Abstract
The original Grunwald-Winstein equation (1948) involved the development of a scale of solvent ionizing power (Y). Subsequent work has refined this scale and involved the development of scales of solvent nucleophilicity (N) and a term to correct for deviations when aromatic rings are present, governed by the aromatic ring parameter (I). These three scales, and the sensitivities towards each, can be related to specific rates of solvolysis through linear free energy relationships (LFERs).
One important area of application of LFERs has been to the solvolyses of tert-alkyl halides. It has been proposed that the solvolysis of tert-butyl chloride involves a nucleophilic component, although other workers have suggested that the effects observed are related to electrophilic not nucleophilic influences. Takeuchi (1997) studied a compound with two of the methyl groups of tert-butyl chloride replaced by neopentyl groups. For this highly-hindered substrate there was no evidence for nucleophilic participation. Liu (1998) and Takeuchi (2001) have reported concerning the solvolyses of additional significantly-hindered tertiary alkyl chlorides. Liu (2009) has presented a parallel study of bromides. Martins (2008) has considered hindered tertiary alkyl halides, mainly with carbon-carbon multiple bonds as substituents. It was proposed that the hI term was of importance, with the sensitivities (h) sometimes positive and sometimes negative. To explain negative values, it was suggested that the I scale might contain a nucleophilicity component. In this review, we bring together, with analysis and commentary, the work of Takeuchi, Liu, Martins and others concerning the solvolyses of tertiary alkyl halides, with emphasis on the relevance of the three scales that have been developed for use in Grunwald-Winstein correlations.
Keywords: Grunwald-Winstein equation, steric hindrance, tertiary alkyl halides, multicollinearity, nucleophilic solvent participation
1. INTRODUCTION
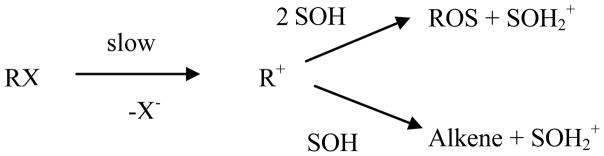
The original Grunwald-Winstein equation was proposed in 1948 for the correlation of solvolysis reactions proceeding by an ionization pathway, with rate-determining formation of a carbocation and its fast subsequent substitution or, when possible, elimination reaction (Scheme 1).
Scheme 1.
The linear free energy relationship (LFER) was developed [1] using tert-butyl chloride as the standard substrate and 80% aqueous ethanol as the standard solvent. The equation has the form shown in Equation 1, where k and k0 are the specific rates of solvolysis of the substrate under study in a given solvent and in the standard solvent, respectively; m is the sensitivity towards changes in the solvent ionizing power (Y), and c is a constant (residual) term.
| (1) |
The Y scale was developed by studying tert-butyl chloride solvolyses in 80% ethanol and a variety of other solvents. Equation 1 was then applied with m = 1 and c = 0, so as to give the series (scale) of Y values for the solvents, anchored by Y = 0 for the standard 80% ethanol solvent. The establishment of the original scale and its development and applications have recently been reviewed [2].
It was realized that the solvolyses of derivatives of cage hydrocarbons could provide improved standard substrates for establishing scales of solvent ionizing power. These solvolyses cannot be subject to rearside nucleophilic participation and elimination will not effectively compete with the substitution reaction (Bredt’s rule) [3]. It was found that different leaving groups (X) in the solvolyses of 1–adamantyl or 2–adamantyl derivatives led to slightly different solvent ionizing power values [4] and listings of Yx values are available [5].

A comparison of the reactivities of tert-butyl substrates with those for 1-adamantyl shows the tert-butyl substrate to be about a thousand times more reactive in ethanol [3] but solvolyses in the weakly nucleophilic 1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP) occur at about the same rate [6–8]. The operation of nucleophilic solvent assistance in the solvolyses of tert-butyl derivatives nicely explains [6–8] these observations. An alternative rationalization of these differences is to assume not enhanced nucleophilic participation by the solvent for the tert-butyl derivative solvolyses but enhanced electrophilic participation by the solvent for the 1-adamantyl derivative solvolyses [9–12]. The observation that the 1-adamantyldimethylsulfonium ion shows little variation in its solvolyses rate in the commonly used solvents [7] but the tert-butyldimethylsulfonium ion shows increasing rate as the nucleophilicity of the solvent is increased [13] gives strong support to the proposed [6–8] nucleophilic solvent assistance to the solvolyses of the tert-butyl derivative. Indeed, the electrophilic participation by the solvent would be of little relevance when the leaving group initially has a formal positive charge on the atom adjacent to the α-carbon, such that it leaves as a neutral molecule. The proposals that the dominant influence is an electrophilic effect have been vigorously rebutted [14].
Surprisingly, the simple one-term equation (1) frequently gives a good correlation for an SN2 reaction in varying compositions of a given binary solvent system. This can occur due to a linear correlation of solvent nucleophilicity and solvent ionizing power values [15]. The m values obtained for SN2 solvolyses are considerably lower than those obtained for SN1 solvolyses [16, 17].
It was realized [18] that a move meaningful application to SN2 reactions would require a second-term within the equation governing the sensitivity to changes in solvent nucleophilicity (Equation 2). In Equation 2, the additional term involves the sensitivity (l) to changes in solvent nucleophilicity (N).
| (2) |
The development of solvent nucleophilicity scales has been briefly reviewed [2] and it has also been reviewed in considerable depth in a book chapter [19]. To avoid a difficult correction for the solvent ionizing power term if a neutral substrate, with an anionic leaving group, is selected as the standard substrate [20, 21] the favored approach is to select a substrate with a neutral molecule as the leaving group. Generally accepted as the standard substrate is the S-methyldibenzothiophenium ion (MeDBTh+), with dibenzothiophene as the leaving group and with attack at the methyl group. The resulting scale scale (l set at unity) is termed the NT scale (Equation 3). The NT scale [19, 22] has had wide application, including bimolecular (addition-elimination) attack at acyl carbon and bimolecular (SN2) attack at phosphorus and sulfur [2].
| (3) |
It was found early in Grunwald-Winstein studies that compounds with aromatic rings at the α-carbon did not correlate well with Y values in situations where a unimolecular reaction would be expected. For this reason, α-phenylethyl chloride was proposed as an alternative standard substrate when a correlation of other α-substituted arylalkyl chlorides, such as diphenylmethyl chloride, was involved [23, 24]. This approach has continued and many “similarity model” standard substrates have been proposed as an improvement on Yx scales when aryl groups are initially present on the α-carbon or are migrating from the β to the α-carbon during solvolysis. Several of these “similarity model” scales have been reviewed [25]. It has been pointed out [26] that, in most instances, only negligible to moderate improvements result from replacing the Yx scale by a “similarity model” scale and the considerable effort involved in developing the new scales frequently does not result in any commiserate reward.
The several solvent ionizing power scales for a given leaving group which have been developed have negative as well as positive aspects. Certainly, a person not working directly in the field would be hesitant to apply the Grunwald-Winstein equation if the choice of a scale is problematic. An alternative approach has been developed involving the application of a third-scale, called the aromatic ring parameter scale (I). This third scale, together with a sensitivity to its variation in value (h) can be added to the equation 2, to give equation 4.
| (4) |
Equation 4 is written with introduction of the NT and the Yx scales, which have been discussed above, and the addition of the hI term. The I scale was developed by consideration of the difference in behavior of the (p-methoxybenzyl)dimethylsulfonium ion and the 1-adamantyldimethylsulfonium ion [27]. Its development and application to the study of benzylic substrates and, also, to the kinetics of the solvent capture of diarylmethyl cations [28] have been reviewed [2]. Frequently, the reaction involves an unassisted ionization and the lNT term can be dropped from the equation 4. For the solvent capture of carbocations [28], the mYx term is not relevant [2].
The equation including the hI term was very usefully applied [2, 29] to reactions involving a 1,2-aryl shift, where it can be shown to be equivalent to the original treatment [30, 31, 32]. It has the advantage that it would not require the development of new solvent ionizing power scales, requiring only the appropriate YX scale, if the study was extended from a p-toluenesulfonate leaving group to other leaving groups.
