Abstract
The norovirus P particle is an octahedral nanoparticle formed by 24 copies of the protrusion (P) domain of the norovirus capsid protein. This P particle is easily produced in Escherichia coli, extremely stable, and highly immunogenic. There are three surface loops per P domain, making a total of 72 loops per particle, and these are potential sites for foreign antigen presentation for immune enhancement. To prove this concept, a small peptide (His tag, 7 amino acids [aa]) and a large antigen (rotavirus VP8, 159 aa) were inserted into one of the loops. Neither insertion affects P particle formation, while both antigens were presented well on the P particle surface. The immune-enhancement effect of the P particle was demonstrated by significantly increased antibody titers induced by the P particle-presented antigens compared to the titers induced by free antigens. In addition, the measured neutralization antibody titers and levels of protection against rotavirus shedding in mice immunized with the VP8 chimeric P particles were significantly higher than those of mice immunized with the free VP8 antigen. Sera from P particle-VP8 chimera-vaccinated animals also blocked norovirus virus-like particle (VLP) binding to the histo-blood group antigen (HBGA) receptors. From these data, the P particle appears to be an excellent vaccine platform for antigen presentation. The readily available three surface loops and the great capacity for foreign antigen insertion make this platform attractive for wide application in vaccine development and antibody production. The P particle-VP8 chimeras may serve as a dual vaccine against both rotavirus and norovirus.
Biomaterials and bioengineering are fast-growing areas that have become critical parts of modern medicine. Because of their versatility and propensity to form arrays, viral structural proteins are ideal substrates in building presentation systems. Through genetic engineering, the self-assembled viral particles have been used as vaccine platforms for antigen presentation. Successful examples have been reported for several viruses, including a Flock House virus (FHV) virus-like particle (VLP) containing an antigen of Bacillus anthracis (20), hepatitis B virus (HBV) capsid-like particle (CLP) containing a surface antigen (OspA) of Borrelia burgdorferi (13, 22, 23), and the cowpea mosaic virus (CPMV) presenting a number of different antigens (4, 5, 10, 16, 17, 25, 26, 38), although limitations in these presentation systems have also been described.
In our previous study of human noroviruses, we discovered a unique subviral particle, the P particle, which can be used for antigen presentation. Noroviruses cause epidemics of acute gastroenteritis in humans. The viruses are nonenveloped, containing an outer protein capsid consisting of a single major structural protein, the capsid protein (VP1). The capsid protein has two major domains, the shell (S) domain forming the interior shell and the protrusion (P) domain constituting the arch-like protruding structures of the virus (28). These two domains, linked by a flexible hinge (8 amino acids [aa]), can be structurally and functionally independent. Expression of the S domain alone forms a smaller, thin-layer particle with a smooth surface without binding function to histo-blood group antigen (HBGA) receptors (1, 31), whereas the P domain forms the P particle, which binds to HBGAs (30, 34). Since the P particle is formed by the surface antigen of a norovirus and contains all the required elements to interact with the viral receptors, it has been proposed as a vaccine candidate against norovirus (30, 34).
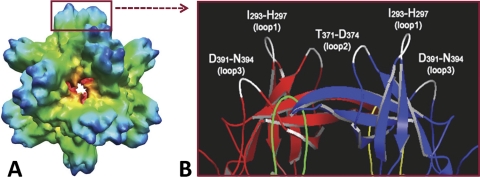
The norovirus P particle is an octahedral nanoparticle with a diameter of ∼20 nm and a molecular mass of ∼840 kDa (Fig. 1A). It assembles spontaneously, with 24 copies of the P monomers organizing into 12 P dimers (30, 34). This P particle is easily produced in Escherichia coli and Pichia pastoris (yeast), extremely stable, and highly immunogenic (30). The crystal structure of the norovirus P protein indicated three loops on the distal surface of each P domain (3), corresponding to the outermost surface of the P particle (Fig. 1) (30). Structure-based sequence alignment suggests that these loops may tolerate large sequence insertions (3), implying that they are potential sites for foreign antigen presentation. Since each P monomer has three surface loops, insertion of a foreign antigen into these loops results in 24 to 72 copies of the antigen on the surface of a P particle, which could greatly enhance the antigenicity and immunogenicity of the antigens. Thus, the P particle may be an excellent nanocarrier for foreign antigen presentation.
FIG. 1.
Norovirus P particle and its surface loops. (A) The structure of norovirus P particle of VA387 (GII.4) reconstructed by cryo-EM. (B) The distal end of a protrusion of the P particle is illustrated in a crystal structure (cartoon model), with the surface loops indicated. Red (P2 domain) and green (P1 domain) indicate one P protomer, while blue (P2 domain) and yellow (P1 domain) indicate the other protomer of a P dimer. Three surface loops of each P monomer that may be suitable sites for antigen insertion are shown in white.
In this study, we evaluated the utility of the norovirus P particle as a carrier for vaccine development and antibody production. We first found that insertion of the His tag as a small epitope to a loop of the P particle enhanced immunogenicity of the inserted His tag in mice. Further study on rotavirus VP8 (159 amino acids) showed that the P particles can tolerate an insertion of large antigens. VP8 is a trypsin-cleaved product of the surface spike protein VP4, which plays an important role in viral infectivity and neutralization of rotavirus, and several neutralizing epitopes have been mapped on its surface (11, 12, 14, 15, 27), indicating that the VP8 may be an excellent subunit vaccine candidate against rotaviruses. In addition to enhanced immunogenicity in mice, antibodies induced by the P particle-VP8 chimeras revealed strong neutralization and protection against rotavirus replication/infection in cell culture and in a murine rotavirus challenge model. The resulting antibody also blocked norovirus VLP binding to HBGA receptors, providing an opportunity to develop a dual vaccine against both noroviruses and rotaviruses. Our data proved the principle that the P particle is a multipurpose platform for vaccine development and antibody production.
MATERIALS AND METHODS
Expression constructs.
The P particle expression vector (30, 34; M. Tan and X. Jiang, U. S. patent application 61, 185, 564) pGEX-4T-1 (glutathione S-transferase [GST] gene fusion system [GE Healthcare Life Sciences]) containing norovirus VA387 (genogroup II, cluster 4 [GII.4]) P domain- and cysteine-containing-peptide-encoding sequences was used as the template for construction of various chimeric P particles. The cysteine-containing peptide was used to enhance and stabilize P particle formation (34). To make the P particle-His tag chimera, the His tag-encoding sequence was inserted into loop 2 between N372 and D374 (Fig. 1; see also Fig. 2A) through site-directed mutagenesis (see below). For the chimeric P particles containing human rotavirus VP8 antigens, a cloning cassette with SpeI and ClaI/EcoRV enzyme sites was first introduced into loop 2 to replace the sequence from T369 to D374 (see Fig. 5A) by site-directed mutagenesis. The VP8 antigen cDNA sequences of a P[8] genotype strain (strain Wa, from amino acids L65 to L223 [GenBank accession no. VPXRWA]) and a P[4] genotype strain (strain DS1, from amino acids L65 to L223 [GenBank accession no. VPXRDS]) were cloned into the cassette. For cloning the cDNA sequence of murine rotavirus (EDIM) VP8 antigen (from amino acids L65 to L222 [GenBank accession no. AF039219]) to the P particle, another cloning cassette with XbaI and BglII sites was inserted between N372 and N373. To make the P dimer-His tag chimera, the His tag was linked to the N terminus of the P domain with the hinge (31, 34). The His-tagged Thermotoga maritima α-l-fucosidase (GenBank accession no. TM0306) expression construct in pDEST17 (Gateway; Invitrogen) (29) was kindly provided by B. Henrissat and Y. Bourne (Architecture et Fonction des Macromolécules Biologiques, UMR 6098, CNRS, and Universités Aix-Marseille I and II, 31 Chemin J. Aiguier, Marseille, France).
