Abstract
Functional defects in cytotoxic CD8+ T cell responses arise in chronic human viral infections, but the mechanisms involved are not well understood. In mice, CD4 cell-mediated interleukin-21 (IL-21) production is necessary for the maintenance of CD8+ T cell function and control of persistent viral infections. To investigate the potential role of IL-21 in a chronic human viral infection, we studied the rare subset of HIV-1 controllers, who are able to spontaneously control HIV-1 replication without treatment. HIV-specific triggering of IL-21 by CD4+ T cells was significantly enriched in these persons (P = 0.0007), while isolated loss of IL-21-secreting CD4+ T cells was characteristic for subjects with persistent viremia and progressive disease. IL-21 responses were mediated by recognition of discrete epitopes largely in the Gag protein, and expansion of IL-21+ CD4+ T cells in acute infection resulted in lower viral set points (P = 0.002). Moreover, IL-21 production by CD4+ T cells of HIV controllers enhanced perforin production by HIV-1-specific CD8+ T cells from chronic progressors even in late stages of disease, and HIV-1-specific effector CD8+ T cells showed an enhanced ability to efficiently inhibit viral replication in vitro after IL-21 binding. These data suggest that HIV-1-specific IL-21+ CD4+ T cell responses might contribute to the control of viral replication in humans and are likely to be of great importance for vaccine design.
CD4+ T cell help is essential to generate long-lived antiviral CD8+ T cell memory (17, 18). Although antigen-specific CD8+ T cells can be primed in the absence of CD4+ T cell help, secondary expansion upon antigen reencounter is inefficient under such circumstances (7, 11, 18, 22). Progressive loss of CD4+ T cells in human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV) infections has been associated with dysfunction of virus-specific CD8+ T cells and ineffective containment of these chronic viral infections. Moreover, under repetitive antigenic stimulation, virus-specific cytotoxic CD8+ T cells (CTL) become increasingly impaired, exhibiting decreased effector functions and upregulation of negative immunoregulatory molecules (2, 19). This dysfunction is likewise more severe in the absence of CD4+ T cell help (21).
The nature of CD4 help required to control chronic human infections remains unclear. In mice, recent studies indicate that CD4+ T cell production of interleukin-21 (IL-21), a common γ-chain cytokine, is required for maintenance of CD8+ T cell function in persistent but not resolving viral infections (3, 4, 23). During lymphocytic choriomeningitis virus (LCMV) infection, expansion of CD4+ T helper cells producing IL-21 is required for sustained CD8+ T cell proliferation and control of viremia. In contrast, mice lacking either IL-21 or the IL-21 receptor are more susceptible to uncontrolled chronic LCMV infection, providing evidence that this cytokine is a key regulator of viral control in a murine model of chronic viral infection.
MATERIALS AND METHODS
Study subjects.
HIV-1-seropositive subjects and uninfected healthy control volunteers were recruited at the Massachusetts General Hospital after providing written informed consent for participation in the study (see Table S1 in the supplemental material). Persons were divided into four groups: HIV-1 controllers (viral loads of <2,000 RNA copies/ml for at least 1 year in the absence of any antiretroviral therapy [ART]), HIV-1 chronic progressors (treatment naïve and with >10,000 RNA copies/ml), subjects on antiretroviral therapy (>6 months of treatment) with fully suppressed viral loads (<50 RNA copies/ml), and HIV-negative individuals (see Table S1 in the supplemental material for patient characteristics). Subjects with acute infection were enrolled at the Jessen-Jessen-Stein HIV clinic in Berlin, Germany, or at the Fenway Community Health Center, Boston, MA. Acute HIV-1 infection was defined as negative anti-HIV p24 antibody enzyme-linked immunosorbent assay (ELISA) or positive ELISA with fewer than three bands in the Western blot. The present study was approved by MGH institutional review boards and was conducted in accordance with the organization's human experimentation guidelines.
Measurement of IL-21 secretion using a combined transwell-microbead capture assay.
CD8+ T cells were depleted from peripheral blood mononuclear cells (PBMCs) using RosetteSep technology (Stemcell Technology) or magnetic beads (MACS). The efficiency of depletion was consistently above 95%. Depletion with lower efficiency was not used in subsequent assays. CD4+ T cells counts in comparison to counts of other PBMCs after depletion was assessed but was not significantly different between the groups. A total of 5 × 105 PBMCs in R10 (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum [FCS] and penicillin-streptomycin) were placed in the bottom chamber of a 96-well HTS transwell permeable support (Corning Inc.; 0.4-μm-pore polycarbonate membranes) and stimulated with pooled HIV peptides (Gag, Pol, Nef, gp120, gp41, and a pool containing the proteins Vif, Vpr, Vpu, Rev, and Tat [VVVRT]) as well as phorbol myristate acetate (PMA)-ionomycin (AG Scientific) as a positive control. For the Gag epitope mapping, cells were stimulated using single overlapping peptides (OLPs) spanning the entire Gag protein (66 peptides synthesized by the MGH Peptide Core Facility). These peptides are 15- to 20-mers overlapping by 10 amino acids and were based on the HIV clade B consensus sequence available from the Los Alamos National Laboratory HIV immunology database (www.hiv.lanl.gov). Luminex anti-IL-21 microbeads (Milliplex MAP kit; Millipore) were added to the upper chamber in the transwell assays. After 40 h of incubation at 37°C with 5% CO2, microbeads were harvested, washed twice, and processed according to the manufacturer's protocol. Plates were run in a Bio-Plex reader (Bio-Plex 100IS; Bio-Rad). Results were analyzed using a 5PL-fit standard curve derived from recombinant cytokine standards.
Measurement of IL-21 secretion by flow cytometry.