2. APPLICATION OF EQUATION 1 TO SOLVOLYSES OF TERTIARY ALKYL HALIDES
(a) Chlorides
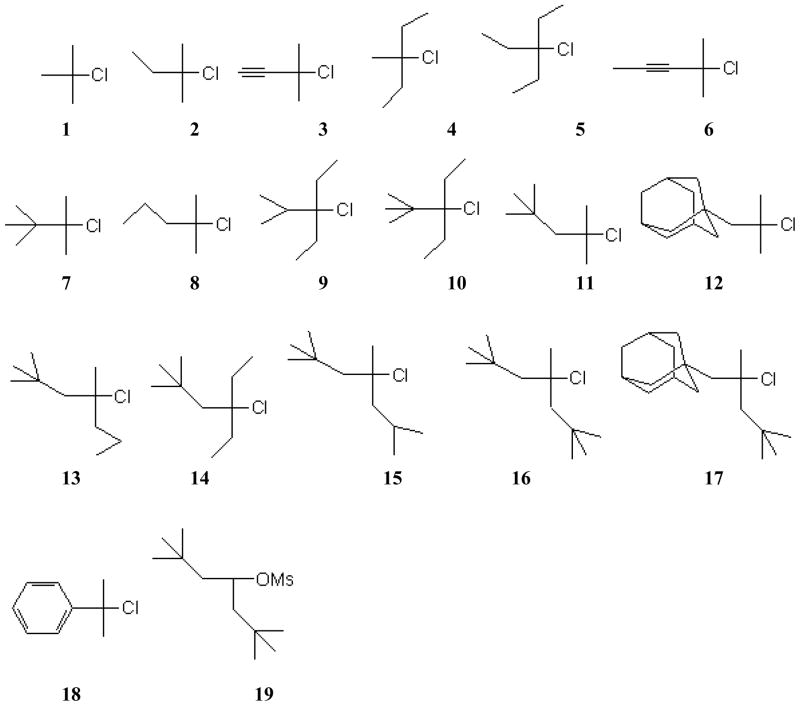
The substrates used in this study are shown in Scheme 2. One of the compounds (19) is a methanesulfonate (mesylate) ester. In the scheme, there is a general trend for the crowding around the α-carbon to increase as the number designating the compound increases. The analyses of the solvolyses of these compounds in terms of the equation 1 are shown in Table 1.
Scheme 2.
Table 1.
Correlations of the specific rates of solvolysis at 25.0 °C of some hindered tertiary alkyl chlorides using the one-term (original) Grunwald-Winstein equation.a
| Substrateb | nc | md | cd | Re | Ff |
|---|---|---|---|---|---|
| 1 | 20g | 0.60±0.06 | −0.07±0.11 | 0.9266 | 109 |
| 1 | 47h | 0.72±0.03 | −0.17±0.09 | 0.9648 | 607 |
| 2 | 20 | 0.67±0.05 | −0.09±0.10 | 0.9451 | 151 |
| 3 | 20 | 0.79±0.05 | −0.23±0.11 | 0.9687 | 274 |
| 4 | 17 | 0.69±0.05 | 0.01±0.09 | 0.9685 | 227 |
| 5 | 15 | 0.72±0.05 | 0.09±0.09 | 0.9749 | 249 |
| 6 | 15 | 0.79±0.02 | −0.01±0.05 | 0.9960 | 1622 |
| 7 | 17 | 0.74±0.02 | 0.07±0.05 | 0.9918 | 906 |
| 8 | 14 | 0.68±0.03 | −0.02±0.05 | 0.9917 | 713 |
| 9 | 16 | 0.87±0.02 | 0.01±0.03 | 0.9982 | 3865 |
| 10 | 18 | 0.90±0.01 | 0.02±0.02 | 0.9989 | 7262 |
| 11 | 24 | 0.74±0.02 | 0.07±0.03 | 0.9955 | 2418 |
| 12 | 17 | 0.73±0.03 | 0.11±0.04 | 0.9906 | 783 |
| 13 | 17 | 0.76±0.03 | 0.18±0.05 | 0.9870 | 566 |
| 14 | 19 | 0.76±0.03 | 0.19±0.05 | 0.9905 | 884 |
| 15 | 15 | 0.71±0.04 | 0.22±0.06 | 0.9751 | 251 |
| 16 | 18 | 0.66±0.06 | 0.39±0.09 | 0.9431 | 129 |
| 17 | 14 | 0.58±0.08 | 0.29±0.09 | 0.9080 | 56 |
| 18 | 16 | 0.69±0.05 | −0.08±0.07 | 0.9695 | 219 |
| 19 (OMs)i | 13 | 0.77±0.04 | 0.05±0.07 | 0.9834 | 324 |
See Scheme 2.
Number of solvents.
From equation 1, using YCl values and with associated standard errors.
Correlation coefficient.
F-test value.
The solvents used for solvolyses of 2.
All available.
A methanesulfonate (mesylate) ester, using YOTs values.
The specific rates of solvolyses in 15 to 10 solvents for 2, 4, 5, 7, 9 and 10 at 25.0°C are abstracted from a report by Liu, Hou, and Tsao [33]. The analyses for compound 1 are using data from a variety of sources. The log (k/ko) values required for application of the equation are the Y values (based on t-butyl chloride solvolysis) listed, with original source indicated, within a review of solvent ionizing power scales [5]. Two additional values for 2,2,2-trifluoroethanol (TFE)-ethanol mixtures [34, 35] and three additional values [36] for highly aqueous acetonitrile-water and dioxane-water mixtures have been added. The 47 solvents used are the 46 used in previous analyses [37] plus a value in 60% TFE-40% EtOH [35].
The specific rates required for the analyses of the specific rates of solvolysis of 3, 6, and 18 are from a recent paper by Reis, Elvas-Leitao, and Martins [38] and those for 11, 12, 13, 14, 15, 16, and 17 solvolyses [35] and for 8 [39] and 19 [40] solvolyses from three papers by Takeuchi and co-workers.
The correlations for the solvolyses of the 19 compounds considered are generally good. The correlations of the solvolyses of 1 are considerably improved when the full 47 specific rates are included, rather than only the values in 20 solvents used in the Liu study [33]. Compound 17 gives the lowest values for the correlation coefficient (R) and the F-test (F) of 0.9080 and 56, respectively. Other correlations have R values in the range of from 0.943 to as high as 0.9989 and F values from 129 to 7262. The correlations of the specific rates of solvolyses of 17 will be discussed in detail in considerations of two-term and three-term correlations. For the other solvolyses, the m values vary from 0.66 ± 0.06 to 0.90 ± 0.01, with 16 of the 19 values lying in the range of 0.66 to 0.79. Since these values are relative to a value 1.00 for the standard 1-adamantyl chloride, they suggest a diminished degree of bond-breaking to the departing chloride-ion in an earlier transition state. Such effects may well be accompanied by some degree of nucleophilic assistance from the solvent for the less hindered substrates. For the more hindered substrates back (B)-strain effects [33, 41] relieving ground-state strain on going to the ionization transition state and/or a greater possibility for charge delocalization in the incipient larger cations lowering the barriers to ionization may operate. Both of these effects would lead to progressively earlier transition states as one moved to bulkier alkyl chlorides.
