Abstract
Viruses depend on the host cell to provide the energy and biomolecular subunits necessary for production of viral progeny. We have previously reported that human cytomegalovirus (HCMV) infection induces dramatic changes to central carbon metabolism, including glycolysis, the tricarboxylic acid (TCA) cycle, fatty acid biosynthesis, and nucleotide biosynthesis. Here, we explore the mechanisms involved in HCMV-mediated glycolytic activation. We find that HCMV virion binding and tegument protein delivery are insufficient for HCMV-mediated activation of glycolysis. Viral DNA replication and late-gene expression, however, are not required. To narrow down the list of cellular pathways important for HCMV-medicated activation of glycolysis, we utilized pharmaceutical inhibitors to block pathways reported to be both involved in metabolic control and activated by HCMV infection. We find that inhibition of calmodulin-dependent kinase kinase (CaMKK), but not calmodulin-dependent kinase II (CaMKII) or protein kinase A (PKA), blocks HCMV-mediated activation of glycolysis. HCMV infection was also found to target calmodulin-dependent kinase kinase 1 (CaMKK1) expression, increasing the levels of CaMKK1 mRNA and protein. Our results indicate that inhibition of CaMKK has a negligible impact on immediate-early-protein accumulation yet severely attenuates production of HCMV viral progeny, reduces expression of at least one early gene, and blocks viral DNA replication. Inhibition of CaMKK did not affect the glycolytic activation induced by another herpes virus, herpes simplex virus type 1 (HSV-1). Furthermore, inhibition of CaMKK had a much smaller impact on HSV-1 replication than on that of HCMV. These data suggest that the role of CaMKK during the viral life cycle is, in this regard, HCMV specific. Taken together, our results suggest that CaMKK is an important factor for HCMV replication and HCMV-mediated glycolytic activation.
It has long been known that infection with numerous evolutionarily divergent viruses results in a general activation of host cell metabolism (4, 9, 10, 17, 21, 25, 30). Furthermore, this metabolic activation can be clinically helpful. For example, a wide variety of antiviral compounds target specific nucleotide metabolic activities to treat numerous different viral infections, such as those caused by hepatitis B virus, HIV, human cytomegalovirus (HCMV), and herpes simplex virus (HSV) (1, 6, 12, 20). While in some instances these activities have proven to be therapeutically beneficial, the identity of most of the specific metabolic activities induced by viral infection and the mechanisms through which they are activated are unclear. The identification of these activities and their associated mechanisms may highlight novel targets for therapeutic intervention given the viral dependence on the host cell metabolic network for the production of viral progeny.
We have previously found that infection with HCMV induces substantial changes to the host cell metabolic network (22, 23). HCMV is a betaherpesvirus containing a large double-stranded DNA genome (∼240-kb) encoding over 200 open reading frames (ORFs). HCMV prevalence is widespread, and infection rarely causes disease in healthy adults. However, in immunosuppressed populations, such as the elderly, transplant recipients, and cancer patients, HCMV is a substantial cause of morbidity (11, 24). HCMV is also a significant cause of birth defects. Congenital HCMV infection occurs in 1 to 2% of all live births (1), with central nervous system damage occurring in the majority of symptomatic newborns (5, 24).
HCMV infection was previously found to increase the cellular glycolytic rate, i.e., glycolytic flux, as well as to increase the activity of a glycolytic rate-determining enzyme, phosphofructokinase (22, 23). The mechanisms through which HCMV infection mediates these changes are currently unclear. Traditional views of metabolic regulation hold that metabolic pathways are largely regulated by the concentrations of allosteric small-molecule effectors on specific rate-limiting enzymes. While these mechanisms of metabolic control undoubtedly still play a role, it is becoming increasingly apparent that metabolic regulation does not rely on allosteric self-regulation alone. Instead, multiple upstream signal transduction networks, for example the phosphatidylinositol 3-kinase (PI3K)/Akt and Ras pathways, play regulatory roles in the control of central carbon and nitrogen metabolism (reviewed in references 8 and 29). As HCMV infection activates numerous signal transduction pathways (reviewed in reference 40), it is possible that viral induction of upstream signal transduction pathways is responsible for downstream metabolic activation.
Here, we have begun to analyze the mechanisms responsible for HCMV-mediated activation of glycolysis. We find that calcium signal transduction is important for HCMV-mediated activation of glycolysis. Specifically, pharmaceutical inhibition of calmodulin-dependent kinase kinase (CaMKK), but not calmodulin-dependent kinase II (CaMKII) or protein kinase A (PKA), blocks HCMV-induced glycolytic activation. HCMV also appears to target calmodulin-dependent kinase kinase 1 (CaMKK1) expression, as HCMV infection was found to increase its mRNA and protein abundance. Furthermore, inhibition of CaMKK severely attenuated the production of infectious progeny. Our results suggest that CaMKK is an important cellular factor for both HCMV-mediated activation of glycolysis and HCMV replication.
MATERIALS AND METHODS
Cell culture and virus infection.
MRC-5 fibroblasts were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum. Cells were grown to confluence in either 60-mm or 6-well tissue culture plates, resulting in a density of 3.2 × 104 cells per cm2. Once confluent, medium was removed and serum-free medium was added. Cells were maintained in serum-free medium for 24 h before infection.
The HCMV strain used in this study was BADwt, a bacterial artificial chromosome (BAC) clone of Ad169 (39). HSV-1 Kos (ATCC) was used for all HSV-1 infections. Mock-infected controls were treated with an equal volume of medium containing the same serum concentrations as virus-treated cells. Virus adsorptions were carried out for 90 min at 37°C, after which viral inocula were aspirated and serum-free DMEM was added back. In experiments utilizing UV-irradiated virus, the viral inocula were exposed to 254 nm light at 0 or 1,920 mJ/cm2 with a model 2400 Stratalinker UV cross-linker prior to infection. Production of infectious virus was measured by a standard viral plaque assay. Toxicity associated with STO-609 treatment was analyzed by a Live/Dead assay (Marker Gene Technologies) according to the manufacturer's protocol.
MRC-5s expressing a kinase-dead variant of CaMKK1 [CaMKK1-KD(K157A)] were generated by recombinant retroviral transduction. The CaMKK1-KD(K157A)/pME18s construct, which expresses rat CaMKK-KD(K157A), was a kind gift from Thomas Soderling and was previously described (38). CaMKK-KD was PCR amplified from this construct with the primers ATCAGAATTCATGGAGCGCAGTCCAGCCGTCTGCTG and TTATCGAATTCGGATGCAGCCTCATCTTCCTGGACT. The resulting product was digested with EcoRI and inserted in-frame into a modified retroviral transduction vector, pLPCX-Flag, which contains a C-terminal double Flag tag. The pLPCX-Flag vector is modified version of pLPCX (Invitrogen) containing a double Flag tag through ligation of the oligonucleotides AATTCGACTACAAAGACCATGACGGTGATTACAAGGATGACGATGACTAAGC and GGCCGCTTAGTCATCGTCATCCTTGTAATCACCGTCATGGTCTTTGTAGTCG into the EcoRI/NotI sites. Recombinant retroviruses were produced by transfecting pLPCX or pLPCX-CaMKK-KD-Flag into Ampho Phoenix cells by use of Fugene (Roche). The resulting retroviral supernatants were used to transduce MRC-5 cells as previously described (18).
