Abstract
We have previously reported that a newly annotated gene of human cytomegalovirus (HCMV), UL21a, encodes an early viral protein termed pUL21a. Most notably, the virions of a UL21a deletion virus had markedly reduced infectivity, indicating that UL21a is required to establish an efficient productive infection. In this study, we infected fibroblasts with equal numbers of DNA-containing viral particles and identified where in the viral life cycle pUL21a acted. The UL21a deletion virus entered cells and initiated viral gene expression efficiently; however, it synthesized viral DNA poorly and accumulated several immediate-early (IE) transcripts at reduced levels at late times of infection. The defect in viral DNA synthesis preceded that in gene expression, and inhibition of viral DNA synthesis reduced the late accumulation of IE transcripts in both wild-type and mutant virus-infected cells to equivalent levels. This suggests that reduced viral DNA synthesis is the cause of reduced IE gene expression in the absence of UL21a. The growth of UL21a deletion virus was similar to that of recombinant HCMV in which pUL21a expression was abrogated by stop codon mutations, and the defect was rescued in pUL21a-expressing fibroblasts. pUL21a expression in trans was sufficient to restore viral DNA synthesis and gene expression of mutant virus produced from normal fibroblasts, whereas mutant virus produced from complementing cells still exhibited the defect in normal fibroblasts. Thus, pUL21a does not promote the functionality of HCMV virions; rather, its de novo synthesis facilitates viral DNA synthesis, which is necessary for the late accumulation of IE transcripts and establishment of a productive infection.
Human cytomegalovirus (HCMV), the prototypic betaherpesvirus, is a ubiquitous pathogen that infects 50 to 90% of the world's population. Upon primary infection, HCMV establishes a lifelong latent or persistent/recurrent infection within its host. Though asymptomatic in most immunocompetent individuals, HCMV can cause severe disease and death in immunocompromised individuals, including AIDS patients and organ transplant recipients. HCMV is also the most common viral cause of birth defects, leading to hearing loss, blindness, and mental retardation in congenitally and perinatally infected infants (32). The economic burden to the U.S. health care system for this virus is estimated at approximately 4 billion dollars annually, with a majority of the costs attributed to long-term sequelae experienced by individuals who acquire congenital HCMV disease (19). A comprehensive understanding of how HCMV interacts with the host to establish both acute and latent infections will be critical for developing an effective vaccine and novel therapeutics to combat HCMV disease (24, 59).
HCMV expresses its genes in a highly regulated temporal cascade during a productive infection (32). The virus first expresses its immediate-early (IE) genes, which appear 2 to 4 h after viral entry and persist throughout the infection. The products of the major immediate-early (MIE) transcript are critical for the establishment of a productive infection and must be downregulated for the virus to establish latency (32). The primary proteins encoded by the MIE transcript are IE1-72 and IE2-86, which are produced by alternative splicing (62, 64, 66). IE1-72 is abundantly expressed during the first few hours of infection. Its abundance then undergoes only a limited increase throughout the remainder of the infection (63). In contrast, the accumulation of IE2-86 is low during the first 12 h of infection, but it increases considerably between 24 and 72 h postinfection (hpi) (62, 64). The reason for this differential MIE expression is unclear, but a specific inhibitor of the cyclin-dependent kinases (CDK) causes a shift in the ratio of IE1 to IE2 during the first 12 h of infection (55), suggesting that CDK activity may play a role in this regulation. The IE2-86 protein is essential for viral replication, while IE1-72 is required at a low multiplicity of infection (MOI) (11, 14, 18, 29, 31, 58). Both proteins transactivate viral promoters and also modulate the cellular environment to be more conducive for viral infection. Additional IE genes, which include TRS1, UL37x1, and US3, help HCMV to overcome innate and adaptive cellular antiviral responses (1, 4, 5, 13, 17, 23, 44).
Transcription of early genes shortly follows IE gene expression, appearing at between 4 and 12 hpi. These genes encode DNA replication enzymes, such as UL44 (processivity factor) and UL54 (viral DNA polymerase), as well as viral regulatory proteins that alter host cells for a cellular environment conducive to viral replication. Late genes are expressed following the onset of viral DNA replication, and many of them encode structural proteins, such as the major capsid protein (MCP) and pp28, which are required for assembly and maturation of the virion. Other late-gene products, such as pp71, are tegument proteins which can antagonize intrinsic cellular defenses and help progeny virus initiate IE gene expression during subsequent infection (20, 51, 52).
HCMV contains a 240-kb double-stranded DNA genome that carries at least 166 putative open reading frames (ORFs) and several microRNAs (miRNAs) (3, 7, 9, 15, 34, 35). With the advent of the infectious bacterial artificial chromosome (BAC) clone-based genetic system for HCMV (2, 73), the functions of HCMV genes have started to be elucidated. Genome-scale mutagenesis approaches have identified a subset of candidate viral genes that are important for HCMV to establish infection in tissue culture models of primary human cells, including fibroblasts (8, 72). More-detailed investigations have begun to uncover the functions of selected viral genes. We have recently identified a 15-kDa viral protein, pUL21a, which is expressed with early kinetics from the newly annotated HCMV gene UL21a (10). pUL21a localizes predominantly to the cytoplasm and undergoes rapid degradation in a ubiquitin-independent, proteasome-dependent manner (10). Analysis of mutant HCMV lacking the UL21a-coding sequence reveals a minor defect in viral DNA replication (∼2- to 3-fold) and a more pronounced reduction in infectivity of progeny viruses (∼10-fold), suggesting that UL21a is required for the establishment of a productive infection (10). It is possible that UL21a promotes the proper tegumentation of the HCMV virion, enabling the virus to effectively initiate productive infection. Alternatively, it may act at the later stages of infection, such as viral gene expression or DNA replication, to drive the progression of the viral life cycle.
In this study, we sought to determine how UL21a drives HCMV infection by analyzing fibroblasts infected with equal numbers of DNA-containing virions of recombinant viruses. We report that UL21a is not required for HCMV to establish the initial events of productive infection, but it facilitates efficient viral DNA synthesis and the late accumulation of several viral IE transcripts. Moreover, we present evidence that DNA synthesis is the cause and not a consequence of the late accumulation of the IE transcripts. We show that pUL21a, the protein product of UL21a, carries out the function of this gene locus. pUL21a expression in trans restores viral DNA synthesis and lytic gene expression of mutant virus that is originally produced from normal fibroblasts. However, mutant virus produced from complementing cells still exhibited reduced lytic gene expression in normal fibroblasts. Collectively, our results indicate that HCMV virions function normally independent of pUL21a, but the de novo synthesis of this protein facilitates viral DNA synthesis, which is necessary for the efficient late accumulation of IE transcripts and the establishment of a productive infection.
MATERIALS AND METHODS
Plasmids and antibodies.
pYD-C160 and pYD-C476 are retroviral vectors derived from pRetro-EBNA. pYD-C160, which carries the coding sequence of the green fluorescent protein (GFP), was previously described (69), and pYD-C476 was created by PCR amplifying the coding sequence of the GFP-UL21a fusion protein from pADinGFP-UL21a (10) and inserting it into the multiple-cloning site of pRetro-EBNA.
The primary antibodies used in the present study included anti-β-actin (AC-15; Abcam); anti-IE2 (mAB8140; Chemicon); anti-acetyl histone H3 (DAM1422332; Upstate); anti-Bip/Grp 78 (H-129; Santa Cruz); anti-UL21a (10); anti-UL38 (69); and anti-IE1, anti-pp28 (60), and anti-TRS1 (49) (generous gifts from Thomas Shenk, Princeton University).
Cells and viruses.
Primary embryonic lung fibroblasts (MRC-5) and human newborn foreskin fibroblasts (HFFs) were propagated with Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, nonessential amino acids, and penicillin-streptomycin. To create MRC-5 cells expressing GFP (MRC-5-GFP) or the GFP/UL21a fusion protein (MRC-5-GFP/UL21a), retrovirus stocks were prepared by transfecting the retroviral vector pYD-C160 or pYD-C476 into Phoenix-Ampho cells (25) using Lipofectamine (Invitrogen) according to the manufacturers' instructions. MRC-5 cells were transduced with retrovirus three times and then allowed to recover for 72 h to generate a pool of cells expressing the protein of interest.
