Abstract
Objectives
To collect normative data, assess differences between demographic groups, and indirectly compare US and Canadian medical systems relative to patient expectations of involvement in cancer treatment decision making.
Study Design
Meta-analysis.
Methods
Individual patient data were compiled across 6 clinical studies among 3491 patients with cancer who completed the 2-item Control Preferences Scale indicating the roles they preferred versus actually experienced in treatment decision making.
Results
The roles in treatment decision making that patients preferred were 26% active, 49% collaborative, and 25% passive. The roles that patients reported actually experiencing were 30% active, 34% collaborative, and 36% passive. Roughly 61% of patients reported having their preferred role; only 6% experienced extreme discordance between their preferred versus actual roles. More men than women (66% vs 60%, P = .001) and more US patients than Canadian patients (84% vs 54%, P <.001) reported concordance between their preferred versus actual roles. More Canadian patients than US patients preferred and actually experienced (42% vs 18%, P <.001) passive roles. More women than men reported taking a passive role (40% vs 24%, P <.001). Older patients preferred and were more likely than younger patients to assume a passive role.
Conclusions
Roughly half of the studied patients with cancer indicated that they preferred to have a collaborative relationship with physicians. Although most patients had the decision-making role they preferred, about 40% experienced discordance. This highlights the need for incorporation of individualized patient communication styles into treatment plans.
Control preferences regarding decision making among patients with cancer have been associated with patient outcomes.1 Several studies have demonstrated that patients who are involved in making medical decisions fare better2,3 and are more satisfied4–6 than patients who do not. Based on the unfolding theory by Coombs,7 Hack and colleagues8 developed a reliable and easy-to-administer instrument to measure the degree of control a patient desires over his or her medical decisions wherein patients were asked to rank 5 different roles in order of preference. The measure was used in multiple nursing investigations. Hack et al found that there was potential for discordance among patients with cancer between the decision-making roles patients preferred and the roles they experienced (or were allowed to have) in the active or collaborative experience. The Control Preferences Scale (CPS) (eAppendix A, available at www.ajmc.com) asks patients 2 questions about how much decision-making control they would like to have and how much decision-making control patients perceived that they actually experienced. There are 5 response categories with associated stick figure drawings for each of the following 5 control options: active, active shared, collaborative, passive shared, and passive. The responses can be further collapsed into the following 3 categories of role responses: active, collaborative, and passive.9 There has been renewed interest in examining patient preferences regarding decision making because of the focus on patient-centered care,10,11 and the CPS is a brief and valid measure of this construct.12
The CPS has been applied in studies across the world, including Sweden,13 the United Kingdom,14 Germany,15 Italy,16 and Norway.17–19 The CPS has been shown to have good psychometric properties.9,20,21 However, to date there has not been a compilation effort to provide collective normative data for others to reference. This article aims to fill that void.
BACKGROUND
Several studies22–24 have attempted to determine what factors are associated with desired levels of control preference in treatment decision making. Many investigations have shown that higher education level is associated with preference for a more active role. In a sample of 1012 Canadian women with breast cancer, Degner et al12 found that patients who were more educated and younger desired a more active role. Education level and length of follow-up were associated with choice of an active or collaborative role among 140 Italian patients with multiple sclerosis.16 In a national survey of 1000 Norwegian women older than 18 years, characteristics associated with more active role preferences were age 45 to 59 years (relative to younger and older age groups), higher education level, better general health status, and a negative attitude toward therapy.18 In a study25 of 5199 adults from Wisconsin, female sex, higher education level, better self-rated health, and fewer prescription medications predicted a significantly higher probability of the most active involvement in decision making. Role preference showed little variability over time. Hence, role preference may be more of an intrinsic personality trait that is consistent over time and situations.
Several other factors are also known to influence role preference. In a community survey of 514 men aged 50 to 70 years in Sydney, Australia, occupational skill level predicted men’s preferences for a passive role.26 Patients with occupational skill levels 3 through 5 were significantly more likely to delegate decision making to physicians than were managers and professionals. Shields and colleagues27 found that patients having prostate or breast cancer, less fatalistic orientation, and professional or managerial occupations preferred a shared or active role compared with patients having other cancers, more fatalistic orientation, and other occupations. In a population-based survey of 2765 adults, age younger than 45 years, excellent self-rated general health, female sex, and white (vs Hispanic) race/ethnicity were associated with preferences being more patient oriented (ie, more active role).28 In a survey of Norwegian women with urinary incontinence, older age and being married were associated with lower likelihood of preferring an active role.17
Rationale for the Pooled Analysis
The differences between US and Canadian healthcare systems have been well documented.29–31 Despite the growing body of evidence for incorporating patient control preferences in medical care, it is unknown if preferences vary by country. This study pooled individual patient data from 6 studies conducted in the United States or Canada and included 3491 patients. The meta-analysis sought to determine (1) estimates of the overall distribution for preferences among patients with cancer in terms of role expectation and experience in treatment decision making, (2) the effect of selected demographic and clinical variables on patient preference and experience in treatment decision making, and (3) differences in control preferences between US and Canadian patients.
