Abstract
Arenaviruses merit interest as clinically important human pathogens and include several causative agents, chiefly Lassa virus (LASV), of hemorrhagic fever disease in humans. There are no licensed LASV vaccines, and current antiarenavirus therapy is limited to the use of ribavirin, which is only partially effective and is associated with significant side effects. The arenavirus glycoprotein (GP) precursor GPC is processed by the cellular site 1 protease (S1P) to generate the peripheral virion attachment protein GP1 and the fusion-active transmembrane protein GP2, which is critical for production of infectious progeny and virus propagation. Therefore, S1P-mediated processing of arenavirus GPC is a promising target for therapeutic intervention. To this end, we have evaluated the antiarenaviral activity of PF-429242, a recently described small-molecule inhibitor of S1P. PF-429242 efficiently prevented the processing of GPC from the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) and LASV, which correlated with the compound's potent antiviral activity against LCMV and LASV in cultured cells. In contrast, a recombinant LCMV expressing a GPC whose processing into GP1 and GP2 was mediated by furin, instead of S1P, was highly resistant to PF-429242 treatment. PF-429242 did not affect virus RNA replication or budding but had a modest effect on virus cell entry, indicating that the antiarenaviral activity of PF-429242 was mostly related to its ability to inhibit S1P-mediated processing of arenavirus GPC. Our findings support the feasibility of using small-molecule inhibitors of S1P-mediated processing of arenavirus GPC as a novel antiviral strategy.
Arenaviruses merit significant interest both as tractable experimental model systems to study virus-host interactions and as clinically important human pathogens (7). Several arenaviruses cause hemorrhagic fever (HF) disease in humans. Thus, the Old World Lassa virus (LASV) and New World (NW) Junin virus (JUNV) are the causative agents of Lassa fever (LF) and Argentine HF disease, respectively (11, 21, 27). In addition, evidence indicates that the globally distributed prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) is a neglected human pathogen of clinical significance in congenital infections (1, 14, 22). Moreover, LCMV infection of immunosuppressed adults can result in severe disease and death (9, 28). Arenaviruses also pose a biodefense threat, and six of them, including LASV, are classified as category A agents (6). Public health concerns about arenavirus infections are aggravated by current arenavirus vaccines being limited to the JUNV live attenuated Candid 1 strain licensed only in Argentina and present antiarenavirus therapy being restricted to the use of the nucleoside analogue ribavirin, which is only partially effective and is associated with significant side effects. Therefore, it is important to develop novel antiviral strategies and drugs to combat human pathogenetic arenaviruses.
Arenaviruses are enveloped viruses with a bisegmented, negative-strand (NS) RNA genome and a life cycle restricted to the cell cytoplasm (7). Each RNA segment uses an ambisense coding strategy to direct the expression of two gene products in opposite orientations and separated by a noncoding intergenic region. The large segment (L; 7.2 kb) encodes the L protein, an RNA-dependent RNA polymerase, and the small RING finger protein Z that is the counterpart of the matrix (M) protein found in many enveloped NS RNA viruses. The small segment (S; 3.5 kb) encodes the viral nucleoprotein (NP) and the glycoprotein (GP) precursor GPC that is posttranslationally processed to yield the peripheral virion attachment protein GP1 and the fusion-active transmembrane protein GP2. Trimers of GP1/GP2 form the spikes that decorate the virus surface and mediate cell entry via receptor-mediated endocytosis (7).
We (16, 34) and others (4, 19) have shown that correct processing of arenavirus GPC by the cellular proprotein convertase sterol regulatory element-binding protein (SREBP) site 1 protease (S1P) is strictly required for production of infectious progeny and cell-to-cell virus propagation and thereby for both intra- and interhost virus propagation (16, 19, 32). S1P mediates GPC processing for all tested arenaviruses (4, 16, 19, 34). Notably, studies on LCMV and JUNV infections of cells deficient in S1P indicated that the appearance of viral variants capable of growing independently of S1P-mediated processing of GPC is highly unlikely. These findings strongly support the idea that inhibitors of S1P would represent promising antiarenaviral drug candidates (20, 35). Because S1P also mediates processing of the major GP precursor of Crimean-Congo HF virus (CCHFV) (39, 44), drugs targeting S1P function are likely to be effective against not only HF arenaviruses but also CCHFV, a tick-borne pathogen that causes HF in humans throughout regions of Africa, Asia, and Europe.
S1P is encoded by the membrane-bound transcription factor S1P gene and is an endoplasmic reticulum (ER)/early Golgi membrane-anchored serine protease (38, 40). Despite its broad consensus sequence, S1P exhibits exquisite substrate specificity and is involved in the proteolytic processing of a defined set of cellular proteins. The key role of S1P in the regulation of lipid metabolism and cholesterol biosynthesis has raised significant interest in developing specific inhibitors of S1P activity. However, so far, a cell-permeating, specific inhibitor of S1P that shows little or no mechanism-based toxicity has not been reported (2, 5). Several peptide- and non-peptide-based S1P inhibitors have provided proof of principle, but the lack of cell-permeating ability would impose severe limitations on their use as antiviral drugs in vivo. More recent efforts focused on the development of S1P inhibitors on the basis of decanoylated chloromethylketone (CMK)-derivatized peptides containing the RRLL recognition sequence of S1P that can act as potent suicide inhibitors of S1P catalytic activity. These drugs cause irreversible inhibition of the catalytic activity of S1P against all targets, host cell and pathogen derived, which might result in unacceptable levels of cellular toxicity. Recently, the small molecule PF-429242 was reported to be a potent S1P inhibitor both in vitro and in cell-based assays (12, 13). Moreover PF-429242-treated mice exhibited reduced expression levels of hepatic SREBP target genes and lower rates of cholesterol and fatty acid synthesis (12, 13). Here, we report that PF-429242 has a potent inhibitory effect on S1P-mediated processing of LCMV and LASV GPC that correlates with a strong inhibition of virus propagation.
MATERIALS AND METHODS
Plasmids.
Plasmids (pCAGGS) expressing LCMV-GPC, LCMV-GPCf, LASV-GPC, LCMV-Z, and LASV-Z have been previously described (23, 35, 43). All GPC and Z constructs were tagged at the C terminus with the Flag epitope.
Cells and viruses.
293T, Vero E6, and BHK-21 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal bovine serum, 2 mM l-glutamine, 100 mg/ml streptomycin, and 100 U/ml penicillin. rLCMV-WT, Armstrong strain, was generated by reverse genetics as described previously (10). rLCMV-GPCf, rLCMV-LASVGPC, and rVSV/LCMV-GPC have been described previously (35, 36). rLCMV/Z-Flag was generated by reverse genetics using a pol-I Lag/Z-Flag plasmid that has a Flag tag epitope at the C terminus of the Z open reading frame. Experiments involving LASV, Josiah strain, were conducted in the Robert E. Shope biosafety level 4 facility at the University of Texas Medical Branch, Galveston.