FIG. 2.
Production and analysis of the P particle-His tag chimera. (A) Expression construct of the P particle-His tag chimera. The His tag was inserted in loops 2 between the N372 and D374 of the P domain. pGEX-4T-1 is an expression vector of the GST gene fusion system. The circled C represents a cysteine-containing peptide (CDCRGDCFC) at the C terminus of the P domain, which is to stabilize P particle formation (34). (B) The distal end of a protrusion of the P particle in the crystal structure (cartoon model) indicates the location of two residues N373 in loop 2 (dot models), where the inserted His tags are expected to be located. (C) Expression and purification of the P domain-His tag chimera. SDS-PAGE analysis revealed that the GST-P domain-His tag fusion protein (GST fusion) is ∼52 kDa. Digestion of the fusion protein in solution by thrombin resulted in GST (∼27 kDa) and the P domain-His tag chimera (PD-His-tag) (∼35 kDa) (left panel). The P domain-His tag chimera can also be released from the purification resin by thrombin digestion (right panel). M represents a prestained protein marker, with bands from top to bottom representing 113, 92, 50, 35, 29, and 21 kDa. (D) The elution curve of a gel filtration chromatography of the thrombin-released P-domain-His tag protein through the Superdex 200 size exclusion column. Three major peaks representing void, P particle-His tag, and P dimer-His tag were indicated, respectively. The sizes of these three peaks were calibrated with blue dextran 2000 (∼2,000 kDa; void), wild-type P particle (∼830 kDa), and wild-type P dimer (∼70 kDa), respectively. mAU, milli-absorbance units. (E) The fractions from gel filtration chromatography (see panel D) were analyzed by SDS-PAGE, and the fractions representing the three peaks are indicated.
Expression and purification of recombinant proteins.
Recombinant proteins were expressed in E. coli (BL21) with an induction of 0.25 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at room temperature (∼25°C) overnight as described elsewhere (34, 35, 37). Purification of the recombinant GST fusion proteins was carried out using resin of glutathione Sepharose 4 Fast Flow medium (GE Healthcare Life Sciences) according to the manufacturer's instructions. GST was removed from the target proteins by thrombin (GE Healthcare Life Sciences) cleavage either on beads or in solution (phosphate-buffered saline [PBS], pH 7.4) followed by a further purification through gel filtration chromatography. Purification of the His-tagged proteins was conducted using Talon His tag purification resins (Clontech, Mountain View, CA) according to the manufacturer's instructions. The His-tagged proteins were eluted from the resin with 250 mM imidazole (Sigma-Aldrich, St. Louis, MO) in PBS (pH 7.4). Further purification of the resin-purified proteins was performed through gel filtration chromatography.
Gel filtration chromatography.
Gel filtration chromatography was carried out through an Akta fast performance liquid chromatography (FPLC) system (GE Healthcare Life Sciences) as described previously (31, 34, 35). Briefly, the affinity column-purified proteins were loaded on a size exclusion column, Superdex 200 (GE Healthcare Life Sciences), powered by an Akta FPLC system (model 920; GE Healthcare Life Sciences). The molecular masses of the eluted fractions were calibrated by gel filtration calibration kits (GE Healthcare Life Sciences). Alternatively, the peaks of void volume, the chimeric P particle, and the P dimer can be determined with blue dextran 2000 (∼2,000 kDa; GE Healthcare Life Sciences) and the wild-type P particle (830 kDa) and the wild-type P dimer (69 kDa) of norovirus VA387 (GII.4), respectively (34). The fractions containing proteins of interest were further analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and/or Western blot analysis.
SDS-PAGE and Western blot analysis.
Recombinant proteins were analyzed by SDS-PAGE using a 10% gel. The specific compositions of recombinant proteins were detected by a Western blot analysis as described elsewhere (31, 37), using hyperimmune sera against norovirus VLPs (VA387 [GII.4]; 1:3,000) or rotavirus VP8 antigen (Wa; 1:3,000). Blotted membrane was blocked by 5% nonfat milk. Secondary antibody-horseradish peroxidase (HRP) conjugates (1:5,000; ICN Pharmaceuticals, Costa Mesa, CA) were used, and the HRP was detected by enhanced chemiluminescence (ECL) Eastern blotting detection reagents (GE Healthcare Life Sciences, Buckinghamshire, England). The ECL signals were captured by Hyperfilm ECL (GE Healthcare Life Sciences, Buckinghamshire, England).
Site-directed mutagenesis.
Site-directed mutagenesis was performed to insert His tag and construct cloning cassettes in loop 2 of the norovirus P domain following the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) procedure provided by the manufacturer as described previously (32). A primer pair, CACCACTGACACAAACCACCACCACCATCATCACCACGATCTTCAAACTGGCC and GGCCAGTTTGAAGATCGTGGTGATGATGGTGGTGGTGGTTTGTGTCAGTGGTG, was used for the insertion of a string of 7 histidines into loop 2 between N372 and D374 (Fig. 1; see also Fig. 2A). In addition, a cloning cassette with three enzyme sites (SpeI and ClaI/EcoRV) in loop 2 of the norovirus P domain was constructed using primer pair GTTCAATACACCACTAGTACAAACATCGATATCCTTCAAACTGGC and GCCAGTTTGAAGGATATCGATGTTTGTACTAGTGGTGTATTGAAC to facilitate the insertion of the coding sequences for human rotavirus VP8 antigens. Another cloning cassette with XbaI and BglII sites was made using another primer pair, TACACCACTGACAVAAACTCTAGACACAGATCTAATGATCTTCAAACTGG and CCAGTTTGAAGATCATTAGATCTGTGTCTAGAGTTTGTGTCAGTGGTGTA, for the insertion of the coding sequence for murine rotavirus VP8 antigens.
Cryo-electron microscopy (cryo-EM) techniques. (i) Cryo-EM image collection.
The procedure used for structure reconstruction of the wild-type P particle described in our previous study (30) was adapted here. Briefly, aliquots (3 to 4 μl) of gel filtration-purified P particle-VP8 (Wa) chimera were flash frozen onto Quantifoil grids in liquid ethane cooled by liquid N2. The sample grids were loaded into the microscope, and low-electron (e)-dose images (∼20 e/Å2) were recorded on films by using a CM200 cryomicroscope with a field emission gun operating at 200 kV. The images were taken at a nominal magnification of ×50,000 and in the defocus range of 2.0 to 4.0 μm. The micrographs were selected and digitized by using a Nikon Super CoolScan 9000ED scanner at step size of 6.35 μm/pixel. The scanned images were binned, resulting in the final sampling of the images at 2.49 Å/pixel for further image processing and three-dimensional (3D) reconstruction.
