Abstract
The Kaposi's sarcoma-associated herpesvirus (KSHV) G protein-coupled receptor (vGPCR) is a constitutively active, highly angiogenic homologue of the interleukin-8 (IL-8) receptors that signals in part via the cytoplasmic protein tyrosine phosphatase Shp2. We show that vGPCR contains a bona fide immunoreceptor tyrosine-based inhibitory motif (ITIM) that binds and constitutively activates Shp2.
Kaposi's sarcoma-associated herpesvirus (KSHV) is a γ2-human herpesvirus and the only human rhadinovirus (8). Seroprevalence varies worldwide, with the highest rates in the Mediterranean basin and Africa, where up to 50% of the population is infected (17). In immunocompromised individuals, KSHV infection leads to Kaposi's sarcoma (KS) (8, 26). KS is the most common cancer in large parts of central Africa and is an increasing problem in the post-organ transplant setting. Despite gaps in our knowledge of KSHV pathobiology, much has been learned about the viral life cycle and how certain viral gene products usurp host cell physiology to generate hyperproliferative states, including the highly angiogenic KS tumor.
Rhadinoviruses, such as KSHV, are adept at pirating useful genes from their hosts during coevolution. We have studied the KSHV G protein-coupled receptor (vGPCR), whose nearest human homologues are the interleukin-8 (IL-8) receptors: CXCR1 and CXCR2. Unlike these normal chemokine receptors, vGPCR signals constitutively via multiple subtypes of G protein (2, 7). This potent constitutive signaling perturbs cellular pathways involved in survival, proliferation, and angiogenesis. In fact, in transgenic mouse models, vGPCR expression causes endothelial tumors with histologic features of KS (12, 14). vGPCR is therefore considered a viral oncogene crucial to KSHV pathobiology and a promising molecular target for the design of anti-KSHV therapeutics.
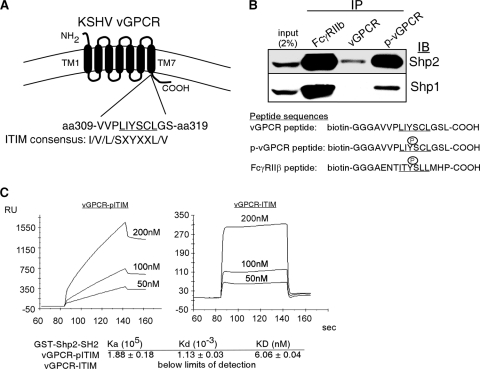
GPCRs signal via the canonical heterotrimeric G protein association. However, several recently discovered noncanonical mechanisms add levels of complexity to GPCR biology (20, 23). These studies have broad implications in that GPCRs are involved in all aspects of cellular physiology, and it is estimated that 30% of clinically available medications target GPCR-driven pathways (1, 10, 25). We have recently published that the protein tyrosine phosphatase (PTP) Shp2 is required for full activation of several KSHV vGPCR-induced signaling events (3). The PTPs are crucial to regulating many basic cellular functions, including growth, survival, and migration, via their contribution to the dynamic process of protein tyrosine phosphorylation and dephosphorylation (16). Shp2 is an Src homology 2 (SH2) domain-containing PTP that positively regulates pathways initiated by many growth factors. Because we recently found that Shp2 is crucial to vGPCR signaling, we hypothesized that vGPCR and Shp2 interacted directly. We identified a short sequence within vGPCR (amino acid 310-VPLIYSCLGS) that fits the consensus immunoreceptor tyrosine-based inhibitory motif (ITIM) I/V/L/S-X-Y-X-X-L/V (4, 6, 19), a motif that was first described in hematopoietic receptors but is now known to trigger activation of some PTPs (16, 18). The location of this putative vGPCR ITIM at the junction of the seventh transmembrane domain (TM7) and the carboxy tail means it could plausibly interact with cytoplasmic proteins (Fig. 1 A).
FIG. 1.
The vGPCR ITIM binds the SH2 domains of Shp1 and Shp2. (A) Schematic of the location and sequence of a putative ITIM at the junction of transmembrane domain 7 (TM7) and the COOH-terminal tail. (B) HEK293 protein lysates were incubated with biotinylated peptide representing the vGPCR ITIM with or without Y314 phosphorylation or with a phosphorylated peptide based on FcγRIIb (positive control). The immunocomplex was blotted for endogenous Shp2. A similar experiment was performed using a BJAB protein lysate for the detection of Shp1 binding. (C) Surface plasmon resonance (SPR). A biotinylated peptide representing the vGPCR ITIM motif with or without Y314 phosphorylation (left and right, respectively) was bound to two different lanes of a streptavidin SPR sensor chip. Recombinant GST-SH2-Shp2 was then flowed across the chip in various concentrations as shown and KD calculated with Biacore software. Note scale differences and that in the right panel, the curve returns to baseline, indicating no binding.