PF-429242.
The compound PF-429242 was synthesized according to previously published methods (13).
GPC cleavage assay.
293T cells (2.5 × 105) were transfected with 0.1 μg of pCAGGS-LASV-GPC, pCAGGS-LCMV-GPC, or pCAGGS-LCMV-GPCf. At 5 h posttransfection, the medium was replaced with fresh medium including the indicated concentration of PF-429242. At 36 h posttransfection, cell lysates were prepared (1% NP-40, 50 mM Tris-HCl [pH 8.0], 62.5 mM EDTA, 0.4% sodium deoxycholate) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting (WB).
In vitro enzymatic assay.
For the in vitro enzymatic assay to assess inhibition of the processing of a LASVGP-derived SKI-1/S1P peptide substrate by PF-429242, we used the previously described methyl coumaride (MCA) peptide Succ-YISRRLL-MCA containing the sequence YISRRLL derived from the S1P recognition site of LASV GPC (24). The fluorogenic peptide was custom made by GenScript Corp. Stock solutions were obtained by dissolving the lyophilized peptide in dimethyl sulfoxide (DMSO) at a 10 mM final concentration. Enzymatic assay was performed as described previously (24). Briefly, conditioned medium from HEK293 stably expressing soluble S1P (sS1P) was used without further purification. For each reaction, 20 μl HEK293-conditioned medium containing sS1P was preincubated with the indicated concentrations of PF-429242 for 20 min prior to the addition of Succ-IYISRRLL-MCA at 20 mM. Each reaction was carried out at room temperature in 100 μl buffer solution (25 mM Tris-HCl, 25 mM morpholineethanesulfonic acid [MES; pH 7.5], 1 mM CaCl2). Enzymatic activity measurements with MCA-conjugated peptides were performed by detecting the liberated 7-amino-4-methylcoumarin (AMC) with a Tristar LB 941 fluorometer (Berthold) with an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Virus titrations.
LCMV titers (focus-forming unit [FFU] counts) were determined by immunofocus assay (3). Briefly, 10-fold serial virus dilutions were used to infect Vero E6 cell monolayers in a 96-well plate, and at 20 h postinfection (hpi), cells were fixed by using 4% formaldehyde in phosphate-buffered saline (PBS). After cell permeabilization by treatment with 0.3% Triton X-100 in PBS containing 3% bovine serum albumin (BSA), cells were stained by using an anti-NP mouse monoclonal antibody and an Alexa Fluor 568-labeled anti-mouse second antibody (Molecular Probes). Vesicular stomatitis virus (VSV) titers (PFU) were determined by plaque assay.
Retroviral pseudotypes.
Retroviral pseudotypes bearing the GPs of LASV, LCMV, and VSV were produced as described previously (37). For inhibition studies, retroviral pseudotypes were preincubated with PF-429242 at the indicated concentrations for 45 min on ice, added to fresh monolayers of BHK-21 cells in 96-well plates (104 cells/well), and then incubated for 1 h in the presence of the drug. Unbound viral particles were removed, the cells were washed twice with DMEM, and fresh medium was added. Pseudotype infection was assessed by determination of luciferase activity after 48 h by Steady-Glo luciferase assay (Promega).
Reverse transcription (RT)-quantitative PCR (qPCR).
RNA was isolated using an RNeasy mini kit (Qiagen catalog no. 74106) and a QIAqube (Qiagen). Briefly, we lysed LCMV-infected cells with a supplied buffer, homogenized them with a QIAshredder, and then applied these samples to a QIAqube. RT was done according to the manual (Qiagen) using a total of 0.5 μg RNA. We used specific primers for LCMV S genome and Hamster glyceraldehyde 3-phosphate dehydrogenase (GAPDH) detection. We performed qPCR using TaqMan 7900HT (Applied Biosystems).
Minigenome rescue assay.
BHK-21 cells were seeded at 4.5 × 105 per M-12 well and on the following day transfected with p-T7, pMG-CAT, pCAGGS-NP, or pCAGGS-L under conditions previously described (17, 18, 26, 29, 30). At 5 h posttransfection, the medium was replaced with fresh medium containing different concentrations of PF-429242. At 48 h posttransfection, cell lysates were prepared to determine levels of chloramphenicol acetyltransferase (CAT) protein by enzyme-linked immunosorbent assay (ELISA) using a CAT ELISA kit (Roche catalog no. 11363727001). Briefly, cells were lysed with 1 ml of lysis buffer (supplied with the kit) and an aliquot (4 ml) was used for ELISA. Dilutions of a known amount of CAT were used to generate a calibration curve. CAT ELISA plates were incubated for 1 h at 37°C and washed twice with wash buffer, and the samples were reacted with an antibody to CAT for 1 h at 37°C. After reaction with the primary antibody, the samples were washed twice and then reacted with the secondary antibody for 1 h at 37°C, followed by two washes prior addition of the substrate. After 20 min at room temperature, samples were analyzed with an ELISA reader (SPECTRA max plus 384; Molecular Devices) to determine the absorbance (405 nm for samples, 490 nm for the reference).
Budding assay.
293T cells (2.5 × 105) were transfected with 0.1 μg of either pC-LCMV-Z or pC-LASV-Z using Lipofectamine 2000 (10 μl/μg DNA). At 5 h after transfection, the medium was replaced with fresh medium containing the indicated PF-429242 concentrations. At 36 h posttransfection, we collected virus-like particle (VLP)-containing tissue culture supernatants (TCS) and cells. After removal of cell debris by centrifugation (1,500 × g; 5 min), VLPs were collected by ultracentrifugation (100,000 × g; 30 min at 4°C) through a 20% sucrose cushion. Cells and VLPs were resuspended in lysis buffer (1% NP-40, 50 mM Tris-HCl [pH 8.0], 62.5 mM EDTA, 0.4% sodium deoxycholate) and analyzed by SDS-PAGE, followed by WB.
Immunoblotting.
Cell lysates or VLP samples were resolved by SDS-PAGE, followed by WB using a rabbit polyclonal serum to Flag (Cayman catalog no. 162150) or a mouse monoclonal antibody to actin (sc-1616-R; Santa Cruz) as the first antibody and horseradish peroxidase (HRP)-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG as the second antibody.
Immunofluorescence assay.
Cells were fixed by using 4% formaldehyde in PBS at the indicated time points after either rLCMV-WT or rLCMV-GPCf infection. After cell permeabilization by treatment with 0.3% Triton X-100 in PBS containing 3% BSA, cells were stained by using an anti-NP mouse monoclonal antibody and an Alexa Fluor 568-labeled anti-mouse second antibody (Molecular Probes).
Cytotoxicity assay.