Chemical reagents.
STO-609 (EMD Biosciences), a specific inhibitor of CaMKK, and HA1004 (Biomol), a specific inhibitor of PKA, were maintained in dimethyl sulfoxide (DMSO) at concentrations of 5 mg/ml and 10 mg/ml, respectively, at −20°C. Cycloheximide (EMD Biosciences), an inhibitor of protein synthesis, was maintained in DMSO at a concentration of 10 mg/ml at 4°C. Phosphonoacetic acid (PAA; MP Biomedicals) was maintained in water at a concentration of 20 mg/ml at room temperature.
Kinetic flux profiling.
Labeled DMEM was prepared from medium lacking glucose by adding 10 mM HEPES and either labeled (13C) or unlabeled (12C) glucose to give a final concentration of 4.5 g liter−1. For flux analysis, samples were switched to fresh, unlabeled serum-free medium 24 h and 1 h before final addition of 13C-labeled medium. Samples were labeled for 1 min, and the reaction was quenched by the addition of −80°C 80% methanol and incubated at −80°C for 10 min. Cells were then scraped in the methanol and centrifuged at 3,000 rpm for 5 min at 4°C, and the supernatant was collected. The pellet was extracted twice more in cold 80% methanol, and all supernatants were subsequently pooled. After extraction, the supernatants were dried down under nitrogen gas and resuspended in 175 μl of 50% methanol. Samples were subsequently spun down at 13,000 × g for 5 min at 4°C, and the remaining supernatant was transferred to high-performance liquid chromatography (HPLC) tubes. The accumulation of fully 13C-labeled fructose-bisphosphate was monitored using liquid chromatography-tandem mass spectrometry (LC-MS-MS) as previously described (22).
LC-MS-MS analysis.
LC-MS-MS was performed using an LC-20AD HPLC system (Shimadzu) and a Synergi Hydro-RP column (150 by 2 mm with a 5-μm particle size; Phenomenex) coupled to a mass spectrometer. The LC parameters were as follows: autosampler temperature, 4°C; injection volume, 20 μl; column temperature, 40°C; flow rate, 200 μl/min. The LC solvents were as follows: solvent A, 100% methanol; solvent B, 10 mM tributylamine and 15 mM acetic acid in 97:3 water-methanol. The gradient conditions were as follows: for t = 0 (negative mode), 100% B; for t = 5, 100% B; for t = 10, 80% B; for t = 20, 80% B; for t = 35, 35% B; for t = 38, 5% B; for t = 42, 5% B; for t = 43, 100% B; and for t = 50, 100% B. Mass spectrometric analyses were performed with a TSQ Quantum Ultra triple-quadrupole mass spectrometer (Thermo Fisher Scientific).
Protein analysis.
Proteins from cell lysates were solubilized in disruption buffer (50 mM Tris [pH 7.0], 2% SDS, 5% 2-mercaptoethanol, and 2.75% sucrose), separated by 10% SDS-PAGE, and transferred to nitrocellulose in Tris-glycine transfer buffer. Blots were then stained with Ponceau S to visualize protein bands and ensure equal protein loading. The membranes were blocked in 5% milk in Tris-buffered saline-Tween 20 (TBST), followed by incubation in primary antibody. After subsequent washes, blots were treated with secondary antibody and protein bands were visualized using the enhanced chemiluminescence (ECL) system (Pierce). The antibodies were specific for viral proteins (IE1 [1B12], UL26 [7H19], and pp28 [10B4-29]) and cellular proteins (tubulin [Epitomics] and the calmodulin-dependent kinase kinase alpha subunit [CaMKKα] [rabbit polyclonal {Santa Cruz Biotechnology, Inc.} and mouse monoclonal {Abcam}] antibodies).
Real-time PCR.
At various time points postinfection, medium was aspirated from cells and RNA was prepared using Trizol as recommended by the manufacturer (Invitrogen). cDNA synthesis was performed using the Superscript first-strand reverse transcriptase system, using random hexamers as designated by the manufacturer's protocol (Invitrogen). Additionally, viral and cellular DNA was harvested at various time points postinfection in lysis buffer (100 mM NaCl, 100 mM Tris-HCl, 25 mM EDTA, 0.5% SDS, 0.1 mg/ml proteinase K, and 40 μg/ml RNase A), and viral DNA was quantified using the UL26 primer set (below). Quantitative PCR (qPCR) was performed using Fast SYBR green master mix, a model 7500 Fast real-time PCR system, and Fast 7500 software (Applied Biosystems). Transcripts were quantified with specific primer pairs as follows: for CaMKKI, 5′-TTTGGCGTCAGCAACCAGTTTGAG-3′ (forward) and 5′-TACATCCAAGGCCTTCCCACTGAA-3′ (reverse); for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-CATGTTCGTCATGGGTGTGAACCA-3′ (forward) and 5′-ATGGCATGGACTGTGGTCATGAGT-3′ (reverse); and for UL26, 5′-AACATCGCGTCGGTGATTTCTTGC-3′ (forward) and 5′-ACAGCTACTTTGAAGACGTGGAGC-3′ (reverse).
RESULTS
Time course of HCMV-glycolytic induction.
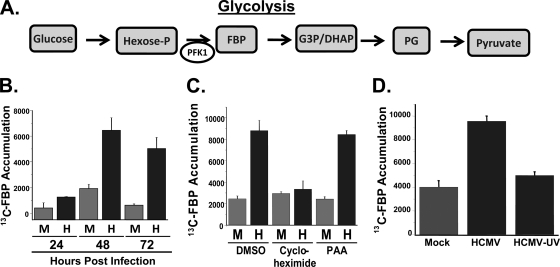
Previously, we have found that at 48 h postinfection (hpi), HCMV activates glycolytic flux and specifically increases the activity of the glycolytic enzyme phosphofructokinase-1 (PFK-1) (22, 23). PFK-1 catalyzes the phosphorylation of fructose-6-phosphate to form fructose-1,6-bisphosphate (FBP) (Fig. 1 A, diagram). This reaction is a key regulatory step of glycolysis that is targeted by diverse signal transduction cascades (reviewed in reference 37). To measure glycolytic activity, 13C-labeled glucose can be utilized as an isotope tracer in which the labeling speed of specific metabolite pools, e.g., FBP, serves as a proxy for metabolic flux (23). To determine how HCMV infection affects the rate of glycolysis over a time course of viral infection, we pulsed HCMV-infected fibroblasts with [13C]glucose and measured the rate of accumulation of 13C-labeled FBP by LC-MS-MS. As shown in Fig. 1B, HCMV infection resulted in only a modest increase in the rate of accumulation of 13C-labeled FBP compared to the level for mock-infected cells at 24 h. However, at 48 h and 72 h, HCMV-infected fibroblasts demonstrated a >3-fold increase in the rate of accumulation of 13C-labeled FBP (Fig. 1B). These time points during infection coincide with a period of intense viral DNA replication and are consistent with a scenario in which virally mediated glycolytic activation provides the increased energy and carbon building blocks for viral replication.