To bind Shp2, an ITIM must be phosphorylated at the central tyrosine residue. We therefore designed biotinylated peptides representing the vGPCR putative ITIM with and without phosphorylation to determine if the sequence is capable of precipitating endogenous Shp2 from an HEK293 cell lysate. Figure 1B (top) clearly shows that the vGPCR ITIM peptide efficiently binds to Shp2 but only when tyrosine phosphorylated. Shp1 is another cytoplasmic phosphatase that interacts with certain ITIM sequences. Shp1 is expressed in hematopoietic cells, so a similar experiment was performed using a BJAB lysate (Fig. 1B, bottom). The vGPCR ITIM did bind Shp1 but less efficiently than Shp2. Since Shp1 is confined to hematopoietic cells and is downregulated in KSHV-infected B cells (unpublished observation), we decided to concentrate on a vGPCR interaction with Shp2.
The tandem N-terminal SH2 domains of Shp2 are responsible for binding ITIMs. Therefore, to confirm that the vGPCR ITIM binds this particular Shp2 domain and to determine the equilibrium dissociation constant (KD) of the interaction, we performed surface plasmon resonance (SPR). Using a Biacore 2000 optical biosensor and BIAevaluation software (Biacore AB, Uppsala, Sweden), the same biotinylated peptides described above were immobilized on streptavidin-coated Biacore sensor chips, over which a truncated form of Shp2 (glutathione S-transferase [GST]-Shp2-SH2; gift of Benjamin Neel) containing only the tandem SH2 domains was flowed. As GST-Shp2-SH2 binds the immobilized peptides, an accumulation of mass is detected by a change in the refractive index. SPR results supported the pulldown experiment in that only the phosphorylated vGPCR ITIM bound with high affinity (KD = 6 nM) to the GST-Shp2-SH2 (Fig. 1C). Taken together, the data in Fig. 1 indicate that the putative vGPCR ITIM binds the tandem SH2 domains of Shp2 as a bona fide ITIM would.
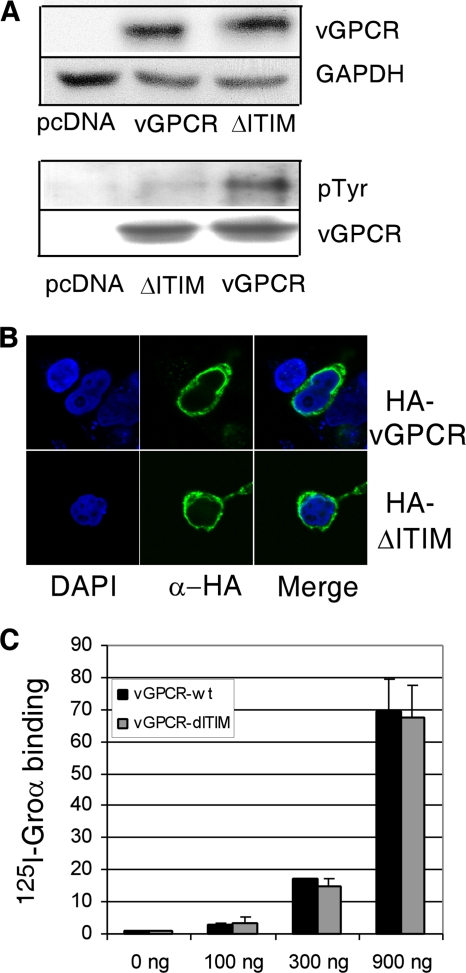
Since we have already shown that Shp2 participates in vGPCR signaling, we wanted next to assess whether an intact ITIM is required for full vGPCR signaling. We therefore constructed a mutant vGPCR in which the required central tyrosine Y314 of the putative ITIM was replaced by phenylalanine, thereby abrogating its potential as an ITIM (ΔITIM). Mutation of GPCRs can cause improper expression and/or localization. Therefore, we performed Western blot analysis to confirm equal protein levels when HEK293 cells were transfected with equal amounts of wild-type (wt) vGPCR or ΔITIM plasmid (Fig. 2 A, top). We were also able to show by Western blot analysis that the ΔITIM mutation caused a decrease in phosphorylated tyrosine levels. The remaining faint band is likely due to other phosphorylated tyrosines within vGPCR (Fig. 2A, bottom). Figure 2B is a confocal image showing that the ΔITIM mutant localizes within the cell in the same distribution as wt vGPCR. Although cytoplasmic localization is obvious from these images, concomitant membrane localization is difficult to appreciate. Therefore, to ensure that similar proportions of ΔITIM and wt vGPCR localize to the cytoplasmic membrane and that the ΔITIM mutant maintains a conformation able to bind ligand, we did binding assays with radiolabeled ligand. Figure 2C shows identical levels of ligand binding at several plasmid doses of wt vGPCR and the ΔITIM mutant. Taken together, these results allow us to conclude that any difference we see in the signaling potency of the ΔITIM mutant versus that of wt vGPCR is not simply due to poor expression or improper localization.