Five BAC-HCMV clones were used in the present study to reconstitute recombinant HCMVs. pAD-GFP carried the GFP-tagged genome of the HCMV AD169 strain and was used to produce wild-type virus ADwt (73). pADsubUL21a carried a GalK/kanamycin dual mutagenic cassette in place of the entire UL21a-coding sequence as previously described (10). pADpmpUL21a carried stop codon mutations at Gln8 and Thr39 engineered to specifically disrupt pUL21a expression (see Fig. 6). It was constructed by replacing the GalK/kanamycin cassette in pADsubUL21a with the PCR fragment of the UL21a gene that contained the desired mutations via linear recombination as previously described (43). These mutations were first generated by using a QuikChange XL kit (Stratagene) with two primer pairs containing the desired mutations and the template plasmid pYD-C417 carrying the HCMV UL21a sequence, resulting in the plasmid pYD-C478 carrying these mutations. The primer pairs are as follows: first pair (Q8X), 5′-GGTAGCCCTGTTCCCTAGCTCACCACCGTCACT-3′ and 5′-AGTGACGGTGGTGAGCTAGGGAACAGGGCTACC-3′; second pair (R39X), 5′-GCCTCCGACGAGCTCACGGCGCTAGAAAGC-3′ and 5′-GCTTTCTACGCGCCGTGAGCTCGTCGGAGGC-3′. pADrevpUL21a was a marker-rescued clone and was created by replacing the mutant UL21a gene in pADpmpUL21a with a wild-type copy of UL21a via two-step linear recombination as previously described (43). pTR is a BAC clone carrying the genome of a clinical HCMV isolate, TR, that was originally isolated from the ocular vitreous fluid of a patient with advanced HIV with CMV retinitis (61) and was used to reconstitute HCMV TR virus (35). All recombinant BACs were verified by restriction digestion, PCR analysis, and direct sequencing.
To reconstitute virus, 2 μg of the BAC-HCMV DNA and 1 μg of the pp71-expressing plasmid were transfected into MRC-5 cells by electroporation. Culture medium was changed 24 h later, and virus stock was prepared by harvesting cell-free culture supernatant when the entire monolayer of cells was lysed. Alternatively, virus stocks were produced by collecting cell-free culture medium from infection of HFFs at a multiplicity of infection (MOI) of 0.05. Virus-containing culture supernatants were then purified by ultracentrifugation through a 20% d-sorbitol cushion at an average relative centrifugal force of 64,000 × g for 1 h, resuspended in DMEM with 10% fetal calf serum, and saved as viral stocks. HCMV titers were determined in duplicate in HFFs by 50% tissue culture infectious dose (TCID50) assay, and their DNA content was determined by real-time quantitative PCR (qPCR) (see below).
Cellular fractionation.
Nuclear and cytoplasmic fractions were derived from infected cells at 2 h postinfection as previously described (27). The cell pellet was resuspended in RSB (10 mM NaCl, 3 mM MgCl2, 10 mM Tris-HCl [pH 7.4]), and 20× detergent (10% sodium deoxycholate and 20% Tween 40 in RSB) was added to a 1× final concentration. Samples were then vortexed and incubated on ice for 10 min. Nuclei were pelleted by centrifugation at 1,000 × g for 3 min at 4°C. The cytoplasmic (supernatant) and nuclear (pellet) fractions were then processed for total DNA and proteins as stated below.
Analysis of virion and intracellular DNAs.
Virion DNA was prepared as previously described (10). Briefly, to prepare virion DNA, 100 μl of cell-free virus (or 10 μl of purified virus) was treated with DNase I (30 U; Roche) at 37°C for 30 min, followed by incubation at 75°C for 20 min to stop the reaction. The samples were then incubated at 55°C overnight in lysis buffer (400 mM NaCl, 10 mM Tris [pH 8.0], 10 mM EDTA, 0.1 mg of proteinase K/ml, 0.2% sodium dodecyl sulfate [SDS]). Virion DNA was extracted with phenol-chloroform and treated with RNase A at 20 μg/ml at 37°C for 1 h. DNA was extracted again with phenol-chloroform, precipitated with ethanol, and resuspended in nuclease-free water (Ambion). To prepare intracellular DNA, HCMV-infected MRC-5 cells were collected at various times postinfection, resuspended in lysis buffer, and incubated at 55°C overnight. DNA was extracted with phenol-chloroform, treated with RNase A, extracted again with phenol-chloroform, precipitated with ethanol, and resuspended in nuclease-free water. Viral DNA was quantified by qPCR as previously described by using a TaqMan probe (Applied Biosystems) and primers specific for the HCMV UL54 gene (41). Cellular DNA was quantified with SYBR green PCR master mix (Clontech) and a primer pair specific for the human β-actin gene as previously described (43). The accumulation of viral DNA was normalized by dividing UL54 gene equivalents by β-actin equivalents.
Protein analysis.
Protein accumulation and subcellular localization were determined by immunoblotting and immunofluorescence, respectively. For immunoblotting, total cell extracts were prepared by lysing phosphate-buffered saline-washed infected cells in SDS-containing sample buffer. Proteins were resolved by electrophoresis on an SDS-containing polyacrylamide gel, transferred to a polyvinylidene difluoride membranes, hybridized with a primary antibody, reacted with the horseradish peroxidase-conjugated secondary antibody, and visualized by using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). For immunofluorescence, cells grown on glass coverslips were fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100 for 5 min, incubated with a primary antibody, and subsequently labeled with the Alexa Fluor 488 secondary antibody (Invitrogen). Labeled cells were counterstained with TO-PRO-3 (Molecular Probes) to visualize the nuclei and then mounted on slides with Prolong Gold antifade reagent (Molecular Probes). Images were captured using Zeiss LSM Image software with a Zeiss LSM 510 META confocal laser scanning microscope.
Reverse transcription-coupled quantitative PCR (RT-qPCR) analysis.
Total RNA was extracted by using the TRIzol reagent (Invitrogen) and treated with the Turbo DNA-free reagent (Ambion) to remove genomic DNA contaminants. cDNA was reverse transcribed from total RNA with random hexamer primers using the High Capacity cDNA reverse transcription kit (Applied Biosystems). cDNA was quantified by qPCR using a TaqMan probe and primers specific for the viral gene IE1 or UL37. Alternatively, cDNA was quantified using SYBR Advantage qPCR premix (Clontech) and primers for the viral genes TRS1, IE2, and exon 3 of the major immediate-early (MIE) transcript or the human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene. Primers and probes specific for IE1 and IE2 spanned the splice junction of these genes, as previously reported (70). All of the primer pairs and TaqMan probes are listed in Table 1. cDNA from infected cells was used to generate a standard curve for each gene examined. The standard curve was then used to calculate the relative amount of specific RNA present in a sample. The amounts of TRS1, UL37, IE2-86, IE1-72, and the MIE transcript were normalized using GAPDH as an internal control.
TABLE 1.
Primers and probes used for RT-qPCR
| Transcript | qPCR type | Primers | 6FAM-TAMRA TaqMan probe |
|---|---|---|---|
| UL122-123 pre-mRNA | SYBR green | 5′-TCTGCCAGGACATCTTTCTCG-3′, 5′-GGAGACCCGCTGTTTCCAG-3′ | Not applicable |
| UL123 (IE1) | TaqMan | 5′-CAAGTGACCGAGGATTGCAA-3′, 5′-CACCATGTCCACTCGAACCTT-3′ | 5′-TCCTGGCAGAACTCGTCAAACAGA-3′ |
| UL122 (IE2) | SYBR green | 5′-TGACCGAGGATTGCAACGA-3′, 5′-CGGCATGATTGACAGCCTG-3′ | Not applicable |
| UL37 | TaqMan | 5′-CCAAGGCGGGAGAGGAT-3′, 5′-CTGGCCTTTTGGTACTTTAGCT-3′ | 5′-TTCAAGGCGTTTTCGCTGGATCC-3′ |
| TRS1 | SYBR green | 5′-CTGTGCAAATGTGGAAGATACCT-3′, 5′-GTCCAGTCCCAGAGCTTGAG-3′ | Not applicable |
RESULTS
The UL21a deletion virus has a more pronounced growth defect in MRC-5 cells than in HFFs.