(b) Bromides
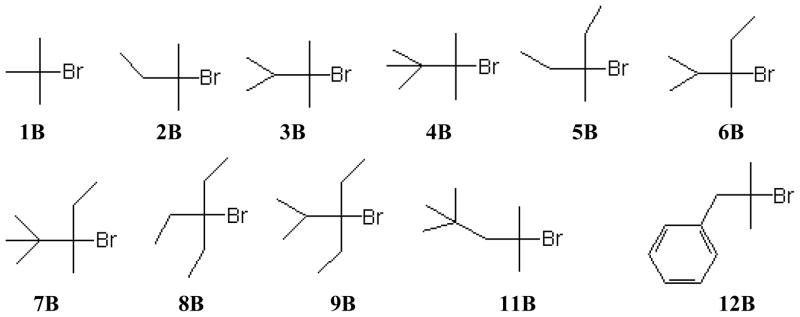
The alkyl bromides whose specific rates of solvolyses are considered are listed in Scheme 3. Except for compound 12B, data from a paper by Martins and coworkers [38], the specific rate values are from a publication by Liu and coworkers [42]. They carried out the study using a series of bromides with gradually increasing steric hindrance to nucleophilic attack at the α-carbon. The data are available for solvolysis of each substrate in from 15 to 21 solvents, usually 19 to 21. For the compounds studied by Liu, we have retained the same reference numbers as used in his publication. Typically, Liu did not use all of the available specific rate measurements in his correlations. In two instances, 9B and 11B, they were all used and, in these instances, our values for m and for R are identical to those reported earlier. In Table 2, in addition to m and R values, c and F values are also reported.
Scheme 3.
Table 2.
Correlation of the specific rates of solvolysis at 25.0°C of some hindered tertiary alkyl bromides using the one-term (original) Grunwald-Winstein equation.
| Substratea | nb | mc | cc | Rd | Fe |
|---|---|---|---|---|---|
| 1B | 20f | 0.58±0.07 | −0.10±0.12 | 0.8775 | 60 |
| 30g | 0.65±0.05 | −0.18±0.09 | 0.9296 | 178 | |
| 32h | 0.67±0.05 | −0.21±0.09 | 0.9383 | 221 | |
| 2B | 21 | 0.64±0.05 | −0.07±0.09 | 0.9394 | 143 |
| 3B | 20 | 0.69±0.04 | −0.04±0.08 | 0.9643 | 239 |
| 4B | 21 | 0.70±0.03 | 0.01±0.05 | 0.9833 | 556 |
| 5B | 21 | 0.65±0.05 | −0.06±0.05 | 0.9542 | 193 |
| 6B | 19 | 0.69±0.04 | 0.05±0.06 | 0.9793 | 397 |
| 7B | 19 | 0.67±0.02 | 0.09±0.04 | 0.9896 | 803 |
| 8B | 20 | 0.67±0.04 | −0.03±0.08 | 0.9641 | 237 |
| 9B | 18i | 0.75±0.02 | 0.06±0.02 | 0.9974 | 3101 |
| 11B | 15 | 0.71±0.01 | 0.05±0.01 | 0.9982 | 3563 |
| 12Bj | 17 | 0.57±0.04 | −0.12±0.06 | 0.9696 | 235 |
Number of solvents.
From equation 1, using YBr values and with associated standard errors.
Correlation coefficient.
F-test value.
From Table 1 of ref. 42.
All available, except AcOH and HCO2H.
All available.
The 70% TFE determination excluded (see ref. 42).
The solvolyses of 9B and 11B are very well correlated by the one-term Grunwald-Winstein equation, with R values of 0.9974 and 0.9982, respectively. In his paper, Liu considers the competition between assistance to ionization from relief of B-strain and from nucleophilic assistance from solvent. He concluded that for these two compounds, and also for 7B, the relative high degree of crowding leads to the dominance of B-strain and for the other substrates nucleophilic assistance is also an important consideration.
For t-butyl bromide (1B), in addition to use of the Liu values, we also report in Table 2 the results from correlations carried out with all available solvents [1, 43–45] and with all available solvents with exclusion of acetic and formic acids. Very little difference is observed on excluding acetic and formic acids, indicating that they are “well behaved” in these solvolyses. As with the correlations of t-butyl chloride (1) reported in Table 1, the correlations are considerably improved, in terms of both R and F values, when the studies in additional solvents are incorporated.
Compound 12B, with a β-phenyl group, gives a rather low m value in its correlation, comparable to that for tert-butyl bromide in a similar number of solvents, and it may be that the introduction of the substituent leads to compensating effects of increased B-strain and reduced electronic stabilization of the developing carbocation.
3. APPLICATION OF EQUATION 2 TO SOLVOLYSES OF TERTIARY ALKYL HALIDES
For application of the equation with two parameters (solvent ionizing power YX and solvent nucleophilicity NT), exactly the same substrates in the same solvents were used as were used in the one-parameter correlations discussed above.
As regards the solvent nucleophilicity scale, Takeuchi and coworkers [40] have used the NT scale but Liu and coworkers [33, 42] and Martins and coworkers [38] have both used the NOTs values. The need to arrive at an approximation for the dependence on solvent ionizing power, so as to subtract the mYOTs value from log (k/ko) in arriving at NOTs values based on methyl p-toluenesulfonate has been extensively discussed [2, 19] and it has been demonstrated that the 0.3 value employed should have been about 0.55, supported by the observation that revised N’OTs values are in excellent agreement with NT values [2, 19]. Further support for the use of NT values comes from the observation that N values based on nucleophilic additions to relatively stable carbocations [28] are in good agreement with NT values. The reason for the choice of the NOTs scale in the paper by Martins appears to be that they wished to carry out a comparison with some of the results obtained by Liu. In turn, the rationale by Liu for use of NOTs appears to be that for solvolyses of several benzylic substrates it gave a slightly better correlation than use of NT. He explained this in terms of the significance of the electrophilic pull on the anionic substrate, which is present in establishing the NOTs scale but absent in the cationic substrate used to establish the NT scale! The difficulty in quantitatively allowing for any electrophilic pull is, of course, exactly the rationale for the choice of the class of substrates used to establish the NT scale. Since the NT scale is used in this review, we cannot directly compare with the previous two-term analyses using the NOTs scale. However, the two scales do have similar values for most solvents [19, 38, 42] and approximate comparisons can be made.
(a) Chlorides
The parent tertiary alkyl chloride, tert-butyl chloride, was the first tertiary compound for which evidence observed for nucleophilic participation by the solvent in the rate-determining step of the solvolysis [2, 5, 6, 19, 37]. In a well designed study of the steric influence of gradually increasing the bulk of the alkyl group, Liu and coworkers [33] studied substrates with one, two, or three ethyl groups replacing methyl groups of 1, the l value of 0.38 ± 0.03 for the solvolyses of 1 in 46 solvents (using the NT values in conjunction with YCl values) [37] falls to values of 0.35 ± 0.05, 0.30 ± 0.03, and 0.25 ± 0.04, respectively (Table 3). When one tert-butyl group is substituted for a methyl group, the l value falls to 0.14 ± 0.03. For the highly hindered 9 and 10, the excellent one-term correlations (R = 0.998, Table 1) were considered to require an l value not appreciably removed from zero. The observed sequence of l values is as would be expected as a consequence of the increased steric hindrance to nucleophilic solvent participation (NSP). It would be much more difficult to explain the orderly trend in l values if the deviations observed in a plot of log (k/ko) values for tert-butyl chloride solvolysis against YCl values were to be due to larger responses to solvent electrophilicity changes for 1-adamantyl chloride than for tert-butyl chloride [9, 10]. We favor a picture involving appreciable stabilization of the developing carbenium ion by a rearside nucleophilic solvation, which falls off as steric hindrance increases, but an alternative view involving similar effects on nucleophilic participation within an SN2 (intermediate) mechanism [6] is also plausible.
Table 3.