FIG. 1.
Impact of HCMV infection on glycolysis. (A) Representation of the major intermediates of the glycolytic pathway. Through a series of enzymatic reactions, glucose is converted into the final product of glycolysis, pyruvate. Both the conversion of glucose to hexose phosphate and the conversion of hexose phosphate to fructose-1,6-bisphosphate (FBP) are ATP-dependent and are rate-limiting steps of glycolysis. Hexose-P, glucose-6 phosphate and its isomers; G3P/DHAP, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate; PG, phosphorylated glycerate isoforms. (B) Time course of glycolytic flux activation. Serum-starved MRC-5 human fibroblast cells were mock infected (M) or infected with HCMV (H) (MOI = 3). Cells were labeled with [13C]glucose containing medium at 24, 48, and 72 h postinfection for 1 min. After the labeling, metabolism was quenched by the addition of cold 80% methanol. Accumulation of [13C]fructose-1,6-bisphosphate was measured upon [13C]glucose pulse via measurement by LC-MS-MS. Values are means ± standard errors (SE) (n = 4). (C) Serum-starved MRC-5 fibroblasts were mock infected or infected with HCMV (MOI = 3). After adsorption, cells were treated with DMSO, an inhibitor of protein synthesis, cycloheximide (10 μg/ml), or an inhibitor of viral DNA replication, PAA (50 μg/ml). At 48 hpi, cells were labeled with [13C]glucose containing DMEM for 1 min, quenched with cold methanol, and processed for LC-MS-MS analysis. Values are means ± SE (n = 4). (D) Serum-starved MRC-5 fibroblasts were mock infected, HCMV infected, or infected with UV-irradiated HCMV (MOI = 3.0). At 48 hpi, cells were labeled with [13C]glucose containing DMEM for 1 min, quenched with cold methanol, and processed for LC-MS-MS analysis. Values are means ± SE (n = 2).
Protein expression, but not DNA replication, is required for HCMV-induced glycolytic activation.
The mechanisms responsible for HCMV-mediated glycolytic activation are currently unclear. To determine if the earliest events of viral infection, i.e., virion binding, envelope fusion, and tegument protein delivery, are sufficient to activate glycolytic flux in the absence of protein expression, we treated mock- or HCMV-infected cells with cycloheximide and assayed for the rate of [13C]FBP accumulation as described above. We found that cycloheximide addition completely blocked the HCMV-mediated induction of glycolysis, reducing it to mock infection levels (Fig. 1C). This result indicates that virion binding and tegument protein delivery in the absence of protein translation are not sufficient for HCMV-activated glycolytic flux. To further test whether viral gene expression is necessary for HCMV-induced activation of glycolysis, fibroblasts were either mock infected, infected with HCMV (multiplicity of infection [MOI] = 3.0), or infected with an equivalent amount of virus that had been UV irradiated. UV irradiation of HCMV blocked the glycolytic induction associated with HCMV infection, indicating that expression of viral genes is necessary for HCMV-mediated glycolytic activation (Fig. 1D).
To determine if later events during viral infection such as DNA replication and late-gene expression are necessary for virally induced activation of glycolysis, we assayed for activation of glycolysis in the presence of phosphonoacetic acid (PAA), an inhibitor of HCMV DNA replication (15). As shown in Fig. 1C, we find that PAA treatment had no effect on HCMV-induced glycolytic activation. These results suggest that DNA replication and de novo expression of late genes are not required for the glycolytic activation induced by HCMV infection.
Pharmaceutical inhibition of CaMKK, but not CaMKII or PKA, blocks HCMV-induced glycolytic activation.
Numerous HCMV viral factors modulate various cellular signal transduction pathways which, in turn, could affect cellular metabolic activity. For example, HCMV encodes constitutively active G protein-coupled receptors whose activities can result in increased adenylate cyclase activity and calcium mobilization (reviewed in reference 2). This increase in adenylate cyclase activity can, in turn, activate the cyclic AMP (cAMP)-dependent kinase, PKA. Activated PKA can activate glycolysis through CREB-mediated transcriptional induction of glycolytic enzymes, or through activation of PFK-2, which results in direct activation of PFK-1 (Fig. 2 A) (reviewed in references 3 and 28). Similarly, activation of calcium-dependent kinases can also result in both CREB activation and activation of specific glycolytic activities (3, 26, 32, 36). To determine if PKA and calcium signaling are important for HCMV-mediated activation of glycolysis, we utilized various inhibitors to target their activity with a subsequent analysis of HCMV-induced glycolytic activation. We found that treatment with HA1004, an inhibitor of PKA (13), had no effect on the rate of accumulation of 13C-labeled FBP in either mock- or HCMV-infected cells compared to the level for DMSO-treated controls (Fig. 2B). To determine whether calcium signaling may be important for HCMV-mediated activation of glycolysis, we treated cells with STO-609, a specific inhibitor of CaMKK (35), and with KN-62, a CaMKII inhibitor (34). CaMKK is an upstream kinase in the calcium-calmodulin kinase cascade, e.g., phosphorylating and activating CaMKI and CaMKIV (33). CaMKII, however, has been reported to autophosphorylate in response to increased calcium levels, thereby making CaMKII independent of CaMKK activity (33). Treatment with KN-62 had no effect on the rate of [13C]FBP accumulation during HCMV infection, suggesting that HCMV-mediated activation of glycolysis is independent of CaMKII (Fig. 2B). Surprisingly, treatment with the CaMKII inhibitor activated glycolysis in mock-infected cells (Fig. 2B). In contrast to what was observed for inhibitors of CaMKII and PKA, treatment of cells with STO-609 completely blocked the glycolytic activation induced by HCMV infection but had no impact on the glycolytic rate of mock-infected cells (Fig. 2B). Our results indicate that pharmaceutical inhibition of CaMKK blocks HCMV-induced glycolytic activation but that inhibition of CaMKII or PKA does not.
FIG. 2.
(A) Regulation of glycolysis by selected kinase cascades. PKA, CaMKK, and CaMKII can activate glycolysis transcriptionally through CREB activation or through direct/indirect phosphorylation/activation of glycolytic regulatory enzymes, e.g., PFK-2 or GAPDH. (B) Pharmaceutical inhibition of CaMKK blocks HCMV-mediated glycolytic activation. Serum-starved MRC-5 human fibroblasts were mock infected or infected with HCMV (MOI = 3). After adsorption, cells were treated with the CaMKK inhibitor STO-609 (10 μg/ml), the PKA inhibitor HA1004 (10 μg/ml), the CaMKII inhibitor KN-62 (7 μg/ml), or DMSO alone. At 48 h postinfection, cells were labeled for 1 min with [13C]glucose containing DMEM, quenched with cold methanol, and processed for LC-MS-MS to measure the accumulation of [13C]fructose-1,6-bisphosphate. Values are means ± SE (n = 4).
Pharmaceutical inhibition of CaMKK attenuates the production of HCMV viral progeny.