A total of 2 × 106 PBMCs were stimulated with HIV peptide pools in R10 containing 50 ng/ml IL-21. Staphylococcal enterotoxin B (SEB) served as a positive control and the respective medium as a negative control. Cells were stimulated for 6 h in the presence of brefeldin A and monensin at 37°C with 5% CO2. Cells were then stained with an amine-live/dead discrimination dye (Invitrogen). After a wash with 2% FCS-phosphate-buffered saline (PBS), cells were stained using anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CCR4, anti-CCR5, anti-CCR6, anti-CXCR3, anti-CXCR4, anti-CXCR5, or anti-IL23R. Cells were then fixed and permeabilized using FixPerm A and B (Caltag, Invitrogen) according to the manufacturer's procedure. Cells were then stained for gamma interferon (IFN-γ), IL-21, IL-17, IL-5, IL-4, and tumor necrosis factor alpha (TNF-α). Samples were acquired on a BD LSRII (BD Biosciences) multicolor flow cytometer and analyzed using FlowJo 8.8.6 software (TreeStar). Initial gating was on the lymphocyte population; a plot of forward scatter width (FSC-W) versus height (FSC-H) was used to remove doublets. CD4+ T cells were defined as viability dye−, CD19−, CD8−, CD3+, and CD4+ lymphocytes.
Quantitation of IL-21R expression on antigen-specific CD8+ T cells using multiparameter flow cytometry.
Cryopreserved PBMCs were thawed, resuspended at 1 × 106 cells/ml in R10 medium, and rested for 2 h at 37°C with 5% CO2. Cells were then washed with PBS and stained with allophycocyanin (APC)-labeled HLA class I tetramers/pentamers (Beckman-Coulter; Proimmune) refolded with the respective CD8+ T cell epitopes. Following 20 min of incubation at room temperature, cells were washed with PBS-2% FCS, resuspended in R10, and stimulated for 5 h at 37°C with 5% CO2 in the presence or absence of IL-21 (50 ng/ml) and in the presence or absence of CD8+ T cell optimal epitopes (2 μg/ml). Cells were then washed with PBS-2% FCS and stained for intracellular amino groups to discriminate between live and dead cells (violet viability dye; Invitrogen). After an additional wash, the following surface antibodies were added: anti-IL-21 receptor (anti-IL-21R)-phycoerythrin (PE) (R&D), anti-CD3-Qdot (Invitrogen), and anti-CD8-APC-Cy7 (BD Biosciences). Cells were washed and fixed with 1% paraformaldehyde. Samples were acquired on a BD LSRII (BD Biosciences) multicolor flow cytometer and analyzed using FlowJo 8.8.6 software (TreeStar). Initial gating was on the lymphocyte population; a plot of forward scatter width (FSC-W) versus height (FSC-H) was used to remove doublets. Expression of IL-21R was measured on HIV-1-specific cells (tetramer+ CD8+ CD3+ CD19− CD14− cells).
Functional analysis of antigen-specific CD8+ T cells by flow cytometry.
The functionality of CD8+ T cells was assessed as previously described (16). Briefly, cells were stimulated in the presence or absence of IL-21 (50 ng/ml) with Gag (each OLP at 2 μg/ml), anti-CD28/anti-CD49d (BD Biosciences) in the presence of anti-CD107a-PE-Cy5 (BD Biosciences), monensin (BD Biosciences), and brefeldin A (Sigma-Aldrich) for 5 h at 37°C. Cells were consecutively washed, stained with viability dye (Invitrogen) and anti-CD3-Qdot605 (Invitrogen), anti-CD8-APC-Cy7 (BD Biosciences), anti-PD1-APC (eBiosicence), and anti-CD4-Qdot655 (Invitrogen). Cells were fixed with 1% paraformaldehyde, washed, permeabilized (Fix Perm B solution; Caltag Invitrogen), and stained intracellularly using a panel of anti-IL-2-fluorescein isothiocyanate (FITC), anti-IFN-γ-PE-Cy7, anti-TNF-α-Alexa700, and anti-MIP1β-PE (all from BD Biosciences). Samples were analyzed as described above.
Perforin expression in antigen-specific CD8+ T cells determined by flow cytometry.
PBMCs were incubated for 5 h in the presence of IL-2, IL-4, IL-7, IL-15, or IL-21. Cells were stained as described above and intracellularly stained using anti-perforin-PE (Tepnel; Lifecodes Corp.).
Measurement of perforin expression by real-time quantitative PCR.
CD8+ T cells were isolated from PBMCs by MACS sorting (Miltenyi Biotec) and cultured in R10 medium in the presence or absence of IL-21 for 5 h at 37°C with 5% CO2. Cells were then resuspended in RNeasy lysis buffer (Qiagen) containing 1% β-mercaptoethanol following the manufacturer's protocol. Briefly, total RNA was isolated using the RNeasy kit (Qiagen) and treated with DNase (Invitrogen), followed by reverse transcription using a high-capacity RNA-to-cDNA master mix (Applied Biosystems). Quantitative real-time PCR was performed using Brilliant SYBR green (Stratagene) on an Mx3005P real-time thermocycler (Stratagene). Each sample included a control without reverse transcriptase, and all reactions were done in triplicate; results were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and expressed as relative mRNA expression level. Primers were designed so that the amplicons span at least one intron, and product specificity was verified by melting temperature (Tm) measurement and standard PCR. Primers were as follows: for GAPDH, 5′-ACCCACTCCTCCACCTTTGA-3′ (forward) and 5′-TGGTGGTCCAGGGGTCTTAC-3′ (reverse); for IL-21, 5′-AGCTCCCAAGGTCAAGATCG-3′ (forward) and 5′-CTGTCTTCTCCCTGCATTTGTG-3′ (reverse); for perforin, 5′-CATGTAACCAGGGCCAAAGTC-3′ (forward) and 5′-ATGAAGTGGGTGCCGTAGTTG-3′ (reverse); for Granzyme A, 5′-CCTCATTCAAGACCCTACATGG-3′ (forward) and 5′-TGGCTCTTCCCTGGTTATTG-3′ (reverse); and for Granzyme B, 5′-GGCTTCCTGATACGAGACGAC-3′ (forward) and 5′-TCCTGCACTGTCATCTTCACC-3′ (reverse).
