Abstract
B cells are one of the targets of Friend virus (FV) infection, a well-established mouse model often used to study retroviral infections in vivo. Although B cells may be effective in stimulating cytotoxic T lymphocyte responses, studies involving their role in FV infection have mainly focused on neutralizing antibody production. Here we show that polyclonal activation of B cells promotes their infection with FV both in vitro and in vivo. Furthermore, we demonstrate that complement opsonization of Friend murine leukemia virus (F-MuLV) enhances infection of B cells, which correlates with increased potency of B cells to activate FV-specific CD8+ T cells.
Recent evidence has shown that complement (C) opsonization of retroviruses can benefit the induction of specific CD8+ T cell responses by dendritic cells (DCs) (4). Here we demonstrate that beyond DCs, B cells can be involved in stimulating CD8+ T cells upon infection with complement-opsonized Friend virus (FV).
FV infection represents a well-established mouse retrovirus model suitable to study specific immune responses upon retroviral infection (18). FV is a complex consisting of two viruses: a nonpathogenic replication-competent helper virus, Friend murine leukemia virus (F-MuLV), and the pathogenic replication-defective spleen focus-forming virus (SFFV). Infection of adult mice with the FV complex results in polyclonal proliferation of erythroid precursor cells, causing massive splenomegaly. Disease progresses to lethal erythroleukemia in susceptible mouse strains, which are unable to mount potent immune responses. Resistant mouse strains are able to control massive viral replication but never completely eradicate the virus. In these animals a chronic infection manifests, which is associated with the induction of regulatory T cells that suppress effector functions of virus-specific CD8+ cytotoxic T lymphocytes (CTLs) (15, 30, 31).
Studies using the surface subunit of F-MuLV envelope fused to green fluorescent protein (GFP) revealed that binding of FV envelope protein occurs through the cationic amino acid transporter mCAT-1, which is expressed on most cells (27). FV targeted TER119+ erythroblasts in vivo, but all hematopoietic cells, including B cells, were shown to interact with FV envelope protein (27). In line with these studies, B cells have been reported as one of the target cell populations for FV infection in vivo (6, 8) and the primary reservoir for chronic virus (17). Inoculation of isolated Ig+ splenic B cells with FV in vitro resulted in productive infection of these cells, which was more pronounced in the presence of a polyclonal B cell activator, lipopolysaccharide (LPS) (6, 8). Since gammaretroviruses like FV require dividing cells for productive infection, polyclonal activation of B cells might be an advantage for viral spread.
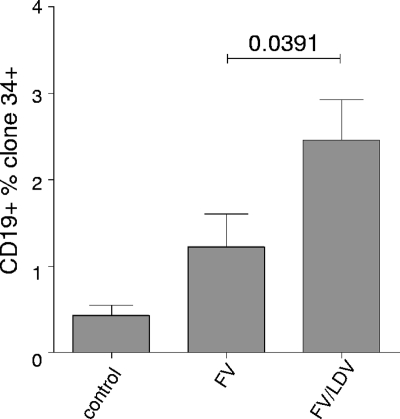
Previous studies have shown that lactate dehydrogenase-elevating virus (LDV) infection caused a polyclonal activation of CD19+ B cells, characterized by the expression of the early activation marker CD69 in vivo (1). With the aim of studying the effect of polyclonal B cell activation on FV infection in vivo, we infected (ABY × C57BL/10)F1 mice (Y10) intravenously either with 3,000 spleen focus-forming units (SFFU) of FV complex alone or with the same amount of FV and 2 × 108 IU of LDV. Y10 mice are characterized by expression of a susceptibility allele at the Fv2 locus (Fv2r/s) but recover from FV-induced leukemia because of their major histocompatibility complex (MHC) background. Therefore, FV infection reaches high levels in these animals and any differences can be followed easily and very accurately. Infected cells were determined by fluorescence-activated cell sorting (FACS) using FV glyco-Gag-specific monoclonal antibody clone 34 as described elsewhere (4). At 14 days postinfection (dpi), we detected a significantly higher percentage of infected spleen B cells in FV/LDV-coinfected mice than in animals infected with FV alone (Fig. 1). LDV-induced polyclonal activation of B cells might participate in enhanced infection of B cells. This hypothesis is further supported by the work of Tsuji-Kawahara et al. (29), who recently showed that the B cells which are infected by FV are also the ones that are activated. These findings provide evidence that activation of B cells is necessary for FV infection. Nevertheless, we cannot exclude the finding that the lower percentage of FV-infected B cells also correlates with the higher frequency of FV-specific CTLs in FV-only-infected mice observed at 7 dpi by Robertson et al. (24).