Given that pharmaceutical inhibition of CaMKK blocks viral activation of glycolysis, we were interested in determining how CaMKK inhibition affected viral replication. Toward this end, we infected fibroblasts with HCMV (MOI = 3.0), treated them with either DMSO or 5 or 10 μg/ml STO-609, and harvested them at various time points postinfection for analysis of the production of viral progeny. As shown in Fig. 3 A, treatment with the CaMKK inhibitor resulted in a dose-dependent decrease in the production of infectious virions at multiple time points of infection. By 120 h postinfection, treatment with 5 and 10 μg/ml STO-609 resulted in >10- and >100-fold defects, respectively (Fig. 3A). These results suggest that in addition to inhibiting viral induction of glycolysis, treatment with a CaMKK inhibitor attenuates HCMV replication.
FIG. 3.
Pharmaceutical inhibition of CaMKK attenuates HCMV viral replication. (A) Serum-starved MRC-5 human fibroblasts were infected with HCMV (MOI = 3). After adsorption, cells were treated with the CaMKK-specific inhibitor STO-609 at concentrations of 5 μg/ml or 10 μg/ml or with DMSO alone. Cells were harvested at 24, 72, and 120 h postinfection, and the production of infectious progeny was measured by a plaque assay. Values are means ± SE (n = 2). Points marked with an asterisk were below the limit of detection (∼20 PFU/ml). (B) Analysis of the potential toxicity of STO-609 treatment. Confluent MRC-5 fibroblasts were mock infected or infected with HCMV (MOI = 3). After adsorption, cells were treated with 10 μg/ml STO-609. At 72 h postinfection, cell viability was measured via a Live/Dead cell viability assay. Green indicates the presence of esterase activity associated with viable cells, while red indicates loss of cellular membrane integrity associated with cell death. As a positive control for staining of a breakdown in membrane integrity, cells were treated with ethanol. The images were obtained by inverted fluorescence microscopy.
In our studies, we had not seen any detectable cell death associated with treatment of mock- or HCMV-infected cells with STO-609; however, we wanted to explore this potential issue further. We treated cells with 10 μg/ml STO-609 and assayed for their viability utilizing the Live/Dead assay, which stains live cells based on a their esterase activity (green) and stains the nucleic acids of dead cells (red) based on the breakdown of membrane integrity. A representative field of cells from the Live/Dead staining is shown in Fig. 3B. Over 15 fields for each condition were counted for green versus red staining, with the percentage of viable cells over 95% for each condition, i.e., >95% of cells exhibited green staining and excluded red staining (Fig. 3B). These data indicate that treatment of mock- or HCMV-infected cells with STO-609 does not induce cellular toxicity.
Impact of CaMKK inhibition on the HCMV viral life cycle.
To further investigate the role that CaMKK plays in viral events, Western blot analysis was performed to observe the effect of CaMKK inhibition on the accumulation of immediate-early (IE1), early (UL26), and late (pp28) proteins throughout infection. Fibroblasts were HCMV infected, treated with STO-609, and harvested at 24, 48, or 72 h postinfection for Western blot analysis. Blots were probed with mouse monoclonal antibodies specific for IE1, UL26, and pp28 as well as a rabbit monoclonal antibody specific for tubulin. Treatment with STO-609 at 5 or 10 μg/ml appeared to have little effect at 24 or 48 h postinfection on the accumulation of the immediate-early protein IE1 (Fig. 4 A). STO-609 treatment at either 5 or 10 μg/ml substantially reduced the accumulation of the early viral protein UL26 relative to the level for the DMSO control at both 48 and 72 h postinfection (Fig. 4A). Decreases were also evident for late genes, as accumulation of pp28 was markedly reduced at 48 and 72 h postinfection at each concentration tested (Fig. 4A). These findings indicate that inhibition of CaMKK does not appear to affect immediate-early-gene accumulation but substantially reduces the accumulation of two proteins representative of early and late kinetic classes. Given that a number of early genes participate in viral DNA replication, a defect in the accumulation of early genes would likely result in a decrease in viral DNA replication. To test this possibility, we utilized quantitative PCR to analyze the replication of viral DNA in the presence of the CaMKK inhibitor. As shown in Fig. 4B, treatment of virally infected cells with STO-609 severely attenuated viral DNA replication. No increase in viral DNA was detectable from 48 to 96 h upon STO-609 treatment, in contrast to the normal increase in viral DNA accumulation apparent upon carrier treatment (DMSO) (Fig. 4B). Our analysis of viral protein and DNA accumulation upon pharmaceutical inhibition suggests that the CaMKK inhibition affects the viral life cycle between the immediate-early-to-early transition and blocks DNA synthesis. The inhibition of viral DNA synthesis could potentially result from the impact of attenuated early-gene expression that occurs when CaMKK is inhibited.
FIG. 4.
Impact of CaMKK pharmaceutical inhibition on viral protein and DNA accumulation. (A) Analysis of the impact of CaMKK inhibition on viral protein accumulation. Serum-starved MRC-5 human fibroblasts were mock infected or infected with HCMV (MOI = 3). After adsorption, cells were treated with the CaMKK-specific inhibitor STO-609 (5 μg/ml or 10 μg/ml) or DMSO alone. Cells were harvested at 24, 48, and 72 h postinfection and analyzed by Western blotting with antisera specific for IE1, UL26, pp28, and tubulin. Mw, molecular mass. (B) Analysis of the impact of CaMKK inhibition on viral DNA accumulation. Serum-starved MRC-5 cells were mock infected or infected with HCMV (MOI = 3). After adsorption, cells were treated with the CaMKK-specific inhibitor STO-609 (10 μg/ml) or DMSO. Viral DNA was extracted from cells that were harvested at 48, 72, and 96 h postinfection and processed for qPCR analysis of viral DNA accumulation. Values are means ± SE (n = 6). (C) Serum-starved MRC-5 human fibroblasts were HCMV infected (MOI = 3). After adsorption, two sets of cells were treated with STO-609 (10 μg/ml) or DMSO, as indicated on the graph. The last set of MRC-5s was treated with STO-609 (10 μg/ml) at 24 hpi and harvested at 96 hpi with the rest of the treatments. Viral replication was measured by a plaque assay. Values are means ± SE (n = 2). An asterisk indicates that the number of infectious virions were below the limit of detection (∼20 PFU/ml). (D) Serum-starved MRC-5 human fibroblasts were mock or HCMV infected (MOI = 3). After adsorption, fresh serum-free medium was placed on the cells. At 24 h postinfection, cells were treated with either DMSO or STO-609. Cells were pulsed at 48 or 72 h postinfection for 1 min with [13C]glucose containing DMEM, quenched with cold methanol, and processed for LC-MS-MS to measure the accumulation of [13C]fructose-1,6-bisphosphate. Values are means ± SE (n = 2).