Correlation of the specific rates of solvolysis at 25.0 °C of some hindered tertiary alkyl chlorides using a two-term (extended) Grunwald-Winstein equation.a
| Substrateb | nc | ld | md | cd | Re | Ff |
|---|---|---|---|---|---|---|
| 1 | 20g | 0.39±0.06 | 0.89±0.06 | −0.06±0.06 | 0.9788 | 194 |
| 43h | 041±0.03 | 0.88±0.02 | −0.04±0.04 | 0.9921 | 1253 | |
| 47i | 0.34±0.04 | 0.84±0.02 | 0.01±0.06 | 0.9863 | 788 | |
| 2 | 20 | 0.33±0.07 | 0.91±0.06 | −0.07±0.07 | 0.9778 | 185 |
| 3 | 20 | 0.36±0.05 | 0.92±0.03 | −0.12±0.06 | 0.9927 | 576 |
| 4 | 17 | 0.26±0.04 | 0.91±0.04 | −0.03±0.05 | 0.9920 | 433 |
| 5 | 15 | 0.22±0.04 | 0.95±0.05 | −0.03±0.06 | 0.9928 | 411 |
| 6 | 15 | 0.15±0.05 | 0.83±0.02 | −0.09±0.04 | 0.9978 | 1355 |
| 7 | 17 | 0.12±0.03 | 0.84±0.03 | 0.04±0.04 | 0.9958 | 824 |
| 8 | 13j | 0.19±0.03 | 0.78±0.02 | 0.02±0.03 | 0.9985 | 1616 |
| 14 | 0.09±0.05 | 0.71±0.03 | 0.03±0.05 | 0.9938 | 438 | |
| 9 | 16 | 0.02±0.02 | 0.89±0.03 | 0.00±0.03 | 0.9983 | 1868 |
| 10 | 18 | −0.02±0.02 | 0.88±0.02 | 0.02±0.02 | 0.9990 | 3586 |
| 11 | 23 | 0.02±0.03 | 0.76±0.02 | 0.05±0.03 | 0.9975 | 1980 |
| 24 | −0.04±0.03 | 0.72±0.02 | 0.04±0.03 | 0.9958 | 1255 | |
| 12 | 17 | −0.10±0.05 | 0.68±0.04 | 0.08±0.04 | 0.9927 | 473 |
| 13 | 17 | −0.16±0.05 | 0.67±0.04 | 0.13±0.04 | 0.9926 | 465 |
| 14 | 19 | −0.11±0.05 | 0.71±.0.03 | 0.16±0.04 | 0.9925 | 528 |
| 15 | 15 | −0.18±0.15 | 0.66±0.06 | 0.18±0.07 | 0.9778 | 131 |
| 16 | 17 | −0.30±−0.11 | 0.52±0.08 | 0.24±0.09 | 0.9630 | 89 |
| 18 | −0.26±0.09 | 0.55±0.06 | 0.25±0.09 | 0.9650 | 102 | |
| 17 | 14 | −0.53±0.32 | 0.46±0.10 | 0.17±0.11 | 0.9272 | 34 |
| 18 | 16 | −0.04±0.09 | 0.67±0.06 | −0.09±0.08 | 0.9700 | 103 |
| 19k | 12j | −0.19±0.05 | 0.59±0.06 | −0.05±0.05 | 0.9939 | 366 |
| 13 | −0.16±0.04 | 0.63±0.05 | −0.05±0.05 | 0.9936 | 386 |
Equation 2, using NT and YCl values.
See Scheme 2.
Number of solvents.
From equation 2, with associated standard errors.
Correlation coefficient.
F-test value.
Same solvents as used for solvolyses of 2.
For comparisons with Table 5 values, using all that have I values also available.
All solvents.
Excluding determination in acetic acid (no I value available).
Mesylate ester, using NT and YOTs values.
Takeuchi [35] also studied several substrates with bulky alkyl groups and he extended the range of compounds studied by Liu [33]. Whereas Liu had found the l values fell essentially to zero, Takeuchi and coworkers found that, for more hindered substrates (12–17), negative values for l could be obtained, especially noticeable for 16 (−0.26 ± 0.09) and 17 (−0.53 ± 0.32). It should, however, be pointed out that these two compounds also gave the poorest correlations (lowest R and F values) of the correlations presented in Table 3. It has been suggested [46] that complications may result from the high degree of elimination reaction observed [40]. For example, in the solvolyses of 16, 94% elimination was observed in methanol and 98% elimination in 2,2,2-trifluoroethanol.
The negative l values suggest even less nucleophilic participation by the solvent than in the solvolyses of 1-adamantyl chloride (l = 0.00, by definition). This was rationalized [40] in terms of a significant part of the solvation being not at the α-carbon but at the six β-hydrogens available for Brønsted-base type solvation for 1-adamantyl chloride, coupled with a steric hindrance even to this type of solvation for the more highly hindered substrates. This is certainly a plausible explanation but two observations would seem to suggest that, if present, these Brønsted-base type solvation effects do not have a large influence on the correlations. The ionizing power values (YOTs) for a p-toluenesulfonate leaving group were determined partially from solvolyses of the 1-adamantyl ester and partially from solvolyses of the 2-adamantyl ester, with the scales combined by an overlap technique. Bentley observed [5] that the two sets of YOTs values can be used interchangeably with no calibrations needed and French workers [47] proposed that only a very small adjustment was needed to put values obtained from both substrates on one scale. This situation would require a fortuitous balancing of the Brønsted-base solvation effects, since while six β-hydrogens are present in the 1-adamantyl ester, the corresponding solvation would be expected to be of the one α-hydrogen and the two β-hydrogens for the 2-adamantyl ester [40]. Similarly, as pointed out earlier [46], the solvolyses of the 1-adamantyldimethylsulfonium ion [51] vary by less than a factor of seven over 41 solvolyses in solvents of widely varying character, with the faster reactions in the least nucleophilic solvents. This, again, suggests that while Brønsted-base type solvation would be expected to exist, its effects on linear free energy relationships are only, at best, minor.
We have shown earlier [46] that the two term correlations of 16 and 19 can be improved by replacing the lNT term by an hI term. It was difficult to come up with a convincing argument as to why a scale developed to consider perturbations due to α-aryl groups (or due to aryl groups migrating to the α-position) should be relevant to reactions carried out in the absence of any π-electrons in the substrate. In a later section, we will return to a consideration of the utility of an hI term in correlating the solvolyses of hindered tertiary alkyl halides, with incorporation of a consideration of several recent correlations of additional substrates [38].
Takeuchi’s logarithmic plots of specific rates for 16 against YCl values and of specific rates for 19 against YOTs values show the points for TFE-H2O and HFIP-H2O solvents with high fluoroalcohol content lying above the plots, as opposed to lying below the plots when NSP is a factor [6, 37]. This gives excellent support to the claim by Liu and coworkers [33] that in going to weakly nucleophilic solvents a change in rate-determining influence from NSP to B-strain can occur. In Table 3, the l values of 0.02 ± 0.02 for solvolyses of 9 and of −0.02 ± 0.02 for solvolyses of 10 strongly support the claim by Liu, based on an excellent one-term correlation, that the l values must be essentially zero.
(b) Bromides
The substrates (Scheme 3) and the solvent employed are identical to those used in the one-term correlations reported in Table 2. The results from the two-term correlations using NT and YBr values are reported in Table 4. While there is a well-chosen range of increasing steric hindrance in moving from 1B to 11B, there are not the equivalents of the substrates with extreme hindrance prepared and studied by Takeuchi and coworkers in the consideration of alkyl chlorides. The l values fall fairly uniformly, starting at values of 0.38 ± 0.04 (for 1B in 32 solvents) and ending with values of 0.02 ± 0.02 (for 9B in 18 solvents) and −0.01 ± 0.02 (for 11B in 15 solvents). As with the chlorides, the trend is consistent with the operation of NSP and with its influence falling as the extent of steric hindrance to backside attack is increased.
Table 4.