METHODS
Data were compiled from 6 studies that administered the CPS to patient populations. The Study of Cancer Survivors I by the American Cancer Society32 was intended to be a national (US) prospective longitudinal study among patients surviving 1 of 10 common cancers, including prostate, female breast, lung, colorectal, bladder, uterine, kidney, ovarian, skin melanoma, and non-Hodgkin’s lymphoma. We included patients from the state of Minnesota for the pooled analysis.
The Mayo Clinic Multi-Disciplinary Quality of Life Study33 investigated interventions to maintain overall quality of life in 103 patients undergoing radiation therapy for advanced-stage cancer. The patients were randomized to standard care or to a structured multidisciplinary intervention that included 8 sessions addressing cognitive, physical, emotional, spiritual, and social functioning domains relative to quality of life.
Degner and colleagues9 studied a consecutive sample of 1012 women with a confirmed diagnosis of breast cancer at 2 tertiary oncology referral clinics and 2 community hospital oncology clinics. They performed a cross-sectional survey querying about patient preferences regarding participation in decision making for their care.
In another study, Degner and Sloan34 investigated patient preferences in decision making among 434 consecutive adult patients with a new cancer diagnosis at 2 tertiary care referral clinics in Winnipeg, Manitoba, Canada. All patients were queried within 6 months of an initial diagnosis of cancer.
Hack and colleagues35 studied the efficacy of audiotaping primary adjuvant therapy consultations on information communication, satisfaction, and quality of life among 628 women with newly diagnosed breast cancer from 6 cancer centers in Canada. The patients were block randomized to 1 of the following 4 consultation groups: (1) standard care control and not audiotaped, (2) audiotaped but no audiotape given, (3) audiotaped and patient given audiotape, or (4) audiotaped and patient offered choice of receiving audiotape or not.
In a similar study, Hack and colleagues36 studied the efficacy of providing an audiotape of primary treatment consultations to 472 men with newly diagnosed prostate cancer undergoing radiation therapy. Patients were recruited from 4 cancer centers in Canada and were block randomized to 1 of 4 consultation groups as described for the breast cancer study.35
Data evaluated in this pooled analysis were CPS findings and patient demographics, including country, sex, and age. Among all studies, patients were categorized as having corcordance or discordance between the role in treatment decision making that they preferred and the role that they experienced. For each CPS question, the distribution of control (role preference and role experience) was summarized by simple proportions and by graphical representations of bar charts. Cross-tabulation of control preferences with demographic variables followed. The Fisher exact test was used to compare role distribution concordance and to measure association with clinical and demographic variables. For ease of interpretation, CPS preferences were categorized as active, collaborative, or passive as in previous studies.9,13,15,17–19 Multivariate logistic regression models were used to assess the effect of the univariately significant variables (age, sex, country, and tumor stage) in combination with each other to predict role preference and concordance between role preference and experience.37
As suggested by a reviewer, we performed additional sensitivity analyses adjusting the aforedescribed multivariate-adjusted models for education level (grade school, high school, more than high school, or not reported) to assess if the country-related and other associations with role preference and concordance were confounded by patient education. In addition, we performed these multivariate analyses considering all 5 CPS categories, instead of the 3 collapsed levels, per the reviewer’s suggestion.
RESULTS
The study cohort consisted of 3491 patients enrolled in 6 different cancer treatment trials (Table 1). There were 2591 patients (74% of the total sample) from Canadian clinical trials and 900 patients (26% of the total sample) from US studies. Overall, the study included 68% female patients, 61% aged 65 years or older, and 29% with tumor stages 0 to 2 (Table 2). Comparisons between the US and Canadian patient cohorts indicate more women, more patients 50 years or older, and more patients with early-stage disease among US patients. There is a considerable amount of missing data on tumor stage, so the latter finding needs to be interpreted with caution. All studies involved CPS assessment at the time of diagnosis or before treatment initiation except for the American Cancer Society study,32 which was a cross-sectional study of survivors.
Table 1.
Studies Included in the Meta-Analysis
| Source | No. of Patients | Tumor Type | Country |
|---|---|---|---|
| Stein et al; American Cancer Society Study of Cancer Survivors I,32 2006 | 797 | Multiple | United States |
| Rummans et al; Mayo Clinic Multi-Disciplinary Quality of Life Study,33 2006 | 103 | Multiple | United States |
| Degner et al,9 1997 | 1012 | Breast | Canada |
| Degner and Sloan,34 1992 | 434 | Multiple | Canada |
| Hack et al,35 2003 | 673 | Breast | Canada |
| Hack et al,36 2007 | 472 | Prostate | Canada |
Table 2.