PF-429242 cytotoxicity was assessed in Vero E6, 293T, and BHK-21 cells using the CellTiter-Glo Luminescent Cell Viability Assay (Promega), which determines the number of viable cells in a culture based on levels of ATP (8). Briefly, 5 × 104 cells were plated per 96-well plate and cultured overnight. The cells were treated with several concentrations of PF-429242, and 24 and 48 h later, CellTiter-Glo reagent was added. Thereafter, the assay was performed according to the manufacturer's recommendations, with a luminometer (Centro LB 960; Berthold Technologies). The viability of DMSO-treated control cells was set at 100%.
RESULTS
Effect of PF-429242 on S1P-mediated processing of LCMV and LASV GPCs.
We first examined whether PF-429242 inhibited the cleavage of LCMV and LASV GPCs in a cell-based transfection assay. For these studies, we used a plasmid expressing an LCMV GPC whose processing into GP1 and GP2 is mediated by furin instead of S1P (GPCf) as a control (35). All of the GPCs used were tagged at the C terminus with the Flag epitope to facilitate the detection of GPC and GP2.
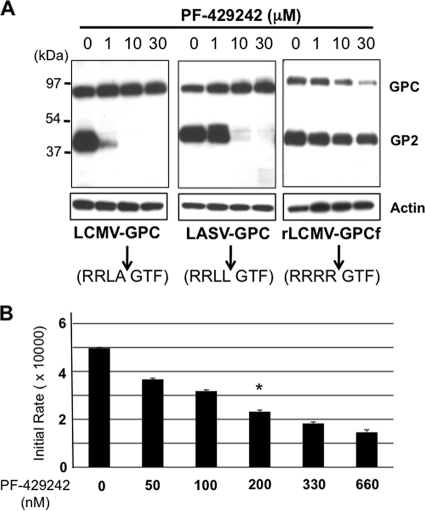
At 36 h posttransfection, we prepared cell lysates and used the same amount of total cell protein from each sample to determine the expression levels of GPC and GP2 by WB using a polyclonal serum to Flag (Fig. 1 A). We observed a dose-dependent inhibition by PF-429242 of LCMV and LASV GPC cleavage. PF-429242 treatment did not inhibit the processing of GPCf, a finding consistent with a specific activity of PF-429242 on S1P. Actin expression levels were similar for all of the samples, suggesting that at the concentrations tested, PF-429242 did not appear to have significant effects on cell viability, which was supported by results of PF-429242-associated toxicity in 293T cells (Table 1). Transfected cells treated with 1 μM PF-429242 showed significantly higher levels of LASV GP2 than LCMV GP2, suggesting that PF-429242 was less efficient in inhibiting S1P-mediated cleavage of GPC from LASV than that from LCMV. However, in an in vitro assay, PF-429242 very efficiently inhibited the cleavage by S1P of a peptide containing the S1P recognition site present in LASV GPC (Fig. 1B). A similar assay could not be done using the equivalent LCMV GPC-derived substrate because previously published work has shown that such a peptide is a very poor substrate for S1P (24).
FIG. 1.
(A) PF-429242 inhibits LCMV and LASV GPC cleavage by S1P. 293T cells (2.5 × 105/M6 well) were transfected with 0.1 μg of pC-LCMV-GPC, pC-LASV-GPC, or pC-LCMV-GPCf using Lipofectamine 2000 (10 μl/μg DNA), and at 5 h posttransfection, the transfection mixture was removed, the cells were washed once, and fresh medium containing PF-429242 (1, 10, or 30 μM) or the vehicle (DMSO) was added. At 36 h posttransfection, GPC and GP2 expression levels in cell lysates were determined by WB using a polyclonal serum to Flag. As a control, we also determined the level of actin in each sample (bottom). The cleavage sequences within GPC recognized by S1P or furin are indicated (arrows). (B) In vitro activity of PF-429242. Soluble S1P was preincubated with the indicated concentrations of PF-429242 for 20 min prior to the addition of the substrate Succ-YISRRLL-MCA (20 mM). The reaction was carried out at room temperature with 25 mM Tris-HCl-25 mM MES (pH 7.5)-1 mM CaCl2, and enzymatic activity measurements were performed by detecting the liberated AMC in a fluorescence plate reader. Plots represent initial rates at the indicated inhibitor concentration with 50% inhibition at circa 200 nM PF-429242 (asterisk).
TABLE 1.
Effect of PF-429242 on cell viabilitya
| Cell line and PF-429242 concn (μM) | Avg % viability ± SD after treatment for |
|
|---|---|---|
| 24 h | 48 h | |
| 293T | ||
| 0 | 100 ± 7.9 | 100 ± 1.2 |
| 1 | 98 ± 4.9 | 84 ± 1.2 |
| 10 | 100 ± 2.9 | 77 ± 8.8 |
| 30 | 89 ± 3.0 | 66 ± 10.9 |
| Vero E6 | ||
| 0 | 100 ± 2.0 | 100 ± 3.9 |
| 1 | 102 ± 3.0 | 85 ± 5.8 |
| 10 | 95 ± 0.8 | 85 ± 5.4 |
| 30 | 84 ± 7.4 | 73 ± 9.8 |
| BHK-21 | ||
| 0 | 100 ± 4.4 | 100 ± 2.9 |
| 1 | 100 ± 2.5 | 77 ± 1.9 |
| 10 | 91 ± 9.9 | 45 ± 10.4 |
| 30 | 80 ± 1.8 | 19 ± 5.4 |
Cell toxicity associated with PF-429242 treatment was assessed by determining cell viability after 24 and 48 h of treatment with different concentrations of PF-429242. Cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega), which measures cell viability based on the quantification of cellular ATP (8). We observed a dose- and cell type-dependent effect of PF-429242 on cell viability after 24 and 48 h of treatment. Inhibition of S1P activity results in impaired synthesis of several lipids that play important roles in cell physiology, which may contribute to PF-429242-associated cell toxicity. We therefore examined whether providing cells with these lipids exogenously influenced PF-429242-associated cell toxicity. Addition of these lipids at the time treatment with PF-429242 was started resulted in only modest increases in cell viability (data not shown).
Effect of PF-429242 on LCMV multiplication in cultured cells.
