Abstract
Sustained combination of HIV prevention strategies is essential to curb the spread of the HIV/AIDS epidemic. The use of highly active antiretroviral therapy (HAART) decreases morbidity and mortality, as well as HIV transmission, among treated individuals. The concept of ‘treatment as prevention’ is dependent on HAART’s ability to sustain HIV-1 RNA virological suppression at the individual and population levels, and has been demonstrated in studies evaluating transmission in mother-to-child, sero-discordant couples and large treated populations. The worldwide expansion of maximally effective antiretroviral drug regimens has been coupled with concerns regarding the magnitude of the financial investment required. However, HAART’s compounding effect on reduced morbidity, mortality and transmission makes the expansion of HAART coverage highly cost-averting. Building on a mathematical model that evaluated the impact of expanded HAART access on viral load in a Canadian setting, we demonstrate that an investment of CA$249 million over the lifetime of treated individuals would result in a net gain of CA$2.1 billion over 30 years. This provides a powerful economic incentive to rapidly scale up HAART access worldwide.
Keywords: Highly active antiretroviral therapy (HAART), HIV, incidence, mortality, cost-aversion
HIV represents a major threat to global health and economic stability. HIV/AIDS is projected to become the third leading cause of mortality worldwide by 2020.1 According to the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO), an estimated 33.2 million people were living with HIV in 2007.2 Despite recent efforts, the roll-out of antiretroviral therapy remains inadequate at a global level. WHO estimates that for every person newly receiving HIV treatment, four people become newly infected.3 Sustained combination HIV prevention strategies are essential to curb the spread of the HIV epidemic.4 These strategies should include aggressive deployment of proven effective strategies, including circumcision and harm reduction for drug users, applied in a culturally sensitive and rights-based fashion.5–7 Progress in other areas, such as curative therapeutic interventions, preventative vaccines and microbicides, has been less than encouraging.8,9 However, research in these areas should continue at a steady pace as part of a comprehensive long-term strategy to eradicate HIV/AIDS. In the interim, we must ensure that resources are specifically targeted to support the aggressive roll-out of proven effective strategies aimed at curbing HIV/AIDS in the short term.5,10 Recently, increased attention has been focused on the added preventative effect of highly active antiretroviral therapy (HAART) as a potent new synergistic strategy to curb the growth of the epidemic worldwide.11 As such, expanded HAART coverage among those in medical need offers the dual benefit of reducing morbidity and mortality among those infected with HIV and decreasing the risk of HIV transmission to those who are susceptible. The sum of these effects makes the expansion of HAART coverage among those in medical need cost-averting, providing yet another powerful motivation to hasten and sustain the roll-out of HAART in both resource-rich and resource-limited settings.
HAART as Prevention
HIV-1 RNA level in plasma has been shown to be a strong determining factor in the risk of HIV transmission in any given setting.12–14 HAART has been shown to predictably reduce HIV-1 RNA levels in plasma, as well as in semen,15 vaginal secretions16 and rectal tissue.17 As a result, HAART can substantially reduce the risk of HIV transmission at the individual and population levels. The impact of HAART on HIV transmission has been most dramatically documented within the context of mother-to-child transmission (MTCT) studies.18 Vertical transmission of HIV has indeed become exceedingly rare wherever HAART-based MTCT prevention programmes have been implemented.19 Similarly, pre- and post-exposure prophylaxis have been proposed as potentially safe and effective means of preventing HIV transmission in selected settings.20 Indeed, post-exposure prophylaxis represents the accepted standard of care for occupational exposure to HIV (i.e. through needle-stick injury) and for mass casualty events.21,22 The European Project on Non-occupational Post-Exposure Prophylaxis for HIV (EURO-NONOPEP) has formulated guidelines for the use of antiretroviral post-exposure prophylaxis in the event of sexual, injection drug or other exposure to HIV.23 The preventative role of HAART has also been described in HIV sero-discordant heterosexual couples. In Uganda, no HIV transmission episodes