Patient Demographics
| Variable | No. (%) |
|---|---|
| Country (n = 3491) | |
| United States | 900 (25.8) |
| Canada | 2591 (74.2) |
| Sex (n = 3489) | |
| Female | 2363 (67.7) |
| Male | 1126 (32.3) |
| Age group, y (n = 2144) | |
| <50 | 809 (37.7) |
| 50–64 | 35 (1.6) |
| ≥65 | 1300 (60.6) |
| Education level (n = 3276) | |
| Grade school | 695 (21.2) |
| High school | 1095 (33.4) |
| >High school | 1463 (44.7) |
| Not reported | 23 (0.7) |
| Tumor stage (n = 1236) | |
| 0–2 | 356 (28.8) |
| 3–5 | 880 (71.2) |
Overall Preferred and Actual Roles in Treatment Decision Making
Four percent of patients preferred an active role in treatment decision making, 22% an active shared role, 49% a collaborative role, 15% a passive shared role, and 9% a passive role (Table 3). Collapsing these into 3 categories indicates that 26% of 3276 patients preferred an active role in treatment decision making, 49% a collaborative role, and 25% a passive role. The percentage of patients preferring an active role ranged from 12% in the multitumor study by Degner and Sloan34 to 36% in the American Cancer Society study.32
Table 3.
Overall Distribution of Preferred Role Versus Actual Role in Treatment Decision Making
| Variable | Preferred Role, % (n = 3276)a | Actual Role, % (n = 2742)b |
|---|---|---|
| Active | 4.7 | 4.1 |
| Active shared | 21.5 | 26.0 |
| Collaborative | 49.1 | 34.3 |
| Passive shared | 15.3 | 13.9 |
| Passive | 9.4 | 21.7 |
215 patients were missing preferred role.
Only the preferred role was assessed by 749 patients in 1 study.
The decision making that patients reported actually experiencing were 4% active, 26% active shared, 34% collaborative, 14% passive shared, and 22% passive (Table 3). Collapsing these into 3 categories indicates that 30% of patients reported that they experienced an active role, 34% a collaborative role, and 36% a passive role. The percentage of patients experiencing an active role ranged from 18% in the Mayo Clinic Multi-Disciplinary Quality of Life Study33 to 40% in the prostate cancer study by Hack et al.36
Role preferences were compared to determine whether preferred roles were in concordance with actual roles (Table 4). Overall, there was roughly 61% concordance (ie, patients’ preferred and actual roles were the same). Only 6% of patients experienced extreme discordance between their preferred and actual roles (ie, wanting an active role and experiencing a passive role, or vice versa). Twenty-six percent of patients preferred an active role, and 30% experienced an active role. Conversely, 36% of patients preferred a passive role, and 25% experienced a passive role. Among 1058 of 2742 patients (39%) reporting discordance between their preferred and actual roles, 453 (43%) had a more active role than they preferred, whereas 605 (57%) had a more passive role than they preferred.
Table 4.
Preferred Role Versus Actual Role Among 2742 Patients Who Had Both Reporteda
| Variable | No. (%) (n = 2742) | |||
|---|---|---|---|---|
| Actual Role | Total | |||
| Active | Collaborative | Passive | ||
| Preferred role | ||||
| Active | 493 (18.0) | 123 (4.5) | 104 (3.8) | 720 (26.3) |
| Collaborative | 271 (10.0) | 696 (25.4) | 378 (13.8) | 1345 (49.1) |
| Passive | 61 (2.2) | 121 (4.4) | 495 (18.1) | 677 (24.7) |
| Total | 825 (30.1) | 940 (34.3) | 977 (35.6) | 2742 (100.0) |
Only the preferred role was assessed by 749 patients in 1 study.
Role Distributions by Sex, Country, Age, Tumor Stage, and Education Level
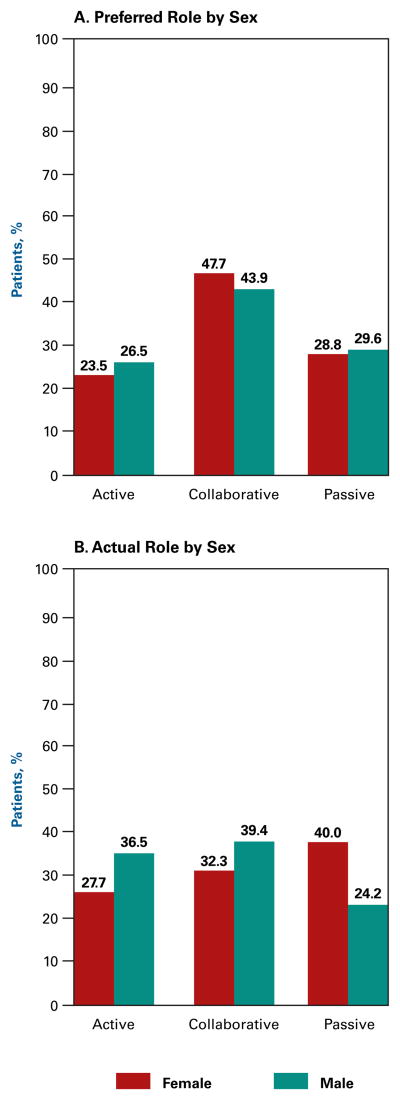
The distribution of patients by the roles they preferred versus actually experienced is broken down by sex in Figure 1. There was little difference in the role distributions preferred by men versus women (Figure 1A). However, there was a significant difference in the distribution of the actual roles patients experienced, with more women than men reporting taking a passive role (40% vs 24%, P <.001) (Figure 1B).