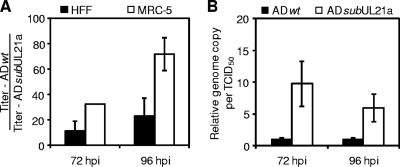
We have previously shown that UL21a is required for efficient HCMV replication in fibroblasts, and interestingly, the defect of the UL21a deletion virus is more pronounced in human embryonic lung fibroblasts (MRC-5) than that in human foreskin fibroblast cells (HFFs) in a multiple-step growth curve (10). In agreement, we found that the UL21a deletion virus also had a greater growth defect in MRC-5 cells than in HFFs in a single-step growth curve (Fig. 1A). In HFFs, the growth of mutant virus was 11- and 23-fold lower than that of wild-type virus at 72 and 96 h postinfection (hpi). In MRC-5 cells, the growth reduction of the mutant virus relative to wild-type virus increased to 32- and 72-fold, respectively. Because of this enhanced growth defect, we used MRC-5 cells in the present study to investigate the role of UL21a during HCMV infection. Importantly, similar to that observed in HFFs (10), the mutant progeny virus produced in MRC-5 had markedly reduced infectivity; the relative ratio of virion DNA to infectious particles of the mutant virus was 5- to 10-fold greater than that of wild-type virus (Fig. 1B). In order to determine why the UL21a deletion virus failed to establish an efficient productive infection, we carried out all of the infection experiments with recombinant viruses using equal input genome number equivalents in this study.
FIG. 1.
UL21a deletion virus has reduced infectivity. (A) Growth of UL21a deletion virus in HFFs and MRC-5 fibroblasts. HFFs or MRC-5 fibroblasts were infected with wild-type virus (ADwt) or UL21a deletion virus (ADsubUL21a) at an MOI of 5, cell-free viruses were collected at 72 and 96 h postinfection (hpi), and their yields were determined by TCID50 assay. Data are presented as the fold reduction in growth of ADsubUL21a relative to ADwt in each cell type. (B) Relative infectivity of progeny virions. MRC-5 cells were infected and cell-free virus was collected as described for panel A. The same volumes of viral samples were measured for their viral genome copies by real-time quantitative PCR (qPCR) and for their infectivity by TCID50 assay. The result is presented as the viral genome copy number per TCID50 unit. The relative genome copy per TCID50 unit of wild-type virus at 72 hpi was set to 1. Shown are representative results from at least two independent experiments. Error bars indicate standard deviations.
HCMV enters host cells efficiently independent of UL21a.
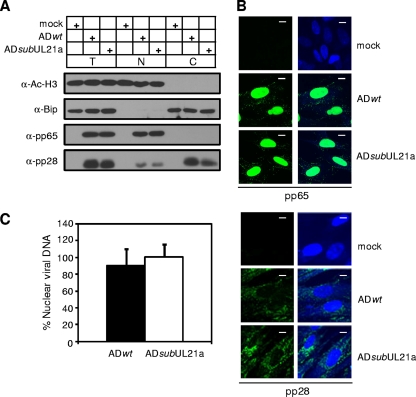
We systematically dissected the life cycle of UL21a deletion virus to define where the defect resided. We first infected MRC-5 cells with wild-type or UL21a deletion virus at equal input genome number equivalents and analyzed the ability of the mutant virus to enter cells by determining the translocation of viral protein and DNA to the nucleus at 2 hpi (Fig. 2). Both immunoblot and immunofluorescence analyses showed that the viral phosphoprotein pp65 translocated to the nucleus in cells infected with the mutant virus as efficiently as in cells infected with wild-type virus (Fig. 2A and B). Moreover, most of the viral protein pp28 stayed in the cytoplasm, as has been previously reported (Fig. 2A and B) (28, 54). The amount of cytoplasmic pp28 and nuclear pp65 in mutant virus infection was equivalent to that in wild-type virus infection. In addition, real-time quantitative PCR (qPCR) analysis of nuclear fractions of infected cells indicated that equivalent amounts of virion DNA had trafficked to the nuclei of cells infected with wild-type and UL21a deletion viruses (Fig. 2C). We conclude that the UL21a deletion virus is competent for viral entry.
FIG. 2.
UL21a deletion virus is competent for viral entry. (A and B) Translocation of tegument proteins pp65 and pp28 delivered into infected cells. MRC-5 cells were infected with ADwt and ADsubUL21a at an input genome number equivalent to 1 or 10 TCID50 units of wild-type virus/cell, collected at 2 hpi, and analyzed by immunoblotting (A) or immunofluorescence (B), respectively. For immunoblotting, total lysate (T), nuclear (N), and cytoplasmic (C) fractions were prepared and analyzed as indicated. Acetyl-histone H3 and Bip were used as nuclear and cytoplasmic fractionation controls, respectively. For immunofluorescence, the staining patterns of nuclei and viral antigens are shown as blue and green, respectively. Scale bar, 10 μm. (C) Nuclear translocation of virion DNA. Intracellular DNA was isolated from both total and nuclear fractions at 2 hpi. The quantity of viral genomes in each fraction was determined by qPCR using primers specific for the viral UL54 gene, and viral genome copies were normalized to cellular genomes using β-actin primers. The percentage of viral DNA translocated into the nucleus was determined by dividing viral genome equivalents in the nuclear fraction by those in the total cell lysate. Shown are representative results from at least two independent experiments. Error bars indicate standard deviations.
UL21a is dispensable for HCMV to initiate IE gene expression but is required for efficient viral DNA synthesis and the late accumulation of viral IE transcripts.
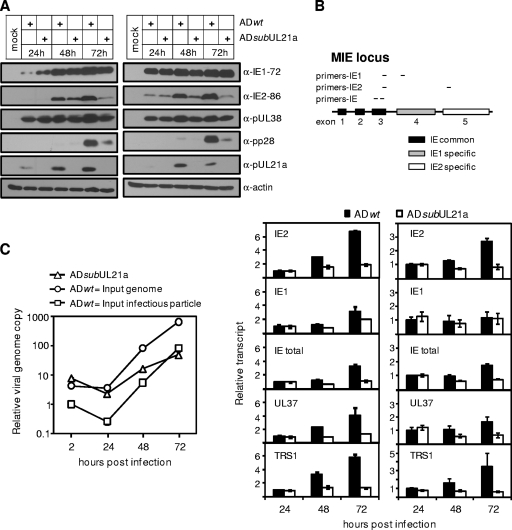
We next compared the profiles of viral gene expression in wild-type and UL21a deletion virus infection. We chose to perform the experiment at both low and high MOIs to ensure an infection condition where the level of viral replication was proportional to the quantity of input virus used. UL21a deletion virus expressed IE1-72 and IE2-86 at levels no less than those for the wild type at 8 (data not shown) and 24 (Fig. 3A) hpi. Moreover, the early HCMV protein pUL38 was also efficiently expressed at 24 hpi during mutant virus infection (Fig. 3A). These results suggest that UL21a deletion virus not only was competent for viral entry but also was able to initiate viral gene expression.
FIG. 3.
HCMV accumulates less IE2-86 protein and fewer IE transcripts at late times and synthesizes viral DNA at reduced levels in the absence of UL21a. (A) Analysis of viral protein accumulation. MRC-5 cells were infected with ADwt or ADsubUL21a at an input genome number equivalent to 0.25 (left panel) or 3 (right panel) TCID50 units of wild-type virus/cell. Cells were collected at the indicated times, and total cell lysates were analyzed by immunoblotting with the indicated antibodies. (B) Analysis of viral transcript accumulation. MRC-5 cells were infected with ADwt or ADsubUL21a at an input genome number equivalent to 0.25 (left panel) or 3 (right panel) TCID50 units of wild-type virus/cell. Total RNA was collected at the indicated times, and amounts of selected viral IE transcripts were measured by reverse transcription-coupled quantitative PCR (RT-qPCR) and normalized to that of GAPDH. Also shown are the genomic structure of the MIE locus and the locations of RT-PCR primers for analyzing IE1, IE2, and total IE transcripts. The normalized amount of viral transcript at 24 hpi during wild-type virus infection was set to 1. (C) Analysis of viral DNA synthesis. MRC-5 cells were infected with ADwt or ADsubUL21a at either an input genome number equivalent to 0.25 TCID50 unit of wild-type virus/cell or an MOI of 0.05. Total intracellular DNA was collected, viral DNA was measured by qPCR, and the amount was normalized to cellular genome copies with primers to β-actin. The normalized amount of viral DNA at 2 hpi during wild-type virus infection was set to 1. Shown are representative results from at least two independent experiments. Error bars indicate standard deviations.