were identified among 51 couples where the index case, who was receiving HAART, had an HIV-1 RNA level <1,500 copies/ml.12 A dose–response effect was also observed in a Thai study, where no cases of HIV transmission were observed in an untreated cohort when the index case’s serum HIV-1 RNA was <1,100 copies/ml.13 An observational study of Spanish sero-discordant couples showed that no HIV seroconversions took place among sexual partners of HAART-treated patients, with HAART being independently associated with an 80% reduction in HIV transmission.24 Further quantitative evidence surrounding the effect of HAART on HIV transmission is expected to emerge from an ongoing randomised controlled trial (RCT) involving 1,750 sero-discordant couples currently under way in sites in Brazil, India, Malawi, Thailand, the US and Zimbabwe.25 At the population level, expanded access to HAART has also been shown to be associated with substantial reductions in HIV transmission.11 A study in Taiwan found a 53% (95% confidence interval [CI] 31–65%) reduction in new positive HIV diagnoses after the widespread introduction of free HAART in 1996.26 This reduction took place in the context of a stable rate of syphilis, used as a surrogate marker of high-risk sexual behaviour. In Uganda, the expansion of HAART access to all clinically eligible individuals was estimated to decrease HIV incidence by 11.2% (interquartile range [IQR] 1.8–21.4%).27 In Canada, new yearly HIV infections in the province of British Columbia (BC) fell by approximately 50% after the introduction of HAART between 1996 and 1998, and has remained unchanged since then. Importantly, in contrast to Taiwan, rates of syphilis have increased steadily in BC each year since 1996.11
The precise extent to which HAART reduces HIV transmission at the population level remains incompletely characterised.28 Indeed, outside of the very specific MTCT setting, limited data are available to quantify the impact of incremental expansions of HAART on HIV transmission. Furthermore, there are important gaps in our understanding of the effect of HAART on HIV transmission among specific transmission groups or socioeconomic environments. Despite these limitations, the available evidence is compelling enough to encourage further rapid scale-up of HAART coverage among those in medical need. This expansion, coupled with close monitoring of the impact of this strategy on HIV transmission in varied settings, should provide further valuable insights into the preventative effect of HAART.
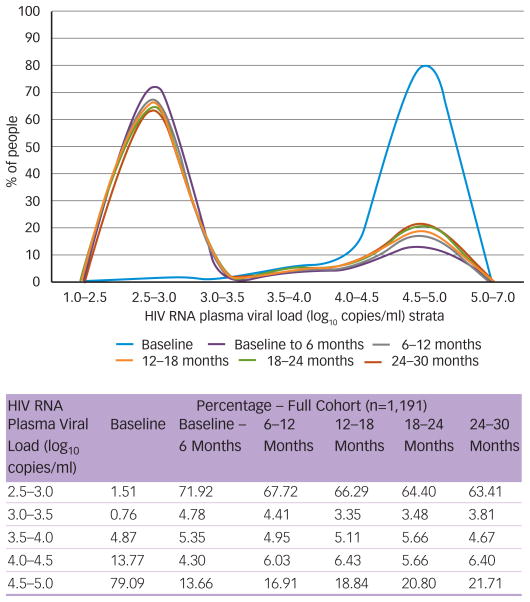
Populational Impact of HAART on Plasma HIV-1 RNA Levels
The risk of HIV transmission is determined by several factors in addition to plasma HIV-1-RNA levels. Among them are the nature of the exposure, increased local HIV burden (as a result of treatment interruptions29 or sexually transmitted diseases [STDs]30) and the susceptibility of the host to transmission.31 In addition, it has been suggested that HIV transmission risk is highest during the primary infection phase.32,33 However, this remains the subject of some controversy, given that a recent large prospective study of sero-discordant couples found that approximately half of all sexual HIV transmission events occurred outside the primary infection phase.33 Furthermore, it has been suggested that the majority of HIV transmission events occur beyond the primary infection phase during the asymptomatic phase of infection, resulting from the relatively longer duration of this phase in the HIV life-cycle.34 Plasma HIV-1 RNA level remains a critical determinant of the risk of HIV transmission in any given setting that is amenable to therapeutic intervention. Specifically, HAART use is expected to have a dramatic effect on plasma HIV-1 RNA level, which can result in a substantial lowering of the populational HIV-1 RNA level.35 Figure 1 presents changes in participants’ plasma viral load distribution over a 30-month period in a longitudinal population-based cohort of 1,119 patients newly receiving HAART. The figure demonstrates that median plasma viral load shifted from 5.0 log10 copies/ml (IQR 4.6–5.0 log10 copies/ml) at baseline to 2.7 log10 copies/ml (IQR 2.7–4.3 log10 copies/ml) at the end of follow-up (p<0.01). Furthermore, the area under the curve for participants with a plasma viral load of 10,000–100,000 copies/ml and >100,000 copies/ml decreased by 84% at six months, 81% at 12 months, 78% at 18 months, 75% at 24 months and 74% at 30 months. These figures provide graphic evidence of the powerful and sustained effect of HAART on plasma viral load, dramatically reducing the pool of highly infectious individuals in this cohort.
