Abstract
Langerhans cells (LCs) and interstitial dendritic cells (IDCs) may be among the first human immunodeficiency virus type 1 (HIV-1) targets after sexual transmission. We generated cells of these types by differentiation of purified CD34+ cord blood cells. After in vitro infection with R5-tropic strains, we obtained similar percentages of infected cells for both dendritic cell (DC) subsets. Moreover, LC infection was not increased by blockage of langerin by antilangerin. These results indicate that, under our experimental conditions, there was no evidence of any preference of HIV replication in LCs versus IDCs. The inhibitory activity of HIV-1-specific IgAs and IgGs against HIV-1 replication in LCs and IDCs was analyzed. We found that neutralizing antibodies inhibit HIV-1 infection of both DC subsets. Interestingly, HIV-1 was inhibited more efficiently by the IgGs than the corresponding IgA, due to an Fcγ receptor-dependent mechanism. Moreover, nonneutralizing inhibitory IgGs were able to inhibit infection of both LCs and IDCs. These results underline the importance of HIV-1 inhibition by the binding of the Fc part of IgGs to Fcγ receptors and suggest that the induction of neutralizing and nonneutralizing inhibitory IgGs in addition to neutralizing IgAs at mucosal sites may contribute to protection against sexual transmission of HIV-1.
Currently, sexual transmission is the major route of new human immunodeficiency virus type 1 (HIV-1) infection. One of the promising new strategies for vaccination against HIV sexual infection is the development of a mucosal vaccine to induce strong local and systemic protective immunity. Such immunity should prevent infection of the first HIV target cells at mucosal sites, particularly Langerhans cells (LCs), interstitial dendritic cells (IDCs), macrophages, and T lymphocytes (32). Five monoclonal neutralizing antibodies (NAbs) have been studied extensively (IgG1 b12, 2F5, 4E10, 447-52D, and 2G12), and additional candidate NAbs have recently been discovered (37, 38). These NAbs inhibit a broad spectrum of HIV-1 strains in vitro, as assessed using conventional neutralization assays with peripheral blood mononuclear cells (PBMCs) or HIV-1-permissive cell lines (TZM-bl) (3, 26, 37). We have previously demonstrated that NAbs have an in vitro inhibitory activity by using two types of antigen-presenting cells (APCs): monocyte-derived macrophages (MDMs) and monocyte-derived dendritic cells (MDDCs) (15, 17). The inhibitory activity of neutralizing antibodies on these target cells was due to two distinct mechanisms of inhibition: (i) classical neutralization of the virus infectivity involving the Fab part of the antibody (Ab) and (ii) an Fcγ receptor (FcγR)-dependent mechanism of inhibition. Moreover, other Abs, which did not show classical neutralizing activity, were able to inhibit HIV-1 replication in these cells by the FcγR-dependent mechanism only. These Abs are referred to as nonneutralizing inhibitory Abs (NNIAbs) (16).
The protective role of neutralizing IgGs has been confirmed in vivo using passive immunization of macaques against a vaginal simian-human immunodeficiency virus (SHIV) challenge (1, 11). More recently, even lower concentrations of NAbs showed protection in a modified challenge protocol involving repeated low doses of simian immunodeficiency virus (SIV) or SHIV (12, 28). Passive-transfer studies of NAbs implicate the Fc part of IgGs in the protection against mucosal infection. Indeed, the protection was significantly lower after passive transfer of the IgG1 b12 mutant LALA (devoid of the capacity to bind to FcγRs) than of the wild-type (WT) b12 strain (11). These results suggest that the Fc region of neutralizing IgGs plays a role in the protection against sexual transmission of HIV.
It has been suggested that Fc-bearing LCs and IDCs in the mucosa are among the first HIV targets following sexual transmission. Although their exact contribution to HIV transmission is controversial, they have been found infected in the mucosal layer (14, 31) and are able to replicate HIV in vitro (4, 14, 19, 24, 25). These FcγR-bearing immune cells display important immune functions. They are involved in the capture and the degradation of HIV-1 IgG immune complexes and in the induction of immune effector functions, such as antigen presentation (2). Thus, inhibiting infection of LCs and IDCs and preserving their immune function may make a valuable contribution to protection against HIV-1 infection and dissemination through the body. Using antibodies to protect these cells from infection should therefore be investigated.
There have been few studies of the inhibitory effect of antibodies on antigen-presenting cells and, to our knowledge, no studies of the inhibitory effects of antibody against infection of LCs and IDCs. These particular dendritic cells (DCs) differentially express HIV-1 alternative receptors compared to MDDCs (for example, C-type lectin receptors [CLRs], such as DC-SIGN or langerin). Moreover, the relative expression of the various FcγRs differs from that in MDDCs. These differences may affect HIV binding to the cells, with consequences for HIV replication and the inhibitory capacity of Abs.
We report an analysis of HIV replication in LCs and IDCs and the neutralizing and Fc-mediated inhibitory activity of Abs on the infection of these cells.