Intriguingly, while the UL21a deletion virus continued to accumulate IE1-72 and pUL38 similarly to wild-type virus throughout the infection, there was a marked reduction in IE2-86 accumulation starting at 48 hpi (Fig. 3A). This defect coincided with a reduced accumulation of the late protein pp28. The reduction in the late accumulation of IE2-86 was not due to an inefficient second round of mutant virus infection, because this reduction was also clear with infection at a high MOI (Fig. 3A). We next sought to determine whether the defect in IE2-86 protein accumulation was the result of the reduced transcript accumulation. We measured the accumulation of the IE2-86 transcript in infections at both low and high MOIs (Fig. 3B) by reverse transcription coupled-qPCR (RT-qPCR) (Fig. 3B) (70). All transcripts detected were specific and were not the result of genomic DNA contamination, as mock-infected cells and reactions done in the absence of reverse transcriptase failed to produce any products (data not shown). Importantly, the IE2 primer pair used in this experiment amplified only the IE2-86 transcript and not any other known late transcripts from this region, such as IE2-60 or IE2-40 (42, 71), as the 5′ primer is located in exon 3, which is upstream of the start site of these two late transcripts. Consistent with the protein expression profile (Fig. 3A), the levels of the IE2 transcript in mutant virus and wild-type virus infection were nearly equivalent at 24 hpi (Fig. 3B). However, by 48 hpi the IE2 transcript accumulation in cells infected with UL21a deletion virus was reduced, and at 72 hpi it was ∼4-fold lower than that with wild-type virus (Fig. 3B). The total MIE transcript was also reduced by ∼3-fold at 72 hpi, suggesting that decreased IE2 expression was mostly due to the reduced accumulation of the MIE transcript at late times of infection (Fig. 3B). Consistent with protein analysis, there was no significant difference in the accumulation of the IE1 transcript in wild-type and mutant virus infection, particularly at a high MOI. This raises the possibility that at late times the IE2 transcript is the major product of MIE transcripts (56). Moreover, the reduced accumulation of late IE transcripts in mutant virus-infected cells was not limited to the MIE transcript. Other IE transcripts, including UL37 and TRS1, were also significantly reduced at late times in UL21a deletion virus-infected cells (Fig. 3B).
We then analyzed viral DNA synthesis in wild-type and UL21a deletion virus-infected cells at both equal numbers of input infectious particles and equal input genome number equivalents (Fig. 3C). When cells were infected with equal numbers of input infectious particles, the UL21a deletion virus started with much higher levels of input viral DNA, consistent with its elevated virion DNA-to-infectious particle ratio (Fig. 1B), but by 72 hpi the two viruses had similar levels of viral DNA. When cells were infected with equal input genome number equivalents, viral DNA levels in both virus infection were similar at 2 and 24 hpi. However, viral DNA synthesis in cells infected with mutant virus was 5-fold lower than in cells infected with wild-type virus at 48 hpi. By 72 hpi, the defect in viral DNA synthesis was ∼20 fold during mutant virus infection. We conclude that the UL21a deletion virus is defective in viral DNA synthesis and the late accumulation of viral IE transcripts.
The defect in viral DNA synthesis precedes that in the late accumulation of IE transcripts during UL21a deletion virus infection.
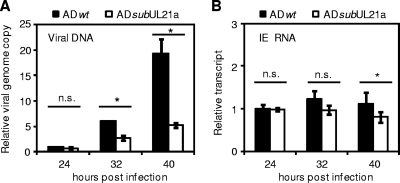
IE2-86 is essential for viral early gene expression and DNA synthesis (18, 29, 57). However, the UL21a deletion virus was able to express IE and early proteins, including IE2-86, at normal levels up to 24 hpi following initiation of viral DNA synthesis. It has been shown that IE2-86 undergoes a significant increase in transcript and protein accumulation between 24 and 72 hpi, which is dependent on DNA synthesis (62, 68). This raises the question of whether the reduced IE2-86 expression in UL21a deletion virus-infected cells is the cause or a consequence of defective viral DNA synthesis. To address this question, we first examined the temporal relationship between the defect in viral DNA synthesis and that in MIE transcript accumulation. In this experiment, a reduction in viral DNA synthesis was clearly evident at 32 hpi, and that reduction reached 4-fold by 40 hpi in mutant virus infection (Fig. 4A). In contrast, the difference in MIE expression between wild-type and mutant virus infection was minimal, particularly at 32 hpi (Fig. 4B). This result led us to hypothesize that the lack of pUL21a results in reduced viral DNA synthesis, which is the cause of the reduced late accumulation of IE transcripts.
FIG. 4.
Defects in viral DNA synthesis precede defects in the late accumulation of viral IE transcripts in UL21a deletion virus-infected cells. (A) Analysis of viral DNA synthesis. MRC-5 cells were infected with ADwt or ADsubUL21a at an input genome number equivalent to 0.25 TCID50 unit of wild-type virus/cell. Total intracellular DNA was collected at the indicated times and analyzed by qPCR as described in the legend to Fig. 3C. The normalized amount of viral DNA at 24 hpi during wild-type virus infection was set to 1. (B) Analysis of viral transcript accumulation. MRC-5 cells were infected as described for panel A, total RNA was collected, and amounts of MIE transcript were analyzed by RT-qPCR as described in the legend to Fig. 3B. The normalized amount of viral transcript at 24 hpi during wild-type virus infection was set to 1. Shown are representative results from at least two independent experiments. n.s., P > 0.05; *, P < 0.05 (by Student's t test). Error bars indicate standard deviations.
Inhibition of viral DNA synthesis reduces the late accumulation of IE transcripts in wild-type and UL21a deletion virus-infected cells to equivalent levels.
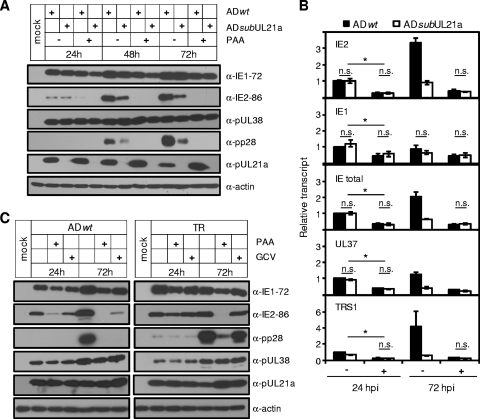
To directly test the hypothesis that the late accumulation of IE transcripts is dependent on UL21a-mediated viral DNA synthesis during HCMV infection, we infected MRC-5 cells with wild-type and UL21a deletion viruses at a high MOI in the presence or absence of the viral DNA synthesis inhibitor phosphonoacetic acid (PAA). The accumulations of IE2-86 and the late protein pp28 were reduced to undetectable levels in both wild-type and mutant virus-infected cells in the presence of PAA, particularly at 48 and 72 hpi (Fig. 5A). Conversely, the expression levels of IE1-72 and early proteins pUL38 and pUL21a were minimally affected. Therefore, the efficient accumulation of IE2-86 protein at late times is dependent on viral DNA synthesis. This effect of viral DNA synthesis on late IE2 expression occurred primarily at the transcriptional level, as PAA reduced the accumulation of IE2 transcript by ∼4- and ∼9-fold during wild-type virus infection at 24 and 72 hpi, respectively (Fig. 5B). Importantly, IE2 was not the only IE gene whose transcript accumulation was affected by inhibition of viral DNA synthesis. This effect was global, as PAA also markedly reduced transcript accumulation of two other IE genes, UL37 and TRS1. As the effect of PAA on IE expression was evident as early as 24 hpi in infection at a high MOI, it was unlikely the result of reduced secondary infection. Moreover, PAA reduced all the IE transcripts examined to equivalent levels in wild-type and UL21a deletion virus infections, even at 72 hpi, when the difference in IE expression between these two virus infections is most notable (Fig. 5B). Collectively, these results suggest that the reduced late IE transcript accumulation in the absence of UL21a is a consequence of reduced viral DNA synthesis.