(ii) Cryo-EM image processing and 3D reconstruction.
The images of the chimeric P particles were selected using EMAN′s boxer program (18). The selected images were manually filtered to exclude false positives. The EMAN′s ctfit program (18) was used to manually determine the contrast-transfer-function (CTF) parameters associated with the set of particle images originating from the same micrograph. Initial models of the chimeric P particles were created using EMAN′s startoct program (18). Then, the EMAN′s refining program (18, 19) was used to iteratively determine the center and orientation of the raw chimeric particles and reconstruct the 3D maps from the two-dimensional (2D) images by the EMAN make3d program until convergence. Octahedron symmetry was imposed during reconstruction of the chimeric P particles.
(iii) Cryo-EM model evaluation and analysis.
The crystal structure of rotavirus VP8 antigen (Wa; PDB accession no. 2DWr, from L65 to L223) (2) was fitted into the extended protrusions of the 3D structure of the P particle-VP8 (Wa) chimera by using UCSF Chimera software (24). Simple rigid body motion was considered to find the best matching of the X-ray structure to the 3D structure of chimeric P particles.
Enzyme immunoassay.
An enzyme immunoassay (EIA) was used to measure immune reactivity and antibody titers of mouse antisera after immunization with P particle-antigen chimeras. Different recombinant antigens were used for different antisera as follows: the His-tagged T. maritima α-l-fucosidase was used for sera after immunization with the P particle-His tag chimera, free VP8 was used for sera induced by the P particle-VP8 chimera, and GST was used for sera induced by the P particle-VP8 chimera, with GST as an internal control. Ninety-six-well microtiter plates were coated with antigen (Dynex Immulon; Dynatech, Franklin, MA). After blocking with 5% nonfat milk, sera at indicated dilutions were incubated with the plates coated with antigens. The bound antibody was detected by the secondary antibody-HRP conjugate as described elsewhere (8, 37). Antigen-specific antibody titers were defined as the end-point dilutions with a cutoff signal intensity of 0.15. Sera from animals that were immunized with wild-type P particle or PBS were used as negative controls.
HBGA binding and blocking assays.
The saliva-based binding assays were carried out basically as described elsewhere (8, 9). Briefly, boiled saliva samples with known HBGA phenotypes were diluted 1,000-fold and used to coat 96-well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA). After blocking with 5% nonfat milk, VLPs or P particles of norovirus (VA387 [GII.4]) were added. The bound VLPs/P particles were detected using our homemade rabbit anti-VA387 VLP antiserum (1:3,300), followed by the addition of HRP-conjugated goat anti-rabbit IgG (ICN Pharmaceuticals, Costa Mesa, CA). The blocking effects of the mouse sera induced by the P particle-VP8 chimera on the norovirus VLP-saliva binding were measured by a preincubation of VLP with diluted serum for 30 min before the VLP was added to the wells coated with saliva. The blocking rates were calculated by comparing the optical densities (ODs) measured with and without blocking with the mouse sera from immunized animals. The blocking rates of the sera from free-VP8-immunized animals were used as negative controls.
Immunizing mice for antibody responses.
Female BALB/c mice at 6 weeks of age (Harlan-Sprague-Dawley, Indianapolis, IN) were immunized with purified chimeric P particles or free antigens at a dose of 5 to 15 μg/mouse three or four times in a 2-week interval. For comparison of the immune responses to the P particle- and P dimer-presented His tags, 5 μg/mouse of recombinant P particle-His tag, P dimer-His tag, or wild-type P particles was administered to mice (n = 5). The same molar amount of free seven-histidine peptide was used to immunize mice as a control. The immunogens were given four times subcutaneously with Freund's adjuvant. For comparison of the immune responses to the P particle-presented VP8 and free VP8, 5 μg/mouse of free VP8 and 15 μg/mouse of P particle-VP8 chimera, in which both immunogens are in the same molar amounts as VP8, were administered to mice (n = 5 to 7) either intranasally without an adjuvant or subcutaneously with Freund's adjuvant for three doses. In the case of GST as an internal control, thrombin-digested GST-P-VP8 fusion protein that contained equal molar amounts of GST and P particle-VP8 chimera were used to immunize mice. Immunization of mice with the chimeric P particles containing murine rotavirus VP8 for protection is described below. Blood was collected by puncture of retro-orbital capillary plexus before immunization and 2 weeks after the final immunization. Sera were processed from blood after overnight stay at 4°C followed by centrifugation.
Rotavirus plaque assay.
A rotavirus plaque assay was performed to determine neutralization of rotavirus replication in cell culture by antisera from mice after immunization with chimeric P particles containing rotavirus VP8 antigen. Tissue culture-adapted rotavirus strain Wa grown in MA104 monkey kidney cells was used in this assay. The MA104 cells were cultivated in 6-well plates, and a rotavirus titer of ∼50 PFU/well was used as the inoculum. For the assay, trypsin-treated rotavirus was incubated with mouse sera at given dilutions for 60 min. The mixture was then added to the MA104 cells in the 6-well plate. After 2 h, the plates were washed and then overlaid with media containing 5 μg/ml trypsin (Invitrogen, Carlsbad, CA) and 0.8% agarose. After a 4-day incubation at 37°C, the plaques in each well were counted. The titer of neutralizing antibody in the sera was defined by the dilution of sera that showed a ∼20% reduction in plaque numbers in the wells treated with antisera relative to the number in untreated control wells.
Murine rotavirus challenge model.
The rotavirus challenge model described in previous studies (6, 7, 21) was followed to examine the protective efficacy of the P particle-VP8 vaccine. Rotavirus-specific-antibody-free BALB/c mice (n = 5 to 7) at 6 weeks of age (Harlan-Sprague-Dawley, Indianapolis, IN) were immunized intranasally three times with the chimeric P particle (15 μg/mouse) containing murine rotavirus (EDIM) VP8 antigen without an adjuvant. Free murine rotavirus VP8 antigen (5 μg/mouse) and the chimeric P particle (15 μg/mouse) containing human rotavirus (Wa) VP8 antigen were included for comparison. All three immunogens contained the same molar amount of the VP8 antigen. In addition, the wild-type P particle (vector control) and PBS were administered as negative controls. Two weeks after the last immunization, mice were challenged by oral gavage with the murine rotavirus EDIM strain at a dose of 4 × 104 focus-forming units (FFU), which is equivalent to 105 50% shedding doses. To measure rotavirus shedding in stools, two fecal pellets were collected from each mouse each day for 6 days following EDIM challenge and kept in 1 ml of Earle's balanced salt solution (EBSS). Samples were stored frozen until analyzed, at which time they were homogenized and centrifuged to remove debris. Quantities of rotavirus antigen in the fecal samples (μg/ml) were determined by an enzyme-linked immunosorbent assay (ELISA) as described previously (21). Since no difference in virus shedding between the two negative-control groups was noted, only the data from animals immunized with the wild-type P particle (vector control) are shown.