Figure 1.
Preferred Role Versus Actual Role by Sex
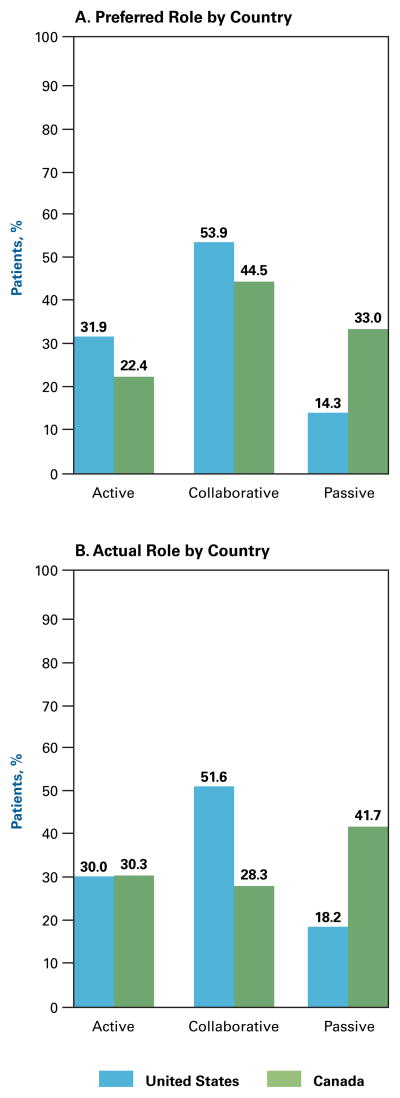
More US patients than Canadian patients preferred active roles (32% vs 22%, P <.001), whereas more Canadian patients than US patients preferred (33% vs 14%, P <.001) and actually experienced (42% vs 18%, P <.001) passive roles (Figure 2A and 2B). American men preferred and actually experienced more active and collaborative roles than Canadian men (eAppendix B1 and B2). Canadian men actually experienced a more active role versus what they preferred (40% versus 24%, P = .004). American women preferred a less passive role than Canadian women (10% vs 33%, P <.001) and actually experienced a less passive role than Canadian women (17% vs 45%, P <.001) (eAppendix B3 and B4).
Figure 2.
Preferred Role Versus Actual Role by Country
The preferred and experienced role distributions varied across age groups. More older patients (≥50 years) than younger patients preferred a passive role in treatment decision making (32% vs 21%, P <.001). More younger patients (<50 years) than older patients preferred an active role (31% vs 22%, P <.001). More older patients than younger patients actually experienced a passive role (38% vs 30%, P <.001). More younger patients than older patients actually experienced an active role (34% vs 29%, P = .02).
In the subset of patients for whom tumor grade was available, there was no significant difference in the preferred role distribution between patients having tumor stages 0 to 2 (24% active and 33% passive) versus patients having tumor stages 3 to 5 (21% active and 36% passive) (P = .50). However, there was a difference in the actual role distribution, with more patients having tumor stages 0 to 2 than those having tumor stages 3 to 5 actually experiencing an active role (30% vs 22%, P = .02).
The preferred role differed by education level. Grade school, high school, and more than high school education levels were associated with preference for a passive role in 49%, 32%, and 22% of patients, respectively (P <.001). A passive actual role was experienced by 64%, 35%, and 23% of patients with grade school, high school, and more than high school education levels, respectively (P <.001).