FIG. 1.
LDV coinfection improves FV infection of B cells in vivo. Compared to FV infection of (ABY × C57BL/10)F1 mice (Y10) at 14 days postinfection (dpi), we detected a significantly higher percentage of infected CD19+ spleen B cells in FV/LDV-coinfected animals. Infected cells were determined by staining with FV glyco-Gag-specific monoclonal antibody clone 34. Infected groups consist of 5 mice each. Data were analyzed by unpaired t test. Error bars represent means ± standard deviations.
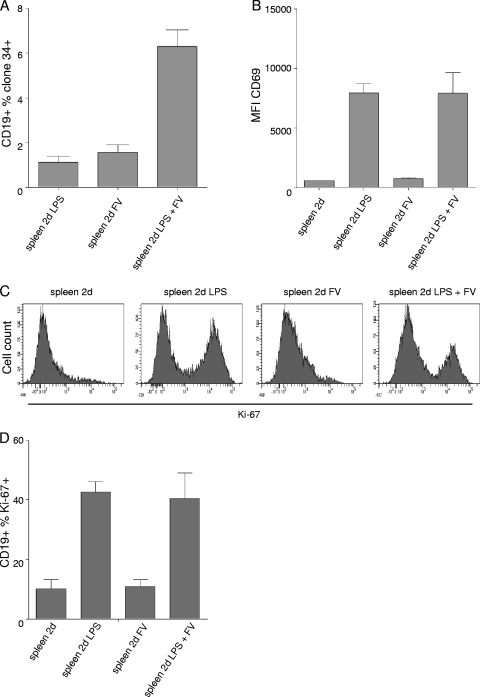
Since LDV does not directly induce polyclonal B cell activation (1), we used LPS, a polyclonal stimulator of B cell activation in our experiments in vitro. Using this experimental model, we could address the influence of B cell activation on FV infection levels. Infection of 106 spleen cells with 10,000 focus-forming units (FFU) of FV resulted in significant infection of B cells only in the presence of LPS (25 μg/ml) as demonstrated by measuring the expression of FV glyco-Gag on B cells 2 dpi by FACS (Fig. 2 A). Levels of FV infection of B cells correlated with activation as shown by the induction of CD69 expression (Fig. 2B) and proliferation (intracellular levels of Ki-67) (Fig. 2C and D). These data further indicate the importance of B cell activation and proliferation for FV infection. Note that FV alone induced neither CD69 expression nor B cell proliferation (Fig. 2B to D) and showed little infection potential (Fig. 2A). Therefore, we used LPS-activated B cells in subsequent experiments to study the role of complement opsonization of FV in infection of B cells.
FIG. 2.
FV infection depends on cell activation. (A) Percentage of infected CD19+ B cells in the presence or absence of LPS in cultures of isolated spleen cells infected with FV in vitro. (B) Expression (mean fluorescence intensity [MFI]) of early activation marker CD69 on CD19+ B cells in the presence or absence of LPS in cultures of isolated spleen cells infected with FV in vitro. (C and D) Expression of intracellular proliferation marker Ki-67 by CD19+ B cells in the presence or absence of LPS in the same cultures. Data represent means ± standard deviations of 5 (A) and 3 (B to D) independent experiments.