FIG. 2.
A Y314F mutation in the vGPCR ITIM motif does not adversely affect vGPCR expression, localization, or ligand binding. (A) HEK293 cells were transfected with pcDNA3.1-vGPCR or pcDNA3.1-ΔITIM as indicated. Immunoblotting for vGPCR (antibody was a gift of Gary Hayward) was done 24 h later. The blot was stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (top). In the bottom panel, lysates were blotted with antibody to phosphorylated tyrosines (Millipore catalogue no. 05-1050X). (B) Cells expressing hemagglutinin (HA)-tagged vGPCR or an HA-tagged Y314F ITIM mutant (ΔITIM). At 24 h, confocal images were obtained. (C) HEK293 cells were transfected with increasing amounts of plasmid encoding vGPCR or the ΔITIM mutant; after 24 h, binding assays using I125-labeled growth-regulated oncogene alpha (Groα) were performed. Results are normalized to untransfected cells.
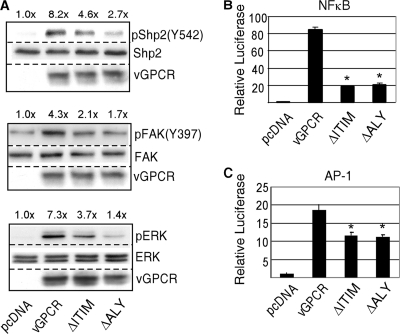
KSHV vGPCR signals via multiple pathways involved in cell survival, proliferation, and migration. We therefore compared ΔITIM vGPCR with wt vGPCR by using immunoblotting to detect phosphorylated forms of extracellular signal-regulated kinase 1 and 2 (ERK1/2), focal adhesion kinase (FAK), and Shp2 itself. In Fig. 3 A, HEK293 cells were plasmid transfected with equal amounts of wt vGPCR or the mutant vGPCR shown; after 48 h, radioimmunoprecipitation (RIPA) lysates were made and Western blotting was performed for the phosphorylated and total levels of Shp2, FAK, and ERK as shown. We also performed reporter assays to assess the mutation's effect on transcription factors activated constitutively by vGPCR, including NF-κB and AP-1. For Fig. 3B, equal amounts of wt vGPCR or mutant vGPCR were transfected into HEK293 cells along with equal amounts of luciferase reporter constructs for the transcription factors shown. Luciferase activity was assayed 48 h posttransfection. In all, Fig. 3 shows that although the ΔITIM mutant activates these pathways above baseline, it is deficient compared to wt vGPCR. For comparison, we have also included an ALY mutant of vGPCR in which the VRY motif at the cytoplasmic end of TM3 has been changed to ALY, thus inhibiting normal interaction with G proteins (5). Taken together, the data in Fig. 1 to 3 indicate that the putative ITIM in vGPCR binds the tandem SH2 domains of Shp2 as expected, and its abrogation results in deficient signaling as predicted by the known dependence of vGPCR on Shp2.
FIG. 3.
KSHV vGPCR requires an intact ITIM for maximal constitutive activation of multiple signaling pathways. (A) Equal amounts of control plasmid, vGPCR, ΔITIM, or a defective vGPCR mutant, ΔALY, were transfected into HEK293 cells. After 48 h, protein lysates were subjected to immunoblotting for phosphorylated forms of the enzymes shown. Blots were stripped and reprobed for total enzyme levels as loading controls. The numbers above the bands represent fold increases in induction compared to that of control cells. (B and C) Cells were transfected with the expression plasmids described above for vGPCR along with a constant amount of luciferase reporter construct for the transcription factors shown as well as a β-galactosidase (β-GAL) construct to which luciferase values were normalized. Bars, standard deviations of results from at least 3 separate experiments; *, P ≤ 0.001 compared to wild-type vGPCR levels.