FIG. 5.
Inhibition of viral DNA synthesis reduces the late expression of IE genes in wild-type and UL21a deletion virus-infected cells to equivalent levels. (A) Analysis of viral protein accumulation in the presence of PAA. MRC-5 cells were infected with ADwt or ADsubUL21a at an input genome number equivalent to 3 TCID50 units of wild-type virus/cell in the presence or absence of PAA (100 μg/ml). Cells were collected at the indicated times, and total cell lysate was analyzed by immunoblotting with the indicated antibodies. (B) Analysis of viral transcript accumulation. MRC-5 cells were infected in the presence (+) or absence (−) of PAA as for panel A, total RNA was collected, and selected IE transcripts were analyzed by RT-qPCR and normalized to GAPDH. The normalized amount of viral transcript at 24 hpi during wild-type virus infection was set to 1. n.s., P > 0.05; *, P < 0.05 (by Student's t test). Error bars indicate standard deviations. (C) Viral protein accumulation in the presence of PAA or ganciclovir (GCV) during infection of HCMV strains AD169 and TR. MRC-5 cells were infected with ADwt (left panel) or TR (right panel) at an MOI of 2 with addition of no drug, PAA (100 μg/ml), or GCV (30 μg/ml). Cells were harvested at the indicated times and analyzed by immunoblotting with the indicated antibodies. Shown are representative results from at least two independent experiments.
Finally, we analyzed viral gene expression in the presence of an additional viral DNA synthesis inhibitor, ganciclovir (GCV), to rule out any potential off-target effects of PAA. In addition, we also examined the gene expression profile of an HCMV clinical isolate to determine whether viral DNA synthesis-dependent late IE gene expression was limited to the laboratory strains of HCMV. MRC-5 cells were infected with either ADwt (Fig. 5C, left panel) or the BAC-derived clinical HCMV isolate TR (Fig. 5C, right panel) and treated with PAA or GCV. As expected, neither drug reduced the levels of the early proteins pUL38 and pUL21a in either viral strain. Importantly, both PAA and GCV significantly reduced the level of IE2-86 and the late protein pp28 in cells infected with ADwt, indicating that reduced IE2-86 levels were not due to any potential off-target effects of PAA (Fig. 5C). During TR infection, GCV had a minimal effect on viral protein expression at all times examined. This is consistent with the fact that TR was isolated from drug-resistant patient with advanced HIV with CMV retinitis (35, 61), though this has not been previously shown in tissue culture. Nonetheless, PAA reduced the accumulation of IE2-86 and pp28 at 72 hpi in TR virus-infected cells, indicating that DNA synthesis-dependent late IE gene expression was not strain specific.
The defect of UL21a deletion virus is due to the loss of the protein product pUL21a.
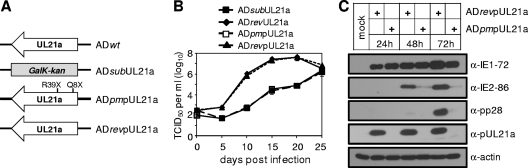
Previously, using various recombinant viruses, we were able to show that the defect of the UL21a deletion virus was due to the loss of the UL21a gene but not the overlapping UL21 ORF or altered regulation of the neighboring genes (10). However, it is formally possible that the defect of the mutant virus was due to other, unidentified functional products or DNA sequence within the UL21a gene rather than the identified protein product, pUL21a. To rule out this possibility, we created a recombinant virus, ADpmpUL21a, in which we introduced two stop codon point mutations at residues Gln8 and Arg39 of the UL21a ORF to abrogate the expression of pUL21a (Fig. 6A). In addition, we created a specific marker-rescued virus for this mutant, ADrevpUL21a (Fig. 6A). We included two stop codon mutations, as the virus was able to produce a truncated protein when only one stop codon mutation was inserted at Gln8 (data not shown). ADpmpUL21a did not express pUL21a (Fig. 6C) and grew indistinguishably from ADsubUL21a in a low-MOI growth curve in MRC-5 cells, while the growth of ADrevpUL21a was nearly identical to that of ADrevUL21a, which was previously shown to grow like the wild type (Fig. 6B) (10). Additionally, when infection was with equal input genome number equivalents, ADpmpUL21a produced markedly reduced amounts of IE2-86 protein compared to the marker-rescued virus in a manner indistinguishable from that of the UL21a deletion virus (Fig. 6C). We conclude that the phenotype of the UL21a deletion virus is due to the loss of pUL21a and not to the loss of any other, unidentified functional gene products or DNA sequence.
FIG. 6.
The growth defect of UL21a deletion virus is due to the loss of the protein product pUL21a. (A) Diagram of the UL21a locus in UL21a deletion virus (ADsubUL21a), a point mutant virus in which two stop codon mutations were introduced into the UL21a ORF (ADpmpUL21a), and its marker-rescued virus (ADrevpUL21a). (B) Growth kinetic analysis of recombinant HCMV viruses. MRC-5 cells were infected with the indicated viruses at an MOI of 0.01, cell-free viruses were collected at different days postinfection, and their yields were determined by TCID50 assay. Error bars indicate standard deviations. (C) Analysis of viral protein accumulation. MRC-5 cells were infected with ADpmpUL21a or ADrevpUL21a at an input genome number equivalent to 0.25 TCID50 unit of wild-type virus/cell, cells were collected at the indicated times, and total lysate was analyzed by immunoblotting. Shown are representative results from at least two independent experiments.
De novo expression of pUL21a is necessary and sufficient for efficient viral gene expression.
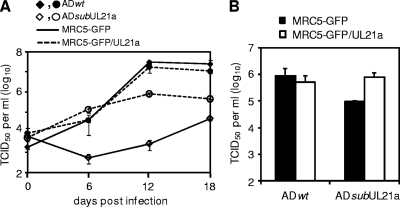
pUL21a may facilitate viral DNA synthesis directly or it may be required for the proper composition of the virion, enabling it to initiate efficient viral DNA synthesis. To differentiate these potential mechanisms, we first attempted to create an inducible expression system to express pUL21a. Multiple approaches were used, including expressing pUL21a within the virus from a tetracycline-inducible promoter or tagging pUL21a with a destabilization domain, ddFKBP (12, 50). However, neither system was able to efficiently regulate the production of pUL21a, possibly due to the highly unstable nature of the protein (data not shown) (10). Instead, we developed a complementation system in which we expressed a functional GFP/UL21a fusion protein (10) in MRC-5 cells by retroviral transduction. The GFP/UL21a fusion protein was abundantly expressed in these cells and appeared as two bands (see Fig. 8B), possibly due to partial degradation or posttranslational modification as we previously reported (10). Overexpression of the GFP/UL21a protein (pGFP/UL21a) complemented growth of the UL21a deletion virus by greater than 200-fold (Fig. 7A). A difference of 10- to 20-fold in the titer of progeny virus remained between ADwt and ADsubUL21a at 12 to 18 dpi when they were grown in pGFP/UL21a-expressing cells. One likely reason for this difference is that we determined the titer of progeny virus in normal fibroblasts in which the mutant virus had reduced infectivity (Fig. 1B). To test this hypothesis, we determined the titer of wild-type and mutant viruses on cells expressing GFP/UL21a or on control cells expressing GFP only. When the titer of virus collected from pGFP/UL21a-expressing cells at 12 dpi was determined on noncomplementing GFP-expressing cells, there was a 10-fold difference in titer between ADwt and ADsubUL21a, similar to that on untransduced cells (Fig. 7A and B). When the titers of these viruses were determined on GFP/UL21a cells, there was no difference between ADwt and ADsubUL21a (Fig. 7B). Thus, we restored the ability of the mutant virus to replicate using pGFP/UL21a-expressing complementing cells.
FIG. 7.