Concordance Between Preferred and Actual Roles by Sex, Country, Age, and Tumor Stage
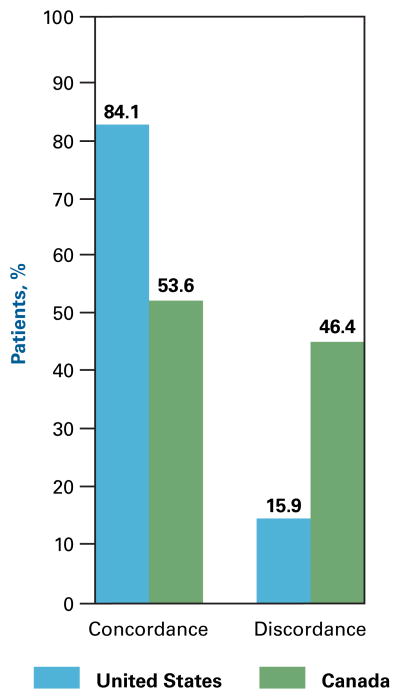
More men than women (66% vs 60%, P = .001) reported concordance between their preferred and actual roles. More US patients than Canadian patients (84% vs 54%, P <.001) reported concordance between their preferred and actual roles (Figure 3). Examination within sex and country of residence shows that US men had greater role concordance than Canadian men (85% vs 52%, P <.001). This was also true for US women compared with Canadian women (85% vs 54%, P <.001). Among patients 65 years or older, 59% reported role concordance compared with 63% among patients aged 50 to 64 years and 62% among patients younger than 50 years (P = .18). Tumor stage did not affect concordance between preferred and actual roles (62% concordance for patients with tumor stages 0 to 2 vs 56% concordance for patients with tumor stages 3 to 5, P = .10). Patients with grade school education had 59% concordance between preferred and actual roles, those with high school education had 59% concordance, and those with more than high school education had 64% concordance (P = .01).
Figure 3.
Concordance and Discordance Between Preferred Role and Actual Role by Country
Multivariate Modeling of Role Preference and Role Concordance
A polytomous logistic regression model was constructed using the preferred trichotomous role categorization (active, collaborative, and passive) to assess which demographic and clinical variables (age, sex, country, and tumor stage) were related to patients’ role preferences. Patients tended to prefer a more active role if they were American (odds ratio [OR], 5.26; 95% confidence interval [CI], 2.86–10.0), female (OR, 2.13; 95% CI, 2.86–10.0), and younger (OR, 1.03; 95% CI, 1.02–1.04) (P <.001 for all, Wald χ2). Collectively, these variables accounted for 62% of the variability in role preferences. Sensitivity analyses that included education level did not change the significance of these associations, as follows: American (OR, 4.27; 95% CI, 2.28–8.00), female (OR, 2.30; 95% CI, 1.42–3.78), and younger (OR, 0.98; 95% CI, 0.97–0.99) (P <.001 for all, Wald χ2). Education level was associated with role preference. Compared with patients having a grade school education level, those having high school (OR, 1.32; 95% CI, 1.01–1.74) and more than high school (OR, 2.16; 95% CI, 1.65–2.82) education levels were more likely to prefer a more active role. Collectively, these variables accounted for 65% of the variability in role preferences.
Considering CPS preference using the original 5-point scale (as suggested by a reviewer), results were consistent with what was seen using the 3-point scale. Patients tended to prefer a more active role if they were American (OR, 3.66; 95% CI, 2.07–6.49), female (OR, 1.73; 95% CI, 1.11–2.71), and younger (OR, 0.97; 95% CI, 0.96–0.98) (P <.01 for all, Wald χ2). These associations changed little in sensitivity analyses that also adjusted for education level. Patients were more likely to prefer a more active role if they were American (OR, 2.90; 95% CI, 1.63–5.18), female (OR, 1.90; 95% CI, 1.21–2.98), and younger (OR, 0.98; 95% CI, 0.97–0.98) and had high school (OR, 1.48; 95% CI, 1.14–1.93) or more than high school (OR, 2.30; 95% CI, 1.77–2.98) education levels (P <.004 for all, Wald χ2). Collectively, these variables accounted for 64% of the variability in role preferences.
A second logistic regression model was constructed using discordance versus concordance indicators to assess which variables were related to patient perception of discordance between preferred and actual roles in the trichotomous categorization (active, collaborative, or passive). Patients who experienced discordance between preferred and actual roles tended to be Canadian (OR, 9.3; 95% CI, 2.2–39.8; P <.001). Sex, age, and tumor stage were unrelated to concordance. Collectively, these variables accounted for 54% of the variability in role preferences. Sensitivity analyses that adjusted for education level indicated that patients with less than high school education experienced a greater likelihood of dissonance compared with patients with high school (OR, 1.30; 95% CI, 1.04–1.63; P = .02) or more than high school (OR, 1.29; 95% CI, 1.03–1.60; P = .02) education levels. However, inclusion of education level in the model did not change the significance of the country association. Greater dissonance was still reported by Canadians (OR, 10.0; 95% CI, 2.3–43.2; P <.001). These variables accounted for 63% of the variability in role preferences.
DISCUSSION
This meta-analysis compiled data among 3491 patients from 6 cancer centers across 2 countries in an attempt to construct normative data in cancer populations for treatment decision making using the CPS. We found that roughly half of the patients preferred a collaborative relationship with their clinician, whereas the remaining half were split almost equally between preferring active and passive roles. Furthermore, although some demographic and clinical influences were evident (accounting for 54%–62% of the combined variability in preferences), patient preference was individualistic. This is consistent with clinical observations regarding patient control preferences in the literature that while older patients tend to be more passive and men more assertive in treatment decision making about tumors unrelated to the reproductive system, women are more assertive about reproductive system tumors. These are tendencies and not prognostic factors. Herein, more women than men experienced a passive role in treatment decision making.