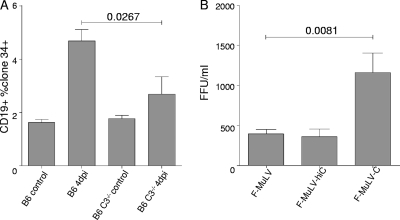
Several studies have demonstrated that retroviral infection of complement receptor-expressing cells can be enhanced by complement opsonization (2, 5, 12, 13, 16, 21, 26, 28). Since mouse B cells express CR1 and CR2, prolonged interactions of B cells with C-opsonized virions are likely (16). This might impact the infection of B cells in vivo. To evaluate this hypothesis, we infected B6 wild-type (wt) and B6 C3-knockout (KO) mice intravenously with 10,000 SFFU of FV. Experiments investigating the role of complement in FV infection of B cells and FV-specific CD8+ T cell activation by B cells had to be performed in C57BL/6 mice, since C3-knockout mice are available only in the C57BL/6 background characterized by the resistant Fv2r/r genotype. FV infection was determined 4 dpi by measuring the expression of FV glyco-Gag on B cells in the spleen. Compared to the infection in B6 wt mice, we found significantly lower levels of infected B cells in B6 C3 KO mice (Fig. 3 A). Of note, we did not observe any significant difference in the infection of complement-receptor-negative TER119+ erythroid cells (data not shown).
FIG. 3.
Complement opsonization improves FV infection of B cells. (A) FV infection of CD19+ B cells in B6 and B6 C3−/− animals determining infected cells on day 4 postinfection by monoclonal Ab clone 34 recognizing FV Gag on infected cells. Data represent means of 8 animals from two independent experiments and were analyzed by unpaired t test. (B) Infection of LPS-stimulated splenic B cells with nonopsonized (F-MuLV and F-MuLV-hiC) and complement-opsonized (F-MuLV-C) F-MuLV. Data represent means ± standard deviations of 10 independent experiments and were analyzed by unpaired t test.
To further study the effects of opsonization on B cell infection, we opsonized F-MuLV in the presence of normal mouse serum (NMS), as a source of complement, at a dilution of 1:10 for 60 min at 37°C (F-MuLV-C). As a control, F-MuLV was incubated in heat-inactivated NMS (F-MuLV-hiC) or buffer alone (F-MuLV). Virus was ultracentrifuged (23,000 × g, 2 h, 4°C), and the virus pellet was resuspended in medium. To confirm the opsonization pattern of the viruses, we applied F-MuLV-C and nonopsonized F-MuLV to a virus capture assay (VCA) as previously described (4). Using antibodies against mouse C3 in the VCA, we captured F-MuLV-C but not F-MuLV or F-MuLV-hiC, revealing the presence of C3 fragments only on effectively opsonized F-MuLV (data not shown). For the subsequent in vitro infection assays, we isolated splenic B cells using a mouse B cell isolation kit (Miltenyi Biotech) according to the manufacturer's instructions. Isolated B cell purity was more than 95%, as determined by flow cytometry. We infected 106 isolated LPS-stimulated (25-μg/ml) splenic B cells with 1,000 FFU of nonopsonized (F-MuLV and F-MuLV-hiC) and complement-opsonized (F-MuLV-C) virus in 100 μl medium for 2 to 3 h at 37°C. Culture medium (400 μl) was then added to each well, and the cells were cultured for another 20 h at 37°C. Input virus was then washed away, and B cells were further cultured in 1 ml RPMI-10% fetal calf serum (FCS) in the presence of LPS (25 μg/ml). Application of culture supernatants from these infected B cells 5 dpi to FV-permissive Mus dunni cells revealed a productive infection of B cells with both F-MuLV and F-MuLV-C. However, significantly higher titers of virus were recovered from culture supernatants of B cells infected with F-MuLV-C (Fig. 3B). Since F-MuLV opsonized in the presence of heat-inactivated serum did not show improved B cell infection, it is likely that complement proteins deposited on the viral surface rather than other serum proteins were responsible for the enhanced infection.