Statistical analysis.
Graphs were made using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) and Microsoft Office Excel 2007. The P values were determined by t test among data groups by using GraphPad Prism version 5.00 for Windows.
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (23a) of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cincinnati Children's Hospital Research Foundation (Animal Welfare Assurance no. A3108-01).
RESULTS
Production of chimeric P particle containing a His tag.
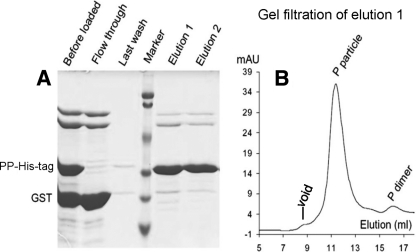
Our study was initiated with an insertion of a small epitope, the polyhistidine (His) tag, into loop 2 of the P particle (Fig. 1 and 2). Expression and purification of this chimeric P protein in E. coli by using a GST gene fusion system resulted in a high yield (>15 mg/liter of culture) of the soluble protein with an expected size (∼35 kDa) following a digestion of the protein to remove the GST tag (∼27 kDa) by use of thrombin (Fig. 2C). The formation of the chimeric P protein into P particles was shown by gel filtration chromatography followed by SDS-PAGE and a Western blot analysis, in which the chimeric P particle formed the major peak at ∼840 kDa (Fig. 2D and E, Fig. 3B, and data not shown). Exposure of the inserted His tag on the chimeric P particle was shown by its specific binding to the Talon resin followed by an elution of the bound P particles with 250 mM imidazole (Fig. 3A, elutions 1 and 2), which resulted in a highly pure preparation of the chimeric P particle. Thus, the P particle-His tag chimera can also be purified from E. coli by using the Talon affinity resin (data not shown).
FIG. 3.
Exposure of the His tag on the P particle (P particle-His tag binding on Talon resin). (A) The GST-P domain-His tag fusion protein was digested by thrombin, resulting in a mixture of the P particle-His tag chimera (PP-His-tag), GST, and other copurified proteins. These mixed proteins (“before loaded”) were loaded onto the Talon resin, and the flowthrough contained all proteins except the P particle-His tag chimera. After being washed (last wash), the P particle-His tag chimera was eluted (elutions 1 and 2) by 250 mM imidazole. “Marker” represents a prestained protein standard, with bands from top to bottom representing 107, 81, 49, 33, 27, and 20 kDa. (B) The elution curve of gel filtration chromatography of the eluted protein (elution 1 of panel A) through the Superdex 200 size exclusion column, indicating that vast majority (>98%) of the eluted protein formed chimeric P particles as shown by a defined peak at ∼840 kDa. The gel filtration column was calibrated as described in the legend to Fig. 2.
Immune enhancement of the His tag by the P particle carrier.
The ability of the P particle carrier to enhance the immune response to the His tag was determined by examination of antibody responses to the His tag in mice (n = 5) following immunization with the P particle-His tag chimera. The His tag-specific antibody titer detected in the mice immunized with the P particle-His tag chimera was significantly higher than that in mice immunized with the P dimer-presented His tag or free seven-histidine peptide (P < 0.05; Fig. 4A and B).
FIG. 4.
Antibody responses of mice to the P particle-presented His tag. (A) Immune reactivity of mouse sera (5 mice) after immunization with equal molar amounts of the P particle-His tag chimera (PP-His), P dimer-His tag chimera (PD-His), free 7×His peptide, and wild-type P particle (WT PP) to recombinant His-tagged-α-fucosidase of T. maritima (His-tag) in EIAs. (B) Antibody titers of the sera described for panel A were determined by an end-point dilution approach. Microtiter plates were coated with antigens at 5 ng/μl for EIAs. Sera at indicated dilutions were used to measure immune reactivity. *, P < 0.05.
Development of a chimeric P particle containing the rotavirus VP8 antigen.
We next examined the capacity of the P particle platform to accommodate a larger polypeptide by an insertion of the rotavirus (Wa) VP8 antigen, containing 159 amino acids. A cloning cassette with three enzyme sites (SpeI and ClaI/EcoRV) in loop 2 of the P particle was constructed (Fig. 5A) to facilitate the VP8 insertion. Expression of the construct in E. coli resulted in a high yield (>20 mg/liter culture) of the GST-P-VP8 fusion protein (∼78 kDa; Fig. 5B, left panel). Released P-VP8 chimera (∼52 kDa) was obtained by thrombin digestion of the GST fusion protein either in solution or on the purification beads (Fig. 5B, middle and right panels, respectively). A high rate (>95%) of P particle formation of the P-VP8 chimeric protein was demonstrated by gel filtration chromatography (Fig. 5C). Western blot analysis showed that the P-VP8 chimeric protein reacted with antibodies against both a norovirus VLP (VA387) and rotavirus (Wa) VP8 (Fig. 5D).
FIG. 5.
Production and analysis of the P particle-VP8 chimera. (A) The P particle-VP8 chimera expression construct based on plasmid pGEX-4T-1 containing the P domain-encoding cDNA sequences. The rotavirus (Wa) VP8 antigen was inserted in loops 2 of the P domain between T368 and L375 through a cloning cassette with enzyme sites SpeI and ClaI/EcoRV. The circled C represents the cysteine-containing peptide (CDCRGDCFC). (B) Expression and purification of the P particle-VP8 chimera. An SDS-PAGE analysis revealed that the GST-P-VP8 fusion protein (GST fusion) is ∼80 kDa (left panel). Digestion of the fusion protein by thrombin results in GST (∼27 kDa) and the P-VP8 chimera (∼52 kDa) (middle panel). The free P-VP8 chimera can also be released from the purification resin by a thrombin digestion (right panel). (C) The elution curve of the gel filtration chromatography of the thrombin-released P-VP8 protein from panel B through the Superdex 200 size exclusion column. An SDS-PAGE analysis of the fractions of the peaks is shown at the top. The single major peak near the void volume indicated that almost all P-VP8 protein formed chimeric P particles. The column was calibrated with blue dextran 2000 (∼2,000 kDa; void), wild-type P particles (∼830 kDa), and wild-type P dimer (∼70 kDa). M, marker. (D) The P-VP8 protein (left panel) reacted to antibodies against rotavirus VP8 (middle panel) and norovirus VLPs (right panel) in a Western blot analysis. In panels B and D, lanes M represent prestained protein markers, with bands from top to bottom representing 113, 92, 50, 35, 29, and 21 kDa.
Cryo-EM and three-dimensional image reconstruction showed that the P particle-VP8 chimera remains in an octahedral symmetry like the wild-type P particle (30), but the chimeric P particle is notably larger (Fig. 6A and B). The extended protrusion is likely the inserted VP8, which can be recognized by a nick at the potential boundary of the P dimer. Fitting of the crystal structure of rotavirus VP8 antigen of the same Wa strain (2) in the density map of the extended protrusions of the chimera confirmed that the extended protrusion is indeed VP8 (Fig. 6C to E).