Figure 1.
HIV RNA Plasma Viral Load at Baseline and During Follow-up in 1,191 Patients First Prescribed HAART
Risk of HIV Transmission Under HAART
The Swiss Commission of AIDS-related Issues recently issued a statement saying that individuals on HAART are sexually ‘non-infectious’ if they are adherent to their regimen, regularly monitored by their physician, have a consistently suppressed plasma HIV-1 RNA (<50 copies/ml) for more than six months and have no concurrent STDs.36 This statement ignited important public health discussions about the residual risk of HIV transmission while on HAART.37 Although the Swiss statement is based solely on sound biological evidence, there has been some concern regarding the impact that this statement may have on the population of HIV-infected individuals at large. As a result, the US Centers for Disease Control and Prevention (CDC), UNAIDS and WHO have released statements asking for further research in this area. Until the impact of HAART on HIV transmission is better characterised, proven HIV prevention strategies – such as correct and consistent condom use,38 timely treatment for STDs39 and harm-reduction strategies among intravenous injection drug users6,40 – should continue to be advocated as an important part of the global prevention effort. However, there is growing recognition that it may prove very difficult to demonstrate that an individual on HAART, with all the appropriate attributes described in the Swiss statement referred to above, is non-infectious. However, there is quite a bit of support for the notion that the same individual will become substantially less infectious under HAART.37 In fact, WHO-based investigators recently published the results of a study involving complex mathematical modelling, concluding that yearly voluntary testing coupled with universal HAART for those testing HIV-positive could eliminate HIV infection from a given jurisdiction as a result of the preventative effect of HAART on HIV transmission.41 In this context, it is worth noting that the recent evolution in the International Guidelines for the use of Antiretroviral Therapy offer a unique opportunity to put these principles into practice, as they widely recommend earlier initiation of HAART.41 Furthermore, it is critical to recognise that in order to maximise the impact of HAART on HIV mortality, morbidity and transmission, the durability of the therapeutic effect and therefore treatment tolerability issues become crucial in both resource-rich and resource-limited settings; basically, it would be short-sighted to save on drug regimen costs if this would lead to poor tolerability and reduced effectiveness.
