2). As such, targeting 4EGi-1-sensitive translational processes offers potent protection from herpesvirus and poxvirus infection with no detectable detrimental effects on the host cell. Given the universal dependence of viruses upon host ribosomes and previous findings that 4EGi-1 also affects translation of reporter genes from eIF4F-independent IRES elements of EMCV and HCV (26), exploring the potential of targeting translation using such compounds may offer a broad-spectrum approach to inhibiting diverse forms of viral infection, a strategy that might also be effective in treating newly emerging pathogens that are poorly understood.
Acknowledgments
We thank Ian Mohr, John Blaho, Richard Roller, Nathanael Gray, and David Sabatini for kindly providing reagents.
This work was supported by grants from Science Foundation Ireland (06 IN.1 B80 and 09-RFP-BMT2130) and the Health Research Board (RP/2007/52) to D.W.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Arias, C., D. Walsh, J. Harbell, A. C. Wilson, and I. Mohr. 2009. Activation of host translational control pathways by a viral developmental switch. PLoS Pathog. 5:e1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beere, H. M., D. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 3.Castelló, A., A. Quintas, E. G. Sánchez, P. Sabina, M. Nogal, L. Carrasco, and Y. Revilla. 2009. Regulation of host translational machinery by African swine fever virus. PLoS Pathog. 5:e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coldwell, M. J., and S. J. Morley. 2006. Specific isoforms of translation initiation factor 4GI show differences in translational activity. Mol. Cell. Biol. 26:8448-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Benedetti, A., and R. E. Rhoads. 1990. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc. Natl. Acad. Sci. U. S. A. 87:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz, J. J., D. Simonin, T. Massé, P. Deviller, K. Kindbeiter, L. Denoroy, and J. J. Madjar. 1993. The herpes simplex virus type 1 US11 gene product is a phosphorylated protein found to be non-specifically associated with both ribosomal subunits. J. Gen. Virol. 74:397-406. [DOI] [PubMed] [Google Scholar]
- 7.Everett, R. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 8.Fan, S., Y. Li, P. Yue, F. R. Khuri, and S. Y. Sun. 2010. The eIF4E/eIF4G interaction inhibitor 4EGI-1 augments TRAIL-mediated apoptosis through c-FLIP down-regulation and DR5 induction independent of inhibition of cap-dependent protein translation. Neoplasia 12:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, P., D. N. Everly, Jr., and G. S. Read. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine-Rodriguez, E. C., T. J. Taylor, M. Olesky, and D. M. Knipe. 2004. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330:487-492. [DOI] [PubMed] [Google Scholar]
- 11.Fukuchi-Shimogori, T., I. Ishii, K. Kashiwagi, H. Mashiba, H. Ekimoto, and K. Igarashi. 1997. Malignant transformation by overproduction of translation initiation factor eIF4G. Cancer Res. 57:5041-5044. [PubMed] [Google Scholar]
- 12.Gingras, A. C., S. P. Gygi, B. Raught, R. D. Polakiewicz, R. T. Abraham, M. F. Hoekstra, R. Aebersold, and N. Sonenberg. 1999. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13:1422-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graff, J. R., B. W. Konicek, R. L. Lynch, C. A. Dumstorf, M. S. Dowless, A. M. McNulty, S. H. Parsons, L. H. Brail, B. M. Colligan, J. W. Koop, B. M. Hurst, J. A. Deddens, B. L. Neubauer, L. F. Stancato, H. W. Carter, L. E. Douglass, and J. H. Carter. 2009. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 69:3866-3873. [DOI] [PubMed] [Google Scholar]
- 14.Graff, J. R., B. W. Konicek, T. M. Vincent, R. L. Lynch, D. Monteith, S. N. Weir, P. Schwier, A. Capen, R. L. Goode, M. S. Dowless, Y. Chen, H. Zhang, S. Sissons, K. Cox, A. M. McNulty, S. H. Parsons, T. Wang, L. Sams, S. Geeganage, L. E. Douglass, B. L. Neubauer, N. M. Dean, K. Blanchard, J. Shou, L. F. Stancato, J. H. Carter, and E. G. Marcusson. 2007. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Invest. 117:2638-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinnebusch, A. G. 2006. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem. Sci. 31:553-562. [DOI] [PubMed] [Google Scholar]
- 16.Hinton, T. M., M. J. Coldwell, G. A. Carpenter, S. J. Morley, and V. M. Pain. 2007. Functional analysis of individual binding activities of the scaffold protein eIF4G. J. Biol. Chem. 282:1695-1708. [DOI] [PubMed] [Google Scholar]
- 17.Imataka, H., A. Gradi, and N. Sonenberg. 1998. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 17:7480-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi-Barve, S., A. De Benedetti, and R. E. Rhoads. 1992. Preferential translation of heat shock mRNAs in HeLa cells deficient in protein synthesis initiation factors eIF-4E and eIF-4 gamma. J. Biol. Chem. 267:21038-21043. [PubMed] [Google Scholar]