MATERIALS AND METHODS
Antibodies and cytokines. (i) MAbs used for LC and IDC staining.
Monoclonal antibodies (MAbs) HLA-DR-phycoerythrin (PE) (G46-6), CD1a-PC5 (BL6), CD11b-allophycocyanin-Cy7 (ICRF44), CD16-PE (3G8), CD83-PE (HB15e), CD206-PC5 (19.2), and DC-SIGN-allophycocyanin (DCN46) were purchased from BD PharMingen (San Diego, CA). MAbs CD14-fluorescein isothiocyanate (FITC) (RMO52), CD64-PC5 (clone 22), CD207-PE (DCGM04), and p24-FITC (KC57) were purchased from Beckman-Coulter (Roissy, France).
(ii) HIV-1-specific Abs.
Anti-HIV-1 human MAbs to gp-120 (IgG1 b12 and 2G12) and to gp-41 (2F5) were obtained from the NIBSC. Human MAb 447-52D and corresponding Fab fragments 240D and 246D were provided by Suzan Zolla-Pazner. Human MAbs 4E10 and IgA b12 were provided by H. Katinger (Polymun Scientific GmbH) and D. Burton, respectively. Nonneutralizing noninhibitory MAbs to gp-41 (5F3 and 3D6) were obtained from the NIH and D. Katinger (Polymun Scientific GmbH), respectively.
(iii) Anti-human langerin Abs.
Mouse monoclonal anti-human langerin (clone DCGM04) was purchased from Beckman-Coulter (Roissy, France). Mouse monoclonal anti-human langerin (clone 343828) and goat polyclonal anti-human langerin were purchased from R&D Systems.
(iv) Cytokines.
All cytokines used for culture and differentiation of the cells were purchased from R&D Systems.
Cell preparation.
PBMCs were obtained by Ficoll-Hypaque sedimentation of human peripheral blood leukocytes, followed by 3 days of phytohemagglutinin (PHA) activation in RPMI medium-10% fetal calf serum (FCS) supplemented with interleukin-2 (IL-2) (10 ng/ml).
Langerhans cells (LCs) and interstitial dendritic cells (IDCs) were obtained by differentiation of purified human cord blood CD34+ stem cells by immunomagnetic bead isolation (AutoMacs; Miltenyi Biotec) after Ficoll-Hypaque sedimentation. Purified CD34+ cells were cultured in RPMI medium-10% FCS supplemented with 50 ng/ml of Flt3 ligand (Flt3-L), 50 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF), 25 ng/ml of stem cell factor (SCF), and 10 ng/ml of thrombopoietine (TPO) at 37°C and under 5% CO2 for 7 days to allow multiplication. Then, the CD34+ cells were differentiated for five additional days in RPMI medium-10% FCS with 50 ng/ml of GM-CSF, 6 ng/ml of transforming growth factor β1 (TGF-β1), and 2 ng/ml of tumor necrosis factor alpha (TNF-α).
All blood or cord blood samples were collected from healthy, HIV-1-seronegative donors. Umbilical cord blood samples were obtained from consenting, full-term pregnant women.
Virus preparation.
HIV-1 primary isolates were produced in human blood leukocytes as previously described (16). Virus stocks were collected at the peak of virus production and were concentrated 80-fold using a 100-kDa cutoff polyethersulfone filter (Centricon 80 Plus Biomax filter; Millipore, Molsheim, France). HIV-1BaL and HIV-1BX08 isolates (subtype B, R5 strains) were provided by S. Gartner, M. Popovic, and R. Gallo from the National Institutes of Health and by H. Fleury, respectively. HIV-1TV1 (subtype C, R5 strain) was obtained from S. Engelbrecht. X4-tropic HIV-1KON was provided by F. Barin.
Infection and neutralization assays.
Aliquots of 25 μl of cells (at 12 × 106 cells/ml, in RPMI medium supplemented with 10% FCS without addition of cytokines) were infected with 25 μl of HIV-1 primary isolate (concentration of 2 to 10 μg/ml p24) in a final volume of 75 μl. The concentration used was adjusted to result in 2 to 5% of infected cells after 72 h. This percentage of infected cells has been shown to be adequate for testing neutralizing antibody activity (22, 23, 33).
The neutralization assay involved preincubation of 25 μl of various concentrations of HIV-specific Abs with 25 μl of virus for 1 h at 37°C, before addition of 25 μl of cells.
After 72 h, the percentage of infected cells was determined by intracellular p24 staining in parallel with membrane staining to allow the discrimination of DC subsets. Flow cytometry analysis was performed, and the neutralizing IC90 (the concentration of Ab which resulted in a 90% reduction in the number of infected cells) was determined. Neutralization was assessed if the percentage of infected cells was >2%. This percentage of infected cells has been shown to be appropriate for the analysis of neutralization by flow cytometry (16, 17, 30).
Flow cytometry analysis.