FIG. 6.
Cryo-EM structure of the P particle-VP8 chimera. (A) A wild-type P particle. (B) A P particle-VP8 chimera. Compared to the wild-type P particle, the chimera shows extended protrusions with nicks in the middle, suggesting the boundary between the P2 subdomain and the inserted VP8 antigen. The radii of the particles in panels A and B are indicated by the same color schemes. (C) Fitting of two copies of the crystal structures (green and blue in the cartoon model) of the rotavirus (Wa) VP8 antigen into the density map of the extended protrusion of the chimera (transparent gray), confirming the exposure of the VP8 antigen on the chimeric P particle. Enlarged side (D) and top (E) views of a protrusion of the P particle-VP8 chimera are shown.
The P particle enhanced immune responses to VP8.
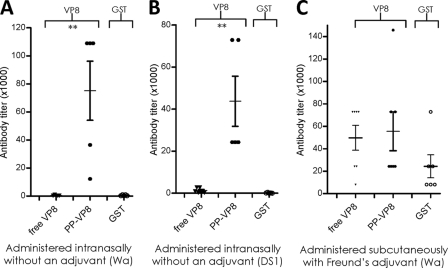
We then studied the immune responses to the P particle-presented VP8 antigen in mice. Following immunization with equal molar amounts of the P particle-VP8 chimera, free VP8, and GST, the resulting mouse sera were examined by an EIA using free VP8 or GST as the antigen (see Materials and Methods). The VP8-specific antibody titer after immunization with the P particle-VP8 chimera was significantly higher than that induced by free VP8 following an intranasal immunization without an adjuvant (P < 0.005). Comparable results were observed for two chimeric P particles containing VP8 antigens of rotaviruses Wa (P[8]) and DS1 (P[4]) and their free-VP8 counterparts (Fig. 7A and B). Only marginal antibody titers against free GST were detected in these animals, further confirming the specific immune enhancement of the P particle-presented VP8s. The immune responses of mice following a subcutaneous immunization of the above-described antigens with Freund's adjuvant were also studied, and we observed a smaller difference between the VP8-specific antibody titers corresponding to the free- and P particle-presented VP8 (Fig. 7C).
FIG. 7.
Immune responses of mice to P particle-presented VP8s. Equal molar amounts of the P particle-VP8 chimera and free VP8 were used to immunize mice, either intranasally without an adjuvant (5 to 7 mice; panels A and B) or subcutaneously with Freund's adjuvant (6 or 7 mice; panel C). Free VP8 and GST were used as antigens for antibody titer determination in EIAs. (A and B) Anti-VP8 and -GST antibody titers of mouse sera after immunization with free VP8 antigen (free VP8) and the chimeric P particles (PP-VP8) containing VP8s of Wa (A) and DS1 (B). (C) Anti-VP8 antibody titers after immunization with free VP8 antigen (free VP8) and the chimeric P particle (PP-VP8) containing VP8 of the Wa strain. In all experiments, an equal molar amount of GST in the immunogens served as an internal control. **, P < 0.01.
The P particle-VP8 chimera induced neutralizing antibodies against rotaviruses.
The hyperimmune antisera induced by the P particle-VP8 (Wa, P[8]) chimera through an intranasal immunization (Fig. 7A) strongly reduced the homologous rotavirus (Wa) replication in cell culture. This effect was significantly stronger than that of immunization with the free VP8 (P < 0.0002) (Fig. 8A). A low level of cross-inhibition of Wa by the sera after immunization with the P particle-VP8 (DS1, P[4]) chimera was also observed (P < 0.05; Fig. 8B). In contrast, sera from animals immunized with the free VP8 of DS1 did not show such cross-inhibition. In addition, the neutralization titers for sera from subcutaneously immunized animals with Freund's adjuvant were also measured. To our surprise, among 4 pairs of mouse sera with similar immune reactivities to free VP8 antigen in EIAs (Fig. 8C), all sera from animals immunized with the P particle-VP8 (Wa) chimera showed neutralization titers significantly higher than those of sera from mice immunized with free VP8 of the homologous Wa strain (P < 0.005; Fig. 8D). These data indicated that the VP8 on the chimeric P particle might be better presented and maintained a proper conformation that is required for a neutralization epitope.
FIG. 8.
Neutralization of rotavirus by mouse sera after immunization with P particle-VP8 chimeras. (A) Mouse sera after intranasal immunization with a P particle-VP8 (Wa, P[8]) chimera without an adjuvant show strong neutralization of the same Wa strain (PP-VP8 sera), while sera after immunization with free VP8 show a significantly lower level of neutralization (VP8 sera). Sera from mice receiving no antigen served as negative controls (control sera). (B) Mouse sera after immunization with the P particle-VP8 (DS1, P[4]) chimera show weak cross-neutralization of Wa (PP-VP8 sera), whereas sera after immunization with free VP8 of DS1 (VP8 sera) and the negative-control sera did not show this cross-neutralization. (C) Immune reactivity against VP8 of sera from mice immunized with free Wa VP8 (VP8 sera) and P particle-VP8 (Wa) chimera (PP-VP8 sera) subcutaneously with Freund's adjuvant. The two antigens induced similar antibody responses. Sera from mice receiving no antigen served as negative controls (control sera). (D) The sera from panel C after immunization with the P particle-VP8 chimera showed significantly higher rotavirus (Wa) neutralization titers (PP-VP8 sera) than sera induced by immunization with free VP8 (VP8 sera). Control sera from mice receiving no antigen served as a negative control (control sera). Asterisks indicate the P values between the neutralization levels of the sera after immunization with the two forms of VP8. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Vaccination with the P particle-VP8 chimera reduced viral shedding after challenge with a murine rotavirus.
We next examined whether a P particle-VP8 chimeric vaccine could provide protection in vivo by using a murine rotavirus model (EDIM strain) (6, 7, 21). To this end, a chimeric P particle containing the EDIM VP8 antigen was constructed and administered to mice (see Materials and Methods). After confirmation of antibody responses to murine rotavirus VP8 (Fig. 9A), the animals were challenged with murine rotavirus, and their levels of virus shedding were measured (Table 1 and Fig. 9B). Mice vaccinated with the P particle-VP8 (EDIM) chimera shed significantly less viral antigen than mice immunized with the wild-type P particle (vector control) (P < 0.05) and mice vaccinated with the P particle-VP8 (Wa) chimera (P < 0.05). The average reduction of viral shedding was 89% in the 6-day period with a particularly high reduction (99.2%) on day 1 after viral challenge. Mice immunized with free murine VP8 had an average reduction of 77%, which is a smaller reduction than that seen in mice immunized with the P particle-VP8 (EDIM) chimera (P > 0.05). Immunization with the chimeric P particle vaccine containing a human rotavirus (Wa) VP8 had a small to modest effect (23%) on viral shedding (Table 1 and Fig. 9B), suggesting some cross-protection against EDIM. This heterologous protection became more apparent on day 6 after the viral challenge (63%; P < 0.05) (Table 1).