Viral inhibition assay.
Autologous CD4 lymphocytes were prepared as described previously (8), infected at day 3 with the NL4-3 HIV-1 isolate at a multiplicity of infection (MOI) of 0.01 for 4 h at 37°C, washed twice, resuspended in medium, and plated at 105 cells/well. Effector cells, consisting of purified CD8+ T cells from the same donor, were separated, rested for 2 days in medium alone, and preincubated in the presence or absence of IL-21 for 5 h. Cells were then washed, added at an effector/target ratio of 1:1, and coincubated in IL-2-supplemented medium at 37°C with 5% CO2. Infected autologous CD4+ T cells alone and uninfected CD4+ T cells served as positive and negative controls, respectively. Supernatant was collected at baseline and days 3, 5, and 7. Viral replication was quantified by p24 ELISA (Perkin-Elmer) in duplicates.
Transwell coincubation assay.
Cryopreserved PBMCs from HIV controllers were CD8+ T cell depleted (MACS), either rested or stimulated with the Gag peptide pool (2 μg/ml) for 1 h at 37°C, and washed, and 1 million cells were plated in the bottom chambers of transwell plates. Three hundred thousand CD8+ T cells from noncontrollers were added in the upper chambers, and when indicated, IL-21 signaling was blocked using anti-IL-21 (10 μg/ml) and anti-IL-21 receptor (25 μg/ml) antibodies. Cells were coincubated for 12 h at 37°C with 5% CO2. CD8+ T cells were harvested, and perforin expression was measured by quantitative PCR.
RESULTS AND DISCUSSION
To explore the potential role of IL-21 as a mediator of CD4+ T cell help in a chronic human viral infection, we analyzed this cytokine in persons infected with HIV-1, examining those with progressive uncontrolled viremia (which is the usual outcome of infection) as well as the rare subset of HIV-1 controllers (13), who spontaneously maintain viral loads at low levels (<2,000 RNA copies/ml plasma) in the absence of antiretroviral therapy.
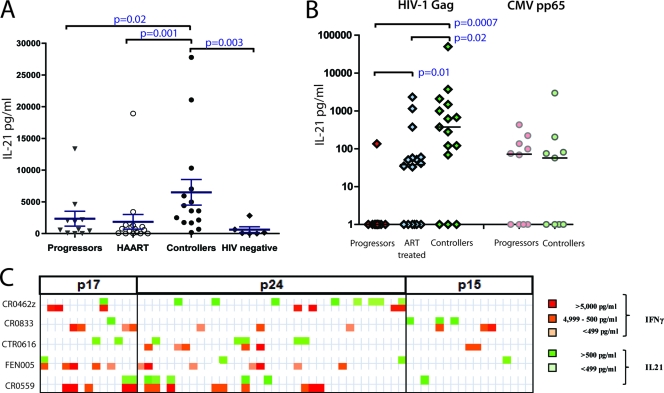
We first developed a novel technique to quantitate IL-21 secretion by CD4+ T cells: coculturing of anti-IL-21 microbeads separated by a 0.4-μm transwell permeable membrane from CD8-depleted PBMCs stimulated in vitro. Released IL-21 was captured by the beads and then measured by multiplex technology. Using this approach (Fig. 1 A), we found significantly elevated levels of IL-21 secretion by CD4+ T cells from HIV-1 controllers upon stimulation with phorbol myristate acetate (PMA) and ionomycin compared to those for HIV-1 infected persons with persistent viremia (P = 0.02), subjects with persistent viremia successfully suppressed with antiretroviral therapy (ART) (P = 0.001), and HIV-1-uninfected individuals (P = 0.003), as previously described (6). However, there was no significant difference in IL-21 secretion between HIV-infected chronic progressors and ART-treated or HIV-1-uninfected individuals.
FIG. 1.
IL-21 production by CD4+ T cells in HIV-1-infected subjects. (A and B) CD8-depleted PBMCs from HIV-1 chronic progressors, ART-treated patients, HIV-1 controllers, and HIV-negative subjects were stimulated with PMA-ionomycin (A) or with HIV Gag peptide pool or the CMV peptide pp65 (B) for 40 h at 37°C in the bottom chamber of a transwell plate with anti-IL-21 Luminex beads coincubated in the upper chamber. Beads were then used for the quantification of secreted IL-21. Mann-Whitney t tests were performed; only significant P values are indicated. Significantly higher IL-21 responses were detected in the controllers than in the other groups. HIV-specific IL-21+ responses were directed against epitopes within the Gag protein in HIV-1 controllers, whereas rare specific responses were detected in the progressors. Error bars indicate standard deviations. (C) Analysis of the secretion of IL-21 and IFN-γ in five HIV-1 controllers following stimulation with individual peptides spanning the HIV Gag proteins p17, p24, and p15. The color intensity indicates the relative magnitude of the IFN-γ response and the IL-21 response to each peptide.