As CaMKK inhibition appeared to affect the viral life cycle between the immediate-early-to-early transition, we wanted to test if viral replication was sensitive to STO-609 treatment at this junction, i.e., at 24 h postinfection, when DNA synthesis is just beginning. As shown in Fig. 4C, addition of STO-609 at 24 h postinfection largely rescued HCMV viral growth, although there was still an ∼1.5-log defect compared to the level for the DMSO-treated control. This suggests that a large proportion of the inhibitory effect of STO-609 treatment on HCMV infection acts prior to 24 h postinfection. Given this result, we were interested to determine how STO-609 treatment at 24 h postinfection affects HCMV-activated glycolysis. As shown in Fig. 4D, treatment of HCMV-infected cells 24 h postinfection did not significantly inhibit the glycolytic activation induced by HCMV infection when assayed at 48 h postinfection. These results suggest that the STO-609 treatment is inhibiting a virally induced event in the first 24 h of infection that is necessary for glycolytic activation. We also assayed how treatment of mock- or HCMV-infected cells at 24 h postinfection affected glycolytic flux at 72 h postinfection. Contrary to what was observed at 48 h postinfection, virally activated glycolytic flux was mostly inhibited at 72 h postinfection when STO-609 was added at 24 h postinfection (Fig. 4D). These results suggest that HCMV-mediated glycolytic activation can largely be reversed when STO-609 is added at later points, i.e., when STO-609 is added at 24 h postinfection, glycolysis is activated at 48 h but is subsequently inhibited by 72 h postinfection. This could reflect a viral or cellular factor responsible for glycolytic activation that continually requires CaMKK activity to maintain activation of glycolysis.
HCMV infection induces the accumulation of CaMKK mRNA and protein.
Our results indicate that CaMKK activity is important both for HCMV-mediated activation of glycolysis and for production of viral progeny. To further investigate how HCMV infection may target CaMKK, we sought to determine how CaMKK expression is affected by HCMV infection. Fibroblasts were mock or HCMV infected and harvested for qPCR and Western blot analysis at 4, 24, 48, and 72 h postinfection. We find that HCMV increases the abundance of CaMKKI mRNA at 24 and 48 h postinfection ∼4-fold (Fig. 5 A). By 72 h postinfection, the levels of CaMKKI fell to those observed during mock infection (Fig. 5A.) Analysis of CaMKKI protein accumulation produced similar results. HCMV-infected fibroblasts exhibited increased levels of CaMKKI protein with respect to mock infection levels at 24 h postinfection (Fig. 5B). The levels of CaMKKI continued to increase from 48 to 72 h postinfection (Fig. 5B). As seen in Fig. 5B, a polyclonal antibody to CaMKKI detected multiple bands that seemed to be induced by HCMV infection. To determine whether these bands could be phosphorylated isoforms of CaMKK, we tested whether they were sensitive to lambda phosphatase treatment. None of the bands showed any migration changes upon lambda phosphatase treatment (data not shown). To further explore the accumulation of CaMKK during HCMV infection, we employed a second CaMKK specific antibody, a mouse monoclonal antibody. An induction of CaMKK levels was again seen at roughly 24 to 72 h postinfection (Fig. 5B). Taken together, our qPCR data as well as the Western analysis suggest that HCMV induces CaMKK expression, consistent with the notion that CaMKK is an important cellular factor for HCMV replication.
FIG. 5.
Impact of HCMV infection on CaMKK1 mRNA and protein levels. (A) Analysis of CaMKK1 mRNA during HCMV infection by qPCR. Serum-starved MRC-5 human fibroblasts were mock infected or infected with HCMV (MOI = 3). Cellular mRNA isolated from cells harvested at 24, 48, and 72 h postinfection was processed for qPCR using CaMKK1-specific primers. Values are plotted as mean relative abundances ± SE observed after normalization to GAPDH (n = 3). (B) Analysis of CaMKK1 protein accumulation over a time course of HCMV infection. Serum-starved MRC-5 human fibroblasts were mock infected or infected with HCMV (MOI = 3). Cells were harvested at 4, 24, 48, and 72 h postinfection and analyzed by Western blotting with two independent CaMKK1-specific antibodies. Tubulin was also blotted for as a loading control.
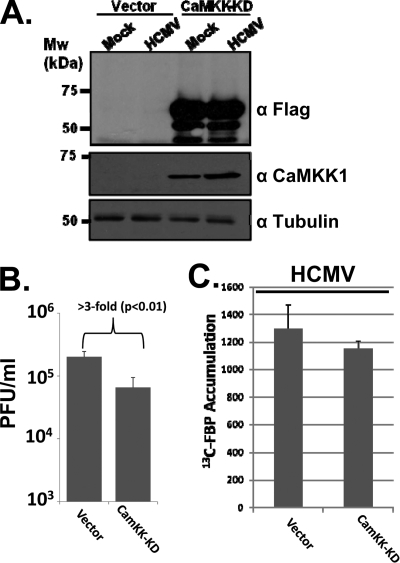
Expression of a kinase-dead variant of CaMKKI inhibits the production of HCMV viral progeny.
The finding that pharmaceutical inhibition of CaMKK attenuates production of HCMV viral progeny suggests that CaMKK is an important cellular factor for HCMV replication. While STO-609 has been demonstrated to be a very specific inhibitor of CaMKK (35), the possibility for off-target effects is always a potential issue with pharmaceutical inhibitors. As an additional means to antagonize CaMKK activity, we employed a kinase-dead variant of CaMKK [CaMKK-KD(K157A)] (38). Fibroblasts were retrovirally transduced with either a control vector or a vector expressing CaMKK-KD. As shown in Fig. 6 A, transduction with the CaMKK-KD construct resulted in abundant expression of CaMKK-KD in contrast to cells transduced with vector alone, in which no CaMKK-KD expression was detectable. To determine if expression of the CaMKK-KD mutant negatively affects HCMV replication, we infected vector-transduced or CaMKK-KD-transduced fibroblasts and assayed for HCMV replication. We find that CaMKK-KD expression results in a >3-fold defect (P < 0.01) compared to the level for control transduced cells (Fig. 6B). While these results were significant and repeatable, the inhibitory effect of expression of the kinase-dead mutant on viral replication was much lower than that observed upon pharmaceutical inhibition of CaMKK. While for some kinases, their kinase-dead alleles are capable of acting as dominant-negative mutants, others do not. To determine whether expression of CaMKK-KD blocked expression of HCMV-mediated glycolysis, we assayed glycolytic labeling in HCMV-infected vector control-transduced or CaMKK-KD-transduced cells. We found that CaMKK-KD expression resulted in a statistically insignificant reduction in [13C]FBP labeling, indicating that the CaMKK-KD expression was not sufficient to inhibit HCMV-mediated activation of glycolysis (Fig. 6C). Our results indicate that pharmaceutical inhibition of CaMKK has a much stronger impact on HCMV infection and on glycolysis than expression of a CaMKK-KD allele. Nevertheless, the results indicating that a kinase-dead variant of CaMKK significantly inhibits viral replication further support the idea that CaMKK plays an important role during HCMV infection.
FIG. 6.