Expression of pUL21a in trans complements the growth of UL21a deletion virus. MRC-5 cells were transduced with retrovirus expressing GFP (MRC5-GFP) or expressing the GFP/UL21a fusion protein (MRC5-GFP/UL21a) and then infected with ADwt and ADsubUL21a at an MOI of 0.2. Cell-free viruses were collected at different days postinfection, and their yields were determined by TCID50 assay in HFFs (A). In addition, the titers of ADwt and ADsubUL21a collected at 12 dpi from MRC5-GFP/UL21a cells were also determined on both MRC5-GFP and MRC5-GFP/UL21a cells to compare their titers measured on complementing and noncomplementing cells (B). Error bars indicate standard deviations.
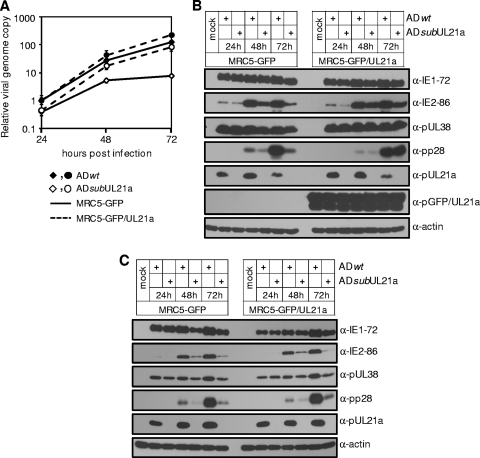
To test the hypothesis that de novo expression of pUL21a facilitates efficient viral replication, we performed two distinct experiments. First, we infected MRC-5 cells expressing either GFP or GFP/UL21a with wild-type or UL21a deletion virus that was produced from normal MRC-5 cells. The expression of pGFP/UL21a but not GFP restored the ability of the UL21a deletion virus to efficiently synthesize viral DNA and produce IE2-86 and pp28, particularly at late times postinfection (Fig. 8A and B).
FIG. 8.
De novo expression of pUL21a is necessary and sufficient for efficient viral DNA synthesis and late accumulation of viral proteins. (A and B) Viral DNA synthesis (A) and protein accumulation (B) in GFP- or GFP/UL21a-expressing cells infected with recombinant virus that is generated in noncomplementing cells. MRC5-GFP cells or MRC5-GFP/UL21a cells were infected with ADwt and ADsubUL21a viruses at an input genome number equivalent to 2 TCID50 units of wild-type virus/cell. Infected cells were collected at the indicated times and analyzed for viral DNA by qPCR (A) and for viral proteins by immunoblotting (B). (C) Viral protein accumulation in normal MRC-5 cells infected with recombinant virus that is generated in GFP- or GFP/UL21a-expressing cells. Normal MRC-5 cells were infected with ADwt or ADsubUL21a virus that was produced from either GFP (MRC5-GFP)- or GFP/UL21a (MRC5-GFP/UL21a)-expressing cells at an input genome number equivalent to 2 TCID50 units of wild-type virus/cell. Infected cells were collected at the indicated times and analyzed for viral proteins by immunoblotting. Shown are representative results from at least two independent experiments. Error bars indicate standard deviations.
In the second experiment, we infected normal MRC-5 cells with wild-type or UL21a deletion virus that was produced from GFP or pGFP/UL21a-expressing cells. We found that even when UL21a deletion virus was produced on complementing cells, it still expressed IE2-86 and pp28 at markedly reduced levels similar to those for the mutant virus that was produced on GFP control cells (Fig. 8C). Therefore, pUL21a is unlikely to be required for the functionality of HCMV virions. Together, these two sets of experiments suggest that the de novo expression of pUL21a is both necessary and sufficient for efficient viral lytic replication.
Collectively, our data indicate that de novo pUL21a expression facilitates HCMV DNA synthesis, leading to efficient late expression of viral IE genes.
DISCUSSION
In a previous study, we found that the HCMV gene UL21a encodes a small, 15-kDa protein, pUL21a, which localizes predominantly to the cytoplasm (10). The UL21a deletion virus has an elevated virion particle-to-infectious virus ratio, suggesting that UL21a is required for HCMV to establish an efficient productive infection (10). In the present study we determined why the mutant virus was unable to establish a productive infection by performing infection analysis using equal numbers of DNA-containing virion particles instead of infectious particles. The UL21a deletion virus entered host cells and initiated its gene expression program like the wild-type virus. However, by 48 to 72 hpi the mutant virus failed to synthesize its DNA efficiently and expressed its IE genes at significantly reduced levels, even though the IE1-72 transcript and protein were not affected (Fig. 3).
Analysis of the UL21a mutant virus infection also provided evidence to clarify the relationship of viral DNA synthesis and late accumulation of viral IE gene products. The immediate-early expression of the viral IE2-86 protein is required for early gene expression and viral DNA synthesis (18, 29, 57). However, two earlier studies have also suggested that the late accumulation of the IE2-86 protein and transcript is dependent on viral DNA synthesis (62, 68). Stenberg and coworkers showed that the late IE2-86 accumulation was eliminated in a temperature-sensitive mutant of HCMV which could not synthesize viral DNA at the restrictive temperature (62). Nonetheless, this mutation in the viral genome has not been mapped, leaving open the possibility that an additional defect prior to DNA synthesis controls the late IE2-86 accumulation. Tenney and Colberg-Poley showed that the late accumulation of the IE2-86-specific transcript was sensitive to the viral DNA synthesis inhibitor phosphonoformic acid (PFA) (68). Nonetheless, it was unclear whether viral DNA synthesis promotes the late expression of only IE2 or the entire IE gene class. In our study, the reduced levels of IE proteins, including IE2-86, seen in UL21a mutant virus were not detected until after 24 hpi, a time when viral DNA synthesis has already initiated. In fact, we could detect reduced DNA synthesis before reduced IE transcript accumulation in mutant virus infection (Fig. 4). Importantly, PAA reduced the expression of IE genes in wild-type and UL21a deletion virus-infected cells to equivalent levels (Fig. 5). Collectively, these data strongly suggests that pUL21a facilitates viral DNA synthesis, which then enhances expression of IE genes, including IE2, at late times during infection.
Inhibition of viral DNA synthesis also reduced the expression of additional IE genes, such as total MIE, TRS1, and UL37, but it had a much smaller or negligible effect on expression of IE1 and the true early gene UL38 (Fig. 5). Our result for TRS1 expression is consistent with previous reports that the accumulation of TRS1 transcript and protein dramatically increases between 24 and 48 hpi (30, 36). Our result for UL37 expression is somewhat different from an earlier report showing no detectable reduction of UL37 transcript levels in the presence of PFA (68). The reason for this discrepancy is unclear, but it is possibly due to different assays used in these studies. We used the more sensitive RT-qPCR method instead of Northern blotting for transcript analysis. Moreover, we used GAPDH instead of total RNA as an internal control, as the GAPDH transcript remains constant throughout infection (33, 47) and is a widely used control for qPCR analysis of HCMV infection (22, 53). Nonetheless, to our knowledge, this is the first report showing that the efficient transcript accumulation of multiple IE genes, in addition to IE2, is dependent on viral DNA synthesis.
Why does inhibition of viral DNA synthesis by PAA treatment or infection in the absence of pUL21a have such a drastic effect on IE2 expression but have little-to-negligible effect on IE1 expression (Fig. 5B)? It has clearly been shown by several groups that IE1 and IE2 proteins accumulate with different kinetics during the viral life cycle. Despite being produced from the same pre-mRNA, IE1 accumulates largely during the very early stages of infection, whereas IE2 accumulates primarily between 24 and 72 hpi, and its accumulation is dependent on DNA synthesis (62, 68) (Fig. 5B). These data suggest that the onset of viral DNA synthesis triggers a viral event that results in a switch from producing IE1 to IE2 transcript. This could be due to alternative splicing or altered stability of one of the MIE transcripts. Interestingly, it has been reported that CDK inhibitors reverse the accumulation of IE1 and IE2 at early stages of infection (55), suggesting that CDK activity may be promoting IE1 accumulation early during infection. CDKs appeared to accomplish this by regulating splicing, as the inhibitors had a similar effect on another spliced IE transcript, UL37, but not on unspliced viral genes (55). It is intriguing to speculate that viral DNA synthesis may somehow alter one or more CDK activities, which results in a change of the splicing pattern of the MIE transcript and the switch from IE1 to IE2 production.