Re-evaluating the Cost-effectiveness of HAART
A potential obstacle to optimally increasing HIV treatment rates is the immediate increase in acquisition costs associated with providing the best antiretroviral therapy regimen to a larger pool of individuals. However, a complete accounting requires that the acquisition costs be offset by the costs averted through avoidance of HIV-related morbidity and mortality and averted new HIV infections. The latter has typically been ignored in previous cost-effectiveness evaluations. Estimating the economic impact of the expansion of HAART with an emphasis on its prevention of HIV transmission is challenging in the absence of a prospective randomised trial. However, important insights can be derived from the use of mathematical modelling. A mathematical model can be created to describe the impact of various prevention efforts on the risk of HIV transmission in a population over time. To estimate the economic impact of treatment as prevention in the Canadian province of BC, we combined an existing mathematical model of HIV transmission42 with estimates of direct medical costs and productivity loss costs associated with HIV disease.43 In this mathematical model, it was estimated that increasing treatment from 50 to 75% of eligible individuals would result in 3,419 averted infections when eligibility for receiving HAART was defined only by having a CD4 cell count <350 cells/mm3.42
Hutchinson et al. provide estimates of the lifetime costs associated with HIV in the US: US$195,318 for treated individuals, US$132,096 for untreated individuals and US$661,100 in productivity losses (estimated using the human capital approach). All costs were discounted at a rate of 3% per year. This implies an additional US$63,222 in lifetime medical costs for a treated individual. We recently converted these costs to CA$ using purchasing power parity44 and applied them to the results of the mathematical model. We applied a second level of discounting at a rate of 3% per year, such that infections averted in the future were valued at less than those averted immediately. Based on these calculations, accounting for direct medical costs and lost productivity from a societal perspective and for averted infections, we estimated that an investment of CA$249 million over the lifetime of currently infected individuals would result in a net gain to BC of CA$2.1 billion over 30 years. This suggests that HAART, which is a cost-effective treatment modality based on morbidity and mortality end-points, becomes a cost-averting modality when the impact of HAART on reduction of HIV transmission is added to the equation.
Future Directions
A recent consensus statement published by WHO and co-sponsored by the World Bank, the Global Fund and the International AIDS Society highlighted the role of HAART in HIV prevention as a top research priority.45 Further research is urgently needed to fully and accurately characterise the impact of HAART on HIV transmission in various settings, populations and environments. The ongoing roll-out of HAART provides a unique opportunity to address these issues. Similarly, the recent change in therapeutic guidelines towards earlier initiation of HAART offers another unique opportunity to further explore these issues.41 In the interim, it is important to recognise that HAART, a cost-effective intervention based on patient-centred outcomes, becomes a cost-avoiding strategy when its added preventative benefit is considered. This should serve as a powerful incentive to urgently expand HAART coverage for those in medical need globally.
Footnotes
Disclosure: Aranka Anema and Karissa Johnston receive funding from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. Viviane D Lima’s research is supported by the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research. Adrian Levy is supported by a Michael Smith Foundation for Health Research Senior Scholar Award and is a shareholder in Oxford Outcomes. Julio SG Montaner has received grants from, served as an ad hoc advisor to or spoken at various events sponsored by Abbott, Argos Therapeutics, Bioject Inc, Boehringer Ingelheim, BMS, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Janssen-Ortho, Merck Frosst, Panacos, Pfizer, Schering, Serono Inc., TheraTechnologies, Tibotec (J&J) and Trimeris, and has received funding from an Avant-Garde Award from the National Institute of Drug Abuse, National Institutes of Health.
References
- 1.Mathers CD, et al. World Health Organization; 2006. [Google Scholar]
- 2. [(accessed 29 September 2008)]; www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/
- 3. [accessed 29 September 2008]; www.who.int/hiv/mediacentre/2008progressreport/en/index.html.
- 4.Coates TJ, et al. Lancet. 2008;372(9639):669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [accessed 5 December 2007]; www.paho.org/English/AD/FCH/AI/New%20Approaches%20to%20HIV%20Prevention.pdf.
- 6. [accessed 15 October 2008]; www.globalhivprevention.org/pdfs/PWG-HIV_prevention_report_FINAL.pdf.
- 7. [accessed 12 November 2008]; search.unaids.org/Results.aspx?q=circumcision&x=0&y=0&o=html&d=en&l=en&s=false.
- 8.Nature. 2007;449(7161):390. doi: 10.1038/449390c. [DOI] [PubMed] [Google Scholar]
- 9. [accessed 5 December 2007]; data.unaids.org/UNA-docs/financingresdevvaccinemicrobicide_report_en.pdf.