FIG. 9.
Protection after immunization of mice with P particle-VP8 chimeric vaccine against a mouse rotavirus infection. (A) Antibody responses of mice to free murine rotavirus VP8 after vaccination with free (free mVP8) and P particle-presented (PP-mVP8) murine rotavirus VP8. The wild-type P particle (WT PP) served as a vector control. **, P < 0.01. (B) Rotavirus shedding (μg/ml) by mice was measured after vaccination with four different vaccines and challenged by murine rotavirus (EDIM). WT PP, mice vaccinated with the wild-type norovirus P particle (vector control; n = 7); PP-hVP8, mice vaccinated with the P particle-VP8 (Wa) chimera (n = 5); free mVP8, mice vaccinated with free murine VP8 (EDIM) antigen (n = 5); PP-mVP8, mice vaccinated with the P particle-VP8 (EDIM) chimera (n = 5). Results of data calculation and statistic analysis are shown in Table 1.
TABLE 1.
Rotavirus shedding in stools of vaccinated mice after challenge with murine rotavirus (EDIM strain)a
| Parameter affected by immunization (no. of mice)b | Value at indicated no. of days after virus challenge |
Overall value | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| WT P particle (control) (7) | |||||||
| Virus shedding | |||||||
| Mean | 692.8 | 4,215.2 | 5,639.9 | 4,474.2 | 3,045.7 | 64.4 | |
| Standard deviation | 869.9 | 2,715.9 | 2,932.7 | 2,063.1 | 2,072.1 | 30.1 | |
| P particle-VP8 from Wa (5) | |||||||
| Virus shedding (μg/ml) | |||||||
| Mean | 801.4 | 4,205.1 | 4,198.7 | 3,768.2 | 1,673.1 | 23.8 | |
| Standard deviation | 254.1 | 1,644.0 | 1,500.6 | 1,205.0 | 758.3 | 6.8 | |
| % reduction in shedding | −11.2 | 0.2 | 25.6 | 15.8 | 45.1 | 63.0 | 23.1 |
| P value | 0.903 | 0.993 | 0.341 | 0.511 | 0.192 | 0.015* | 0.101 |
| Free VP8 from EDIM (5) | |||||||
| Virus shedding (μg/ml) | |||||||
| Mean | 143.9 | 949.1 | 1,227.0 | 809.8 | 211.8 | 29.6 | |
| Standard deviation | 151.8 | 988.6 | 1,328.2 | 311.5 | 57.2 | 29.3 | |
| % reduction in shedding | 79.2 | 77.5 | 78.2 | 81.8 | 93.1 | 54.0 | 77.3 |
| P value | 0.201 | 0.029* | 0.011* | 0.003** | 0.013* | 0.073 | 0.019* |
| P particle-VP8 from EDIM (5) | |||||||
| Virus shedding (μg/ml) | |||||||
| Mean | 5.4 | 575.3 | 662.4 | 875.6 | 139.0 | 9.2 | |
| Standard deviation | 12.0 | 1,095.1 | 648.4 | 334.5 | 187.9 | 11.9 | |
| % reduction in shedding | 99.2 | 86.3 | 84.2 | 80.4 | 95.4 | 85.7 | 88.5 |
| P value | 0.048* | 0.018* | 0.004** | 0.005** | 0.011* | 0.003** | 0.018* |
Mice were immunized intranasally as described in Materials and Methods. Two weeks after the last immunization, mice were challenged with 105 50% shedding doses of murine EDIM. Stools were collected from each mouse for 6 days after challenge and analyzed for the quantity of rotavirus antigen.
Antigen shedding is per mouse per day in each group. Reduction in shedding is presented as the percent reduction in shedding relative to the control group, either by day or over 6 days. P values were calculated by comparison to the control group vaccinated with the wild-type (WT) P particle. A single asterisk indicates a statistically significant difference (P < 0.05), while a double asterisk indicates a statistically very significant difference (P < 0.01).
Immunization with the P particle-VP8 chimera induced antibody that blocked norovirus binding to HBGA receptors.
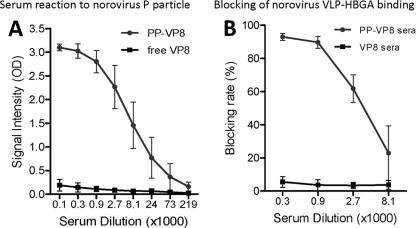
The role of the P particle backbone in immune responses in mice was also examined following immunization with the P particle-VP8 (Wa) chimera. As expected, the mouse sera after immunization with chimeric vaccines reacted strongly with norovirus VLPs and P particles as determined by EIAs (Fig. 10A and data not shown). More importantly, these sera blocked norovirus VLP binding to HBGA receptors (Fig. 10B and data not shown). As negative controls, sera from animals immunized with the free VP8 (Wa) did not show such a blockade. This result indicates that the P particle-VP8 chimera could be a dual vaccine against both rotavirus and noroviruses.
FIG. 10.
Mouse sera after immunization with P particle-VP8 chimeras block binding of norovirus VLPs to HBGA receptors. (A) Mouse sera after immunization with the P particle-VP8 (Wa) chimeras reacted strongly to norovirus P particle (PP-VP8), while sera after immunization with the free VP8 did not show this reactivity (free VP8). (B) Mouse sera from panel A blocked binding of norovirus VLPs to HBGA receptors (type A saliva, PP-VP8 sera), while sera after immunization with free VP8 did not show this blockade (VP8 sera).
DISCUSSION
Our previous studies showed that the norovirus P particle is easily produced, extremely stable, and highly immunogenic and can be used as a subunit vaccine against noroviruses (30, 34). In this study, we further demonstrated that the P particle can also be used as a novel vaccine platform for immune enhancement of a foreign antigen. We have shown (i) that the surface loops of the P particle are excellent sites for foreign antigen insertion without affecting the formation and production of the P particle, (ii) that the P particle tolerates a foreign antigen in a size up to at least 159 amino acids, and (iii) enhanced immune responses to inserted antigens demonstrated by both in vitro neutralization and in vivo protection experiments. The P particle-VP8 chimera served as a promising dual vaccine against both rotavirus and norovirus. Thus, the simple procedure to generate chimeric particles and the multiple surface loops with potential for multipolyvalent foreign insertion make the P particle an attractive vaccine platform for antigen presentation for infectious diseases or other medical conditions that would benefit from an efficient vaccine.
A primary goal of this study was to examine whether the P particle can enhance the immunogenicity of a small polypeptide antigen that generally has a low level of immunogenicity. We first studied this issue by using the His tag as a model and obtained excellent results. Two major factors may be responsible for the observed immune enhancement, namely, multicopy number and surface exposure of the inserted antigens. The P particle is composed of 24 copies of P monomers, which may explain its enhanced immune responses compared with a free His tag peptide and the His tag fused to a P dimer (Fig. 4A). Thus, the P particle may act as an adjuvant by its large size (830 kDa) and proper presentation of a foreign antigen that otherwise has low immunogenicity. The increased multicopy number of an antigen per particle is another feature that may explain the increased immune responses.