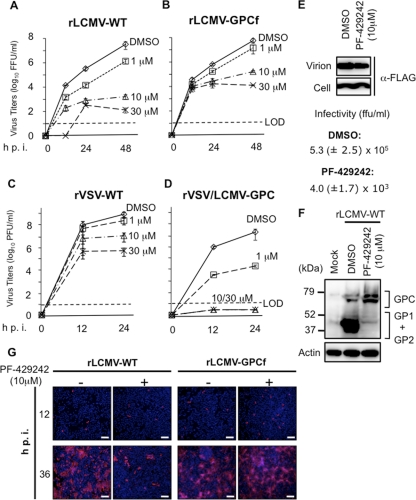
We next asked whether the inhibitory effect of PF-429242 on GPC processing by S1P correlated with an inhibitory effect on virus multiplication. For this, we infected BHK-21 cells (multiplicity of infection [MOI] of 0.01) with recombinant LCMVs, generated via reverse genetics, expressing either WT GPC (rLCMV-WT) or GPCf (rLCMV-GPCf) (35) in the presence of different concentrations (0, 1, 10, and 30 μM) of PF-429242. At the indicated times postinfection (p.i.), we determined titers of infectious virus in TCS (Fig. 2 A and B). We observed a dose-dependent inhibitory effect of PF-429242 on the multiplication of both rLCMV-WT and rLCMV-GPCf, but the magnitude of the inhibitory effect on rLCMV-WT was much greater than that on rLCMV-GPCf. Thus, at 48 hpi in the presence of 10 μM PF-429242, production of infectious rLCMV-WT and rLCMV-GPCf was reduced by 4 and 2 logs, respectively, compared to the titers produced by the corresponding infected but mock-treated cells. After 48 h in the presence of 10 μM PF-429242, the viability of BHK-21 cells was close to 50%, indicating that the 4-log reduction in the production of infectious rLCMV-WT could not be explained by PF-429242-associated toxicity (Table 1). The finding that PF-429242 was able to inhibit the multiplication of rLCMV-GPCf was consistent with previously published data showing that peak titers of rLCMV-GPCf are significantly lower in S1P−/− cells than in S1P+/+ cells (35).
FIG. 2.
PF-429242 inhibits multiplication of LCMV in cultured cells. (A to D) Effect of PF-429242 on production of infectious progeny. Cells were infected with rLCMV-WT (A), rLCMV-GPCf (B), rVSV-WT (C), or rVSV/LCMV-GPC (D) (MOI of 0.01). After 90 min of adsorption time, the inoculum was removed, the cell monolayers were washed, and fresh medium containing PF-429242 (0, 1, 10, or 30 μM) was added. At the indicated times p.i., virus titers were determined in TCS by an immunofluorescence (LCMV) or a plaque (VSV) assay. LOD, limit of detection. (E) PF-429242 treatment causes a reduction in the PFU/total viral particle ratio. Cells were infected with rLCMV/Z-Flag (MOI of 1) in the absence or presence of PF-429242 (10 μM), and at 24 hpi, TCS were collected. Infectious virus titers in TCS were determined by counting FFU. To assess levels of total virion particles, TCS samples (equal volumes) were clarified at low-speed centrifugation and total virion particles were collected by ultracentrifugation and subjected to WB using an antibody to Flag. (F) Treatment with PF-429242 inhibits GPC processing in LCMV-infected cells. Cells were infected with rLCMV-WT (MOI of 3) and treated with either PF-429242 (10 μM) or the vehicle (DMSO). At 24 hpi, cell lysates were prepared and analyzed by WB using a mixture of mouse monoclonal antibodies to GP1 and GP2. The faster GPC band corresponds to nonglycosylated GPC species. (G) PF-429242 inhibits cell-to-cell propagation of LCMV. Cells were infected with either rLCMV-WT or rLCMV-GPCf (MOI of 0.01) in the presence of the indicated concentration of PF-429242, and at 12 and 36 hpi, the numbers of virus antigen-positive cells were determined by immunofluorescence assay using an antibody to NP. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Bars, 200 μm.
To further confirm that the effect of PF-429242 on LCMV multiplication was related to its ability to inhibit the S1P-mediated processing of the arenavirus GPC, we examined the effect of PF-429242 on the multiplication of recombinant VSVs, generated via reverse genetics, that expressed either WT VSV G (rVSV-WT) or LCMV GPC instead of VSV G (rVSV/LCMV-GPC) (31). We infected BHK-21 cells (MOI of 0.01) with either rVSV/LCMV-GPC or rVSV-WT in the presence of the indicated PF-429242 concentrations, and at 0, 12, and 24 hpi, we determined levels of infectious virus in TCS (Fig. 2C and D). At the lowest concentration of PF-429242 used (1 μM), we observed a dramatic inhibition (>3 logs) of the production of infectious titers of rVSV/LCMV-GPC, whereas the titers of rVSV-WT were not significantly affected. At higher concentrations of PF-429242 (10 and 30 μM), levels of infectious progeny of rVSV/LCMV-GPC were below the level of detection (>7-log reduction). At these higher concentrations, PF-429242 also caused a reduction in the infectious titers of rVSV-WT of about 1.5 logs (10 μM PF-429242) and 3 logs (30 μM PF-429242). Whether the effect of higher PF-429242 concentrations on rVSV-WT multiplication reflect off-target effects or a role for S1P in the life cycle of VSV, or both, remains to be determined.
Reduced production of infectious LCMV progeny in PF-429242-treated cells is predicted to reflect the inability of unprocessed GPC to be incorporated into arenavirus particles (16, 19, 34), which should result in a decreased PFU/total viral particle ratio in TCS from LCMV-infected cells and delayed virus spread following infection at a low MOI in the presence of PF-429242. We therefore first compared the PFU/total viral particle ratios of TCS from LCMV-infected cells (MOI of 1) in the absence or presence of PF-429242. For this experiment, we used a recombinant LCMV carrying a Flag-tagged version of Z (rLCMV/Z-Flag) to facilitate the detection of virion particles by WB. PF-429242 treatment resulted in a decreased PFU/total viral particle ratio in TCS from infected cells (Fig. 2E). Consistent with these findings, processing of GPC was inhibited in LCMV-infected cells treated with PF-429242 (Fig. 2F). Likewise, propagation of rLCMV-WT, but not of rLCMV-GPCf, was strongly reduced following infection (MOI of 0.01) in the presence of PF-429242, as determined by the numbers of LCMV antigen-positive cells (Fig. 2G).
Effect of PF-429242 on early steps of LCMV cell entry.
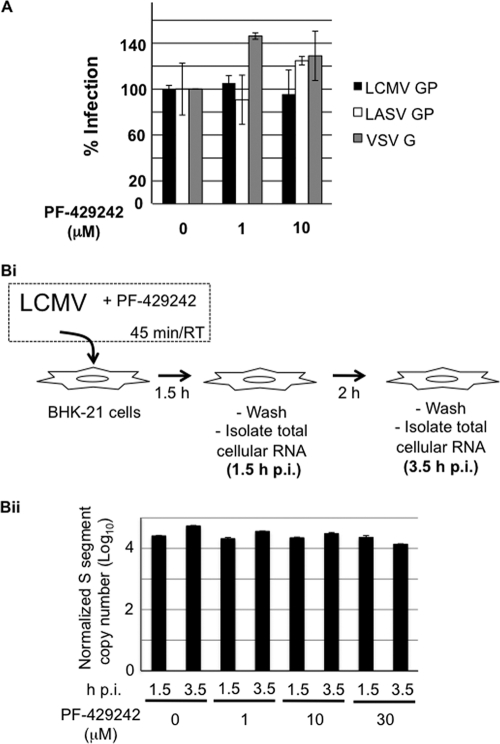
PF-429242 could have had a virucidal effect on LCMV or affected very early steps of LCMV multiplication that might have contributed to the antiviral activity of PF-429242. To specifically assess the effects of PF-429242 on cell entry of LASV and LCMV, we used recombinant retroviruses that bear the GPs of LCMV, LASV, and VSV in their envelope and contain a luciferase reporter in their genome (37). Since cell attachment and entry of arenaviruses are mediated exclusively by the viral GP, these retroviral pseudotypes allow the detection of possible interference of PF-429242 with these early steps in arenavirus infection. Retroviral pseudotypes were preincubated with increasing concentrations of PF-429242 for 45 min, followed by infection of BHK-21 cells in the presence of the drug. At 48 hpi, pseudotype infection was determined by detection of luciferase activity. As shown in Fig. 3 A, PF-429242 did not significantly affect infection with any of the pseudotypes, suggesting that the drug acts at a postentry step of infection.