Correlation of the specific rates of solvolysis at 25.0 °C of some hindered tertiary alkyl bromides using a two-term Grunwald-Winstein equation.
| Substratea | nb | lc | mc | cc | Rd | Fe |
|---|---|---|---|---|---|---|
| 1B | 20e | 0.44±0.06 | 0.92±0.06 | −0.05±0.06 | 0.9732 | 152 |
| 30g | 0.43±0.04 | 0.89±0.03 | −0.01±0.05 | 0.9861 | 477 | |
| 32h | 0.38±0.04 | 0.85±0.03 | 0.00±0.05 | 0.9852 | 478 | |
| 2B | 21 | 0.33±0.05 | 0.88±0.05 | −0.03±0.05 | 0.9831 | 259 |
| 3B | 20 | 0.24±0.04 | 0.87±0.04 | −0.03±0.05 | 0.9870 | 321 |
| 4B | 21 | 0.15±0.04 | 0.81±0.04 | 0.03±0.04 | 0.9911 | 498 |
| 5B | 21 | 0.28±0.04 | 0.86±0.04 | −0.03±0.05 | 0.9857 | 308 |
| 6B | 19 | 0.19±0.02 | 0.85±0.02 | 0.06±0.03 | 0.9960 | 992 |
| 7B | 19 | 0.08±0.03 | 0.74±0.03 | 0.09±0.03 | 0.9925 | 529 |
| 8B | 20 | 0.24±0.04 | 0.86±0.04 | −0.02±0.04 | 0.9895 | 399 |
| 9B | 18i | 0.02±0.02 | 0.76±0.02 | 0.07±0.02 | 0.9975 | 1519 |
| 11B | 15 | −0.01±0.02 | 0.70±0.02 | 0.05±0.01 | 0.9982 | 1684 |
| 12B | 17j | 0.29±0.06 | 0.67±0.04 | −0.04±0.05 | 0.9821 | 190 |
This study of the specific rates of solvolysis of 12B is from the paper of Martins and coworkers considered [38]. This substrate can be considered as tert-butyl bromide with one β-hydrogen replace by a phenyl group. The l value of 0.29 ± 0.06 (17 solvents) is comparable to the value of 0.33 ± 0.05 for 2B (in 21 solvents), where one β-hydrogen of tert-butyl bromide is replaced by a methyl group.
4. APPLICATION OF EQUATION 4 TO SOLVOLYSES OF TERTIARY ALKYL HALIDES
This three-term equation involves the aromatic-ring parameter as well as solvent nucleophilicity and solvent ionizing power. It is generally considered [48] that five data points are needed for each parameter incorporated in the LFER. Accordingly, for consideration of this equation, we should have a minimum of 15 specific rates of solvolysis in 15 well-chosen solvents. All of the bromides considered meet this criterion and, for the chlorides, all meet the criterion except for 17 (14 solvents), 8 (13 solvents), and 19 (12 solvents).
Although we discussed the use of the three-term equation in an earlier consideration of data for solvolyses of 16, and 19 [40], there was insufficient data points, especially for 16, available at that time. Additional data points have since become available [35]. We earlier found excellent (R > 0.995) correlations for both compounds, with a two-term equation involving mYX and hI terms applied to 10 solvents for 16 and 12 solvents for 19.
More recently, Martins and coworkers [38] have applied one-, two-, and three-term Grunwald-Winstein equation correlations to the solvolyses of five substrates (2, 3, 6, 18, and 12B). Three of these involve π-electrons which are capable of conjugating with the developing positive charge (3, 6, 18), one involves remote π-electrons (12B), and one is a conventional tertiary alkyl chloride (2).
In applying the three-term equation, it was found [38] that two gave positive and three gave negative h values, including the tertiary alkyl chloride 2. It was suggested that the negative h values arose because I was not a pure parameter and it included a solvent nucleophilicity component. Although it was not demonstrated to be the case, it was assumed [38] that, in the correlations, this would lead to a downward deviation of the h values, pushing them away from zero.
In reality, the inclusion of a solvent nucleophilicity component in the I scale has quite different consequences to those claimed. Assuming a nucleophilic component to the I scale, we can modify equation 4 to log (k/k0) = lNT + mYX + h(I + xNT) + c, where x governs the extent of incorporation of the
| (5) |
solvent nucleophilicity component. This equation can be rewritten as in equation 5 and the effect of the incorporation of the xNT component into the I scale is entirely on the l value and a modified l value (l′) can be expressed as l′ = l + hx. Several calculations, involving varying the x value for a given substrate, confirm that the predicted behavior is actually observed and, in particular, the h and m values are independent of the arbitrarily chosen x values. One observation concerning the five correlations carried out by Martins and coworkers [38] deserves to be returned to when we move forward to consider the 18 three-term correlations for tertiary alkyl chlorides in Table 5 and the 11 three-term correlations for tertiary alkyl bromides in Table 6. This observation is that the negative h values (use of equation 4) are accompanied by a reduction in the values for both l and m from their two-term values (obtained with use of equation 2) and exactly the reverse is observed for positive h values. This suggest that, indeed, Martins and coworkers were correct to assume that the I scale was not totally independent of the NT and YX scales. Two possible interrelationships were investigated [38] in terms of possible collinearity between I and N or between I and Y and the intercorrelations were found to be very weak (R2 < 0.3). These requirements are, however, overrestrictive and, as Bentley recently emphasized [14], multicollinearity can occur if one parameter in a multi-term LFER correlates with any linear combination of two or more of the other parameters; in this case, if I can be correlated with any linear combination of NT and YX values. This broader basis for multicollinearity has frequently been overlooked. For example, in a review of the treatments of solvent effects in organic chemistry using multiparameter equations, collinearity was discussed only in terms of a correlation between any two of the parameters [48].
Table 5.
Correlation of the specific rates of solvolysis at 25.0 °C of some hindered tertiary alkyl chlorides using a three-term Grunwald-Winstein equation.a
| Substrateb | nc | ld | md | hd | cd | Re | Ff |
|---|---|---|---|---|---|---|---|
| 1 | 20g | 0.21±0.04 | 0.80±0.03 | −0.73±0.11 | −0.05±0.03 | 0.9946 | 491 |
| 1 | 43h | 0.20±0.03 | 0.78±0.01 | −0.71±0.07 | −0.06±0.02 | 0.9978 | 2985 |
| 2 | 20 | 0.16±0.06 | 0.82±0.05 | −0.65±0.15 | −0.06±0.05 | 0.9901 | 264 |
| 3 | 20 | 0.22±0.04 | 0.82±0.03 | −0.53±0.11 | −0.06±0.04 | 0.9970 | 876 |
| 4 | 17 | 0.19±0.04 | 0.88±0.04 | −0.32±0.10 | −0.05±0.04 | 0.9954 | 465 |
| 5 | 15 | 0.14±0.04 | 0.90±0.04 | −0.32±0.10 | −0.03±0.04 | 0.9962 | 474 |
| 6 | 15 | 0.24±0.05 | 0.89±0.02 | 0.38±0.13 | −0.05±0.03 | 0.9988 | 1515 |
| 7 | 17 | 0.07±0.04 | 0.81±0.04 | −0.15±0.11 | 0.05±0.04 | 0.9963 | 785 |
| 8 | 13h | 0.18±0.04 | 0.78±0.02 | −0.07±0.13 | 0.02±0.03 | 0.9985 | 1002 |
| 9 | 16 | 0.03±0.03 | 0.90±0.03 | 0.05±0.08 | 0.00±0.03 | 0.9983 | 1196 |
| 10 | 18 | 0.01±0.02 | 0.89±0.02 | 0.11±0.06 | 0.03±0.02 | 0.9992 | 2780 |
| 11 | 23 | 0.01±0.03 | 0.76±0.02 | −0.06±0.08 | 0.05±0.03 | 0.9976 | 1289 |
| 12 | 17 | −0.05±0.06 | 0.71±0.04 | 0.25±0.14 | 0.05±0.04 | 0.9942 | 369 |
| 13 | 17 | −0.11±0.06 | 0.70±0.04 | 0.26±0.15 | 0.11±0.04 | 0.9940 | 357 |
| 14 | 19 | −0.01±0.06 | 0.77±0.04 | 0.39±0.14 | 0.14±0.04 | 0.9951 | 502 |
| 15 | 15 | 0.13±0.14 | 0.79±0.06 | 0.65±0.19 | 0.15±0.05 | 0.9893 | 168 |
| 16 | 17 | −0.12±0.11 | 0.63±0.08 | 0.80±0.27 | 0.16±0.08 | 0.9779 | 95 |
| 17 | 14 | 0.02±0.19 | 0.70±0.07 | 1.07±0.19 | 0.13±0.05 | 0.9829 | 95 |
| 18 | 16 | 0.09±0.08 | 0.76±0.06 | 0.63±0.21 | −0.11±0.06 | 0.9832 | 116 |
| 19 (OMs)i | 12h | −0.06±0.05 | 0.69±0.05 | 0.53±0.15 | −0.03±0.03 | 0.9976 | 559 |
Equation 4, using NT, YCl, and I values.