Acquisition of multicolor samples was performed with a cytometer (LSRII Sorp; BD). Cytometer setup and tracking (CST) calibration particles (BD) were used to ensure consistency of fluorescence intensity measurements throughout all experiments. A flow cytometry CompBeads kit (BD) was used for compensation. Forward angle and side scatter light gating were used to exclude dead cells and debris from the analysis. Forward width and forward area were used to exclude doublet cells. FACSDiva software (BD) was used for the final analysis and graphical output.
RESULTS
Phenotypes of LCs and IDCs derived from CD34+ cord blood cells.
To analyze the inhibitory activity of antibodies against HIV infection of LCs and IDCs, we generated these cells from CD34+ stem cells in vitro. As the recovery of CD34+ cells from cord blood is low, we first amplified them for 7 days before the 5-day differentiation step to generate LCs and IDCs (see Materials and Methods). Under these conditions, we obtained LCs and IDCs in coculture. Each cell population in the coculture was characterized by immunophenotyping (Fig. 1); LCs and IDCs were distinguished according to their specific cell surface expression markers, langerin (CD207) and CD11b, respectively (6, 34).
FIG. 1.
Phenotypes of Langerhans cells (LCs; pink) and interstitial dendritic cells (IDCs; blue) obtained after differentiation of CD34+ cord blood cells. (A) LCs and IDCs were distinguished by their expression of langerin and CD11b, respectively. (B) The expression of various markers was determined by flow cytometry (LSRII Sorp; BD) with gated populations of langerin-positive/CD11b− LCs and langerin-negative/CD11b+ IDCs. Dot plots are representative of 4 different donors and 7 independent experiments.
The CD11b+/langerin-negative IDCs (Fig. 1, blue population) expressed low levels of CD1a and CD14 (10 and 22%, respectively). In contrast, 73% of CD11b−/langerin-positive LCs (Fig. 1, pink population) expressed CD1a, but few were positive for CD14. A third cell population, negative for both CD11b and langerin markers, was also detected in the coculture (see Fig. SA1a in the supplemental material, gray population). We extensively analyzed these cells. They were also negative for the CD14-specific monocyte marker, for CD4 and CCR5, and for all other surface markers tested (FcγRs, FcαR, the C-type lectins DC-SIGN and mannose receptor, CD1a, and HLA-DR).
Like APCs, LCs and IDCs strongly expressed HLA-DR. The mannose receptor was detected at the cell surface of most IDCs but not LCs, and the expression of DC-SIGN was very weak on IDCs and absent on LCs. To assess the ability of LCs and IDCs to bind HIV-1 IgG immune complexes through FcγRs, we studied the expression of the different types of this receptor family. LCs and IDCs differentially expressed FcγRI (4 and 18%, respectively) and FcγRII (24 and 54%, respectively), but both expressed FcγRIII only weakly. The expression of FcαR (IgA receptor) was very weak on IDCs and not detectable on LCs (not shown).
The receptor and coreceptor for R5-tropic HIV-1 strains, CD4 and CCR5, respectively, were expressed at the surface of both DC subsets, whereas no expression of CXCR4 (coreceptor for X4-tropic HIV-1 strains) could be detected (not shown). LCs and IDCs did not express the maturation marker CD83 and were therefore considered to be immature.
The LCs and IDCs in coculture obtained by in vitro differentiation of cord blood stem cells thus showed a phenotype very similar to the phenotype previously described for DCs residing in mucosal sites (13, 35).
Attempts to purify LCs and IDCs.
We tried to separate IDCs from LCs and also to improve their purity in culture (see Fig. SA2 in the supplemental material).
First, we modified the culture conditions and the cytokine cocktail used. Without adding TGF-β to the culture, we obtained pure IDCs, without LCs (see Fig. SA2c in the supplemental material). However, these IDCs showed a phenotype similar to that of MDDCs; in particular, unlike other IDCs, they expressed DC-SIGN. IDCs obtained in the presence of TGF-β did not express DC-SIGN (probably due to the effect of the cytokine, as reported by Relloso et al. [27]) and therefore show a phenotype closer to that of IDCs purified from the derma.
Purification by cell sorting was also attempted (see Fig. SA2b in the supplemental material). However, the size and the morphology of the LCs obtained on days 10 to 12 were not appropriate for magnetic cell sorting (the cells stuck to the column and were recovered with deformities). Moreover, staining of the cells on day 12 before cell sorting induced rapid maturation of the cells, making them resistant to R5 HIV infection. Purification of LCs at earlier times was not feasible because langerin was expressed only weakly before day 12.
As we could not improve the purity of LCs or IDCs, cocultures were used for infection experiments.
Productive HIV-1 infection of LCs and IDCs.