Given the association between IL-21 production and disease outcome described above, we next evaluated whether IL-21 production might be triggered by virus-specific immune cells. We stimulated CD8-depleted T cells from each group with overlapping HIV Gag peptides, which are known to be the major stimulus for CD4+ T cell IFN-γ production in chronic HIV-1 infection (9, 14). The same capture assay reproducibly detected HIV-1-specific IL-21+ CD4+ T cell responses in HIV-1 controllers (Fig. 1B) as well as a nearly complete lack of these responses in HIV-1-infected progressors (P < 0.001). In contrast, these HIV-1-infected progressors exhibited robust IL-21+ CD4+ T cell responses against the cytomegalovirus (CMV) peptide pp65 at levels similar to those observed in HIV controllers, pointing to an HIV antigen-specific loss of IL-21-secreting CD4+ T cells in chronic, progressive HIV-1 infection (Fig. 1B). Although Gag was the strongest inducer, IL-21 production was occasionally triggered by other viral proteins, including gp41, gp120, and Pol. These responses were present particularly in subjects on fully suppressive antiretroviral therapy; however, no significant differences were observed between the patient groups (see Fig. S1 in the supplemental material). In addition, a small number of HIV controllers did not mount a detectable IL-21+ CD4+ T cell response. While the reason for this occurrence is not straightforward, several potential explanations are plausible, including viral escape from IL-21+ CD4+ T cell responses, IL-21 responses below the limit of detection, or alternative, IL-21-independent mechanisms of antiviral function in these subjects.
Given the prominent role of Gag in the induction of HIV-specific CD4+ T cell responses, we next assessed the relative secretion of IL-21 and IFN-γ upon antigenic stimulation with individual overlapping 15- to 20-mer Gag peptides in CD8-depleted T cells from five HIV-1 controllers. In each individual tested, multiple different Gag peptides were able to induce IL-21 secretion; although some peptides elicited both IL-21 and IFN-γ, the majority of immunogenic peptides were discordant for these responses, indicating that the effector populations were largely nonoverlapping (Fig. 1C). These responses were enriched in the Gag region p24 when overlapping peptides spanning the entire Gag region was tested (Fig. 1C; see Table S2 in the supplemental material).
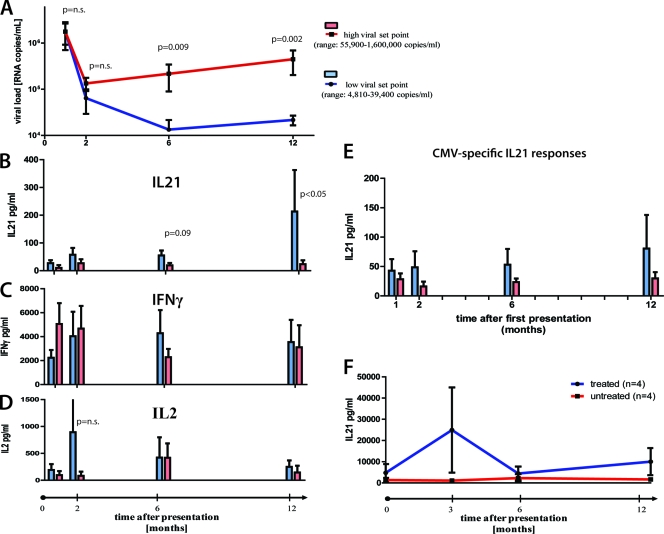
Given the robust differences in IL-21 CD4+ T cell frequencies between individuals with controlled versus progressive disease, we were interested in whether there is a causative link between HIV-1-specific IL-21+ CD4+ T cell response induction and viral control. We therefore selected 12 individuals with acute HIV infection, following them over 1 year into the chronic phase of infection. All subjects had been diagnosed with symptomatic acute HIV-1 infection, with a negative or indeterminate Western blot (<3 bands positive), in the presence of detectable viremia (average viral load, 2,951,214 RNA copies/ml; range, 250,000 to 10,000,000 RNA copies/ml). Gag-specific IL-21+ CD4+ T cell responses were detectable in most patients within 6 months of acute HIV-1 infection. However, six subjects were able to maintain robust IL-21+ CD4+ T cell responses in the first 12 months of the infection and had a significantly lower viral load than subjects lacking these responses (4,810 to 39,400 copies/ml versus 55,900 to 1,600,000 RNA copies/ml [P = 0.002]) (Fig. 2A and B), yet the two groups did not differ in their viral loads at baseline level (1,780,667 versus 2,042,800 [P = 0.98]). Moreover, the same individuals maintained stable frequencies or decreasing IFN-γ- or IL-2-secreting Gag-specific CD4+ T cell responses over the course of 1 year (Fig. 2C and D), suggesting a preferential expansion of HIV-1-specific IL-21+ CD4+ T cells in HIV-1 controllers. Interestingly, subjects able to maintain their IL-21 response show a trend, but not a significant peak, of HIV-specific IL-2+ CD4+ T cell responses, which warrants further investigation. The selective expansion of IL-21-secreting CD4+ T cells was HIV specific, as CMV-specific IL-21+ CD4+ T cells remained unchanged from acute to chronic infection (Fig. 2E). It is important to note, however, that IL-21 responses alone most likely do not explain enhanced control of viral replication but rather contribute to an enhanced ability to control viral replication. Interestingly, viral suppression by ART restored IL-21 secretion of Gag-specific CD4+ T cells, although the levels were significantly lower than in HIV-1 controllers (P = 0.02). Longitudinal analysis following treatment in acute HIV-1 infection showed that these responses initially expanded but then declined (Fig. 2F). Thus, our data indicate that the expansion of HIV-specific IL-21+ CD4+ T cells contributes to enhanced viral control early in infection and that the loss of IL-21-secreting CD4+ T cells is characteristic of progressive HIV disease.
FIG. 2.