See Scheme 2.
Number of solvents.
From equation 4, with associated standard errors.
As in Table 3.
All available with determined NT, YCl, and I values.
Mesylate ester, YOTs used in place of YCl.
Table 6.
Correlation of the specific rates of solvolysis at 25.0 °C of some hindered tertiary alkyl bromides using a three-term Grunwald-Winstein equation.
| Substratea | nb | lc | mc | hc | cc | Rd | Fe |
|---|---|---|---|---|---|---|---|
| 1B | 20f | 0.23±0.03 | 0.77±0.03 | −0.82±0.08 | 0.02±0.02 | 0.9969 | 843 |
| 1B | 30g | 0.27±0.03 | 0.81±0.02 | −0.66±0.09 | −0.05±0.03 | 0.9956 | 976 |
| 2B | 21 | 0.17±0.04 | 0.78±0.03 | −0.60±0.10 | −0.01±0.03 | 0.9944 | 506 |
| 3B | 20 | 0.14±0.05 | 0.82±0.04 | −0.39±0.13 | −0.03±0.04 | 0.9916 | 314 |
| 4B | 21 | 0.07±0.05 | 0.76±0.04 | −0.29±0.12 | 0.04±0.04 | 0.9934 | 425 |
| 5B | 21 | 0.15±0.04 | 0.78±0.04 | −0.51±0.11 | −0.01±0.03 | 0.9937 | 448 |
| 6B | 19 | 0.12±0.02 | 0.80±0.02 | −0.29±0.05 | 0.07±0.02 | 0.9989 | 2061 |
| 7B | 19 | 0.09±0.04 | 0.74±0.04 | 0.02±0.12 | 0.09±0.04 | 0.9925 | 331 |
| 8B | 20 | 0.16±0.04 | 0.81±0.04 | −0.34±0.11 | −0.01±0.04 | 0.9933 | 394 |
| 9B | 18h | 0.02±0.02 | 0.76±0.02 | 0.00±0.06 | 0.07±0.02 | 0.9975 | 945 |
| 11B | 15 | −0.01±0.02 | 0.71±0.02 | 0.02±0.05 | 0.05±0.02 | 0.9982 | 1045 |
| 12Bi | 17 | 0.16±0.04 | 0.63±0.03 | −0.31±0.11 | −0.02±0.03 | 0.9949 | 422 |
(a) Chlorides
The correlations resulting from use of equation 4 are outlined in Table 5. Again the last listed substrate (19) is a mesylate ester rather than a chloride. In view of the observations for the five compounds correlated by Martins and coworkers [38] of a relationship between the sign of the h value and the increase or decrease of the l and m values (relative to the values from the two-term correlation), it is of considerable interest to extend these observations using the values in Table 3 and 5. In every instance, it is found that the behavior observed in the Martins and coworkers correlations [38] continues to apply. This strongly suggests that there is a relationship between the I values and a linear combination of the NT and YCl values.
We have repeated the correlations of the individual parameters using 43 solvents used to study the solvolysis of tert-butyl chloride for which all three parameters are available.
For the correlation of I against NT, we obtain:
For the correlation of I against YCl, we obtain:
For the correlation of I against a linear combination of NT and YCl, we obtain equation 6.
| (6) |
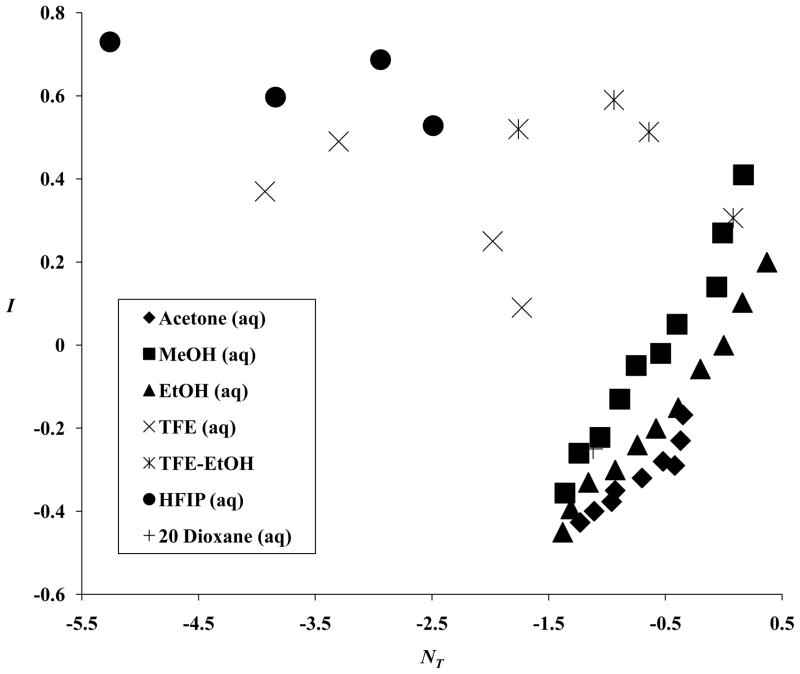
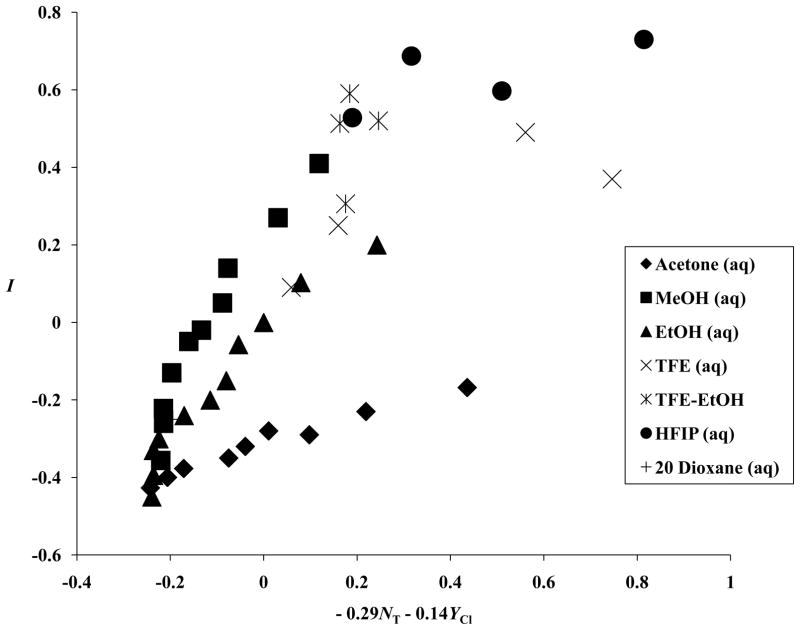
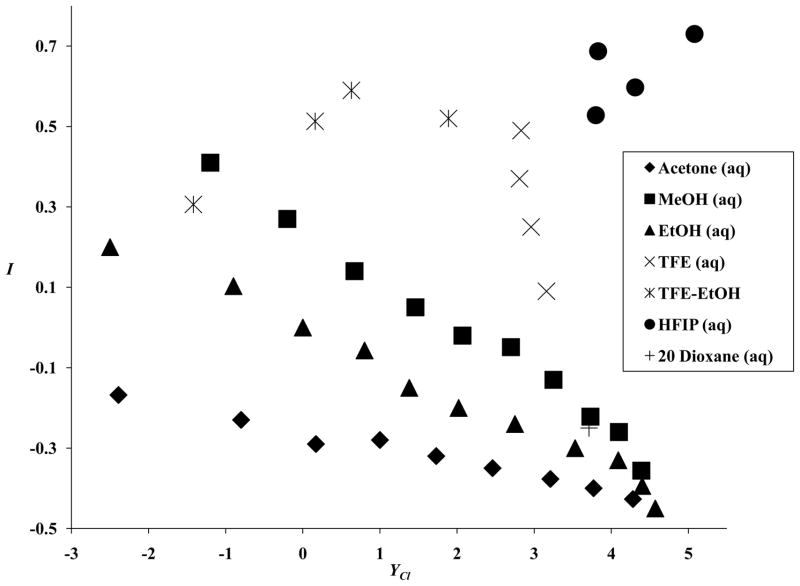
The three correlations are presented in Figures 1–3. The best correlation is against the linear combination of NT and YCl. This is not an outstanding correlation but, considering that the hI term makes (at best) only a minor contribution to the overall LFER, it may well be sufficient to explain the behavior observed. We can conclude that I values can safely be used (as regards collinearity) together with either NT or YCl values but use with both NT and YCl may well lead to multicollinearity problems.