To analyze the infection of LCs and IDCs by different HIV-1 strains, we used flow cytometry to detect, in each population of DCs, intracellular p24 viral antigen, which is a reliable early indicator of productive infection (33). Flow cytometry was also used to detect the cell-specific markers langerin and CD11b for the phenotypic characterization of infected LCs and IDCs, respectively. This method discriminated between infected LCs and infected IDCs in the coculture, which is not possible by enzyme-linked immunosorbent assay (ELISA) (dosage of p24 in the supernatant). Intracellular p24 was analyzed at various times after infection (Fig. 2C) . After 24 and 48 h, the percentages of LCs and IDCs infected were negligible. However, 72 h postinfection, the percentages of LCs and IDCs infected were 2 to 5% for the three R5-tropic strains (HIV-1BaL, HIV-1BX08, and HIV-1TV1), but no productive infection was detected with X4 HIV-1KON (Fig. 2A). Although the percentage of infected IDCs was slightly higher than that of LCs, the difference was not significant for HIV replication in LCs and IDCs from various donors (P > 0.34 by analysis of variance [ANOVA]) (Fig. 2B). The percentage of infected cells increased with time (at days 4 and 5) (Fig. 2C) and correlated with virus released into the medium, as assessed by p24 ELISA (not shown).
FIG. 2.
HIV-1 replication in LCs and IDCs. (A) Contour plot representation of the percentages of p24-positive langerin-positive/CD11b− LCs, langerin-negative/CD11b+ IDCs, and langerin-negative/CD11b− cells. Intracellular p24 was detected 72 h after infection with R5-tropic HIV-1BaL, HIV-1TV1, or HIV-1BX08 or X4-tropic HIV-1KON. AZT (10 mM) was added to inhibit productive HIV-1 replication. (B) Box plot representation of the percentages of HIV-1BaL infection of LCs and IDCs from 7 different donors. (C) Kinetics of infection of LCs and IDCs. DCs were infected with HIV-1BaL, and the infection was stopped at 24, 48, 72, 96, and 120 h postinfection. The percentages of infected LCs and IDCs were scored at each time. (D) Percentages of LCs and IDCs infected by HIV-1BaL in the presence or absence of saquinavir. Saquinavir (5 ng/ml) was added directly after addition of the virus. Cells were incubated for 72 h, and the percentage of infected cells was determined by intracellular p24 measurement. (E) Kinetics of HIV-1BaL replication. AZT (10 mM) was added at different times postinfection, and the percentages of infected LCs and IDCs were scored at 72 h. Error bars indicate standard deviations.
To determine whether the infection detected at 72 h corresponded to a single cycle or several cycles of replication, cells were treated with saquinavir (a protease inhibitor). Saquinavir treatment decreased the numbers of infected LCs and IDCs by 25% and 30%, respectively (Fig. 2D). This indicates that virus replication detected at 72 h corresponded mainly to a single cycle of HIV replication in both DC types. To measure the kinetics of the early reverse transcription step, we added zidovudine (AZT) at various times after infection and measured the percentages of infected cells at 72 h. AZT had no detectable inhibitory effect when added up to 24 h after the virus. This indicates that the reverse transcription step of HIV-1 replication needs about 24 h to be completed in these cells (Fig. 2E). These findings also suggest that the early kinetics of replication were similar in LCs and IDCs.
No infection was detected in langerin-negative/CD11b− cells, and these cells could therefore be considered nonpermissive to HIV-1 infection (see Fig. SA1b in the supplemental material). Analysis of the inhibitory activity of antibodies after 72 h was chosen, corresponding to a single cycle of infection and minimizing the inhibitory activity due to cytokine production or other factors.
Effect of antilangerin on HIV-1 infection of LCs.
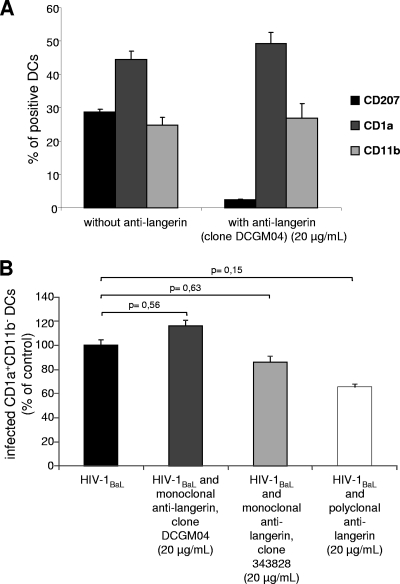
Langerin is expressed exclusively on LCs and may participate in the degradation of viral particles, limiting HIV replication in these cells (8). The role of langerin in HIV-1 infection was assessed in our model by competition experiments using three antibodies directed against human langerin CD207 (one polyclonal and two monoclonal Abs). Anti-human langerin Ab (20 μg/ml) was preincubated with LCs and IDCs for 30 min before infection with a primary isolate of HIV-1BaL. These Abs impeded antilangerin-PE staining by targeting the same epitope or by steric hindrance, demonstrating the efficacy of their binding (Fig. 3 A). We analyzed the productive HIV-1 infection in LCs identified as CD11b− CD1a+ (Fig. 3B). The presence of antilangerin Abs (either monoclonal or polyclonal) did not increase the percentages of LCs (or IDCs) infected after 3 days (Fig. 3B). Indeed, in the presence of antilangerin antibodies, the percentage of HIV-infected CD1a+ LCs was slightly but not statistically significantly lower than that for controls. Under these conditions, we found no evidence of langerin being involved in LC infection. In addition, there was no significant difference between the percentages of HIV-infected LCs and IDCs (Fig. 2B), indicating that LCs were infected with an efficiency similar to that for the IDCs also present in the coculture.