In addition to inserting the His tag peptide, we have successfully inserted a number of other small peptides into the P particles, including the T cell epitope of murine cytomegalovirus (9 aa), the Epi8 epitope of Pseudomonas aeruginosa (14 aa), the T cell epitope of murine rotavirus VP6 (14 aa), and the M2 extracellular epitope of influenza virus (M2e, 23 aa) (M. Tan and X. Jiang, unpublished). Significantly increased immune response and protection via the M2e antigen through the P particle platform has also been demonstrated in a mouse model (M. Xia, M. Tan, and X. Jiang, unpublished). These data suggested that the P particle may be readily useful for immune enhancement with a wide variety of small polypeptide antigens. The successful insertion of different rotavirus VP8s (159 aa) and the green fluorescent protein (GFP; 238 aa) into P particles (this report; M. Tan and X. Jiang, unpublished) has greatly extended the application of the P particle platform for a wider range of larger foreign antigens. The rotavirus VP8 is a spike protein on the viral capsid and is believed to be important for rotavirus infectivity. It is also one of two rotavirus antigens that induce neutralizing antibodies. A number of neutralizing epitopes have been identified on the VP8 protein (11, 12, 14, 15, 27). The success of the P particle-VP8 chimera in inducing a neutralizing antibody response and providing protection against rotavirus shedding in mice suggested that these epitopes have been preserved on the P particle carrier.
Future study to develop the P particle-VP8 chimera into a useful vaccine against rotaviruses is warranted. While the two recently introduced rotavirus vaccines are highly effective, new-generation vaccines may be needed for potentially new emerging viruses. Noninfectious subunit vaccines do not have a risk of reversion to virulent strains. The development of a VLP vaccine for rotaviruses has been proposed for years. However, a rotavirus VLP vaccine faces the challenges of low-efficiency expression and high cost of manufacturing because of the requirement for cotransfection of several capsid genes to the baculovirus host. In contrast, generation of the P particle-VP8 chimera requires only a routine E. coli-based cloning and expression procedure, which is highly efficient and low in cost. In addition, cross-neutralization epitopes on VP8 have been described. We have shown in this study that immunization of mice with a chimeric P particle containing VP8 from a P[4] virus conferred cross-neutralization of a P[8] rotavirus (Fig. 8B). A cocktail vaccine containing a minimal number of P types may also be cost-effective.
The ability of antibodies induced by the P particle-VP8 chimera to block the binding of norovirus VLPs to HBGAs is unexpected. As shown in Fig. 6, the distal surface of the P dimers, including the HBGA binding interfaces (3, 36) of the P particle, is most likely to be covered by the inserted VP8s. This leads to the loss of the capability of the chimera to bind to HBGA receptors (data not shown). One possibility for the continued blocking ability seen is that the epitopes of the HBGA binding interfaces of the P particle-VP8 chimera are still accessible for host immune response even though they are covered by the inserted VP8 antigens. Alternatively, the observed carbohydrate blockage may be due to an antibody binding in the vicinity of the carbohydrate binding site. No matter which mechanism is involved, the ability of the chimera-induced antibody to block binding of norovirus VLPs to HBGAs adds additional value to the P particle platform. The concept of the P particle-VP8 chimera as a dual vaccine against both norovirus and rotavirus may be particularly valuable for specific populations at risk for both infections.
Although only data on loop 2 of the P particle (Fig. 1) are reported here, success with various antigen insertions in the other two loops has been demonstrated (L. Y. Wang, M. Tan, and X. Jiang, unpublished). The availability of three surface loops per P monomer provides opportunities for versatile vaccine designs. For example, to increase immune responses, the same epitope or antigen can be inserted in all three loops to reach 72 copies of the antigen per particle. An even higher copy number can also be generated by insertion of tandem repeats of individual antigens. Alternatively, different antigens can be inserted into each of the three loops, resulting in a multivalent vaccine against different pathogens. Additional vaccine templates may also be generated by insertion of functional tags for different purposes. For example, insertion of a His tag would further simplify the purification procedure. Special ligands or signal molecules may also be used to stimulate immune responses by targeting the vaccine to special organs, tissues, or cells of the host. A potential drawback of using a norovirus particle vaccine platform could be the preexisting antibodies to noroviruses in humans. Thus, further studies are required to clarify this issue.
A further application of the P particle platform is for production of antibodies against small peptides for research and diagnostic uses. A variety of disease biomarkers (mainly peptide epitopes) has been identified, and antibodies against these biomarkers are important for diagnostic purposes. Small peptides can be easily inserted into a loop of the P particle by a simple DNA cloning procedure. We have developed convenient P particle vectors containing cloning cassettes that would further facilitate the process. Following expression of the recombinant chimeric P particle in bacteria, high-titer antibodies specific to the inserted peptide antigens can be made by immunization of laboratory animals with the chimeric P particle according to the established procedure in this study. Antibody production in this way will avoid the costly steps of peptide synthesis and conjugation of the peptide to a macromolecule such as keyhole limpet hemocyanin (KLH) for immune enhancement. Since the norovirus P domain has a unique sequence that shares no homology with any other proteins, cross-reactivity with other proteins should not be a concern. Thus, the P particle vaccine platform can be used as a convenient tool for antibody production in many areas of biomedical research.
Acknowledgments
The research described in this article was supported by the National Institutes of Health, the National Institute of Allergy and Infectious Diseases (grants R01 AI37093 and R01 AI055649), and the Department of Defense (grant PR033018) (grants to X.J.). This study was also supported by an Institutional Clinical and Translational Science Award, NIH/NCRR grant 1UL1RRO26314-01 to M.T. In addition, the Infectious Disease Grant (given to M.T.) sponsored by the Nipert Foundation contributed to this research.