- 10.Anema A, et al. CMAJ. 2008;179(1):13–14. doi: 10.1503/cmaj.071809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaner JSG, et al. Lancet. 2006;368:531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 12.Quinn TC, et al. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 13.Tovanabutra S, et al. J Acquir Immune Defic Syndr. 2003;3:275–83. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Dickover RE, et al. AMA. 1996;275(8):599–605. [PubMed] [Google Scholar]
- 15.Vernazza PL, et al. AIDS. 1997;11:1249–54. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Cu-Uvin S, et al. AIDS. 2000;14:415–21. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 17.Kotler DP, et al. AIDS. 1998;12(597):597–604. doi: 10.1097/00002030-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Fang G, et al. Proc Natl Acad Sci U S A. 1995;92(26):12100–4. doi: 10.1073/pnas.92.26.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Cock KM, et al. JAMA. 2000;9:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 20.Gay CL, et al. Curr Infect Dis Rep. 2008;10(4):323–31. doi: 10.1007/s11908-008-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panlilio AL, et al. MMWR Recomm Rep. 2005;54(RR-9):1–17. [PubMed] [Google Scholar]
- 22.Chapman LE, et al. Disaster Med Public Health Prep. 2008;2(3):150–65. doi: 10.1097/DMP.0b013e318187ac66. [DOI] [PubMed] [Google Scholar]
- 23. [accessed 20 October 2008]; www.inmi.it/news/LineeGuida/Recommendations.NONOCC.htm.
- 24.Castilla J, et al. J Acquir Immune Defic Syndr. 2005;40:96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 25. [accessed 17 Ocober 2008]; clinicaltrials.gov/ct2/show/NCT00074581?term=HPTN+052&rank=1.
- 26.Fang CT, et al. J Infec Dis. 2004;190:879–85. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 27.Abbas UL, et al. J Acquir Immune Defic Syndr. 2006;41(5):632–41. doi: 10.1097/01.qai.0000194234.31078.bf. [DOI] [PubMed] [Google Scholar]
- 28.Cohen MS, et al. Ann Intern Med. 2007;146(8):591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi E, et al. J Acquir Immune Defic Syndr. 2001;27:209. doi: 10.1097/00126334-200106010-00017. [DOI] [PubMed] [Google Scholar]
- 30.Galvin SR, et al. Nat Rev Microbiol. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 31.Royce R, et al. J Infect Dis. 2007;195(7):951–9. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 33.Wawer MJ, et al. Uganda J Infec Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 34.Hollingsworth TD, et al. J Infect Dis. 2008;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 35.Lima VD, et al., Sydney, Australia, 22–25 July 2007.
- 36.Vernazza PL, et al. Schweizerische Arztezeitung/Bulletin des medecins suisses/Bollettino dei medici svizzeri. 2008;89:5. [Google Scholar]
- 37.Garnett GP, et al. Lancet. 2008;372:270–72. doi: 10.1016/S0140-6736(08)61089-2. [DOI] [PubMed] [Google Scholar]
- 38. [accessed 17 October 2008]; www.cdc.gov/hiv/resources/press/020108.htm.
- 39. [accessed 17 October 2008]; 209.85.173.104/search?q=cache:SDmLtmROLiMJ:data.unaids.org/pub/PressStatement/2008/080201_hivtransmission_en.pdf+Antiretroviral+therapy+and+sexual+transmission+of+HIV+UNAIDS+and+WHO+statement.&hl=en&ct=clnk&cd=1&gl=ca.
- 40.Hammer SM, et al. JAMA. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 41.Granich RM, et al. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 42.Lima VD, et al. J Infect Dis. 2008;198(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 43.Hutchinson AB, et al. J Acquir Immune Defic Syndr. 2006;43(4):451–57. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 44.Health Data 2007. 2007 [Google Scholar]
- 45.World Health Organization (WHO) WHO Consensus Statement. Aug 4, 2008. [Google Scholar]