FIG. 3.
Blockade of langerin on LCs. (A) Immunophenotyping in the presence of mouse anti-human langerin IgG (clone DCGM04) (20 μg/ml). CD1a and CD11b expression was not affected by the antilangerin treatment. (B) Cells were treated or not treated with various mouse or goat IgGs directed against langerin (20 μg/ml for 30 min) and then infected with HIV-1BaL. Intracellular p24 was measured in CD1a+ CD11b− LCs after 72 h. Values are representative of 4 independent experiments performed on cells from 3 different donors. Error bars indicate standard deviations.
Inhibition of HIV-1 replication in DCs by HIV-1-specific Abs.
The inhibitory activity of a panel of HIV-1-specific Abs on HIV-1 replication in LCs and IDCs was evaluated. Anti-HIV-1 Abs were incubated with R5-tropic HIV-1BaL or HIV-1TV1 for 1 h before the addition of LCs and IDCs. After 72 h, the percentages of infected LCs and DCs were determined and the inhibition of HIV-1 replication after Ab treatment was calculated. The IC90 of the Abs (i.e., the concentration of Ab which resulted in a 90% reduction in the number of infected cells) was determined for infection of LCs and IDCs with either HIV-1Bal or HIV-1TV1 (Table 1). The five well-known NAbs tested strongly inhibited HIV-1BaL and HIV-1TV1 replication in LCs and IDCs, and their inhibitory activities were stronger than those measured on PBMCs (Table 1). Interestingly, NNIAbs 240D and 246D, which do not exhibit neutralizing activity on PBMCs, reduced the number of HIV-1BaL-infected LCs and IDCs by 90% (39). The nonneutralizing noninhibitory Abs (NNNIAbs) 5F3 and 3D6, directed against gp41, which did not exhibit any inhibitory activity in previous assays, also had no inhibitory effects in our model system (17). Under these experimental conditions, no induction of the maturation marker CD83 at the surface of the LCs or IDCs could be detected (not shown).
TABLE 1.
Inhibition of HIV-1BaL and HIV-1TV1 by NAbs and NNIAbs when LCs/IDCs were used as target cells
| Monoclonal antibody | IC90 (μM)a |
|||||
|---|---|---|---|---|---|---|
| HIV-1BaL |
HIV-1TV1 |
|||||
| LCs | IDCs | PBMCs | LCs | IDCs | PBMCs | |
| NAbs | ||||||
| 447-52D | 6 | 3 | 150 | NDb | ND | NIc |
| 4E10 | 30 | 9 | 150 | 45 | 9 | 300 |
| IgG1 b12 | 9 | 9 | 25 | 6 | 6 | NI |
| 2G12 | 30 | 27 | 150 | 36 | 27 | NI |
| 2F5 | 24 | 21 | 90 | 30 | 30 | 150 |
| IgA b12 | 75 | 90 | 165 | ND | ND | ND |
| NNIAbs | ||||||
| 240D | 24 | 30 | NI | ND | ND | NI |
| 246D | 225 | 210 | NI | ND | ND | NI |
| NNNIAbs | ||||||
| 5F3 | NI | NI | NI | NI | NI | NI |
| 3D6 | NI | NI | NI | NI | NI | NI |
IC90, the concentration of Ab which resulted in a 90% reduction in the number of infected cells. Values are means from 4 to 7 independent experiments.
ND, not done.
NI, no inhibitory activity detected at 300 μM Ab.
Participation of FcγRs in the inhibition of HIV-1 replication by HIV-1-specific IgGs.
As FcγRs are expressed on LCs and IDCs, we studied the role of the Fc part of Abs in binding to their cognate FcγRs. We first analyzed the inhibitory activity of the Fab part of the neutralizing monoclonal IgG 447-52D (Fig. 4 A). The inhibitory activity of the Fab part of this NAb was only 1/10 that of the whole NAb. However, when PHA-stimulated PBMCs were used as target cells, the inhibition by the Fab part of 447-52D was only slightly lower than that of the whole IgG (Fig. 4A). This suggests not only that the much greater inhibition of DC infection by whole IgG than by the Fab fragment was due to a higher avidity of divalent than monovalent antibodies but also that the Fc domain of the NAb contributes substantially to the mechanism of inhibition of HIV-1 replication in LCs and IDCs. To test this possibility further, we prevented the binding of the NAb to the corresponding FcγRs by preincubating LCs and IDCs with 10 μg/ml each of anti-FcγRI, anti-FcγRII, and anti-FcγRIII before addition of HIV-1 IgG immune complexes. Blocking the binding of the NAb to the FcγRs present on the cell surface of the DCs reduced the inhibitory activity of the IgG 447-52D (Fig. 4B). Although increased inhibitory activity was less clear for NAb b12 than for 447-52D (Table 1), blocking the binding of b12 to the FcγRs also induced a significant and reproducible decrease of the inhibitory activity on LCs and IDCs (not shown). These results clearly show that an FcγR-mediated inhibitory activity contributes to the inhibition of HIV-1 infection of LCs and IDCs.