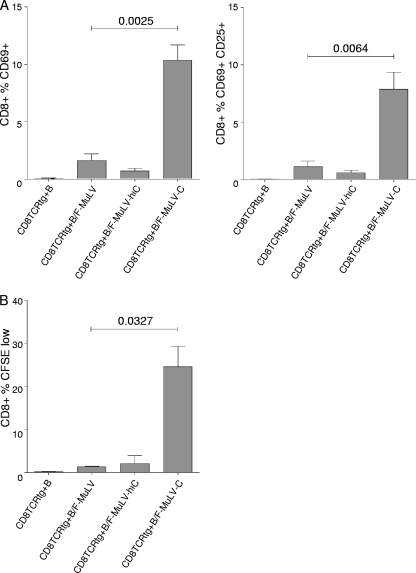
The effective control of FV infections in vivo involves both T cell and B cell responses, since FV-specific cytotoxic CD8+ T lymphocytes (CTLs), CD4+ T cells, and neutralizing antibodies (Abs) are required for recovery from infection (18). The efficient induction of a CTL response is widely accepted to be a result of antigen presentation by DCs (3). Studies investigating the role of other professional antigen-presenting cells, especially B cells for the induction of CTL responses, are still underrepresented (10, 11, 14, 22, 23, 25). Productive infection of B cells by FV suggests that viral proteins produced intracellularly are subject to presentation to CD8+ T cells in an MHC class I context. Therefore, we determined the capacity of spleen B cells loaded with nonopsonized or C-opsonized F-MuLV to activate naïve transgenic CD8+ T cells expressing a T cell receptor specific for an FV Gag peptide (TCRtg CD8+ T cells) in vitro (9). Coculture of 1 × 106 TCRtg CD8+ T cells with 1 × 106 LPS-stimulated B cells exposed to 1,000 FFU of F-MuLV or F-MuLV-hiC induced a slight expression of the early activation marker CD69 on CD8+ T cells compared to nonloaded control B cells (Fig. 4 A, left panel). However, CD8+ T cell activation was significantly enhanced when FV-specific CD8+ T cells were cocultured with B cells loaded with C-opsonized F-MuLV (Fig. 4A, left panel). In addition to CD69, another activation marker, CD25, was coexpressed on CD8+ T cells when activated by F-MuLV-C-pulsed B cells (Fig. 4A, right panel) after 48 h of coculture. To visualize proliferation of FV-specific CD8+ T cells induced by virus-loaded B cells, we isolated FV-specific TCRtg CD8+ T cells and stained them with carboxyfluorescein succinimidyl ester (CFSE) prior to coculture with F-MuLV-pulsed B cells. After 4 days of coculture both non- and C-opsonized F-MuLV-loaded B cells induced proliferation of FV-specific CD8+ T cells. However, the proliferation response of CD8+ T cells induced by F-MuLV-C-loaded B cells was significantly more pronounced (Fig. 4B). These results suggest that B cells infected with F-MuLV are able to stimulate FV-specific CD8+ T cells. Complement opsonization of F-MuLV enhanced infection of B cells and thereby amplified virus-specific CD8+ T cell responses. Infection of B cells seems to be critical for effective CD8+ T cell activation, since B cells loaded with either UV-inactivated F-MuLV or F-MuLV-C were not able to provide any CD8+ T cell stimulation (data not shown).
FIG. 4.
Activation of FV-specific T cells by F-MuLV-loaded B cells. (A) Bars indicate mean percentages of FV-specific CD8+ T cells expressing activation markers following coculture with B cells loaded with nonopsonized (CD8TCRtg+B/F-MuLV and CD8TCRtg+B/F-MuLV-hiC) and C-opsonized (CD8TCRtg+B/F-MuLV-C) F-MuLV (expression of CD69, left panel; dual expression of CD69 and CD25, right panel). (B) CFSE dilution as measured by flow cytometry was used to analyze proliferation of FV-specific TCRtg CD8+ T cells in response to variously loaded B cells. Data represent means ± standard deviations of 5 (A) and 3 (B) independent experiments and were analyzed by unpaired t test.