FIG. 3.
(A) PF-429242 does not interfere with GP-mediated arenavirus cell entry. Retroviral pseudotypes bearing the GPs of LASV, LCMV, and VSV were preincubated with PF-429242 at the indicated concentrations for 45 min on ice and then added to fresh monolayers of BHK-21 cells for 1 h in the presence of the drug. Pseudotype infection was assessed by luciferase assay after 48 h. Data are luminescence levels normalized to those of untreated samples means ± standard deviations (n = 3). (B) PF-429242 does not significantly affect levels of viral genome RNA at early times during infection. (Bi) Schematic of the experiment. LCMV was incubated with PF-429242 (0,1, 10, or 30 μM) for 45 min before infection of BHK-21 cells (MOI of 3). At 1.5 hpi, infected cells were washed with PBS and fresh medium without PF-429242 was added. At 1.5 and 3.5 hpi, total cellular RNA was isolated. (Bii) Levels of S genome RNA. RNA (0.5 μg) from each sample was analyzed by RT-qPCR. Copy numbers of S genome RNA were normalized on the basis of GAPDH mRNA copy numbers. Data are averages and standard deviations from three independent experiments (P < 0.002).
We also used RT-qPCR to measure levels of cell-associated LCMV S genome RNA at 1.5 and 3.5 hpi (MOI of 3) of BHK-21 cells on the presence of the indicated concentrations of PF-429242 during the first 1.5 h of infection (Fig. 3B). Levels of S genome RNA were normalized with respect to levels of GAPDH mRNA also determined by RT-qPCR in the same sample. Levels of S genome RNA were very similar among all of the samples at 1.5 hpi, further indicating that PF-429242 did not affect the efficiency of virus cell internalization. However, PF-429242 caused a modest but significant (P < 0.002) dose-dependent decrease in levels of S genome RNA at 3.5 hpi.
Effect of PF-429242 on LCMV RNA replication.
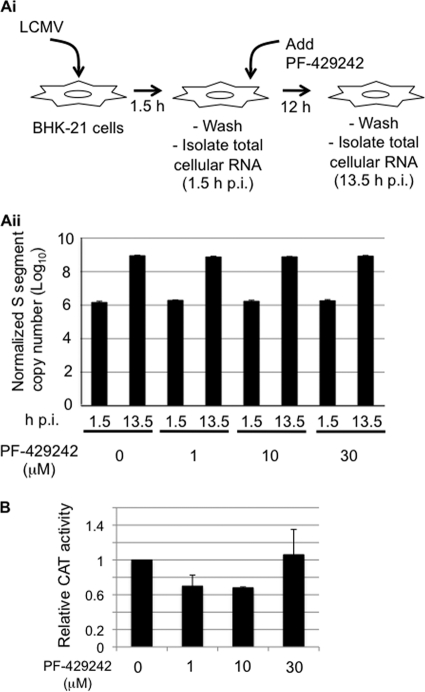
To examine the effect of PF-429242 on virus RNA replication, we infected BHK-21 cells with LCMV (MOI of 3) in the absence of PF-429242 during the first 1.5 h of infection, followed by addition of PF-429242 at the indicated concentrations. Levels of S genome RNA were similar among all of the samples at both 1.5 and 13.5 hpi (Fig. 4 A), suggesting that PF-429242 does not interfere with the steps involved in the initiation of viral RNA synthesis directed by the virus ribonucleoprotein (RNP) following its release in the cytoplasm of the cell upon completion of the virus cell entry process. Accordingly, we observed that PF-429242 did not significantly affect the expression levels of the CAT reporter gene produced by an LCMV minigenome (Fig. 4B).
FIG. 4.
PF-429242 does not affect arenavirus RNA replication and gene expression. (A) PF-429242 does not affect levels of arenavirus genome RNA replication. (Ai) Schematic of the experiment. BHK-21 cells were infected with LCMV (MOI of 3). At 1.5 hpi, the virus inoculum was removed, cells were washed with PBS, and fresh medium containing PF-429242 (0, 1, 10, or 30 μM) was added. At 1.5 and 13.5 hpi, total cellular RNA was isolated. (Aii) Levels of S genome RNA. RNA (0.5 μg) from each sample was analyzed by RT-qPCR. Copy numbers of S genome RNA were normalized on the basis of GAPDH mRNA copy numbers. Data are averages and standard deviations from three independent experiments. (B) Effect of PF-429242 on LCMV minigenome expression. BHK-21 cells were transfected with pC-T7, pMG-CAT, pC-NP, and pC-L under previously described conditions (17, 18, 26, 29, 30), and 5 h after transfection, the transfection media were replaced with fresh media containing PF-429242 (0, 1, 10, or 30 μM). At 48 h posttransfection, cell lysates were prepared for CAT ELISA. The CAT activity obtained from vehicle (DMSO)-treated cells was set to 1.0 to normalize CAT activities from the other samples. Data are averages and standard deviations from three independent experiments.
Effect of PF-429242 on Z-mediated budding.
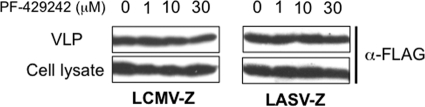
To examine whether PF-429242 affected virus budding, we used a cell-based assay for Z-mediated budding. This assay is based on the bona fide budding activity of Z that allows it to produce VLPs in the absence of other viral gene products (25, 41-43). For this assay, we transfected 293T cells with 0.25 μg of either pC-LASV-Z or pC-LCMV-Z, and at 5 h posttransfection, the medium was replaced with fresh medium including PF-429242 or a DMSO control. At 36 h posttransfection, we collected TCS and prepared cell lysates. Z-containing VLPs were isolated from TCS as described in Materials and Methods and previously (43). Levels of Z in cell lysates and VLP preparations were determined by WB using an antibody to Flag. PF-429242 treatment affected neither cell expression levels of LASV or LCMV Z nor Z-mediated budding (Fig. 5).
FIG. 5.