Figure 1.
A plot of the aromatic-ring parameter (I) values against the solvent nucleophilicity (NT) values for 43 solvents.
Figure 3.
A plot of the aromatic-ring parameter (I) values against (−0.29 NT–0.14 YCl) for 43 solvents.
The collinearity relationship seems to be a robust one and restricting to a set of 12 solvents (acetic acid omitted, since no I value) studied by Takeuchi [40] gives an only slightly altered relationship:
In general, we can now write: log (k/ko) = lNT + mYCl + h[l’NT + m’YCl + c′] + c, where l′ and m′ are the sensitivities towards variations in NT and YCl values as the I value is varied, and c′ is the residual in that relationship. The equation results from substitution of the general form of equation 6 into equation 4. We can rearrange the equation to arrive at the two-term equation 7.
| (7) |
Since l′ and m′ are both negative (equation 6), it follows that a positive h value will lead to [l + hl′] and [m + hm′] values, corresponding to the l and m values of the two-term equation (equation 2), which are lower than the l and m values from equation 4. The reverse will hold with negative h values. Exactly these trends are observed in comparing the relevant values of Table 3 and 5.
To quantitatively demonstrate the influence of the multicollinearity, we have substituted for I using equation 6. The l′ (0.29 ± 0.04), m′ (0.14 ± 0.02) and c′ (0.03 ± 0.05) values are used within equation 7, together with the l, m, h, and c values (with standard errors) from Table 5. In Table 7, we report the [l + hl′] and [m + hm′] values, and the associated standard errors, as the calculated l and m values. These represent the first two columns of the table. For comparison, in columns 3 and 4 of the table are given the values obtained from a direct application of equation 2, abstracted from the data of Table 3.
Table 7.
Comparison of the l and m values obtained indirectly from values in Table 5, by substituting (−0.289 NT–0.136YCl) for I values, with those obtained directly from equation 2 (as listed in Table 3).
| Substratea | nb | lc | mc | lc | mc |
|---|---|---|---|---|---|
| (calc. from Table 5) | (from Table 3) | ||||
| 1 | 20 | 0.42±0.07 | 0.90±0.05 | 0.39±0.06 | 0.89±0.06 |
| 1 | 43 | 0.41±0.06 | 0.88±0.03 | 0.41±0.03 | 0.88±0.02 |
| 2 | 20 | 0.35±0.09 | 0.91±0.06 | 0.33±0.07 | 0.91±0.06 |
| 3 | 20 | 0.37±0.06 | 0.89±0.04 | 0.36±0.05 | 0.92±0.03 |
| 4 | 17 | 0.28±0.05 | 0.92±0.05 | 0.26±0.04 | 0.91±0.04 |
| 5 | 15 | 0.23±0.05 | 0.94±0.05 | 0.22±0.04 | 0.95±0.05 |
| 6 | 15 | 0.13±0.06 | 0.84±0.03 | 0.15±0.05 | 0.83±0.02 |
| 7 | 17 | 0.11±0.05 | 0.83±0.04 | 0.12±0.03 | 0.84±0.03 |
| 8 | 13 | 0.20±0.04 | 0.79±0.02 | 0.19±0.03 | 0.78±0.02 |
| 9 | 16 | 0.02±0.03 | 0.89±0.03 | 0.02±0.02 | 0.89±0.03 |
| 10 | 18 | −0.02±0.02 | 0.88±0.02 | −0.02±0.02 | 0.88±0.02 |
| 11 | 23 | 0.03±0.03 | 0.77±0.02 | 0.02±0.03 | 0.76±0.02 |
| 12 | 17 | −0.12±0.07 | 0.68±0.05 | −0.10±0.05 | 0.68±0.04 |
| 13 | 17 | −0.19±0.07 | 0.66±0.05 | −0.16±0.05 | 0.67±0.04 |
| 14 | 19 | −0.12±0.07 | 0.72±0.05 | −0.11±0.05 | 0.71±0.03 |
| 15 | 15 | −0.06±0.17 | 0.70±0.07 | −0.18±0.15 | 0.66±0.06 |
| 16 | 17 | −0.35±0.14 | 0.52±0.10 | −0.30±0.11 | 0.52±0.08 |
| 17 | 14 | −0.29±0.23 | 0.55±0.09 | −0.53±0.32 | 0.46±0.10 |
| 18 | 16 | −0.09±0.10 | 0.67±0.07 | −0.04±0.09 | 0.67±0.06 |
| 19 | 12 | −0.21±0.07 | 0.62±0.06 | −0.19±0.05 | 0.59±0.06 |
The excellent agreement between the values of columns 1 and 3 and those of columns 2 and 4 are consistent with the concept that the observation of small contributions from the hI term on application of equation 4 is an artifact, directly related to the multicollinearity that exists between the I values and a linear combination of NT and YCl values.
(b) Bromides
The analyses carried out immediately above for the chlorides have been repeated for the bromides. Very good three-term correlations are obtained with R values in excess of 0.992 and F-test values in excess of 300. Again, there is a very poor correlation between I and YBr values [I = 0.01(± 0.04)YBr + 0.04(± 0.07); R = 0.040; F = 0.045] and a considerably improved correlation between I and a linear combination of NT and YBr values (equation 8).
| (8) |
The correlation involves 30 solvents available for the study of the solvolyses of tert-butyl bromide for which NT, YBr, and I values are all available. As one would anticipate due to the similarity of YCl and YBr scales [5], the sensitivity values are essentially identical to those for the similar treatment of chlorides (equation 6).
We have applied the sensitivities of equation 8 to again substitute for I in equation 4. In Table 8, the calculated two-term values obtained in this way are, as in Table 7, shown to be in excellent agreement with those calculated directly using equation 2 and abstracted from Table 4. These analyses of the specific rates of solvolysis of several tertiary alkyl bromides strongly support the conclusion already reached from parallel experiments with chlorides that the presence of an hI term for substrates not having appropriately placed π-electrons is an artifact due to the multicollinear relationship between I values and a linear combination of NT and YBr values.
Table 8.