IL-21+ CD4+ T cell responses in acute HIV infection. (A) Twelve individuals with acute HIV infection were grouped based on their viral load set points at 12 month after presentation (high, 55,900 to 1,600,000 RNA copies/ml; low, 4,810 to 39,400 RNA copies/ml). PBMCs from these individuals were obtained at baseline and 2, 6, and 12 months after initial presentation. (B to D) PBMCs were CD8 depleted and stimulated with Gag peptide pool, and IL-21 (B), IL-2 (C), and IFN-γ (D) secretion was measured by Luminex. (E) Following the same protocol, CMV-specific IL-21 secretion was assessed at the same time points in all individuals. (F) Longitudinal assessment of IL-21 production by Gag-stimulated CD4+ T cells in treated (n = 4) and untreated (n = 4) subjects during acute HIV-1 infection, showing an initial increase in the early phase of acute infection in treated subjects which then decreased. Error bars indicate standard deviations.
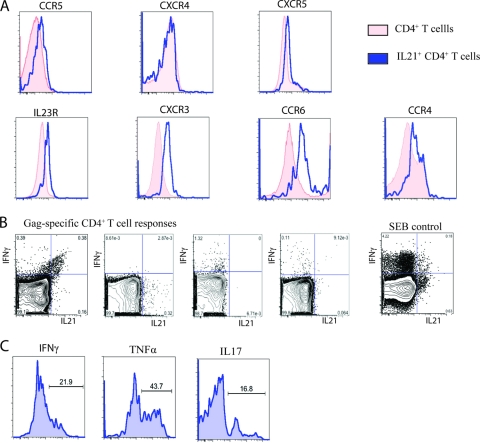
To further characterize the properties of the IL-21+ CD4+ T cells, we analyzed the phenotype of the HIV-1-specific IL-21+ CD4+ T cells in controllers by cytokine flow cytometry (Fig. 3 A). We were able to visualize clear but discrete Gag-specific IL-21+ CD4+ T cell populations in these individuals after stimulation with Gag peptides in IL-21-supplemented medium using intracellular cytokine staining. Addition of IL-21 was necessary to induce the previously described autocrine feedback regulation of IL-21 secretion (12). We compared HIV-1-specific CD4+ T cells that secrete either IFN-γ or IL-21 and observed that the IL-21-secreting population exhibited higher expression levels of the HIV coreceptor CCR5, which has been linked to greater susceptibility to infection in vivo (1). In addition, the IL-21+ CD4+ T cells also showed increased expression of IL23R, CXCR3, CCR6, and CCR4, which have been previously described to also be increased on effector CD4+ T cells such as Th1 or Th17 cells (Fig. 3A) (5, 12).
FIG. 3.
Phenotypic and functional characteristics of HIV-1-specific IL-21+ CD4+ T cells. (A and B) Phenotypic (A) and functional (B) characterization of HIV-1-specific IL-21+ CD4+ T cells (representative for six controllers). For assessing intracellular cytokine secretion, cells of four HIV controllers were stimulated as indicated with the Gag peptide pool or SEB for 5 h in IL-21-supplemented medium (50 ng/ml). (C) Functional characterization of IL-21+ CD4+ T cells after SEB stimulation.
In addition, we confirmed our previous finding that IL-21 and IFN-γ are not necessarily cosecreted by using flow cytometry for different Gag-specific CD4+ T cell responses (Fig. 3B). To further characterize the functional properties of IL-21-secreting CD4+ T cells in humans and to investigate whether IL-21+ CD4+ T cells are able to secrete additional cytokines, we stimulated PBMCs from HIV-1 controllers for 6 h with staphylococcal enterotoxin B (SEB) in IL-21-supplemented medium. Interestingly, while a fraction of the IL-21+ CD4+ T cells were simultaneously secreting IFN-γ or TNF-α, a majority of these cells stained negative for both cytokines. Furthermore, we also detected IL-21+ CD4+ T cells cosecreting the proinflammatory cytokine IL-17, confirming previous findings from the murine model (Fig. 3C) (12). Although we cannot fully exclude that the addition of exogenous recombinant IL-21 might skew the responses seen in vitro, these findings confirm via a separate approach the results of our multiplex bead based capture assay described above. Moreover, a recent publication by Iannello et al. also suggests higher IL-21 expression in CD4+ T cells from HIV controllers than in those from progressors (6), which is in contrast to a publication by Yue et al. describing lower levels of IL-21-secreting CD4+ T cells in long-term nonprogressors (<200 RNA copies/ml) (24). While the explanation for this discrepancy is not immediately apparent, it is interesting to note that Yue et al. observed the highest level of HIV-specific IL-21-secreting CD4+ T cells in individuals with “relative control” (defined as having viral loads between >200 and 20,000 RNA copies/ml). The divergent findings of the latter paper may also be due to detection limitations of the specific assay used.
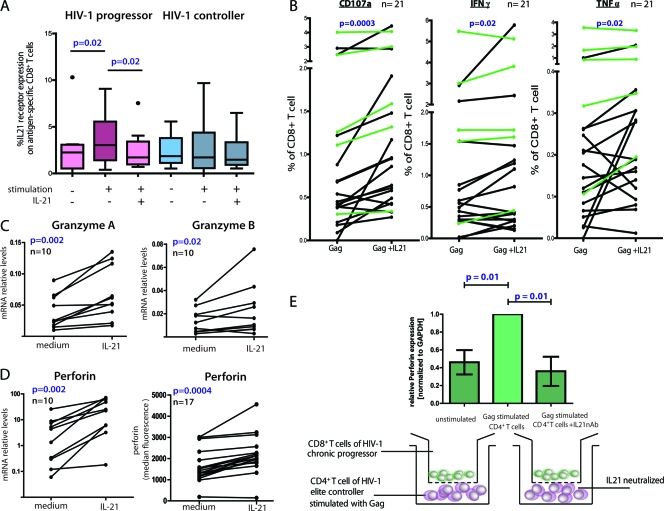
Because IL-21+ CD4+ T cells are major contributors to control in the setting of LCMV infection through the modulation of virus-specific CD8+ T cells and because these cells are markedly enriched in the setting of controlled HIV-1 infection, we next examined the influence of these responses on HIV-1-specific CD8+ T cells. We first determined IL-21 receptor (IL-21R) expression on tetramer-positive HIV-specific CD8+ T cells of HIV-1 controllers compared to HIV-1-infected chronic progressors. We did not observe any differences in the expression levels of IL-21R at baseline (Fig. 4 A), but we observed significant upregulation of IL-21R on HIV-1-specific CD8+ T cells of chronic progressors following a 5-h stimulation with the respective optimal peptide (P = 0.02); the expression levels of IL-21R on HIV-1-specific CD8+ T cells of controllers did not significantly change. When we then added recombinant IL-21 together with the cognate peptide, the expression of IL-21R significantly decreased on HIV-1-specific CD8+ T cells of chronic progressors (P = 0.02), but this had no impact on the expression level of IL-21R on HIV-1-specific CD8+ T cells of controllers. Thus, the expression levels of IL-21R on HIV-1-specific CD8+ T cells upon antigen stimulation are sensitive to external IL-21, and the presence of IL-21-secreting CD4+ T cells in HIV controllers versus the lack of these cells in progressors might explain the discordant expression levels of IL-21R in the two groups.