FIG. 4.
Inhibition by NAb of HIV-1BaL replication in LCs and IDCs. Values correspond to the numbers of infected cells in the presence of various concentrations of Ab, calculated as percentages of the totals without Ab. (A) Inhibition of HIV-1 replication by monoclonal NAb 447-52D (whole IgG) and its corresponding Fab fragment on LCs, IDCs, and PBMCs. (B) Inhibition of HIV-1 replication by monoclonal NAb 447-52D with or without a 30-min preincubation of the cells with 10 μg/ml of each mouse monoclonal IgG directed against human FcγRI, FcγRII, and FcγRIII. (C) Inhibition of HIV-1 replication by neutralizing IgG1 b12 versus monomeric IgA b12. Data are representative of 3 to 5 independent experiments. Error bars indicate standard deviations.
In the mucosa, LCs and IDCs may encounter IgA in addition to IgGs. We evaluated inhibition by antibody b12 of the IgA type of LC and IDC infection; IgA b12 is a recombinant human IgA2 that carries the heavy plus light (H+L) chain variable domains of IgG1 b12 (21). Monomeric IgA b12 inhibited HIV-1 replication in DCs, but the inhibition was weaker than that by the corresponding IgG1 b12 (Fig. 4C and Table 1). Dimeric and polymeric IgA b12 had similar inhibitory activities (data not shown). Moreover, the inhibitory activity of IgA b12 on LCs and IDCs was of the same order of magnitude as that recorded when PBMCs were the HIV targets (Table 1) (21). These results suggest that the Fc part of IgA does not participate in inhibition of HIV and provide further support to the notion of an FcγR-mediated mechanism of HIV inhibition by IgGs.
DISCUSSION
We studied the capacity of HIV-1-specific Abs to inhibit HIV-1 replication in LCs and IDCs. LCs and IDCs were obtained by differentiation of cord blood CD34+ stem cells. Immunophenotyping of these cells showed that DCs generated under these conditions expressed the surface markers detected on DC subsets present in the mucosa. These in vitro-differentiated cells therefore appear to be a good model for the analysis of HIV-1 infection of LCs and IDCs from the mucosa and neutralization by antibodies.
We analyzed the first step of HIV replication in these cells, which have contrasting patterns of expression of cell surface markers, also different from that of DCs obtained after differentiation of blood monocytes (17, 29, 35). We found that the reverse transcription step and the kinetics of HIV replication were similar in LCs and IDCs and were comparable to those previously reported for MDDCs (10, 17). Treatment with saquinavir demonstrated that HIV-infected cells detected after 72 h corresponded mostly to a single cycle of virus replication. Thus, under conditions where HIV was incubated with both LCs and IDCs, the first steps of virus entry and replication were similar in both DC subtypes and there was no evidence of any preference of HIV replication.
These similarities in HIV-1 replication in LCs and IDCs were unexpected, as these cells express different C-type lectin receptors (CLRs). LCs express langerin, reported to be involved in the degradation of HIV-1 particles (8). de Witte et al. showed that antilangerin Abs block HIV-1 degradation. Therefore, we anticipated that IDCs would be preferentially infected in our culture conditions, but the percentages of infection in LCs and IDCs were similar. To analyze the role of langerin, we performed competition experiments with three antilangerin antibodies. The antilangerin clone DCGM04, also used by de Witte's group, did not modify HIV-1 replication in LCs, as previously reported (8). However, the two other Abs tested (the monoclonal antilangerin Ab from another clone and polyclonal antilangerin Ab) also failed to increase HIV-1 replication in LCs. The lack of effect of these antilangerin Abs on HIV-1 replication may be because those described by de Witte et al. as able to block HIV-1 degradation in LCs recognize a very specific epitope, distinct from that recognized by our commercial Abs. Another possibility is that the large amount of virus required to detect infection of these cells may overwhelm the inhibitory activity of langerin. This point is difficult to assess because using lower concentrations of virus did not allow detection of infected cells by flow cytometry after a single cycle of infection (not shown). Moreover, the origins of the DC populations used were different in these two studies (LCs derived from CD34+ progenitors were used in our model, and skin-purified LCs were used by de Witte et al. [8]), and this may have consequences for the langerin inhibitory activity. Therefore, our results show (i) similar infection rates of LCs and IDCs and (ii) the absence of increased infection after langerin blockage. These results are not in accordance with the anti-HIV function of langerin proposed by van der Vlist and Geijtenbeek (36).