Similar to our results in B cells, complement opsonization of F-MuLV or HIV was also shown to enhance the induction of virus-specific CTL responses by DCs both in vitro and in vivo (4). Although infected B cells are able to efficiently activate FV-specific CD8+ T cells, studies on lymphocytic choriomeningitis virus (LCMV) suggest that DCs initially induce primary CTL responses (19). LCMV infects DCs, B cells, and macrophages, but the priming of LCMV-specific CTL responses depends mainly on DCs (20). However, specific depletion of DCs does not completely abolish the CTL response against FV (7). Thus, all infected antigen-presenting cells, including B cells and macrophages, might help amplify the in vivo CTL response by stimulating primed CTLs to become activated.
Taken together, we showed that polyclonal activation of B cells promotes their infection with FV both in vitro and in vivo. Furthermore, complement opsonization of F-MuLV enhances infection of B cells. The enhanced infection correlates with a significant increase in the capacity of virus-loaded B cells to activate FV-specific CD8+ T cells. The data shown here prove that complement opsonization serves as a natural adjuvant in the induction of antiviral CTL responses.
Acknowledgments
We thank Manfred P. Dierich for his helpful discussion, Lindsay Hohsfield for critical reading of the manuscript, and L. Hahn for her secretarial support.
We are supported by the Austrian Science Fund (FWF; P21508 to Z.B.), the Innsbruck Medical University (MFI; 6400 to Z.B.), and the European Union (DEC-VAC 018685 to H.S.). Part of this work was supported by a grant from the Deutsche Forschungsgemeinschaft (TRR60 project B4 to U.D.).
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Ammann, C. G., R. J. Messer, K. E. Peterson, and K. J. Hasenkrug. 2009. Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One 4:e6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajtay, Z., C. Speth, A. Erdei, and M. P. Dierich. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 173:4775-4778. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Bánki, Z., W. Posch, A. Ejaz, V. Oberhauser, S. Willey, C. Gassner, H. Stoiber, U. Dittmer, M. P. Dierich, K. Hasenkrug, and D. Wilflingseder. 2010. Complement as an endogenous adjuvant for dendritic cell-mediated induction of retrovirus-specific CTLs. PLoS Pathog. 6:e1000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouhlal, H., N. Chomont, M. Réquena, N. Nasreddine, H. Saidi, J. Legoff, M. D. Kazatchkine, L.Bélec, and H. Hocini. 2007. Opsonization of HIV with complement enhances infection of dendritic cells and viral transfer to CD4 T cells in a CR3 and DC-SIGN-dependent manner. J. Immunol. 178:1086-1095. [DOI] [PubMed] [Google Scholar]
- 6.Bowen, D. L., D. D. Isaak, and J. Cerny. 1979. Inhibition of in vitro Friend murine leukemia virus infection of lipopolysaccharide-activated B-cells with concanavalin A. J. Natl. Cancer Inst. 62:1497-1502. [PubMed] [Google Scholar]
- 7.Browne, E. P., and D. R. Littman. 2009. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 5:e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerny, J., P. A. Hensgen, S. H. Fistel, and L. M. Demler. 1976. Interactions of murine leukemia virus (MuLV) with isolated lymphocytes. II. Infections of B and T cells with Friend virus complex in diffusion chambers and in vitro: effect of polyclonal mitogens. Int. J. Cancer 18:189-196. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin, C. M., B. A. Vance, S. A. Grupp, and R. H. Vonderheide. 2004. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 103:2046-2054. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, A., M. Macleod, T. Schumacher, L. Corlett, and D. Gray. 2006. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J. Immunol. 176:3498-3506. [DOI] [PubMed] [Google Scholar]
- 12.Delibrias, C. C., A. Mouhoub, E. Fischer, and M. D. Kazatchkine. 1994. CR1(CD35) and CR2(CD21) complement C3 receptors are expressed on normal human thymocytes and mediate infection of thymocytes with opsonized human immunodeficiency virus. Eur. J. Immunol. 24:2784-2788. [DOI] [PubMed] [Google Scholar]