We thank B. Henrissat and Y. Bourne at the Architecture et Fonction des Macromolécules Biologiques in France for providing the expression construct for His-tagged Thermotoga maritima α-l-fucosidase.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Bertolotti-Ciarlet, A., L. J. White, R. Chen, B. V. Prasad, and M. K. Estes. 2002. Structural requirements for the assembly of Norwalk virus-like particles. J. Virol. 76:4044-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard, H., X. Yu, B. S. Coulson, and M. von Itzstein. 2007. Insight into host cell carbohydrate-recognition by human and porcine rotavirus from crystal structures of the virion spike associated carbohydrate-binding domain (VP8*). J. Mol. Biol. 367:1215-1226. [DOI] [PubMed] [Google Scholar]
- 3.Cao, S., Z. Lou, M. Tan, Y. Chen, Y. Liu, Z. Zhang, X. C. Zhang, X. Jiang, X. Li, and Z. Rao. 2007. Structural basis for the recognition of blood group trisaccharides by norovirus. J. Virol. 81:5949-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterji, A., L. L. Burns, S. S. Taylor, G. P. Lomonossoff, J. E. Johnson, T. Lin, and C. Porta. 2002. Cowpea mosaic virus: from the presentation of antigenic peptides to the display of active biomaterials. Intervirology 45:362-370. [DOI] [PubMed] [Google Scholar]
- 5.Chatterji, A., W. Ochoa, L. Shamieh, S. P. Salakian, S. M. Wong, G. Clinton, P. Ghosh, T. Lin, and J. E. Johnson. 2004. Chemical conjugation of heterologous proteins on the surface of Cowpea mosaic virus. Bioconjug. Chem. 15:807-813. [DOI] [PubMed] [Google Scholar]
- 6.Choi, A. H., M. Basu, M. M. McNeal, J. Flint, J. L. VanCott, J. D. Clements, and R. L. Ward. 2000. Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. J. Virol. 74:11574-11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, A. H., M. M. McNeal, M. Basu, J. A. Bean, J. L. VanCott, J. D. Clements, and R. L. Ward. 2003. Functional mapping of protective epitopes within the rotavirus VP6 protein in mice belonging to different haplotypes. Vaccine 21:761-767. [DOI] [PubMed] [Google Scholar]
- 8.Huang, P., T. Farkas, S. Marionneau, W. Zhong, N. Ruvoen-Clouet, A. L. Morrow, M. Altaye, L. K. Pickering, D. S. Newburg, J. LePendu, and X. Jiang. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188:19-31. [DOI] [PubMed] [Google Scholar]
- 9.Huang, P., T. Farkas, W. Zhong, M. Tan, S. Thornton, A. L. Morrow, and X. Jiang. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J., T. Lin, and G. Lomonossoff. 1997. Presentation of heterologous peptides on plant viruses: genetics, structure, and function. Annu. Rev. Phytopathol. 35:67-86. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs-Nolan, J., and Y. Mine. 2006. Tandem copies of a human rotavirus VP8 epitope can induce specific neutralizing antibodies in BALB/c mice. Biochim. Biophys. Acta 1760:1884-1893. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs-Nolan, J., D. Yoo, and Y. Mine. 2003. Fine mapping of sequential neutralization epitopes on the subunit protein VP8 of human rotavirus. Biochem. J. 376:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratz, P. A., B. Bottcher, and M. Nassal. 1999. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc. Natl. Acad. Sci. U. S. A. 96:1915-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larralde, G., and M. Gorziglia. 1992. Distribution of conserved and specific epitopes on the VP8 subunit of rotavirus VP4. J. Virol. 66:7438-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larralde, G., B. G. Li, A. Z. Kapikian, and M. Gorziglia. 1991. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J. Virol. 65:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, T., and J. E. Johnson. 2003. Structures of picorna-like plant viruses: implications and applications. Adv. Virus Res. 62:167-239. [DOI] [PubMed] [Google Scholar]
- 17.Lomonossoff, G. P., and J. E. Johnson. 1996. Use of macromolecular assemblies as expression systems for peptides and synthetic vaccines. Curr. Opin. Struct. Biol. 6:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludtke, S. J., P. R. Baldwin, and W. Chiu. 1999. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128:82-97. [DOI] [PubMed] [Google Scholar]
- 19.Ludtke, S. J., J. Jakana, J. L. Song, D. T. Chuang, and W. Chiu. 2001. A 11.5 A single particle reconstruction of GroEL using EMAN. J. Mol. Biol. 314:253-262. [DOI] [PubMed] [Google Scholar]
- 20.Manayani, D. J., D. Thomas, K. A. Dryden, V. Reddy, M. E. Siladi, J. M. Marlett, G. J. Rainey, M. E. Pique, H. M. Scobie, M. Yeager, J. A. Young, M. Manchester, and A. Schneemann. 2007. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 3:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeal, M. M., M. N. Rae, J. A. Bean, and R. L. Ward. 1999. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J. Virol. 73:7565-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassal, M., C. Skamel, P. A. Kratz, R. Wallich, T. Stehle, and M. M. Simon. 2005. A fusion product of the complete Borrelia burgdorferi outer surface protein A (OspA) and the hepatitis B virus capsid protein is highly immunogenic and induces protective immunity similar to that seen with an effective lipidated OspA vaccine formula. Eur. J. Immunol. 35:655-665. [DOI] [PubMed] [Google Scholar]
- 23.Nassal, M., C. Skamel, M. Vogel, P. A. Kratz, T. Stehle, R. Wallich, and M. M. Simon. 2008. Development of hepatitis B virus capsids into a whole-chain protein antigen display platform: new particulate Lyme disease vaccines. Int. J. Med. Microbiol. 298:135-142. [DOI] [PubMed] [Google Scholar]
- 23a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC.
- 24.Pettersen, E. F., T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, and T. E. Ferrin. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605-1612. [DOI] [PubMed] [Google Scholar]
- 25.Porta, C., V. E. Spall, T. Lin, J. E. Johnson, and G. P. Lomonossoff. 1996. The development of cowpea mosaic virus as a potential source of novel vaccines. Intervirology 39:79-84. [DOI] [PubMed] [Google Scholar]
- 26.Porta, C., V. E. Spall, J. Loveland, J. E. Johnson, P. J. Barker, and G. P. Lomonossoff. 1994. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology 202:949-955. [DOI] [PubMed] [Google Scholar]
- 27.Prasad, B. V., J. W. Burns, E. Marietta, M. K. Estes, and W. Chiu. 1990. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature 343:476-479. [DOI] [PubMed] [Google Scholar]
- 28.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 29.Sulzenbacher, G., C. Bignon, T. Nishimura, C. A. Tarling, S. G. Withers, B. Henrissat, and Y. Bourne. 2004. Crystal structure of Thermotoga maritima alpha-L-fucosidase. Insights into the catalytic mechanism and the molecular basis for fucosidosis. J. Biol. Chem. 279:13119-13128. [DOI] [PubMed] [Google Scholar]
- 30.Tan, M., P. Fang, T. Chachiyo, M. Xia, P. Huang, Z. Fang, W. Jiang, and X. Jiang. 2008. Noroviral P particle: structure, function and applications in virus-host interaction. Virology 382:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan, M., R. S. Hegde, and X. Jiang. 2004. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J. Virol. 78:6233-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan, M., P. Huang, J. Meller, W. Zhong, T. Farkas, and X. Jiang. 2003. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J. Virol. 77:12562-12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Tan, M., and X. Jiang. 2005. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J. Virol. 79:14017-14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan, M., J. Meller, and X. Jiang. 2006. C-terminal arginine cluster is essential for receptor binding of norovirus capsid protein. J. Virol. 80:7322-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, M., M. Xia, S. Cao, P. Huang, T. Farkas, J. Meller, R. S. Hegde, X. Li, Z. Rao, and X. Jiang. 2008. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology 379:324-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan, M., W. Zhong, D. Song, S. Thornton, and X. Jiang. 2004. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 74:641-649. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, K. M., T. Lin, C. Porta, A. G. Mosser, H. A. Giesing, G. P. Lomonossoff, and J. E. Johnson. 2000. Influence of three-dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J. Mol. Recognit. 13:71-82. [DOI] [PubMed] [Google Scholar]