Impact of expression of a CaMKK kinase-dead allele on HCMV replication. (A) Analysis of Flag-CaMKK-KD protein expression in fibroblasts. MRC-5 fibroblasts were transduced with an empty vector or a vector expressing a Flag-tagged kinase-dead allele of CaMKK (CaMKK-KD). Fibroblasts containing the CaMKK-KD variant or an empty-vector control were mock infected or infected with HCMV (MOI = 0.1). Cells were harvested 10 days postinfection (α-Flag blot) or 48 h postinfection (mouse α-CaMKKα and tubulin blots). The presence of the CaMKK-KD or tubulin was monitored by Western blotting with the indicated antibodies. (B) Impact of CaMKK-KD expression on HCMV viral replication. Serum-starved MRC-5 fibroblasts expressing either CaMKK-KD or an empty vector were infected with HCMV (MOI = 0.1). Cells were harvested at 10 days postinfection, and viral replication was measured by a viral plaque assay. Values are plotted as means ± SE (n = 6). (C) Impact of CaMKK-KD expression on glycolysis in HCMV-infected cells. Serum-starved MRC-5 fibroblasts expressing either CaMKK-KD or an empty vector were infected with HCMV (MOI = 3.0). At 48 h postinfection, cells were labeled for 1 min with [13C]glucose containing DMEM, quenched with cold methanol, and processed for LC-MS-MS to measure the accumulation of [13C]fructose-1,6-bisphosphate. Values are means ± SE (n = 2).
Inhibition of CaMKK does not inhibit HSV-mediated glycolytic activation.
As all viruses rely on the cellular metabolic network to provide the energy and biomolecular subunits necessary for viral replication, it is possible that viral activation of glycolysis could be a common hallmark of viral infection. To begin to examine this issue, we analyzed the impact of an alphaherpesvirus, herpes simplex virus type 1 (HSV-1), on glycolysis. We pulsed fibroblasts that had been infected with HSV-1 for 12 h with [13C]glucose and measured the rate of FBP accumulation. We find that HSV-1 increases the speed of FBP labeling compared to the level for mock infection (Fig. 7 A). However, in contrast to what we observed with HCMV infection, pharmacological inhibition of CaMKK had no impact on the glycolytic activation induced by HSV-1 infection (Fig. 7A). These results suggest that while both HCMV and HSV-1 induce glycolytic activation, HCMV-mediated glycolytic activation is dependent on the CaMKK cascade, whereas HSV-1 mediated activation of glycolysis is not. This indicates that HCMV and HSV-1 employ different strategies to activate glycolysis.
FIG. 7.
Impact of CaMKK inhibition on HSV viral replication and glycolytic activation. (A) Impact of CaMKK inhibition on HSV viral replication. Serum-starved MRC-5 human fibroblasts were infected with HSV (MOI = 10). After adsorption, cells were treated with DMSO or STO-609 (10 μg/ml). Cells were harvested at 24 h postinfection, and viral replication was measured by a plaque assay. Values are means ± SE (n = 4). (B) Impact of CaMKK inhibition on HSV-mediated activation of glycolysis. MRC-5 fibroblasts were mock infected or infected with HSV (MOI = 10). After adsorption, cells were treated with DMSO or STO-609 (10 μg/ml). Cells were harvested at 12 h postinfection, and flux analysis was performed using LC-MS-MS. Values are means ± SE (n = 4).
To determine if HSV-1 replication is sensitive to pharmaceutical inhibition of CaMKK, fibroblasts were infected in the presence of DMSO or STO-609 (10 μg/ml) and harvested at 24 h postinfection to determine the production of viral progeny. As shown in Fig. 7B, pharmaceutical inhibition of CaMKK resulted in an ∼10-fold reduction of HSV titers. While this reduction in production of infectious HSV-1 is significant, similar drug concentrations resulted in a >100-fold defect in HCMV titers (Fig. 3A). These results suggest that HSV-1 and HCMV infections differ in their sensitivity to CaMKK inhibition, both in their activation of glycolysis and in their attenuation of viral growth. HCMV growth is more sensitive to inhibition of CaMKK than HSV-1 growth is, which correlates with the increased impact of CaMKK inhibition on glycolysis during HCMV infection relative to that observed during HSV-1 infection.
Inhibition of glycolysis attenuates HCMV and HSV replication.
We find that pharmaceutical inhibition of CaMKK attenuates HCMV-induced activation of glycolysis and production of infectious HCMV. If the inhibition of glycolysis is responsible for the growth defect observed when cells are treated with a CaMKK inhibitor, one would predict that treatment with an inhibitor of glycolysis would attenuate viral growth to an extent similar to that observed upon CaMKK inhibition.
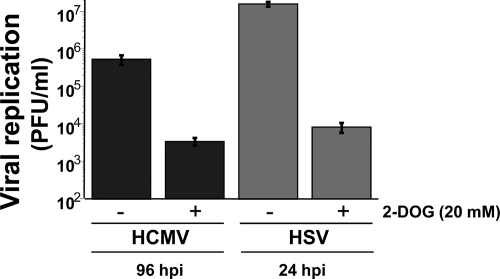
Previous reports have indicated that inhibition of glycolysis attenuates the replication of both HCMV and HSV-1 (7, 27). To confirm these findings in our cells and to determine how the potential growth defect associated with glycolytic inhibition compares to that of CaMKK inhibition, we assayed for the production of HCMV and HSV viral progeny in the presence of the glycolytic inhibitor 2-deoxyglucose (2-DOG). Fibroblasts were infected with HCMV or HSV-1 in the presence of 2-DOG (20 mM) and harvested for analysis of viral replication via a plaque assay. As seen in Fig. 8, 2-DOG treatment resulted in a >100-fold defect in infectious HCMV production, similar to the level of attenuation observed upon CaMKK inhibition (Fig. 3A). While only a correlation, the similar levels of viral attenuation associated with glycolytic and CaMKK inhibition are consistent with a scenario in which HCMV-induced CaMKK activity is necessary for activation of glycolysis and subsequent production of wild-type levels of infectious virions. For HSV-1 infection, 2-DOG treatment resulted in a >1,000-fold defect (Fig. 8). This attenuation was much greater than the observed impact on HSV-1 replication upon CaMKK inhibition (Fig. 7B) and is consistent with our results showing a lack of a role for CaMKK in glycolytic activation during HSV-1 infection.
FIG. 8.
Impact of glycolytic inhibition on HCMV and HSV viral replication. MRC-5 human fibroblasts were infected with HCMV (MOI = 3) or HSV (MOI = 10). After adsorption, cells were treated with DMSO or 2-deoxyglucose (20 mM), a glycolytic inhibitor. HSV-infected cells were harvested at 24 h postinfection, while HCMV-infected cells were harvested at 96 h postinfection. Viral replication was measured by a viral plaque assay. Values are means ± SE (n = 2).
DISCUSSION
We have previously reported that HCMV infection induces numerous changes to the metabolic network; among these changes is the activation of glycolysis (22, 23). In this study, we began to elucidate the mechanisms responsible for HCMV-mediated glycolytic activation. We find that viral envelope fusion and tegument protein delivery are not sufficient for glycolytic activation; instead, de novo protein synthesis is necessary for HCMV-mediated glycolytic activation (Fig. 1). While it is currently unclear which viral factors are responsible for the activation of glycolysis, our results indicating that inhibition of viral DNA replication does not affect glycolytic activation suggest that late proteins, whose expression is dependent on DNA replication, are not involved.