- 13.Delibrias, C. C., M. D. Kazatchkine, and E. Fischer. 1993. Evidence for the role of CR1 (CD35), in addition to CR2 (CD21), in facilitating infection of human T cells with opsonized HIV. Scand. J. Immunol. 38:183-189. [DOI] [PubMed] [Google Scholar]
- 14.DiLillo, D. J., K. Yanaba, and T. F. Tedder. 2010. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. 184:4006-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittmer, U., H. He, R. J. Messer, S. Schimmer, A. R. Olbrich, C. Ohlen, P. D. Greenberg, I. M. Stromnes, M. Iwashiro, S. Sakaguchi, L. H. Evans, K. E. Peterson, et al. 2004. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity 20:293-303. [DOI] [PubMed] [Google Scholar]
- 16.Gras, G., Y. Richard, P. Roques, R. Olivier, and D. Dormont. 1993. Complement and virus-specific antibody-dependent infection of normal B lymphocytes by human immunodeficiency virus type 1. Blood 81:1808-1818. [PubMed] [Google Scholar]
- 17.Hasenkrug, K. J., and U. Dittmer. 2007. Immune control and prevention of chronic Friend retrovirus infection. Front. Biosci. 12:1544-1551. [DOI] [PubMed] [Google Scholar]
- 18.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4(+) T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Probst, H. C., and M. van den Broek. 2005. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J. Immunol. 174:3920-3924. [DOI] [PubMed] [Google Scholar]
- 21.Pruenster, M., D. Wilflingseder, Z. Bánki, C. G. Ammann, B. Muellauer, M. Meyer, C. Speth, M. P. Dierich, and H. Stoiber. 2005. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. Eur. J. Immunol. 35:2691-2698. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie, D. S., J. Yang, I. F. Hermans, and F. Ronchese. 2004. B-lymphocytes activated by CD40 ligand induce an antigen-specific anti-tumour immune response by direct and indirect activation of CD8(+) T-cells. Scand. J. Immunol. 60:543-551. [DOI] [PubMed] [Google Scholar]
- 23.Rivera, A., C. C. Chen, N. Ron, J. P. Dougherty, and Y. Ron. 2001. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13:1583-1593. [DOI] [PubMed] [Google Scholar]
- 24.Robertson, S. J., C. G. Ammann, R. J. Messer, A. B. Carmody, L. Myers, U. Dittmer, S. Nair, N. Gerlach, L. H. Evans, W. A. Cafruny, and K. J. Hasenkrug. 2008. Suppression of acute anti-Friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J. Virol. 82:408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultze, J. L., S. Grabbe, and M. S. von Bergwelt-Baildon. 2004. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 25:659-664. [DOI] [PubMed] [Google Scholar]
- 26.Spear, G. T., M. Hart, G. G. Olinger, F. B. Hashemi, and M. Saifuddin. 2001. The role of the complement system in virus infections. Curr. Top. Microbiol. Immunol. 260:229-245. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, T., S. Aizawa, and H. Ikeda. 2001. Expression of receptor for ecotropic murine leukemia virus on hematopoietic cells. Arch. Virol. 146:507-519. [DOI] [PubMed] [Google Scholar]
- 28.Thieblemont, N., N. Haeffner-Cavaillon, A. Ledur, J. L'Age-Stehr, H. W. Ziegler-Heitbrock, and M. D. Kazatchkine. 1993. CR1 (CD35) and CR3 (CD11b/CD18) mediate infection of human monocytes and monocytic cell lines with complement-opsonized HIV independently of CD4. Clin. Exp. Immunol. 92:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji-Kawahara, S., T. Chikaishi, E. Takeda, M. Kato, S. Kinoshita, E. Kajiwara, S. Takamura, and M. Miyazawa. 2010. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in Friend virus-infected mice. J. Virol. 84:6082-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelinskyy, G., A. R. Kraft, S. Schimmer, T. Arndt, and U. Dittmer. 2006. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur. J. Immunol. 36:2658-2670. [DOI] [PubMed] [Google Scholar]
- 31.Zelinskyy, G., K. K. Dietze, Y. P. Hüsecken, S. Schimmer, S. Nair, T. Werner, K. Gibbert, O. Kershaw, A. D. Gruber, T. Sparwasser, and U. Dittmer. 2009. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 114:3199-3207. [DOI] [PubMed] [Google Scholar]