PF-429242 does not affect Z-mediated budding. 293T cells (2.5 × 105) were transfected with 0.25 μg of either pC-LCMV-Z or pC-LASV-Z, and at 5 h posttransfection, the media were replaced with fresh media containing PF-429242 (0, 1, 10, or 30 μM). At 36 h posttransfection, TCS were collected and total cell lysates were prepared. VLPs were isolated from TCS as described previously (43). Levels of Z protein in total cell lysates and VLPs were detected by WB using an antibody to Flag (Cayman catalog no. 162150).
Effect of PF-429242 on LASV multiplication in cultured cells.
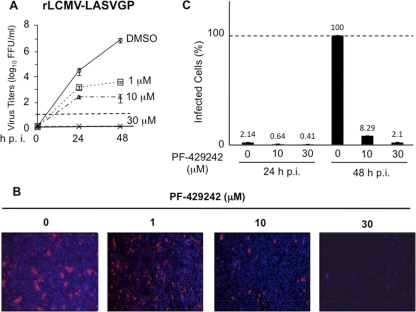
Inhibition of LCMV GPC cleavage by PF-429242 correlated with the ability of PF-429242 to inhibit the propagation of LCMV in cultured cells. We therefore examined whether this finding could be extended to highly pathogenic LASV. For this, we first examined the effect of PF-429242 on the propagation of a recombinant LCMV where LASV GPC was substituted for LCMV GPC (rLCMV-LASVGP) (36). We infected BHK-21 cells with rLCMV-LASVGP (MOI of 0.01) in the presence of different amounts of PF-429242, and at the indicated time points p.i., we determined the titers of infectious virus in TCS. PF-429242 treatment significantly reduced the production of infectious virus compared to that in DMSO-treated control cells (Fig. 6 A). Virus titers at 48 hpi were reduced by 3 and almost 5 logs by treatment with 1 μM and 10 μM, respectively. We could not detect infectious virus at 48 hpi in samples treated with 30 μM PF-429242. Consistent with these results, PF-429242 at a concentration (10 μM) associated with a modest decrease in cell viability also exhibited a strong inhibitory effect on the multiplication of LASV in Vero E6 cells (Fig. 6B and C and Table 1).
FIG. 6.
PF-429242 inhibits multiplication of LASV in Vero E6 cells. (A) BHK-21 cells were infected with rLCMV-LASVGP (MOI of 0.01). At 1.5 hpi, the virus inoculum was removed, cells were washed, and fresh medium containing PF-429242 (0, 1, 10, or 30 μM) was added. At the indicated times p.i., virus titers in TCS were determined. (B) Vero E6 cells were infected with LASV (MOI of 0.01). At 1.5 hpi, the virus inoculum was removed, cells were washed, and fresh medium containing PF-429242 (0, 1, 10, or 30 μM) was added. At 24 and 48 hpi, cells were fixed (4% paraformaldehyde-PBS) and infected cells were identified by immunofluorescence assay using a polyclonal antibody raised against LASV (kindly provided by Robert Tesh), followed by a secondary antibody labeled with Alexa Fluor 594. Nuclei were visualized by DAPI staining. (C) Quantitation of immunofluorescence assay data shown in panel B.
DISCUSSION
Individuals succumbing to LASV infection generate very limited or no anti-LASV immune responses, and histological examination of LF cases has revealed minimal immune cell infiltrates and tissue damage (45). These findings support the idea that the morbidity and mortality associated with LASV infection are due to the failure of the host's innate defense mechanisms to limit virus multiplication early during infection and to facilitate the initiation of an effective adaptive immune response capable of controlling and eliminating the infection. Accordingly, viremia levels are a good predictor of the outcome of LF patients (15). Notably, a similar scenario has been described in recent cases of fatal LCMV infection associated with organ transplantation (9, 28). Since arenavirus GPC processing by the cellular protease S1P is critical for productive arenavirus infection (16, 19, 32), the inhibition of this late step in the virus life cycle should slow down virus propagation, thus providing the host's immune system with a window of opportunity for the generation of an efficient antiviral immune response. Therefore, S1P-mediated processing of arenavirus GPC is an attractive target for therapeutic intervention against human pathogenic arenaviruses.
Previous studies have shown that expression in mammalian cells of S1P-adapted α1-antitrypsin variants resulted in the inhibition of S1P-mediated processing of the envelope GP of LASV (20) and also the human pathogen Crimean Congo HF virus (33). Since these protein-based S1P inhibitors have very poor cell-permeating ability, their application for antiviral therapy in vivo may be difficult. Recently, the peptide-based suicide inhibitor of the S1P catalytic activity decanoyl-RRLL-CMK was shown to be able to interfere with LCMV multiplication in cultured cells (35). However, the irreversible nature of this inhibition, also preventing the processing of S1P cellular targets, may pose difficulties for an optimal therapeutic approach aimed at specifically inhibiting S1P-mediated processing of arenavirus GPC while minimizing side effects on cell physiology. Moreover, CMK is very unstable and has a short half-life. Therefore, the identification of nontoxic and stable small-molecule inhibitors of S1P-mediated processing of arenavirus GPC could provide a novel type of antiarenaviral drugs. For this approach, a first and necessary step would be to prove that a small-molecule inhibitor of S1P can inhibit arenavirus multiplication in cultured cells under conditions associated with acceptable levels of cell toxicity. Here we have used PF-429242, a recently described reversible, competitive aminopyrrolidineamide inhibitor of S1P (12, 13), to provide proof of concept for the feasibility of using small-molecule inhibitors of S1P to inhibit the propagation of arenavirus in infected patients.
We confirmed that PF-429242 was able to inhibit the S1P-mediated processing of LCMV and LASV GPCs efficiently (Fig. 1). The specificity of this inhibition was supported by the observation that PF-429242 did not inhibit the processing of LCMV GPCf (Fig. 1A). In cell-based transfection assays, PF-429242 inhibited LASV GPC less efficiently than LCMV GPC. At 1 μM PF-429242, LCMV GPC, but not LASV GPC, cleavage was strongly inhibited (Fig. 1A). The reasons for these differences remain to be determined. However, it should be noted that S1P-mediated processing of LASV and LCMV GPCs takes place in the ER and Golgi compartments of the cell, respectively (4, 46). It is plausible that differences between these two subcellular environments could influence the activity of PF-429242 or its access to S1P or the S1P-GPC complex. Consistent with this possibility, PF-429242 very efficiently inhibited in vitro cleavage of a peptide containing the S1P recognition sequence present in LASV GPC (Fig. 1B).