Compared with older patients, younger patients preferred and experienced a more active role in treatment decision making. This confirms similar results from a breast cancer study.12 Other studies have reported similar findings that patients 60 years or older preferred a more passive role than younger patients in decision making for urinary incontinence therapy17,18 and for hormone therapy.18
Most patients experience a role in treatment decision making that is consistent with their preferences. Only 6% of patients herein experienced extreme discordance between their preferred and actual roles. This is important for the science of healthcare delivery since discordance between patient expectations and experiences may lead to reduced patient satisfaction with care and to overall decreased quality of life. Because roughly 39% of patients herein experienced discordance between their preferred and actual roles in treatment decision making, research is needed to identify effective communication strategies. Where discrepancies exist, patients were almost equally likely to have experienced a more passive or active role than what they expected, with a slight preponderance (6%) being more passive. Hence, the issue is not that the healthcare system needs to encourage or discourage patient input in general. The real key to improving care is to deliver the type of experience that the patient prefers in terms of his or her decision-making role. Trying to engage patients in decision making when they prefer to be passive is just as harmful as disallowing sufficient input in the process when they prefer to be active.
This article presents a rare cross-country comparison of patient control preferences between the United States and Canada. Conclusions from such analyses must be drawn carefully because of myriad potentially confounding concomitant influences that we did not explore in our study. For example, differences between the US and Canadian studies evaluated in the meta-analysis could be influenced by other causes of variation not collected, such as differences in the populations being surveyed, possible secular trends, and the number of uninsured individuals in the American samples. This cross-country comparison is not intended to be definitive. Rather, it is a first attempt to explore whether there is any evidence that the amount of input that patients want in treatment decision making might be a function of cultural, societal, and structural differences between the 2 healthcare systems. The effect of structural differences is particularly of interest given the current debate on healthcare reform in the United States. One of the key questions herein is whether the differences observed are truly due to country or system differences or to a combination of demographic and socioeconomic variables. Many authors have described differences between the United States and Canada in general social and economic terms and relative to healthcare delivery systems. The sharp differences in healthcare philosophy have been delineated in recent contentious debates about healthcare reform in which the Canadian system has alternatively been demonized and praised for its differences from the American system, depending on the perspectives and motives of the writer. No single study can disentangle such complex interactions to arrive at definitive attributions for the differences that we observed. Hence, we simply characterize the observations herein as differences between samples drawn from each country. These results must be interpreted in the context that role preference remains a primarily individualistic phenomenon, as repeatedly found in previous investigations.
We found that US patients preferred and experienced a more active role in their treatment decision making than their Canadian counterparts (ie, tend to be more assertive than Canadian patients). Many possible reasons exist for these differences, albeit within the limitations indicated in the previous paragraph. There may be more trust between physicians and patients in the Canadian healthcare system because there is patient expectation of relative uniformity of care, although slight differences in resources and care delivery exist across the Canadian provinces.38 The 2 healthcare systems differ in many additional respects in terms of healthcare access, needs, and costs, which may also affect patients’ role preferences and the roles they actually experience in their care. Compared with Canadians, Americans are less likely to have a regular physician and are more likely to have unmet health needs, pay higher prices, and have shorter waiting times for the same medical services.30,39 Compared with older Canadians, older Americans are more likely to undergo procedures and are less likely to receive evaluation and management services.40 Compared with Canadians and with insured Americans, uninsured Americans have significantly greater healthcare access barriers to use of hospital or physician services, and lower satisfaction.41 In the United States, patients must make an active choice with respect to their healthcare insurance and delivery system and are individually responsible for the insurance co-payments and other costs incurred. This would logically encourage a more active role in choosing healthcare providers similar to that involved in purchasing other goods and services. Because patients are required to have a more active role in seeking healthcare, it is reasonable to suggest that this may translate into a more active role preference in all aspects of healthcare such as treatment decision making.
The multivariate modeling confirmed our univariate results that country, sex, and age showed some relationship to differing role preference distributions in treatment decision making. However, variability among individuals remained the dominant factor, so that none of the variables by themselves are sufficient to predict a patient’s role preference.
Our study has several limitations. Only certain types of cancers are well represented in this analysis (prostate and breast); therefore, the findings may not be generalizable to patients with other, less common cancers. Because most studies were performed at tertiary care referral centers, they tend to recruit patients living in urban or suburban areas and may have low representation of patients living in rural settings or patients receiving care in community healthcare settings. Therefore, our results may not be generalizable to those groups of patients.