FIG. 4.
CD8+ T cells responses to IL-21 stimulation. (A) PBMCs from eight HIV-1-infected progressors and nine controllers were cultured for 5 h in the presence or absence of HIV CD8+ optimal peptides and IL-21 (50 ng/ml). Box plots represent the IL-21R expression on antigen-specific (tetramer-positive) CD8+ T cells detected by flow cytometry. (B) PBMCs of 16 HIV-infected progressors (black) and 5 HIV controllers (green) were cultured with or without IL-21, and the percentage of CD8+ T cells expressing CD107a, IFN-γ, or TNF-α was assessed by flow-cytometry using intracellular cytokine staining. (C) Granzyme A, granzyme B, and perforin mRNA upregulation measured by quantitative PCR on bulk CD8+ T cells after IL-21 stimulation of PBMCs from 10 untreated HIV-infected subjects. (D) IL-21-mediated perforin upregulation in tetramer-positive CD8+ T cells of 17 untreated HIV-infected subjects was measured by flow cytometry. These results together demonstrate the ability of IL-21 to increase both the bulk and HIV-specific CD8+ T cell cytotoxic potential of chronic progressors. (E) CD8-depleted PBMCs from six controllers were prestimulated with Gag peptides for 1 h or left unstimulated. The cells were thoroughly washed and coincubated for 12 h in the bottom chamber of a transwell plate with CD8+ T cells from a chronic progressor in the upper chamber. IL-21 blocking was performed by adding neutralizing anti-IL-21 and anti-IL-21 receptor antibodies to the CD8+ T cells in the upper chamber. After the incubation, CD8+ T cells were harvested and perforin mRNA relative levels were assessed by quantitative PCR (bar diagrams). Error bars indicate standard deviations.
To further investigate whether HIV-1-specific IL-21+ CD4+ T cell responses affect the functional profile of HIV-1-specific CD8+ T cells, we next stimulated PBMCs of HIV-1 controllers and progressors with pooled Gag peptides in the presence or absence of IL-21-supplemented medium (see Fig. S3 in the supplemental material). After a 5-h coincubation period, we observed a significant increase in LAMP1 (CD107a) expression on HIV-1-specific CD8+ T cells from controllers as well as progressors, demonstrating the augmented ability of CD8+ T cells to degranulate upon peptide stimulation in the presence of IL-21(P = 0.0003) (Fig. 4B; see Fig. S3 in the supplemental material); degranulation was more pronounced in HIV-1-infected progressors (black lines; P = 0.002) than in controllers (green lines; not significant [n.s.]). In addition, we observed a similar effect for other effector cytokines such as IFN-γ and TNF-α. In contrast, no difference in CD8+ T cell secretion of IL-2 or MIP1β was observed (data not shown), suggesting that IL-21 specifically increases the expression of antiviral effector molecules of HIV-specific CD8+ T cells.
Based on the observation of enhanced degranulation of HIV-specific CD8+ T cells in the presence of IL-21, we next determined whether IL-21 also changes the granule composition of perforin and granzyme in these cells. Previous reports have demonstrated that HIV-1-specific CD8+ T cells, under persistent antigenic stimulation, become dysfunctional and nonresponsive to external signals (21). Thus, we assessed whether the mRNA expression levels of typical granule content, such as granzyme A and B as well as perforin, change in HIV-1-specific CD8+ T cells after incubation with IL-21 (Fig. 4C). Indeed, CD8+ T cells significantly upregulated both granzyme A and perforin mRNA expression levels after a short coincubation with recombinant IL-21 (P = 0.002 for both), while the upregulation of granzyme B was less pronounced but still significant (P = 0.02). We next confirmed perforin upregulation on the protein level as measured by intracellular cytokine staining in tetramer+ HIV-1-specific CD8+ T cells (P = 0.0004) (Fig. 4D; see Fig. S4 in the supplemental material). These changes were independent of whether the CD8+ T cells were derived from HIV-1 controllers or progressors (ratio for controllers, 1.34 ± 0.28; ratio for progressors, 1.38 ± 0.33).
To exclude the possibility that perforin upregulation is nonspecifically mediated via γ-chain signaling, we repeated the experiment with three subjects, stimulating with a number of γ-chain cytokines: IL-2, IL-4, IL-7, IL-15, and IL-21 (see Fig. S5 in the supplemental material). Perforin was preferentially upregulated following IL-15 and IL-21 incubation. Overall, these data demonstrate that IL-21 enhances the effector functions of HIV-1-specific CD8+ T cells independently of their exhaustion profile or the disease stage of the individual subjects.