The inhibitory activity of PF-429242 on S1P-mediated processing of LCMV GPC in transfection assays correlated with the compound's ability to inhibit the propagation of LCMV (Fig. 2A) and rVSV/LCMV-GPC (Fig. 2D) in cultured cells. We observed that multiplication of rLCMV-GPCf was also inhibited, though to lesser degree, by PF-429242 (Fig. 2B), a finding consistent with our previous observation that peak titers of rLCMV-GPCf are about 2 logs lower in S1P−/− cells than in S1P+/+ cells (35). Our new results further support the possibility that S1P plays other roles, in addition to the processing of GPC, in the arenavirus life cycle, which may also contribute to the barrier to the emergence of S1P-independent viral variants. For reasons as yet unknown, we observed a significantly stronger inhibitory effect of PF-429242 on the multiplication of rVSV/LCMV-GPC than on that of LCMV (compared Fig. 2A and D). On the other hand, the observed effect of PF-429242 on rVSV-WT multiplication may reflect off-target effects of the compound. However, we cannot exclude the possibility that S1P, via the processing of one or several of its cellular substrates, may play a role in the life cycle of VSV.
Treatment of LCMV-infected cells with PF-429242 caused a decreased in the PFU/total viral particle ratio in the TCS that was associated with reduced levels of intracellular processing of GPC. These findings strongly support the conclusion that inhibition of S1P-mediated processing of GPC played a key role in the antiviral activity associated with PF-429242. To further assess the specificity of the mechanism of action by which PF-429242 exerted its inhibitory effect on LCMV multiplication in cultured cells, we examined the effects of PF-429242 on several critical steps, other than GPC processing, of the virus life cycle, including very early steps of virus cell entry, amplification of genome RNA via replication, and budding. These studies indicated that PF-429242 does not affect GP-mediated arenavirus cell entry (Fig. 3) or virus RNA replication (Fig. 4) or budding (Fig. 5). Intriguingly, when PF-429242 treatment started at the time of virus adsorption, we observed a modest reduction in the levels of S genome RNA at 3.5 hpi in cells infected with rLCMV-WT (Fig. 3B). However, the same PF-429242 treatment schedule did not affect the numbers of rLCMV-GPCf-infected cells, as determined by detection of virus antigen expression (Fig. 2G). These results suggest a possible effect, though modest, of PF-429242 on an early entry event after the GP-mediated fusion event that releases the virus RNP into the cytoplasm of the infected cell to initiate virus RNA synthesis.
PF-429242 exhibited a stronger inhibitory effect on the propagation of rLCMV/LASV-GPC than on that of LCMV (compare Fig. 2A and 6A) following infection of BHK-21 cells with each virus at the same MOI. A possible explanation for these differences relates to the slower growth kinetics of rLCMV/LASV-GPC than LCMV, which may facilitate a more efficient inhibition by PF-429242 of S1P-mediated GPC processing. This, in turn, could result in lower infectious progeny output levels per cell, which would negatively impact virus propagation following infection at a low MOI. Results from cell-based transfection assays revealed that PF-429242 inhibited the S1P-mediated processing of LASV less efficiently than that of LCMV GPC. Consistent with this result, we observed that inhibition of the propagation of bona fide LASV in Vero E6 cells required 10 μM or a higher concentration of PF-429242 (Fig. 6B and C). Nevertheless, treatment with 10 μM PF-429242 resulted in a dramatic reduction of LASV antigen-positive cells at 48 hpi.
The key role of S1P-mediated processing of GPC in productive arenavirus infection, together with a low probability of the emergence of S1P-independent escape variants, makes S1P a promising cellular target for the development of novel and potent antiarenaviral drugs. Since S1P plays important roles in a number of physiological processes, the development of inhibitors that specifically target the processing of arenavirus GPC while having minimal effects on the processing of other cellular substrates of S1P would provide the ideal type of therapeutic drug. Here, we have documented that the small-molecule S1P inhibitor PF-429242 also inhibits the S1P-mediated processing of arenavirus GPC, which correlates with a potent antiviral activity in cell-based infectivity assays. Our results provide strong support for the implementation of screening efforts aimed at identifying additional small-molecule inhibitors of S1P-mediated GPC processing that could be developed into antiviral drugs to combat infection by arenaviruses pathogenic to humans. To this end, future efforts should be focused on examining the antiviral activity of PF-429242 in animal models of arenavirus infection.
Acknowledgments
We are grateful to B. Cubitt and S. Emonet for a helpful discussion and support and thank Cylia Rochat for her experimental contributions.
This work was supported by NIH grant R01 AI079665 to J.C.T. S.U. is supported by NIH grant T32-AI0735419. S.K. and A.P. were supported by Swiss National Science Foundation grant 3100A0-120250/1 to S.K.
This is the Scripps Research Institute paper 20830 from the Department of Immunology and Microbial Science.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Barton, L. L., M. B. Mets, and C. L. Beauchamp. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187:1715-1716. [DOI] [PubMed] [Google Scholar]
- 2.Basak, S., N. A. Stewart, M. Chretien, and A. Basak. 2004. Aminoethyl benzenesulfonyl fluoride and its hexapeptide (Ac-VFRSLK) conjugate are both in vitro inhibitors of subtilisin kexin isozyme-1. FEBS Lett. 573:186-194. [DOI] [PubMed] [Google Scholar]
- 3.Battegay, M. 1993. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 well plates. ALTEX 10:6-14. [PubMed] [Google Scholar]
- 4.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodvard, K., J. Mohlin, and W. Knecht. 2007. Recombinant expression, purification, and kinetic and inhibitor characterisation of human site-1-protease. Protein Expr. Purif. 51:308-319. [DOI] [PubMed] [Google Scholar]
- 6.Borio, L., T. Inglesby, C. J. Peters, A. L. Schmaljohn, J. M. Hughes, P. B. Jahrling, T. Ksiazek, K. M. Johnson, A. Meyerhoff, T. O'Toole, M. S. Ascher, J. Bartlett, J. G. Breman, E. M. Eitzen, Jr., M. Hamburg, J. Hauer, D. A. Henderson, R. T. Johnson, G. Kwik, M. Layton, S. Lillibridge, G. J. Nabel, M. T. Osterholm, T. M. Perl, P. Russell, and K. Tonat. 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391-2405. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier, M. J., C. J. Peters, and J. C. de la Torre. 2007. Arenaviridae: the viruses and their replication, p. 1792-1827. In D. L. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 8.Crouch, S. P., R. Kozlowski, K. J. Slater, and J. Fletcher. 1993. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160:81-88. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, S. A., M. B. Graham, M. J. Kuehnert, C. N. Kotton, A. Srinivasan, F. M. Marty, J. A. Comer, J. Guarner, C. D. Paddock, D. L. DeMeo, W. J. Shieh, B. R. Erickson, U. Bandy, A. DeMaria, Jr., J. P. Davis, F. L. Delmonico, B. Pavlin, A. Likos, M. J. Vincent, T. K. Sealy, C. S. Goldsmith, D. B. Jernigan, P. E. Rollin, M. M. Packard, M. Patel, C. Rowland, R. F. Helfand, S. T. Nichol, J. A. Fishman, T. Ksiazek, and S. R. Zaki. 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 354:2235-2249. [DOI] [PubMed] [Google Scholar]