We find that inhibition of CaMKK but not PKA or CaMKII ablates HCMV-induced glycolytic activation (Fig. 2). HCMV has been shown to mobilize cellular calcium stores (14), which suggests that HCMV infection should activate calmodulin-dependent kinase cascades. Viral proteins that have been implicated in calcium signaling, e.g., UL37 (31), could play a role in CaMKK activation and therefore viral induction of glycolysis.
Interestingly, inhibitors of CaMKII did not block HCMV-mediated glycolytic activation compared to the level for DMSO-treated infected cells. This would suggest that CaMKII is not important for HCMV-mediated glycolytic activation. However, strict conclusions from this result are difficult since treatment with the CaMKII inhibitor KN-62 resulted in the elevation of glycolysis in mock-infected cells (Fig. 2B). This was surprising given the reports suggesting that CaMKII can activate glycolysis (3, 32). One possibility is that HCMV infection attenuates CaMKII activity, resulting in increased glycolysis. In this scenario, the magnitude of virally mediated glycolytic activation might be insensitive to pharmaceutical inhibition of CaMKII since it is already inhibited by HCMV infection. Another scenario is that CaMKII inhibition elevates the basal levels of glycolysis for both mock- and HCMV-infected cells, potentially to a high enough level that HCMV cannot induce it further. Such scenarios as well as their importance for HCMV infection could be tested through future analysis of CaMKII activity during the HCMV viral life cycle.
An alternative to CaMKII-dependent signaling that is consistent with our results would be CaMKK-mediated activation of CaMKIV. CaMKIV can activate CREB, resulting in transcriptional activation of cAMP response element (CRE)-containing genes (3). CREB has been reported to activate several glycolytic enzymes (16, 19), and we have previously reported that viral infection activates the expression of several glycolytic enzymes as well (22). Taken together, these results suggest a potential scenario in which upstream CaMKK-mediated transcriptional activation of glycolytic genes could be responsible for HCMV-mediated activation of glycolysis. Other mechanisms of CaMKK-mediated activation of glycolysis include CaMKK-mediated activation of CaMKI. CaMKI has been reported to phosphorylate phosphfrucktokinase-2 (PFK-2) (26), a glycolytic control enzyme whose phosphorylation results in glycolytic activation through activation of PFK-1 activity (reviewed in reference 28). Additionally, the AMP-activated kinase is another enzyme that can be activated by CaMKK and is capable of phosphorylating/activating PFK-2 (reviewed in reference 36). These possibilities are not mutually exclusive, and future work will determine their respective roles in HCMV-mediated glycolytic activation.
Our results indicate that pharmaceutical inhibition of CaMKK attenuates viral infection (Fig. 3A). Additionally, expression of a kinase-dead variant of CaMKK also attenuated HCMV infection, albeit to a lesser extent (Fig. 6B). The discrepancy between these two methods of CaMKK inhibition poses a challenge with respect to data interpretation. On one hand, small-molecule pharmaceuticals can have off-target effects which can be controlled for through analysis of secondary methods of inhibition. On the other hand, results involving a kinase-dead variant, i.e., an ATP-binding mutant, are totally dependent on the extent to which the mutant allele actually inhibits wild-type CaMKK activity. Such inhibition requires both substantial overexpression of the dominant-negative mutant and the subsequent dilution of crucial concentration-limited secondary interacting factors that are necessary for wild-type CaMKK activity. With respect to this point, to our knowledge, there have been no reports indicating that expression of a kinase-dead CaMKK variant can attenuate CaMKK's glycolytic phenotypes. Our results indicating that CaMKK-KD expression did not affect HCMV-mediated glycolysis support this possibility. Regardless, both our pharmaceutical approaches and our genetic-based approaches indicate that targeted inhibition of CaMKK attenuates HCMV replication. Further analysis of players downstream of CaMKK, using additional pharmaceutical and genetic tools, will further illuminate the role of this pathway during HCMV infection.
We find that pharmaceutical inhibition of glycolysis results in a magnitude of viral growth inhibition similar to that observed for pharmaceutical CaMKK inhibition. These results are consistent with a scenario in which HCMV infection is dependent on CaMKK activity for glycolytic activation. It is currently unclear what aspects of glycolysis contribute to viral replication. Non-mutually exclusive possibilities include an increased rate of ATP generation, production of glycosylation moieties for proteins, and production of acetyl-CoA for fatty acid biosynthesis, which we have previously reported to be increased during HCMV infection (23). Another possibility is that the viral induction of glycolysis is necessary to maintain a basal level or floor of pentose-phosphate pathway flux, an activity we find is actually decreased during HCMV infection (23). This could be required so that the virus can maintain pentose production for nucleotide biosynthesis. Further dissection of these metabolic pathways with subsequent analysis of their impact on the metabolic network and on viral replication will begin to answer these questions.
Our data indicate a correlation between CaMKK inhibition, glycolytic inhibition, and viral growth. Despite this correlation between the viral attenuation resulting from the inhibition of CaMKK and glycolysis, the impact on viral replication in the face of CaMKK inhibition may be independent of its impact on virally induced glycolysis. Additionally, at this point we also cannot rule out the possibility that CaMKK inhibition may block HCMV-mediated glycolytic induction by preventing the accumulation of a viral factor whose expression is necessary for glycolytic activation. It does appear, however, that continued CaMKK activity is necessary for maintenance of glycolytic activation, as we find that delayed treatment with STO-609 allows virally mediated glycolysis activation at 48 h postinfection but subsequently inhibits glycolytic activation at 72 h postinfection. Our data also indicate that HCMV infection induces the accumulation of CaMKK mRNA and protein (Fig. 5). While the exact mechanism through which CaMKK contributes to HCMV replication needs to be elucidated, the attenuation of HCMV replication in the face of CaMKK inhibition and the induction of CaMKK expression observed during HCMV infection suggest that CaMKK is targeted by HCMV infection.
Our results indicate that, like HCMV infection, HSV-1 infection induces glycolysis (Fig. 7A). However, HSV-1 mediated induction of glycolysis is independent of CaMKK inhibition (Fig. 7A). Pharmaceutical inhibition of CaMKK also resulted in a reduction in HSV-1 viral replication (Fig. 7B), but to a much smaller extent than that of the observed impact on HCMV replication (Fig. 3A). These results suggest that HSV-1 and HCMV employ different mechanisms to activate cellular glycolytic flux.
In conclusion, CaMKK is an important cellular factor for both HCMV-mediated activation of glycolysis and HCMV replication, and the expression of CaMKK is induced by viral infection. Future work will elucidate the mechanisms through which CaMKK contributes to both virally mediated glycolytic activation and viral replication. Given the dependence of viral replication on these mechanisms, their specific identification could have therapeutic potential.