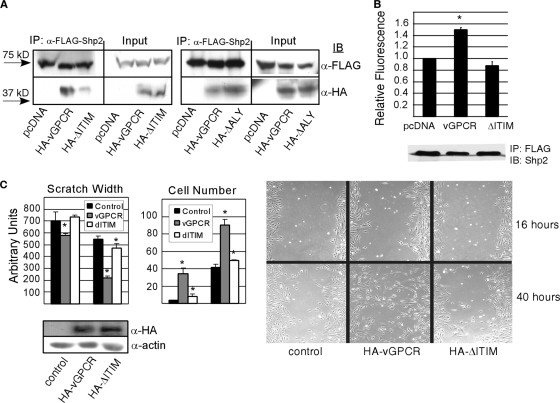
If the vGPCR ITIM is truly functional, it should allow full-length vGPCR to bind to endogenous Shp2 and to activate its phosphatase activity. Immunoprecipitating GPCRs intact with binding partners is notoriously problematic. However, using specialized detergent conditions determined by the Myszka lab (15), we show that despite similar expression levels, wt vGPCR coprecipitated with Shp2 to a greater extent than the ΔITIM mutant (Fig. 4 A). To show that it is specifically the ITIM mutation responsible for decreased binding, we also performed coimmunoprecipitation (co-IP) with the vGPCR ΔALY mutant and saw no difference in binding to Shp2. Some residual association of the ΔITIM mutant with Shp2 is not surprising since coimmunoprecipitation indicates that the proteins are merely in the same complex but not necessarily binding directly. Together with the data in Fig. 1, this allows us to conclude that the vGPCR ITIM helps localize Shp2 to vGPCR, although some vGPCR is present via another mechanism, perhaps via binding to other signaling partners that in turn interact with Shp2. Indeed, vGPCR is certainly part of a large multifunctional protein complex, including G proteins and their effectors and Shp2 and its many binding partners; therefore, abrogating just one domain-domain interaction is unlikely to cause complete absence of vGPCR from this complex. We have published that vGPCR induces phosphorylation of Shp2 on regulatory tyrosines, but there is controversy over whether such events correlate with Shp2 enzymatic activity. Since a bona fide ITIM should activate Shp2, we performed phosphatase assays. Figure 4B shows that wt vGPCR but not the ΔITIM mutant constitutively activates Shp2 enzymatic activity beyond its baseline. The scratch assay in Fig. 4C shows that the vGPCR-ITIM mutant is also deficient at stimulating endothelial cell migration, consistent with a role for Shp2 in angiogenesis (3, 13).
FIG. 4.
KSHV vGPCR requires an intact ITIM to effectively bind and activate Shp2. (A) HEK293 cells were transfected with either HA-vGPCR, HA-ΔITIM, or HA-ΔALY along with a FLAG-Shp2 construct. FLAG-Shp2 was immunoprecipitated, and complexes were blotted with an anti-HA antibody to detect the wt HA-vGPCR and mutants. (B) HEK293 cells were transfected with equal amounts of plasmid expressing FLAG-Shp2 and either pcDNA3.1-vGPCR or pcDNA3.1-ΔITIM. Twenty-four hours later, cells were serum starved overnight followed by immunoprecipitation of FLAG-Shp2 and phosphatase assay using DiFMUP as a fluorescent substrate. The blot beneath the graph shows that equal amounts of FLAG-Shp2 were precipitated in each case. Bars, standard deviations; *, P = 0.017 (Student's t test) relative to the pcDNA3.1 control. There was no statistical difference between the control and the ΔITIM mutant. (C) Primary endothelial cells were transfected with lentivirus expressing wt vGPCR or the ΔITIM mutant or control lentivirus and grown to confluence. A scratch wound was introduced and medium changed to M199 with 1% fetal bovine serum (FBS). The number of cells migrating into the wound was determined as was wound width at 16 h and 40 h postwounding as described previously (3). Bars, standard deviations; *, P ≤ 0.005 (Student's t test).