- 10.Flatz, L., A. Bergthaler, J. C. de la Torre, and D. D. Pinschewer. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. U. S. A. 103:4663-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert, T. W., and P. B. Jahrling. 2004. Exotic emerging viral diseases: progress and challenges. Nat. Med. 10:S110-S121. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins, J. L., M. D. Robbins, L. C. Warren, D. Xia, S. F. Petras, J. J. Valentine, A. H. Varghese, I. K. Wang, T. A. Subashi, L. D. Shelly, B. A. Hay, K. T. Landschulz, K. F. Geoghegan, and H. J. Harwood, Jr. 2008. Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J. Pharmacol. Exp. Ther. 326:801-808. [DOI] [PubMed] [Google Scholar]
- 13.Hay, B. A., B. Abrams, A. Y. Zumbrunn, J. J. Valentine, L. C. Warren, S. F. Petras, L. D. Shelly, A. Xia, A. H. Varghese, J. L. Hawkins, J. A. Van Camp, M. D. Robbins, K. Landschulz, and H. J. Harwood, Jr. 2007. Aminopyrrolidineamide inhibitors of site-1 protease. Bioorg. Med. Chem. Lett. 17:4411-4414. [DOI] [PubMed] [Google Scholar]
- 14.Jahrling, P. B., and C. J. Peters. 1992. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch. Pathol. Lab. Med. 116:486-488. [PubMed] [Google Scholar]
- 15.Johnson, K. M., J. B. McCormick, P. A. Webb, E. S. Smith, L. H. Elliott, and I. J. King. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:456-464. [DOI] [PubMed] [Google Scholar]
- 16.Kunz, S., K. H. Edelmann, J. C. de la Torre, R. Gorney, and M. B. Oldstone. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168-178. [DOI] [PubMed] [Google Scholar]
- 17.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de Latorre. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, K. J., M. Perez, D. D. Pinschewer, and J. C. de la Torre. 2002. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 76:6393-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenz, O., J. ter Meulen, H. D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. U. S. A. 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maisa, A., U. Stroher, H. D. Klenk, W. Garten, and T. Strecker. 2009. Inhibition of Lassa virus glycoprotein cleavage and multicycle replication by site 1 protease-adapted alpha(1)-antitrypsin variants. PLoS Negl. Trop. Dis. 3:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever, p. 75-110. In M. B. Oldstone (ed.), Arenaviruses I, vol. 262. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 22.Mets, M. B., L. L. Barton, A. S. Khan, and T. G. Ksiazek. 2000. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am. J. Ophthalmol. 130:209-215. [DOI] [PubMed] [Google Scholar]
- 23.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 24.Pasquato, A., P. Pullikotil, M. C. Asselin, M. Vacatello, L. Paolillo, F. Ghezzo, F. Basso, C. Di Bello, M. Dettin, and N. G. Seidah. 2006. The proprotein convertase SKI-1/S1P. In vitro analysis of Lassa virus glycoprotein-derived substrates and ex vivo validation of irreversible peptide inhibitors. J. Biol. Chem. 281:23471-23481. [DOI] [PubMed] [Google Scholar]
- 25.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. U. S. A. 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez, M., and J. C. de la Torre. 2003. Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 77:1184-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters, C. J. 2002. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 262:65-74. [DOI] [PubMed] [Google Scholar]
- 28.Peters, C. J. 2006. Lymphocytic choriomeningitis virus—an old enemy up to new tricks. N. Engl. J. Med. 354:2208-2211. [DOI] [PubMed] [Google Scholar]
- 29.Pinschewer, D. D., M. Perez, and J. C. de la Torre. 2005. Dual role of the lymphocytic choriomeningitis virus intergenic region in transcription termination and virus propagation. J. Virol. 79:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinschewer, D. D., M. Perez, and J. C. de la Torre. 2003. Role of the virus nucleoprotein in the regulation of lymphocytic choriomeningitis virus transcription and RNA replication. J. Virol. 77:3882-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinschewer, D. D., M. Perez, E. Jeetendra, T. Bachi, E. Horvath, H. Hengartner, M. A. Whitt, J. C. de la Torre, and R. M. Zinkernagel. 2004. Kinetics of protective antibodies are determined by the viral surface antigen. J. Clin. Invest. 114:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinschewer, D. D., M. Perez, A. B. Sanchez, and J. C. de la Torre. 2003. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 100:7895-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pullikotil, P., M. Vincent, S. T. Nichol, and N. G. Seidah. 2004. Development of protein-based inhibitors of the proprotein of convertase SKI-1/S1P: processing of SREBP-2, ATF6, and a viral glycoprotein. J. Biol. Chem. 279:17338-17347. [DOI] [PubMed] [Google Scholar]
- 34.Rojek, J. M., A. M. Lee, N. Nguyen, C. F. Spiropoulou, and S. Kunz. 2008. Site 1 protease is required for proteolytic processing of the glycoproteins of the South American hemorrhagic fever viruses Junin, Machupo, and Guanarito. J. Virol. 82:6045-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojek, J. M., G. Pasqual, A. B. Sanchez, N. T. Nguyen, J. C. de la Torre, and S. Kunz. 2010. Targeting the proteolytic processing of the viral glycoprotein precursor is a promising novel antiviral strategy against arenaviruses. J. Virol. 84:573-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojek, J. M., A. B. Sanchez, N. T. Nguyen, J. C. de la Torre, and S. Kunz. 2008. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J. Virol. 82:7677-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojek, J. M., C. F. Spiropoulou, K. P. Campbell, and S. Kunz. 2007. Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan's host-derived ligands. J. Virol. 81:5685-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai, J., A. Nohturfft, J. L. Goldstein, and M. S. Brown. 1998. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J. Biol. Chem. 273:5785-5793. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez, A. J., M. J. Vincent, and S. T. Nichol. 2002. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J. Virol. 76:7263-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidah, N. G., S. J. Mowla, J. Hamelin, A. M. Mamarbachi, S. Benjannet, B. B. Toure, A. Basak, J. S. Munzer, J. Marcinkiewicz, M. Zhong, J. C. Barale, C. Lazure, R. A. Murphy, M. Chretien, and M. Marcinkiewicz. 1999. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc. Natl. Acad. Sci. U. S. A. 96:1321-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strecker, T., R. Eichler, J. Meulen, W. Weissenhorn, H. Dieter Klenk, W. Garten, and O. Lenz. 2003. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urata, S., T. Noda, Y. Kawaoka, H. Yokosawa, and J. Yasuda. 2006. Cellular factors required for Lassa virus budding. J. Virol. 80:4191-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urata, S., J. Yasuda, and J. C. de la Torre. 2009. The Z protein of the New World arenavirus Tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. J. Virol. 83:12651-12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent, M. J., A. J. Sanchez, B. R. Erickson, A. Basak, M. Chretien, N. G. Seidah, and S. T. Nichol. 2003. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J. Virol. 77:8640-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]
- 46.Wright, K. E., R. C. Spiro, J. W. Burns, and M. J. Buchmeier. 1990. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology 177:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]