The efficiency, validity, and reliability of the CPS have been demonstrated across many clinical settings as indicators of patient preferences and experiences with respect to the role they have in treatment decision making. Sex and cross-county differences exist, although the preference for any particular patient remains individualistic. Healthcare practice has paid more attention to patient preferences in recent years, and most patients experience their preferred role. However, further research is needed because 4 of 10 patients experience a role in treatment decision making that is different from what they would have preferred. The effect of discordance between patients’ preferred and actual roles on treatment outcomes highlights the need for individualized patient communication styles to be incorporated into treatment plans.
Take-Away Points.
Roughly 50% of patients with cancer preferred to have a collaborative relationship with physicians in treatment decision making, and the remaining were split equally between preferring active and passive roles.
Most patients had the decision-making role they preferred, but about 40% of patients experienced discordance between their preferred and actual roles.
Being American and younger was associated with patient preference for a more active role.
Being Canadian was associated with greater discordance between preferred and actual roles experienced by the patient.
This study highlighted that individualized patient communication styles should be incorporated into treatment plans.
Acknowledgments
Funding Source: Supported by clinical and translational science award 1 KL2 RR024151-01 from the National Institutes of Health (to the Mayo Clinic Center for Clinical and Translational Research).
Footnotes
Author Disclosures: Dr Singh reports serving as a paid consultant to Colcrys and Savient Pharmaceuticals, Inc. The other authors (JS, PJA, TS, TFH, MMH, TAR, MMC, BD, and LFD) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
Authorship Information: Concept and design (JS, PJA, TAR, MMC, LFD); acquisition of data (JS, PJA, TS, TFH, TAR, MMC, BD, LFD); analysis and interpretation of data (JAS, JS, PJA, TFH, MMH, TAR, MMC, BD); drafting of the manuscript (JAS, JS, PJA, TFH, TAR, MMC); critical revision of the manuscript for important intellectual content (JAS, JS, PJA, TS, TFH, TAR); statistical analysis (JAS, JS, PJA, MMH, BD); provision of study materials or patients (TFH, TAR, MMC); obtaining funding (TS, TAR, LFD); and supervision (TAR).
References
- 1.Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575–580. doi: 10.1136/bmj.301.6752.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33(5):951–956. [PubMed] [Google Scholar]
- 3.Greenfield S, Kaplan S, Ware JE., Jr Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528. doi: 10.7326/0003-4819-102-4-520. [DOI] [PubMed] [Google Scholar]
- 4.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52(12):1865–1878. doi: 10.1016/s0277-9536(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 5.Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early-stage breast cancer: should surgeons match patients’ desired level of involvement? J Clin Oncol. 2002;20(6):1473–1479. doi: 10.1200/JCO.2002.20.6.1473. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JR, Brundage MD, Feldman-Stewart D. Lung cancer treatment decisions: patients’ desires for participation and information. Psychooncology. 1999;8(6):511–520. doi: 10.1002/(sici)1099-1611(199911/12)8:6<511::aid-pon415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Coombs CH. A Theory of Data. New York, NY: John Wiley & Sons; 1964. [Google Scholar]
- 8.Hack TF, Degner LF, Dyck DG. Relationship between preferences for decisional control and illness information among women with breast cancer: a quantitative and qualitative analysis. Soc Sci Med. 1994;39(2):279–289. doi: 10.1016/0277-9536(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 9.Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485–1492. [PubMed] [Google Scholar]
- 10.Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 11.Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25(9):1067–1073. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- 12.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 13.Florin J, Ehrenberg A, Ehnfors M. Clinical decision-making: predictors of patient participation in nursing care. J Clin Nurs. 2008;17(21):2935–2944. doi: 10.1111/j.1365-2702.2008.02328.x. [DOI] [PubMed] [Google Scholar]
- 14.Beaver K, Luker KA, Owens RG, Leinster SJ, Degner LF, Sloan JA. Treatment decision making in women newly diagnosed with breast cancer. Cancer Nurs. 1996;19(1):8–19. doi: 10.1097/00002820-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rothenbacher D, Lutz MP, Porzsolt F. Treatment decisions in palliative cancer care: patients’ preferences for involvement and doctors’ knowledge about it. Eur J Cancer. 1997;33(8):1184–1189. doi: 10.1016/s0959-8049(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 16.Giordano A, Mattarozzi K, Pucci E, et al. Participation in medical decision-making: attitudes of Italians with multiple sclerosis. J Neurol Sci. 2008;275(1–2):86–91. doi: 10.1016/j.jns.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell M, Hunskaar S. Preferences for involvement in treatment decision-making among Norwegian women with urinary incontinence. Acta Obstet Gynecol Scand. 