The presence of functional ITIMs in GPCRs has so far received little attention (9, 22, 24). However, we speculate that this noncanonical GPCR signaling mechanism may be widespread, particularly in light of recent combinatorial chemistry work suggesting that the consensus ITIM sequence might be expanded to T/V/I/Y-X-Y-A/S/T/V-X-I/V/L for the amino SH2 domain of Shp2 and I/L/V-X-Y-T/V/A-X-I/V/L/F for the carboxy SH2 domain (11, 21). Furthermore, given the common “NPXXY” motif found in many GPCRs of the rhodopsin family at the cytoplasmic end of TM7, an overlapping consensus ITIM motif may indeed be very common. Table 1 shows a list of other herpesviral GPCRs with a putative Shp2-binding ITIM at the distal part of TM7. Of note, using the broader ITIM consensus sequence, the mammalian homologues of KSHV vGPCR contain putative ITIMs at the same location as KSHV vGPCR. Whether these are bona fide ITIMs and whether ligand binding is required for interaction with Shp2 or other ITIM-binding proteins needs to be studied.
TABLE 1.
GPCRs related to KSHV vGPCR with putative Shp2-binding ITIM (I/V/L/S-X-Y-X-X-L/V, T/V/I/Y-X-Y-A/S/T/V-X-I/V/L, or I/L/V-X-Y-T/V/A-X-I/V/L) located at the junction of transmembrane domain 7 and the carboxy taila
| Organism | GPCR | UniProt accession no. | Putative ITIM | Category |
|---|---|---|---|---|
| Human | CXCR1 | P25024 | aa303-IIYAFI | Mammalian GPCR |
| Human | CXCR2 | P25025 | aa312-LIYAFI | Mammalian GPCR |
| KSHV | ORF74 | Q9QEV2 | aa312-LIYSCL | Human rhadinovirus |
| HVS | ORF74 | Q01035 | aa291-LYYCML | Nonhuman rhadinovirus |
| MHV68 | ORF74 | O41975 | None | Nonhuman rhadinovirus |
| AtHV3 | ORF74 | Q9YTJ2 | aa291-LLYCLL | Nonhuman rhadinovirus |
| RRV H26-95 | ORF74 | Q77NH5 | aa313-VVYSGL | Nonhuman rhadinovirus |
| HCMV | US28 | Q910P5 | aa289-LLYVFV | Other human herpesviruses |
| HCMV | UL33 | Q6SW98 | aa304-ILYALL | Other human herpesviruses |
| HCMV | UL78 | P16751 | None | Other human herpesviruses |
| HCMV | US27 | P09703 | None | Other human herpesviruses |
| HHV6 | U12 | P52380 | None | Other human herpesviruses |
| HHV6 | U51 | P52382 | None | Other human herpesviruses |
| HHV7 | U12 | P52381 | None | Other human herpesviruses |
| HHV7 | U51 | P52383 | None | Other human herpesviruses |
| EBV | BILF2 | Q777B4 | None | Other human herpesviruses |
KSHV, Kaposi's sarcoma-associated virus; HVS, herpesvirus saimiri; MHV68, murine gammaherpesvirus 68; AtHV3, ateline herpesvirus 3; RRV, rhesus rhadinovirus; HCMV, human cytomegalovirus; HHV6, human herpesvirus 6; HHV7, human herpesvirus 7; EBV, Epstein-Barr virus; aa, amino acid. Domain prediction was performed using the Swiss-Model repository (http://swissmodel.expasy.org/).
In summary, we present here the first report of a GPCR that constitutively activates Shp2 via an ITIM. In addition to the many growth-related pathways involving Shp2 mentioned above, recent data also support a role for Shp2 in angiogenesis (13). Indeed, one of the most striking functional consequences of vGPCR signaling in endothelial cells in vitro and in animal models is angiogenesis. Inhibition of vGPCR is a promising approach to inhibit KSHV-induced pathobiology, but to date there are no convincing reports of ligand-induced inhibition of a constitutively active GPCR. Therefore, better understanding of the proximal events initiated by vGPCR is crucial to designing such an approach. Closer scrutiny of virally encoded GPCRs also stands to teach us about normal mammalian GPCRs since during the course of viral evolution crucial mutations that affect GPCR signaling are selected for and retained.