Note that the IDCs that are in coculture with LCs are different from MDDCs; in particular, expression of DC-SIGN was very weak. DC-SIGN efficiently binds HIV particles; this binding may favor close proximity between HIV and neighboring CD4 receptors and, in this way, trigger virus fusion (5). This may not happen on our IDCs, which lack this CLR. Nonetheless, IDCs were successfully infected under our coculture conditions.
The similarities in HIV-1 replication in LCs and IDCs allowed us to study the inhibitory activities of Abs in the cultures containing both DC subsets. We demonstrated for the first time that NAbs efficiently inhibit HIV-1 infection of LCs and IDCs. To determine the involvement of the Fc part of IgG in HIV inhibition, we compared the activity of whole IgG 447-52D to that of its corresponding Fab fragments by using PBMCs and LCs/IDCs. When CD4 T cells were the targets, the inhibitory activity of Fab was about half that of whole IgG. As CD4 T lymphocytes do not express FcγR (whereas DC subsets do), this small difference in inhibitory activity of Fab can be attributed to the monovalent structure of Fab, compared to that of whole IgG. In contrast, when LCs and IDCs were used as targets instead of PBMCs, the inhibitory activity of the Fab was less than 1/10 that of the whole IgG, strongly implicating the Fc fragment in the inhibitory activity of IgGs. Thus, the lower inhibitory activity of Fab was due not only to fewer epitopes but also to an FcγR-dependent mechanism of inhibition. Also, the NNIAbs 240D and 246D inhibited infection of LCs and IDCs, confirming the existence of an FcγR-mediated inhibitory mechanism in these cell subtypes.
We previously showed that FcγRI was mainly involved on macrophages whereas FcγRII was mainly involved on MDDCs (17). The relative expression levels of FcγRI, FcγRII, and FcγRIII differ among LCs, IDCs, and MDDCs. Nevertheless, we found that NAbs and NNIAbs had very comparable HIV-1 inhibitory activities for these three types of DCs (17). The relative contribution of each of the different FcγRs needs to be assessed further. After binding to their cognate FcγRs, HIV-1 IgG immune complexes may undergo degradation. The activation pathways that lead to the degradation of opsonized viral particles remain obscure. Immune complexes may be responsible for the activation of phagocytosis followed by the degradation of the pathogen in macrophages or monocytes (7, 18). As phagocytosis is impaired when these cells are infected by HIV-1, HIV-1-specific IgG should be induced early before acute infection and at the site of virus entry to exploit the FcγR-dependent inhibition mechanism maximally (20).
The mucosal immune response is characterized by the induction of specific IgA. We studied the inhibitory activity of this Ab class against infection of LCs and IDCs. Neutralization with b12 Abs of the IgA type showed a potent inhibitory activity against HIV-1 replication in LCs and IDCs, but this activity was nevertheless lower than that for the corresponding IgG1. In our LCs and IDCs generated from cord blood, expression of FcαR at the surface was negligible. Our findings are in accordance with a report by Geissmann et al. (9) that shows the expression of FcαR only in a subpopulation of IDCs in the derma and not in LCs in the epidermis. Note that we also failed to detect any efficient FcαR-mediated inhibitory activity on monocyte-derived macrophages (MDMs) (15), which, unlike LCs, strongly express FcαR. FcγR-mediated inhibitory activity has been detected for human MDMs and MDDCs and now also for LCs and IDCs (15, 17). We propose that this mechanism may be common to all APCs bearing specific FcγRs. As a consequence, FcγR-mediated inhibition may have a physiological relevance for APCs present at the mucosal site by contribution to HIV-1 inhibition after sexual transmission. Overall, these results strongly suggest that induction, in the mucosa, of IgG type Abs in addition to IgA may be highly beneficial in protecting APCs from HIV infection, thereby limiting HIV transmission to the neighboring cells, particularly CD4 T lymphocytes.
The inhibitory activity of NNIAbs on APCs provides new opportunities to induce Abs able to recognize “nonneutralizing” epitopes, in addition to NAbs. As the epitopes recognized by the NNIAbs 246D and 240D are both well conserved and very immunogenic, the design of immunogens able to induce such Abs should be feasible. To date, most assays used to measure Abs induced by vaccination involve PBMCs or T cell lines. Consequently, the presence of nonneutralizing IgGs after vaccination may have been missed. We therefore propose extending neutralization assays to other cell types, notably macrophages and DCs, which would allow the identification of these NNIAbs. In addition, optimization of the design of the Fc part of IgGs for their FcγRs should be considered (30).
By using Fc mutated IgGs, Hessell et al. revealed the importance of the Fc part of IgGs in protection against SHIV infection in a macaque model of experimental challenge by the vaginal route (11, 12). Our results support the importance of FcγR-mediated inhibition of HIV-1 infection of numerous target cells present at mucosal sites. Further characterization of the type of humoral immune response that should be induced in the mucosa to protect from HIV sexual infection will potentially be very valuable.