Acknowledgments
We thank Thomas Shenk for the generous gift of HCMV-specific antibodies and Thomas Soderling for the generous gift of a plasmid expressing CaMKK1-KD. We also thank Nat Moorman for scientific discussions and feedback regarding the current work.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (R01AI081773). J.M. is a Damon Runyon-Rachleff Innovator supported (in part) by the Damon Runyon Cancer Research Foundation (DRR-09-10).
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Andrei, G., E. De Clercq, and R. Snoeck. 2008. Novel inhibitors of human CMV. Curr. Opin. Invest. Drugs 9:132-145. [PubMed] [Google Scholar]
- 2.Beisser, P. S., H. Lavreysen, C. A. Bruggeman, and C. Vink. 2008. Chemokines and chemokine receptors encoded by cytomegaloviruses. Curr. Top. Microbiol. Immunol. 325:221-242. [DOI] [PubMed] [Google Scholar]
- 3.Bito, H., and S. Takemoto-Kimura. 2003. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 34:425-430. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, P. L. 1957. Metabolism of pyruvate and alpha-ketoglutarate in virus-infected mouse brain. Nature 180:1418-1419. [DOI] [PubMed] [Google Scholar]
- 5.Burny, W., C. Liesnard, C. Donner, and A. Marchant. 2004. Epidemiology, pathogenesis and prevention of congenital cytomegalovirus infection. Expert Rev. Anti Infect. Ther. 2:881-894. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane, A. B. 2006. Antiviral dosing and efficacy for prophylaxis of cytomegalovirus disease in solid organ transplant recipients. Am. J. Health Syst. Pharm. 63:S17-S21. [DOI] [PubMed] [Google Scholar]
- 7.Courtney, R. J., S. M. Steiner, and M. Benyesh-Melnick. 1973. Effects of 2-deoxy-glucose on herpes simplex virus replication. Virology 52:447-455. [DOI] [PubMed] [Google Scholar]
- 8.DeBerardinis, R. J., J. J. Lum, G. Hatzivassiliou, and C. B. Thompson. 2008. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7:11-20. [DOI] [PubMed] [Google Scholar]
- 9.Fioretti, A., T. Furukawa, D. Santoli, and S. A. Plotkin. 1973. Nonproductive infection of guinea pig cells with human cytomegalovirus. J. Virol. 11:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa, T., S. Tanaka, and S. A. Plotkin. 1975. Resticted growth of human cytomegalovirus in UV-irradiated WI-38 human fibroblasts. Proc. Soc Exp. Biol. Med. 148:1249-1251. [DOI] [PubMed] [Google Scholar]
- 11.Gerna, G., F. Baldanti, and M. G. Revello. 2004. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum. Immunol. 65:381-386. [DOI] [PubMed] [Google Scholar]
- 12.Goodrich, J. M., et al. 1993. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann. Intern. Med. 118:173-178. [DOI] [PubMed] [Google Scholar]
- 13.Hidaka, H., M. Inagaki, S. Kawamoto, and Y. Sasaki. 1984. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036-5041. [DOI] [PubMed] [Google Scholar]
- 14.Himpens, B., et al. 1995. Human cytomegalovirus modulates the Ca2+ response to vasopressin and ATP in fibroblast cultures. Cell Calcium 18:111-119. [DOI] [PubMed] [Google Scholar]
- 15.Huang, E. S. 1975. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J. Virol. 16:1560-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungmann, R. A., D. Huang, and D. Tian. 1998. Regulation of LDH-A gene expression by transcriptional and posttranscriptional signal transduction mechanisms. J. Exp. Zool. 282:188-195. [PubMed] [Google Scholar]
- 17.Kaplan, A. S., and T. Ben-Porat. 1961. The action of 5-fluorouracil on the nucleic acid metabolism of pseudorabies virus-infected and noninfected rabbit kidney cells. Virology 13:78-92. [DOI] [PubMed] [Google Scholar]
- 18.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 19.Lee, M. G., and P. L. Pedersen. 2003. Glucose metabolism in cancer: importance of transcription factor-DNA interactions within a short segment of the proximal region og the type II hexokinase promoter. J. Biol. Chem. 278:41047-41058. [DOI] [PubMed] [Google Scholar]
- 20.Lee, W. A., and J. C. Martin. 2006. Perspectives on the development of acyclic nucleotide analogs as antiviral drugs. Antiviral Res. 71:254-259. [DOI] [PubMed] [Google Scholar]
- 21.Litman, R. M., and A. B. Pardee. 1956. Production of bacteriophage mutants by a disturbance of deoxyribonucleic acid metabolism. Nature 178:529-531. [DOI] [PubMed] [Google Scholar]
- 22.Munger, J., S. U. Bajad, H. A. Coller, T. Shenk, and J. D. Rabinowitz. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munger, J., et al. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Williams and Wilkins, New York, NY.
- 25.Pearson, H. E., and R. J. Winzler. 1949. Oxidative and glycolytic metabolism of minced day-old mouse brain in relation to propagation of Theiler's GD VII virus. J. Biol. Chem. 181:577-582. [PubMed] [Google Scholar]
- 26.Qin, H., B. Raught, N. Sonenberg, E. G. Goldstein, and A. M. Edelman. 2003. Phosphorylation screening identifies translational initiation factor 4GII as an intracellular target of Ca(2+)/calmodulin-dependent protein kinase I. J. Biol. Chem. 278:48570-48579. [DOI] [PubMed] [Google Scholar]
- 27.Radsak, K. D., and D. Weder. 1981. Effect of 2-deoxy-D-glucose on cytomegalovirus-induced DNA synthesis in human fibroblasts. J. Gen. Virol. 57:33-42. [DOI] [PubMed] [Google Scholar]
- 28.Rider, M. H., et al. 2004. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem. J. 381:561-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robey, R. B., and N. Hay. 2009. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salzman, N. P., R. Z. Lockart, Jr., and E. D. Sebring. 1959. Alterations in HeLa cell metabolism resulting from poliovirus infection. Virology 9:244-259. [DOI] [PubMed] [Google Scholar]
- 31.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. U. S. A. 103:19117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, P., M. Salih, J. J. Leddy, and B. S. Tuana. 2004. The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J. Biol. Chem. 279:35176-35182. [DOI] [PubMed] [Google Scholar]
- 33.Swulius, M. T., and M. N. Waxham. 2008. Ca(2+)/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 65:2637-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokumitsu, H., et al. 1990. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 265:4315-4320. [PubMed] [Google Scholar]
- 35.Tokumitsu, H., et al. 2002. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277:15813-15818. [DOI] [PubMed] [Google Scholar]
- 36.Towler, M. C., and D. G. Hardie. 2007. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100:328-341. [DOI] [PubMed] [Google Scholar]
- 37.Yalcin, A., S. Telang, B. Clem, and J. Chesney. 2009. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp. Mol. Pathol. 86:174-179. [DOI] [PubMed] [Google Scholar]
- 38.Yano, S., H. Tokumitsu, and T. R. Soderling. 1998. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396:584-587. [DOI] [PubMed] [Google Scholar]
- 39.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurochko, A. D. 2008. Human cytomegalovirus modulation of signal transduction. Curr. Top. Microbiol. Immunol. 325:205-220. [DOI] [PubMed] [Google Scholar]