How does pUL21a augment viral DNA synthesis? Our analysis of the mutant virus infection in complementing cells indicates that that de novo expression of pUL21a is necessary and sufficient for the establishment of a productive infection (Fig. 8). Moreover, the mutant virus was competent for entry and initiation of gene expression (Fig. 2 and 3). These results, together with the facts that pUL21a is undetectable in the HCMV virion and does not localize to cytoplasmic viral assembly centers (10), argue strongly against its potential involvement in virion assembly. pUL21a has a diffuse cytoplasmic distribution in both infected and overexpressing cells and shares no homology to any known DNA replication proteins. Furthermore, it is not expressed from genomic loci required for transient complementation of HCMV oriLyt-dependent replication (39), suggesting that this protein is unlikely to act directly as a core DNA replication enzyme to synthesize viral DNA. pUL21a may play other roles, for instance, by increasing concentrations of deoxynucleoside triphosphates (dNTPs) within infected cells, enhancing accumulation of viral and cellular DNA replication enzymes required for viral DNA synthesis, shuttling critical factors in or out of the nucleus, or blocking cellular antiviral defenses against viral DNA synthesis. We are currently testing these potential roles for pUL21a, identifying viral or cellular factors with which it may interact, and investigating the mechanistic basis of its function.
Little is known about regulation of immediate-early gene expression by viral DNA synthesis in herpesvirus infection. It is well understood that the expression of subsets of early (β2, delayed-early) and late (γ1, early-late) viral genes is augmented by viral DNA replication during lytic infection of herpesviruses (48). Early evidence suggested that expression of the IE2 gene could be also augmented by viral DNA synthesis in HCMV infection (62, 68). In addition, the expression of the IE1 protein in MCMV infection and the expression of an IE protein, ICP-27, during herpes simplex virus type 1 (HSV-1) infection of trigeminal ganglion and rat superior cervical ganglia neurons have also been shown to be enhanced by viral DNA synthesis (26, 38, 45). For HSV-1, the studies led to the hypothesis that DNA synthesis represents a critical decision point in the choice between latent and lytic infection. In neurons, HSV-1 expresses limited IE genes during the initial phase of the infection. If robust viral DNA synthesis follows, the accumulation of the IE genes is dramatically increased, allowing the virus to establish a lytic infection. Conversely, such as in the case of a thymidine kinase (TK) mutant or in the presence of PAA, viral DNA synthesis is inhibited, no additional IE genes are produced, and the virus will establish a latent infection (26, 38). It is intriguing to speculate that DNA synthesis may also be critical for HCMV to establish a latent or lytic infection in appropriate cell types, such as in CD34+ cells or undifferentiated THP-1 cells. When these cells were treated with histone deacetylase (HDAC) inhibitors, HCMV initiated IE1-72 expression and progressed to early gene expression, but the infection aborted prior to late gene expression (51, 53). Thus, in addition to a block at IE gene expression, HCMV perhaps is also unable to initiate efficient viral DNA replication in these cells, both of which may contribute to the establishment of its latency.
Finally, it will be important to identify the function(s) of the late-accumulating IE proteins, particularly the IE2-86 protein. It is clear that expression of IE2-86 at immediate-early times is essential for early viral gene expression and viral DNA synthesis in HCMV infection (18, 29, 57). Almost paradoxically, the greatest increase in IE2-86 accumulation comes at late times of infection, and it is not entirely clear what, if any, role this late-accumulating IE2-86 protein plays during viral replication. It has been shown that IE2-86 is involved in autorepression of the MIEP during the late stages of infection by its interactions with histone-modifying enzymes (46). Mutation within the cis repression sequence (crs) in the MIEP increases the abundance of both IE transcripts and protein at late times of infection (46). However, mutations within the crs also resulted in defects in the early events of viral infection, including reduced MIE gene expression and viral DNA replication, thus complicating the analysis defining the precise role of this IE autorepression during HCMV infection (21). Another potential function for the newly synthesized IE2-86 is to transactivate the late promoters of HCMV. Late promoters remain largely associated with repressive chromatin marks and only begin to become acetylated starting at 24 hpi (6, 16). Binding of IE2-86 to HDACs 1, 2, and 3 and multiple histone methyltransferases (HMTs) during the late stages of infection may activate transcription from late promoters (37, 40, 46). However, it is also possible that IE2-86 transactivates late promoters through its interactions with many other viral and cellular partners or that the late-accumulating IE2-86 is responsible for one of the many other functions attributed to this protein (65). In any event, it is sensible for the virus to couple DNA synthesis with expression of transactivator proteins that are required for late gene expression. This will ensure that late proteins, such as capsid and tegument proteins, will accumulate along with viral DNA synthesis, enhancing the production of infectious viral particles and facilitating the ability of the virus to spread to new host cells.
Acknowledgments
We thank Herbert Virgin and the members of his laboratory for helpful discussions and invaluable advice, Caroline Kulesza (University of Colorado School of Medicine) for the nuclear fractionation protocol, Thomas Shenk for the antibodies, and members of the Yu lab for critical reading of the manuscript.
This study was supported by a Public Health Service grant (R01 CA120768) and in part by a grant from the American Heart Association (09GRNT2290199) and a grant from Children's Discovery Institute at Washington University. D.Y. holds an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund. A.R.F. was a Morse/Berg Fellow of the Department of Molecular Microbiology, Washington University School of Medicine, and is supported by Institutional Training Grant T32-AI007172.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. U. S. A. 101:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chee, M. S., S. C. Satchwell, E. Preddie, K. M. Weston, and B. G. Barrell. 1990. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature 344:774-777. [DOI] [PubMed] [Google Scholar]
- 4.Child, S. J., M. Hakki, K. L. De Niro, and A. P. Geballe. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Child, S. J., S. Jarrahian, V. M. Harper, and A. P. Geballe. 2002. Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus. J. Virol. 76:4912-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuevas-Bennett, C., and T. Shenk. 2008. Dynamic histone H3 acetylation and methylation at human cytomegalovirus promoters during replication in fibroblasts. J. Virol. 82:9525-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan, A., C. Cunningham, R. D. Hector, A. F. Hassan-Walker, L. Lee, C. Addison, D. J. Dargan, D. J. McGeoch, D. Gatherer, V. C. Emery, P. D. Griffiths, C. Sinzger, B. P. McSharry, G. W. Wilkinson, and A. J. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85:1301-1312. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn, W., P. Trang, Q. Zhong, E. Yang, C. van Belle, and F. Liu. 2005. Human cytomegalovirus expresses novel microRNAs during productive viral infection. Cell. Microbiol. 7:1684-1695. [DOI] [PubMed] [Google Scholar]
- 10.Fehr, A. R., and D. Yu. 2010. Human cytomegalovirus gene UL21a encodes a short-lived cytoplasmic protein and facilitates virus replication in fibroblasts. J. Virol. 84:291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gawn, J. M., and R. F. Greaves. 2002. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J. Virol. 76:4441-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass, M., A. Busche, K. Wagner, M. Messerle, and E. M. Borst. 2009. Conditional and reversible disruption of essential herpesvirus proteins. Nat. Methods 6:577-579. [DOI] [PubMed] [Google Scholar]
- 13.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72:366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grey, F., A. Antoniewicz, E. Allen, J. Saugstad, A. McShea, J. C. Carrington, and J. Nelson. 2005. Identification and characterization of human cytomegalovirus-encoded microRNAs. J. Virol. 79:12095-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groves, I. J., M. B. Reeves, and J. H. Sinclair. 2009. Lytic infection of permissive cells with human cytomegalovirus is regulated by an intrinsic ‘pre-immediate-early’ repression of viral gene expression mediated by histone post-translational modification. J. Gen. Virol. 90:2364-2374. [DOI] [PubMed] [Google Scholar]
- 17.Hakki, M., E. E. Marshall, K. L. De Niro, and A. P. Geballe. 2006. Binding and nuclear relocalization of PKR by human cytomegalovirus TRS1. J. Virol. [DOI] [PMC free article] [PubMed]