We then further examined the IL-21-induced mechanism responsible for alterations in CD8+ T cell function. As previous reports have suggested that IL-21 can induce apoptosis, we simultaneously assessed the expression of perforin and annexin V binding sites on HIV-1-specific CD8+ T cells over 24 h in the presence of added IL-21. Both perforin loading and annexin V expression peaked within 2 h on HIV-1-specific CD8+ T cells (see Fig. S6 in the supplemental material). These findings suggest that IL-21 signaling can affect CD8+ T cells by inducing apoptosis or by increasing effector functions, supporting a model in which CD4+ T helper cells can mediate the fine-tuned regulation of virus-specific CD8+ T cell responses through IL-21.
We next determined whether HIV-1-specific CD4+ T cells from controllers can directly exert the same effect as exogenous recombinant IL-21 on CD8+ T cells through IL-21 secretion. PBMCs from six controllers with strong IL-21 responses were depleted of CD8+ T cells and then pulsed with Gag peptide for 1 h. The remaining peptide was thoroughly removed by repetitive washes. We placed the peptide-pulsed CD8-depleted PBMCs from each individual in the bottom chamber of a transwell plate and compared their impact on CD8+ T cells from noncontrollers, which were added to the upper chamber. Unstimulated CD8-depleted PBMCs in the lower chamber with CD8+ T cells in the upper chamber served as a negative control (Fig. 4E). The Gag-stimulated CD8-depleted PBMCs of controllers induced robust upregulation of perforin mRNA expression in the CD8+ T cells (P = 0.01) (Fig. 4E). Addition of a neutralizing antibody to IL-21 significantly abrogated the effect (P = 0.01), demonstrating the direct modulating role of IL-21 on CD8+ T cell effector functions. However, a potential inhibitory effect on the autocrine IL-21 secretion of CD4+ T cells might contribute as well. Taken together, these data demonstrate that HIV-1-specific IL-21-secreting CD8-depleted PBMCs induce important effector functions in HIV-1-specific CD8+ T cells. This effect can be induced by the HIV-1-specific CD4+ T cells of HIV controllers and can improve the functionality of CD8+ T cells of progressors.
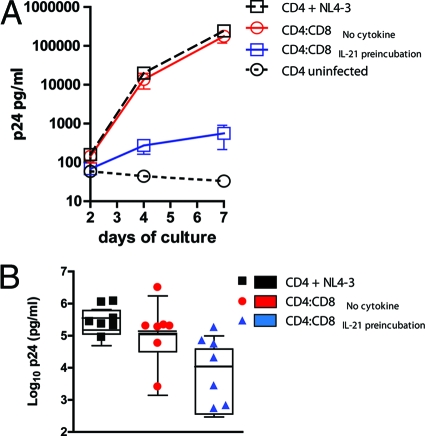
Finally, we assessed whether IL-21 secretion can improve the ability of HIV-1-specific CD8+ T cells to suppress viral replication in vitro. We selected seven HIV-1-infected individuals for whom we had previously determined that the CD8+ T cells were minimally capable of inhibiting viral replication in vitro. CD8+ T cells were separated from PBMCs and rested in the absence of mitogens or cytokines for 2 days. Five hours before the coculture experiment, CD8+ T cells were incubated in the presence or absence of IL-21 (50 ng/ml). As expected, HIV-infected CD4+ T cells alone showed a high level of viral replication, and we observed no differences in the level of viral replication when autologous bulk CD8+ T cells were added (Fig. 5 A, showing a representative result from one individual). However, addition of CD8+ T cells preincubated with low levels of IL-21 for 5 h resulted in a 1,000-fold increase in the ability of the CD8+ T cells to inhibit viral replication. We repeated this with six more subjects for whom we had previously confirmed minimal inhibitory capacity of the peripheral CD8+ T cells. This revealed heterogeneity among study subjects in their ability to suppress viral replication after preincubation with IL-21 (Fig. 5B). Although some individuals benefited strongly from the IL-21 signal, others showed only minimal improvement in CD8+ T cell-mediated viral inhibition. Interestingly, those individuals who exhibited enhanced viral inhibition after IL-21 pretreatment tended to be the subjects who upregulated perforin expression the greatest as determined by flow cytometry (data not shown). Thus, IL-21 has the ability to substantially enhance the potential of HIV-1-specific CD8+ effector cells to lyse HIV-1-infected cells by increasing the perforin content of cytolytic granules.
FIG. 5.
IL-21 induces suppression of HIV-1 replication by bulk CD8+ T cells. In order to test whether IL-21 can improve the viral suppressive capacity of bulk CD8+ T cells, we selected subjects with a known lack of CD8+ T cell viral inhibition (assessed previously) to understand the impact of IL-21 on their inhibitory capacity. (A) Representative results from one individual. Bulk CD8+ T cells, isolated from frozen PBMCs by positive selection and rested for 2 days, failed to inhibit replication of HIV-1 virus NL4-3 (X4 tropic) in autologous CD4+ T cells at a CD8-to-CD4 T cell ratio of 1:1. However, preincubation of the CD8 T cells with IL-21 (50 ng/ml) led to a 1000 fold viral inhibition. (B) Comparison of the viral suppressive capacities of bulk CD8+ T cells with or without preincubation with IL-21 in seven subjects selected based on a previous assessment of their inhibitory capacity. First we confirmed that in the presence of CD8+ T cells, viral replication was not significantly reduced (log10 p24 [pg/ml], 5.18 ± 0.36 versus 4.63 ± 0.76 for CD4+ controls and CD4-CD8 cocultures, respectively [n.s.]). However, preincubation of the CD8+ T cells with IL-21 improved the inhibitory effect significantly (log10 p24 [pg/ml], 3.71 ± 1.11 [P < 0.05]). Error bars indicate standard deviations.