2007;86(11):1370–1376. doi: 10.1080/00016340701622310. [published correction appears in Acta Obstet Gynecol Scand. 2008;87(4):484] [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell M, Hunskaar S. Preferences for involvement in treatment decision-making generally and in hormone replacement and urinary incontinence treatment decision-making specifically. Patient Educ Couns. 2007;68(3):243–251. doi: 10.1016/j.pec.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell M, Monz B, Hunskaar S. General preferences for involvement in treatment decision making among European women with urinary incontinence. Soc Sci Med. 2007;64(9):1914–1924. doi: 10.1016/j.socscimed.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Degner LF, Russell CA. Preferences for treatment control among adults with cancer. Res Nurs Health. 1988;11(6):367–374. doi: 10.1002/nur.4770110604. [DOI] [PubMed] [Google Scholar]
- 21.Adams JR, Drake RE, Wolford GL. Shared decision-making preferences of people with severe mental illness. Psychiatr Serv. 2007;58(9):1219–1221. doi: 10.1176/ps.2007.58.9.1219. [DOI] [PubMed] [Google Scholar]
- 22.Davison BJ, Degner LF. Feasibility of using a computer-assisted intervention to enhance the way women with breast cancer communicate with their physicians. Cancer Nurs. 2002;25(6):417–424. doi: 10.1097/00002820-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Hawley ST, Lantz PM, Janz NK, et al. Factors associated with patient involvement in surgical treatment decision making for breast cancer. Patient Educ Couns. 2007;65(3):387–395. doi: 10.1016/j.pec.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deber RB, Kraetschmer N, Urowitz S, Sharpe N. Do people want to be autonomous patients? preferred roles in treatment decision-making in several patient populations. Health Expect. 2007;10(3):248–258. doi: 10.1111/j.1369-7625.2007.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med. 2006;63(5):1158–1169. doi: 10.1016/j.socscimed.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattellari M, Ward JE. Measuring men’s preferences for involvement in medical care: getting the question right. J Eval Clin Pract. 2005;11(3):237–246. doi: 10.1111/j.1365-2753.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Shields CG, Morrow GR, Griggs J, et al. Decision-making role preferences of patients receiving adjuvant cancer treatment: a University of Rochester cancer center community clinical oncology program. Support Cancer Ther. 2004;1(2):119–126. doi: 10.3816/SCT.2004.n.005. [DOI] [PubMed] [Google Scholar]
- 28.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making: a national study of public preferences. J Gen Intern Med. 2005;20(6):531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Globerman S, Hodges H, Vining A. Canadian and the United States’ health care systems performance and governance: elements of convergence. Appl Health Econ Health Policy. 2002;1(2):75–88. [PubMed] [Google Scholar]
- 30.Bell CM, Crystal M, Detsky AS, Redelmeier DA. Shopping around for hospital services: a comparison of the United States and Canada. JAMA. 1998;279(13):1015–1017. doi: 10.1001/jama.279.13.1015. [DOI] [PubMed] [Google Scholar]
- 31.Zia MI, Goodman SG, Peterson ED, et al. Paradoxical use of invasive cardiac procedures for patients with non-ST segment elevation myocardial infarction: an international perspective from the CRUSADE Initiative and the Canadian ACS Registries I and II. Can J Cardiol. 2007;23(13):1073–1079. doi: 10.1016/s0828-282x(07)70876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein K, Smith T, Kim Y, et al. American Cancer Society. The American Cancer Society’s studies of cancer survivors: the largest, most diverse investigation of long-term cancer survivors so far. Am J Nurs. 2006;106(3 suppl):83–85. doi: 10.1097/00000446-200603003-00029. [DOI] [PubMed] [Google Scholar]
- 33.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24(4):635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 34.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 35.Hack TF, Pickles T, Bultz BD, et al. Impact of providing audio-tapes of primary adjuvant treatment consultations to women with breast cancer: a multisite, randomized, controlled trial. J Clin Oncol. 2003;21(22):4138–4144. doi: 10.1200/JCO.2003.12.155. [DOI] [PubMed] [Google Scholar]
- 36.Hack TF, Pickles T, Bultz BD, Ruether JD, Degner LF. Impact of providing audiotapes of primary treatment consultations to men with prostate cancer: a multi-site, randomized, controlled trial. Psychooncology. 2007;16(6):543–552. doi: 10.1002/pon.1094. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- 38.Allin S. Does equity in healthcare use vary across Canadian provinces? Healthc Policy. 2008;3(4):83–99. [PMC free article] [PubMed] [Google Scholar]
- 39.Lasser KE, Himmelstein DU, Woolhandler S. Access to care, health status, and health disparities in the United States and Canada: results of a cross-national population-based survey. Am J Public Health. 2006;96(7):1300–1307. doi: 10.2105/AJPH.2004.059402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch WP, Verrilli D, Katz SJ, Latimer E. A detailed comparison of physician services for the elderly in the United States and Canada. JAMA. 1996;275(18):1410–1416. [PubMed] [Google Scholar]
- 41.Kennedy J, Morgan S. Health care access in three nations: Canada, insured America, and uninsured America. Int J Health Serv. 2006;36(4):697–717. doi: 10.2190/EC30-KP22-RA84-RAL4. [DOI] [PubMed] [Google Scholar]