- 19.Kahvejian, A., Y. V. Svitkin, R. Sukarieh, M. N. M'Boutchou, and N. Sonenberg. 2005. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 19:104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khaleghpour, K., S. Pyronnet, A. C. Gingras, and N. Sonenberg. 1999. Translational homeostasis: eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol. Cell. Biol. 19:4302-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimball, S. R., A. N. Do, L. Kutzler, D. R. Cavener, and L. S. Jefferson. 2008. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J. Biol. Chem. 283:3465-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudchodkar, S. B., Y. Yu, T. G. Maguire, and J. C. Alwine. 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 78:11030-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larralde, O., R. W. Smith, G. S. Wilkie, P. Malik, N. K. Gray, and J. B. Clements. 2006. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J. Virol. 80:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masutani, M., N. Sonenberg, S. Yokoyama, and H. Imataka. 2007. Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 26:3373-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon, R., and D. Walsh. 2008. Efficient quiescent infection of normal human diploid fibroblasts with wild-type herpes simplex virus type 1. J. Virol. 82:10218-10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moerke, N. J., H. Aktas, H. Chen, S. Cantel, M. Y. Reibarkh, A. Fahmy, J. D. Gross, A. Degterev, J. Yuan, M. Chorev, J. A. Halperin, and G. Wagner. 2007. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128:257-267. [DOI] [PubMed] [Google Scholar]
- 27.Mohr, I. 2006. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 119:89-99. [DOI] [PubMed] [Google Scholar]
- 28.Mokas, S., J. R. Mills, C. Garreau, M. J. Fournier, F. Robert, P. Arya, R. J. Kaufman, J. Pelletier, and R. Mazroui. 2009. Uncoupling stress granule assembly and translation initiation inhibition. Mol. Biol. Cell 20:2673-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moorman, N. J., and T. Shenk. 2010. Rapamycin-resistant mTORC1 activity is required for herpesvirus replication. J. Virol. 84:5260-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss, B. 2007. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 31.Nathan, C. O., S. Franklin, F. W. Abreo, R. Nassar, A. De Benedetti, and J. Glass. 1999. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J. Clin. Oncol. 17:2909-2914. [DOI] [PubMed] [Google Scholar]
- 32.Proud, C. G. 2005. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 16:3-12. [DOI] [PubMed] [Google Scholar]
- 33.Pyronnet, S., H. Imataka, A. C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 18:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramŕez-Valle, F., S. Braunstein, J. Zavadil, S. C. Formenti, and R. J. Schneider. 2008. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J. Cell Biol. 181:293-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinker-Schaeffer, C. W., J. R. Graff, A. De Benedetti, S. G. Zimmer, and R. E. Rhoads. 1993. Decreasing the level of translation initiation factor 4E with antisense RNA causes reversal of ras-mediated transformation and tumorigenesis of cloned rat embryo fibroblasts. Int. J. Cancer 55:841-847. [DOI] [PubMed] [Google Scholar]
- 36.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex viruses, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 37.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnow, P., R. C. Cevallos, and E. Jan. 2005. Takeover of host ribosomes by divergent IRES elements. Biochem. Soc. Trans. 33:1479-1482. [DOI] [PubMed] [Google Scholar]
- 39.Silvera, D., S. C. Formenti, and R. J. Schneider. 2010. Translational control in cancer. Nat. Rev. Cancer 10:254-266. [DOI] [PubMed] [Google Scholar]
- 40.Smith, M. R., M. Jaramillo, Y. L. Liu, T. E. Dever, W. C. Merrick, H. F. Kung, and N. Sonenberg. 1990. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 2:648-654. [PubMed] [Google Scholar]
- 41.Sonenberg, N., and A. G. Hinnebusch. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamburini, J., A. S. Green, V. Bardet, N. Chapuis, S. Park, L. Willems, M. Uzunov, N. Ifrah, F. Dreyfus, C. Lacombe, P. Mayeux, and D. Bouscary. 2009. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 114:1618-1627. [DOI] [PubMed] [Google Scholar]
- 43.Thoreen, C. C., S. A. Kang, J. W. Chang, Q. Liu, J. Zhang, Y. Gao, L. J. Reichling, T. Sim, D. M. Sabatini, and N. S. Gray. 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284:8023-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh, D., C. Arias, C. Perez, D. Halladin, M. Escandon, T. Ueda, R. Watanabe-Fukunaga, R. Fukunaga, and I. Mohr. 2008. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol. Cell. Biol. 28:2648-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh, D., and I. Mohr. 2006. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev. 20:461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18:660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, D., C. Perez, J. Notary, and I. Mohr. 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 79:8057-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2:135-140. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, J. E., T. V. Pestova, C. U. T. Hellen, and P. Sarnow. 2000. Initiation of protein synthesis from the A site of the ribosome. Cell 102:511-520. [DOI] [PubMed] [Google Scholar]
- 51.Yueh, A., and R. J. Schneider. 2000. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14:414-421. [PMC free article] [PubMed] [Google Scholar]
- 52.Zaborowska, I., and D. Walsh. 2009. PI3K signaling regulates rapamycin-insensitive translation initiation complex formation in vaccinia virus-infected cells. J. Virol. 83:3988-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]