Supplementary Material
Acknowledgments
We thank G. Laumond, A. Proust, and J. Penichon for technical assistance and R. El Habib for fruitful discussions.
This work was supported by funds from EuroNeut 41 (FP7-HELTH-2007-A-201038), EuroPrise (LSHP-CT-2006-037611), Dormeur Investment Services Ltd., and ANRS. M.P. and X.K. were supported by scholarships from Sidaction and from EuroPrise, respectively.
We declare no competing interests.
Footnotes
Published ahead of print on 17 November 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Baba, T. W., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Binley, J. M., et al. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broliden, K., A. T. Haase, S. K. Ahuja, G. M. Shearer, and J. Andersson. 2009. Introduction: back to basics: mucosal immunity and novel HIV vaccine concepts. J. Intern. Med. 265:5-17. [DOI] [PubMed] [Google Scholar]
- 5.Burleigh, L., et al. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J. Virol. 80:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caux, C., et al. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 184:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David, A., et al. 2006. The engagement of activating FcgammaRs inhibits primate lentivirus replication in human macrophages. J. Immunol. 177:6291-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Witte, L., et al. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367-371. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann, F., et al. 2001. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J. Immunol. 166:346-352. [DOI] [PubMed] [Google Scholar]
- 10.Goujon, C., et al. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hessell, A. J., et al. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101-104. [DOI] [PubMed] [Google Scholar]
- 12.Hessell, A. J., et al. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirbod, T., et al. 2009. Abundant and superficial expression of C-type lectin receptors in ectocervix of women at risk of HIV infection. J. Acquir. Immune Defic. Syndr. 51:239-247. [DOI] [PubMed] [Google Scholar]
- 14.Hladik, F., et al. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holl, V., et al. 2004. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 173:6274-6283. [DOI] [PubMed] [Google Scholar]
- 16.Holl, V., et al. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holl, V., et al. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, V. C., J. M. Lynch, D. J. Bucher, J. Le, and D. W. Metzger. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166:7381-7388. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura, T., et al. 2008. Significant virus replication in Langerhans cells following application of HIV to abraded skin: relevance to occupational transmission of HIV. J. Immunol. 180:3297-3304. [DOI] [PubMed] [Google Scholar]
- 20.Kedzierska, K., et al. 2003. Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. J. Clin. Virol. 26:247-263. [DOI] [PubMed] [Google Scholar]
- 21.Mantis, N. J., et al. 2007. Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region. J. Immunol. 179:3144-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascola, J. R. 2004. Neutralizing antibody quantification by flow cytometry. Methods Cell Biol. 75:709-716. [DOI] [PubMed] [Google Scholar]
- 23.Mascola, J. R., et al. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, C. J., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59-67. [DOI] [PubMed] [Google Scholar]
- 25.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polonis, V. R., et al. 2008. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 375:315-320. [DOI] [PubMed] [Google Scholar]
- 27.Relloso, M., et al. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 168:2634-2643. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds, M. R., et al. 2010. Macaques vaccinated with SIVmac239Δnef delay acquisition and control replication after repeated low-dose heterologous SIV challenge. J. Virol. 84:9190-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozis, G., A. Benlahrech, S. Duraisingham, F. Gotch, and S. Patterson. 2008. Human Langerhans' cells and dermal-type dendritic cells generated from CD34 stem cells express different Toll-like receptors and secrete different cytokines in response to Toll-like receptor ligands. Immunology 124:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siberil, S., C. A. Dutertre, W. H. Fridman, and J. L. Teillaud. 2007. FcgammaR: the key to optimize therapeutic antibodies? Crit. Rev. Oncol. Hematol. 62:26-33. [DOI] [PubMed] [Google Scholar]
- 31.Smed-Sorensen, A., et al. 2005. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79:8861-8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava, I. K., J. B. Ulmer, and S. W. Barnett. 2005. Role of neutralizing antibodies in protective immunity against HIV. Hum. Vaccin. 1:45-60. [DOI] [PubMed] [Google Scholar]
- 33.Turville, S. G., M. Aravantinou, H. Stossel, N. Romani, and M. Robbiani. 2008. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nat. Methods 5:75-85. [DOI] [PubMed] [Google Scholar]
- 34.Valladeau, J., et al. 2000. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12:71-81. [DOI] [PubMed] [Google Scholar]
- 35.Valladeau, J., and S. Saeland. 2005. Cutaneous dendritic cells. Semin. Immunol. 17:273-283. [DOI] [PubMed] [Google Scholar]
- 36.van der Vlist, M., and T. B. Geijtenbeek. 2010. Langerin functions as an antiviral receptor on Langerhans cells. Immunol. Cell Biol. 88:410-415. [DOI] [PubMed] [Google Scholar]
- 37.Walker, L. M., et al. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, X., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 65:4832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.