Acknowledgments
This work was funded by American Cancer Society grant RSG-10-060-01 to M.L.C.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Armbruster, B. N., and B. L. Roth. 2005. Mining the receptorome. J. Biol. Chem. 280:5129-5132. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 3.Bakken, T., M. He, and M. L. Cannon. 2010. The phosphatase Shp2 is required for signaling by the Kaposi's sarcoma-associated herpesvirus viral GPCR in primary endothelial cells. Virology 397:379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billadeau, D. D., and P. J. Leibson. 2002. ITAMs versus ITIMs: striking a balance during cell regulation. J. Clin. Invest. 109:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger, M., J. A. Burger, R. C. Hoch, Z. Oades, H. Takamori, and I. U. Schraufstatter. 1999. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 163:2017-2022. [PubMed] [Google Scholar]
- 6.Burshtyn, D. N., A. M. Scharenberg, N. Wagtmann, S. Rajagopalan, K. Berrada, T. Yi, J. P. Kinet, and E. O. Long. 1996. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity 4:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 9.Duchene, J., J. P. Schanstra, C. Pecher, A. Pizard, C. Susini, J. P. Esteve, J. L. Bascands, and J. P. Girolami. 2002. A novel protein-protein interaction between a G protein-coupled receptor and the phosphatase SHP-2 is involved in bradykinin-induced inhibition of cell proliferation. J. Biol. Chem. 277:40375-40383. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins, A. L., and C. R. Groom. 2002. The druggable genome. Nat. Rev. Drug Discov. 1:727-730. [DOI] [PubMed] [Google Scholar]
- 11.Imhof, D., A. S. Wavreille, A. May, M. Zacharias, S. Tridandapani, and D. Pei. 2006. Sequence specificity of SHP-1 and SHP-2 Src homology 2 domains. Critical roles of residues beyond the pY+3 position. J. Biol. Chem. 281:20271-20282. [DOI] [PubMed] [Google Scholar]
- 12.Jensen, K. K., D. J. Manfra, M. G. Grisotto, A. P. Martin, G. Vassileva, K. Kelley, T. W. Schwartz, and S. A. Lira. 2005. The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi's sarcoma. J. Immunol. 174:3686-3694. [DOI] [PubMed] [Google Scholar]
- 13.Mannell, H., N. Hellwiga, T. Gloea, C. Plankc, H. Sohnb, L. Groessera, B. Walzoga, U. Pohla, and F. Krötza. 2008. Inhibition of the tyrosine phosphatase SHP-2 suppresses angiogenesis in vitro and in vivo. J. Vasc. Res. 45:153-163. [DOI] [PubMed] [Google Scholar]
- 14.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 15.Navratilova, I., J. Sodroski, and D. G. Myszka. 2005. Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal. Biochem. 339:271-281. [DOI] [PubMed] [Google Scholar]
- 16.Neel, B. G., H. Gu, and L. Pao. 2003. The ′Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284-293. [DOI] [PubMed] [Google Scholar]
- 17.Olsen, S. J., Y. Chang, P. S. Moore, R. J. Biggar, and M. Melbye. 1998. Increasing Kaposi's sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi's sarcoma endemic region, Zambia in 1985. AIDS 12:1921-1925. [DOI] [PubMed] [Google Scholar]
- 18.Poole, A. W., and M. L. Jones. 2005. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell. Signal. 17:1323-1332. [DOI] [PubMed] [Google Scholar]
- 19.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 290:84-89. [DOI] [PubMed] [Google Scholar]
- 20.Ribas, C., P. Penela, C. Murga, A. Salcedo, C. Garcia-Hoz, M. Jurado-Pueyo, I. Aymerich, and F. Mayor, Jr. 2007. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim. Biophys. Acta 1768:913-922. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney, M. C., A. S. Wavreille, J. Park, J. P. Butchar, S. Tridandapani, and D. Pei. 2005. Decoding protein-protein interactions through combinatorial chemistry: sequence specificity of SHP-1, SHP-2, and SHIP SH2 domains. Biochemistry 44:14932-14947. [DOI] [PubMed] [Google Scholar]
- 22.Vatinel, S., A. Ferrand, F. Lopez, A. Kowalski-Chauvel, J. P. Esteve, D. Fourmy, M. Dufresne, and C. Seva. 2006. An ITIM-like motif within the CCK2 receptor sequence required for interaction with SHP-2 and the activation of the AKT pathway. Biochim. Biophys. Acta 1763:1098-1107. [DOI] [PubMed] [Google Scholar]
- 23.Violin, J. D., and R. J. Lefkowitz. 2007. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 28:416-422. [DOI] [PubMed] [Google Scholar]
- 24.Voisin, T., A. El Firar, C. Rouyer-Fessard, V. Gratio, and M. Laburthe. 2008. A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J. 22:1993-2002. [DOI] [PubMed] [Google Scholar]
- 25.Wettschureck, N., and S. Offermanns. 2005. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85:1159-1204. [DOI] [PubMed] [Google Scholar]
- 26.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, and A. S. Denton. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]