- 18.Heider, J. A., W. A. Bresnahan, and T. E. Shenk. 2002. Construction of a rationally designed human cytomegalovirus variant encoding a temperature-sensitive immediate-early 2 protein. Proc. Natl. Acad. Sci. U. S. A. 99:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heineman, T. (ed.). 2007. Human cytomegalovirus vaccines. Cambridge University Press, Cambridge, United Kingdom. [PubMed]
- 20.Hwang, J., and R. F. Kalejta. 2007. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp71 protein in human cytomegalovirus-infected cells. Virology 367:334-338. [DOI] [PubMed] [Google Scholar]
- 21.Isomura, H., M. F. Stinski, A. Kudoh, S. Nakayama, T. Murata, Y. Sato, S. Iwahori, and T. Tsurumi. 2008. A cis element between the TATA box and the transcription start site of the major immediate-early promoter of human cytomegalovirus determines efficiency of viral replication. J. Virol. 82:849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juckem, L. K., K. W. Boehme, A. L. Feire, and T. Compton. 2008. Differential initiation of innate immune responses induced by human cytomegalovirus entry into fibroblast cells. J. Immunol. 180:4965-4977. [DOI] [PubMed] [Google Scholar]
- 23.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 24.Khanna, R., and D. J. Diamond. 2006. Human cytomegalovirus vaccine: time to look for alternative options. Trends Mol. Med. 12:26-33. [DOI] [PubMed] [Google Scholar]
- 25.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 26.Kosz-Vnenchak, M., J. Jacobson, D. M. Coen, and D. M. Knipe. 1993. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J. Virol. 67:5383-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulesza, C. A., and T. Shenk. 2004. Human cytomegalovirus 5-kilobase immediate-early RNA is a stable intron. J. Virol. 78:13182-13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landini, M. P., B. Severi, G. Furlini, and L. Badiali De Giorgi. 1987. Human cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65-69 by immunoelectron microscopy. Virus Res. 8:15-23. [DOI] [PubMed] [Google Scholar]
- 29.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall, E. E., C. J. Bierle, W. Brune, and A. P. Geballe. 2009. Essential role for either TRS1 or IRS1 in human cytomegalovirus replication. J. Virol. 83:4112-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mocarski, E. S., G. W. Kemble, J. M. Lyle, and R. F. Greaves. 1996. A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc. Natl. Acad. Sci. U. S. A. 93:11321-11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mocarski, E. S., T. Shenk, and R. F. Pass (ed.). 2007. Cytomegaloviruses, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA.
- 33.Munger, J., S. U. Bajad, H. A. Coller, T. Shenk, and J. D. Rabinowitz. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy, E., I. Rigoutsos, T. Shibuya, and T. E. Shenk. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevels, M., W. Brune, and T. Shenk. 2004. SUMOylation of the human cytomegalovirus 72-kilodalton IE1 protein facilitates expression of the 86-kilodalton IE2 protein and promotes viral replication. J. Virol. 78:7803-7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. U. S. A. 101:17234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1996. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J. Virol. 70:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, J. J., Y. E. Kim, H. T. Pham, E. T. Kim, Y. H. Chung, and J. H. Ahn. 2007. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J. Gen. Virol. 88:3214-3223. [DOI] [PubMed] [Google Scholar]
- 41.Petrik, D. T., K. P. Schmitt, and M. F. Stinski. 2006. Inhibition of cellular DNA synthesis by the human cytomegalovirus IE86 protein is necessary for efficient virus replication. J. Virol. 80:3872-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puchtler, E., and T. Stamminger. 1991. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J. Virol. 65:6301-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian, Z., B. Xuan, T. T. Hong, and D. Yu. 2008. The full-length protein encoded by human cytomegalovirus gene UL117 is required for the proper maturation of viral replication compartments. J. Virol. 82:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 45.Reddehase, M. J., M. R. Fibi, G. M. Keil, and U. H. Koszinowski. 1986. Late-phase expression of a murine cytomegalovirus immediate-early antigen recognized by cytolytic T lymphocytes. J. Virol. 60:1125-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reeves, M., J. Murphy, R. Greaves, J. Fairley, A. Brehm, and J. Sinclair. 2006. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin-remodeling enzymes by IE86. J. Virol. 80:9998-10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinhardt, B., P. Schaarschmidt, A. Bossert, A. Luske, G. Finkenzeller, T. Mertens, and D. Michel. 2005. Upregulation of functionally active vascular endothelial growth factor by human cytomegalovirus. J. Gen. Virol. 86:23-30. [DOI] [PubMed] [Google Scholar]
- 48.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, vol. 2. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 49.Romanowski, M. J., and T. Shenk. 1997. Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1 whose product antagonizes transcriptional activation. J. Virol. 71:1485-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rupp, B., Z. Ruzsics, T. Sacher, and U. H. Koszinowski. 2005. Conditional cytomegalovirus replication in vitro and in vivo. J. Virol. 79:486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saffert, R. T., and R. F. Kalejta. 2007. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J. Virol. 81:9109-9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saffert, R. T., R. R. Penkert, and R. F. Kalejta. 2010. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. J. Virol. 84:5594-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez, V., A. K. McElroy, J. Yen, S. Tamrakar, C. L. Clark, R. A. Schwartz, and D. H. Spector. 2004. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J. Virol. 78:11219-11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez, V., and D. H. Spector. 2008. Subversion of cell cycle regulatory pathways. Curr. Top. Microbiol. Immunol. 325:243-262. [DOI] [PubMed] [Google Scholar]
- 57.Sanders, R. L., C. L. Clark, C. S. Morello, and D. H. Spector. 2008. Development of cell lines that provide tightly controlled temporal translation of the human cytomegalovirus IE2 proteins for complementation and functional analyses of growth-impaired and nonviable IE2 mutant viruses. J. Virol. 82:7059-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders, R. L., C. J. Del Rosario, E. A. White, and D. H. Spector. 2008. Internal deletions of IE2 86 and loss of the late IE2 60 and IE2 40 proteins encoded by human cytomegalovirus affect the levels of UL84 protein but not the amount of UL84 mRNA or the loading and distribution of the mRNA on polysomes. J. Virol. 82:11383-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schleiss, M. R. 2008. Cytomegalovirus vaccine development. Curr. Top. Microbiol. Immunol. 325:361-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva, M. C., Q. C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, I. L., I. Taskintuna, F. M. Rahhal, H. C. Powell, E. Ai, A. J. Mueller, S. A. Spector, and W. R. Freeman. 1998. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch. Ophthalmol. 116:178-185. [DOI] [PubMed] [Google Scholar]
- 62.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenberg, R. M., and M. F. Stinski. 1985. Autoregulation of the human cytomegalovirus major immediate-early gene. J. Virol. 56:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stenberg, R. M., P. R. Witte, and M. F. Stinski. 1985. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J. Virol. 56:665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stinski, M. F., and D. T. Petrik. 2008. Functional roles of the human cytomegalovirus essential IE86 protein. Curr. Top. Microbiol. Immunol. 325:133-152. [DOI] [PubMed] [Google Scholar]
- 66.Stinski, M. F., D. R. Thomsen, R. M. Stenberg, and L. C. Goldstein. 1983. Organization and expression of the immediate early genes of human cytomegalovirus. J. Virol. 46:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenney, D. J., and A. M. Colberg-Poley. 1991. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology 182:199-210. [DOI] [PubMed] [Google Scholar]
- 68.Tenney, D. J., and A. M. Colberg-Poley. 1991. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection. J. Virol. 65:6724-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terhune, S., E. Torigoi, N. Moorman, M. Silva, Z. Qian, T. Shenk, and D. Yu. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J. Virol. 81:3109-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White, E. A., C. L. Clark, V. Sanchez, and D. H. Spector. 2004. Small internal deletions in the human cytomegalovirus IE2 gene result in nonviable recombinant viruses with differential defects in viral gene expression. J. Virol. 78:1817-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White, E. A., C. J. Del Rosario, R. L. Sanders, and D. H. Spector. 2007. The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus. J. Virol. 81:2573-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]