Understanding exactly how CD4+ T helper cells coordinate the immune system and respond to immune challenges is crucial for the development of an effective HIV vaccine (15, 20). Several previous studies have focused on a potential role of CD4+ T cell help in HIV-specific CD8+ T cell responses. It has been demonstrated that in the chronic phase of the infection, CD8+ T cells lose their ability to proliferate upon antigen stimulation, but the presence of CD4+ T cells derived from cryopreserved PBMCs was able to rescue their proliferative capacity (10) in a manner that has been attributed primarily to the secretion of IL-2. Interestingly, in the absence of other cytokines such as IL-15, IL-21 does not induce proliferation of HIV-specific CD8+ T cells (25). However, our data suggest that a different CD8+ T cell effector function, namely, cytolytic antiviral activity, can be rescued through IL-21 secretion by CD4+ T cells. Nevertheless, it is important to note that a limitation of both these studies is that a singular helper function of CD4+ T cells has been assessed. It is likely that several additional, and possibly unknown, helper functions act in concert to provide optimal stimulation for CD8+ T cells in vivo.
Our data indicate that the ability of HIV-1-specific CD4+ T cells to improve the antiviral efficacy of HIV-1-specific CD8+ T cells via IL-21 signaling is a central part of virus-specific immunity. This effect is conserved early on in individuals with controlled infection and is preferentially lost in subjects with chronic progressive HIV-1 infection, and it likely contributes to the controller phenotype through augmentation of CD8+ T cell effector function. Other factors, including epitope processing, antigen recognition, synapse formation, target cell adhesion, and signal transduction, are also critical elements in successful target cell lysis and are not likely to be affected by IL-21. This not only may help to explain the variability in IL-21 production in a small proportion of the controller patients but also provides a basis for the heterogeneous effects of IL-21 on the ability of CD8+ T cells to kill infected CD4+ T cells in these subjects. However, the ability of IL-21 produced by HIV-1-specific CD4+ T cells of HIV-1 controllers to improve the effector functions of HIV-1-specific CD8+ T cells of HIV-1-infected progressors suggests that the induction and preservation of these responses are likely to be an important cornerstone for the generation of an effective HIV-1 vaccine.
Supplementary Material
Acknowledgments
This study was funded by grant NIH 1R01AI091450-01, the Bill and Melinda Gates Foundation, and the Susan and Philip T. Ragon Foundation.
Footnotes
Published ahead of print on 3 November 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 2.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 3.Elsaesser, H., K. Sauer, and D. G. Brooks. 2009. IL-21 is required to control chronic viral infection. Science 324:1569-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frohlich, A., J. Kisielow, I. Schmitz, S. Freigang, A. T. Shamshiev, J. Weber, B. J. Marsland, A. Oxenius, and M. Kopf. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576-1580. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin, A., P. Monteiro, N. Chomont, F. Diaz-Griffero, E. A. Said, S. Fonseca, V. Wacleche, M. El-Far, M. R. Boulassel, J. P. Routy, R. P. Sekaly, and P. Ancuta. 2010. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 184:1604-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannello, A., M. R. Boulassel, S. Samarani, O. Debbeche, C. Tremblay, E. Toma, J. P. Routy, and A. Ahmad. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114-126. [DOI] [PubMed] [Google Scholar]
- 7.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 8.Julg, B., K. L. Williams, S. Reddy, K. Bishop, Y. Qi, M. Carrington, P. J. Goulder, T. Ndung'u, and B. D. Walker. 2010. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84:5540-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann, D. E., P. M. Bailey, J. Sidney, B. Wagner, P. J. Norris, M. N. Johnston, L. A. Cosimi, M. M. Addo, M. Lichterfeld, M. Altfeld, N. Frahm, C. Brander, A. Sette, B. D. Walker, and E. S. Rosenberg. 2004. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J. Virol. 78:4463-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurieva, R., X. O. Yang, G. Martinez, Y. Zhang, A. D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S. S. Watowich, A. M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448:480-483. [DOI] [PubMed] [Google Scholar]
- 13.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 14.Ramduth, D., C. L. Day, C. F. Thobakgale, N. P. Mkhwanazi, C. de Pierres, S. Reddy, M. van der Stok, Z. Mncube, K. Nair, E. S. Moodley, D. E. Kaufmann, H. Streeck, H. M. Coovadia, P. Kiepiela, P. J. Goulder, and B. D. Walker. 2009. Immunodominant HIV-1 CD4+ T cell epitopes in chronic untreated clade C HIV-1 infection. PLoS One 4:e5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saez-Cirion, A., G. Pancino, M. Sinet, A. Venet, and O. Lambotte. 2007. HIV controllers: how do they tame the virus? Trends Immunol. 28:532-540. [DOI] [PubMed] [Google Scholar]
- 16.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 20.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320:760-764. [DOI] [PubMed] [Google Scholar]
- 21.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams, M. A., B. J. Holmes, J. C. Sun, and M. J. Bevan. 2006. Developing and maintaining protective CD8+ memory T cells. Immunol. Rev. 211:146-153. [DOI] [PubMed] [Google Scholar]
- 23.Yi, J. S., M. Du, and A. J. Zajac. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue, F. Y., C. Lo, A. Sakhdari, E. Y. Lee, C. M. Kovacs, E. Benko, J. Liu, H. Song, R. B. Jones, P. Sheth, D. Chege, R. Kaul, and M. A. Ostrowski. 2010. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 185:498-506. [DOI] [PubMed] [Google Scholar]
- 25.Zeng, R., R. Spolski, E. Casas, W. Zhu, D. E. Levy, and W. J. Leonard. 2007. The molecular basis of IL-21-mediated proliferation. Blood 109:4135-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.