Comparison of the l and m values obtained indirectly from values in Table 6, by substituting (−0.247NT–0.129YBr) for I values, with those calculated directly from equation 2 (as listed in Table 4).
| Substratea | nb | lc | mc | lc | mc |
|---|---|---|---|---|---|
| (calc. from Table 6) | (from Table 4) | ||||
| 1B | 20 | 0.43±0.07 | 0.88±0.06 | 0.44±0.06 | 0.92±0.06 |
| 30 | 0.43±0.06 | 0.90±0.05 | 0.43±0.04 | 0.89±0.03 | |
| 2B | 21 | 0.32±0.07 | 0.86±0.05 | 0.33±0.05 | 0.88±0.05 |
| 3B | 20 | 0.24±0.07 | 0.87±0.06 | 0.24±0.04 | 0.87±0.04 |
| 4B | 21 | 0.14±0.07 | 0.80±0.05 | 0.15±0.04 | 0.81±0.04 |
| 5B | 21 | 0.28±0.07 | 0.85±0.06 | 0.28±0.04 | 0.86±0.04 |
| 6B | 19 | 0.19±0.04 | 0.84±0.03 | 0.19±0.02 | 0.85±0.02 |
| 7B | 19 | 0.09±0.04 | 0.74±0.04 | 0.08±0.03 | 0.74±0.03 |
| 8B | 20 | 0.24±0.06 | 0.85±0.05 | 0.24±0.04 | 0.86±0.04 |
| 9B | 18 | 0.02±0.02 | 0.76±0.02 | 0.02±0.02 | 0.76±0.02 |
| 11B | 15 | −0.01±0.02 | 0.71±0.02 | −0.01±0.02 | 0.70±0.02 |
| 12B | 17 | 0.24±0.06 | 0.67±0.04 | 0.29±0.06 | 0.67±0.04 |
5. CONCLUSIONS
The one-term equation, using YCl values based on 1-adamantyl chloride solvolyses, when applied to tert-butyl chloride solvolyses shows the points for fluoroalcohols lying below the Grunwald-Winstein plot of other solvents. This has been assigned to nucleophilic participation in the solvolyses of tert-butyl chloride lowering the specific rates in the solvents of low nucleophilicity. It has also been suggested that the deviations result from enhanced electrophilicity effects for 1-adamantyl chloride in the highly electrophilic fluoroalcohols. Observations favoring the nucleophilic solvent participation (NSP) rationalization include the observation that, while the 1-adamantyldimethylsulfonium ion shows almost constant solvolysis rates over a wide variety of solvents, the tert-butyldimethylsulfonium ion shows rates that increase as the nucleophilicity of the solvent increases.
An alternative approach is to vary the size of the alkyl group in the tertiary alkyl halide. In such a study, the one-term correlation (equation 1) shows improved correlation as one increases the steric hindrance (Tables 1 and 2), consistent with a movement towards reduced perturbations due to NSP. In the two-term correlation (equation 2), the sensitivity (l) towards changes in solvent nucleophilicity (NT) falls from a value of about 0.4 to zero as steric hindrance is increased. This is nicely consistent with Liu’s postulate of a competition between NSP and (backside) B-strain, with NSP favored at lower degrees of steric hindrance and B-strain increasingly favored as the degree of steric hindrance (crowding) increases.
A complication to this approach is that, in applying equation 2, Takeuchi found that for very highly hindered substrates the l values fell below zero. These negative values were rationalized in terms of Brønsted-base interactions at hydrogens beta to the leaving group in 1-adamantyl chloride, this type of interaction will be much weaker in the presence of a high degree of steric hindrance to approach of the solvent to the hydrogens at or near the reaction site, such that the overall NSP is lower for the substrate under study than for the (l = 0) standard substrate. However, an appreciable contribution from this type of Brønsted-base interaction is rendered unlikely by the observation that YOTs values are essentially identical when based on either 1- or 2-adamantyl p-toulenesulfonate solvolyses and by the observation that the rates of solvolysis of the 1-adamantyldimethylsufonium ion vary only slightly with solvent variation and the faster reactions are in the least nucleophilic (basic) solvents. The origin of the negative l values remains unclear. It may be possibly related, in some way, to the fact that the more negative l values are for solvolysis proceeding with over 90% elimination to alkene.
Martins applied the three-term correlation (equation 4) to the specific rates of solvolysis of five moderately-hindered tertiary alkyl halides and found the sensitivities (h) to changes in the aromatic-ring parameter (I) were sometimes positive and sometimes negative. In this present review, it is shown that the claim that this is a consequence of a nucleophilic component to the I scale cannot (even if such a component exists) be the cause of the reduction of the h values to below zero and, indeed, such a component would influence only the sensitivity to changes in solvent nucleophilicity (l value). We have extended the three term-treatment to data for 18 tertiary alkyl chlorides (Table 5) and 11 tertiary alkyl bromides (Table 6). Both positive and negative h values continue to be observed. In all cases, negative h values are accompanied by a reduction in both the l and m values to values below those obtained by use of the two-term equation 2 (as reported in Tables 3 and 4), with exactly the reverse behavior being observed when positive h values are obtained.
These qualitative observations suggest that, despite the fact that we could confirm Martins’ finding of only a low degree of collinearity between the I scale and either the NT or YX scales, there is a multicollinearity relating I values to a linear combination of NT and YX values (equations 6 and 8). Substituting these expressions for I into equation 4 leads to a return to exactly the l and m values of the two-term equation (Tables 7 and 8). Although the application of the three-term equation does in some cases lead to improved F-test values, it appears that the apparent utility of the hI term in these three-term analyses, including application to solvolyses of substrates without appropriately situated π electrons, is an artifact resulting from the multicollinearity that is present between the I values and a linear combination of NT and YX values.
Figure 2.
A plot of the aromatic-ring parameter (I) values against the solvent ionizing power (YCl) values for a chloride-ion leaving group for 43 solvents.
Acknowledgments
This research was supported by grant number 2 P2O RR016472-09 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This IDeA Network of Biomedical Research Excellence (INBRE) grant to the state of Delaware was obtained under the leadership of the University of Delaware, and the authors sincerely appreciate their efforts.
References
- 1.Grunwald E, Winstein S. The correlation of solvolysis rates. J Am Chem Soc. 1948;70:846–854. [Google Scholar]
- 2.Kevill DN, D’Souza MJ. Sixty years of the Grunwald-Winstein equation: Development and recent applications. J Chem Res. 2008:61–66. [Google Scholar]
- 3.Schleyer PvR, Nicholas RD. The reactivity of bridgehead compounds of adamantane. J Am Chem Soc. 1961;83:2700–2707. [Google Scholar]
- 4.Bentley TW, Schleyer PvR. Medium effects on the rates and mechanisms of solvolytic reactions. Adv Phys Org Chem. 1977;14:32–40. [Google Scholar]
- 5.Bentley TW, Llewellyn G. Yx scales of solvent ionizing power. Prog Phys Org Chem. 1990;17:121–158. [Google Scholar]
- 6.Bentley TW, Carter GE. The SN2-SN1 spectrum. 4. Mechanism for solvolyses of tert-butyl chloride: A revised Y scale of solvent ionizing power based on solvolyses of 1-adamantyl chloride. J Am Chem Soc. 1982;104:5741–5747. [Google Scholar]
- 7.Kevill DN, Anderson SW. Essentially solvent-independent rates of solvolysis of the 1-adamantyldimethylsulfonium ion. Implications regarding nucleophilic assistance in solvolyses of tert-butyl derivatives and the NKL solvent nucleophilicity scale. J Am Chem Soc. 1986;108:1579–1585. [Google Scholar]
- 8.Bentley TW, Roberts K. Weakly nucleophilic leaving groups. Solvolyses of 1-adamantyl and t-butyl heptafluorobutyrates and trifluoroacetates. J Chem Soc Perkin II. 1989:1055–1060. [Google Scholar]
- 9.Fărcasciu D, Jähme J, Rüchardt C. Relative reactivity of bridgehead adamantyl and homoadamantyl substrates from solvolyses with heptafluorobutyrate as a highly reactive carboxylate leaving group. Absence of SN2 character of solvolysis of tert-butyl derivatives. J Am Chem Soc. 1985;107:5717–5722. [Google Scholar]
- 10.Gajewski JJ. Is the tert-butyl chloride solvolysis the most misunderstood reaction in organic chemistry? Evidence against nucleophilic solvent participation in the tert-butyl chloride transition state and for increased hydrogen bond donation to the 1-adamantyl chloride solvolysis transition state. J Am Chem Soc. 2001;123:1087–10883. doi: 10.1021/ja010600d. [DOI] [PubMed] [Google Scholar]
- 11.Dvorko GF, Ponomareva EA, Ponomarev ME. Role of nucleophilic solvation and the mechanism of covalent bond heterolysis. J Phys Org Chem. 2004;17:825–836. [Google Scholar]
- 12.McManus SP, Somani S, Harris JM, McGill RA. A solvolysis model for 2-chloro-2-methyladamantane based on the linear solvation energy approach. J Org Chem. 2004;69:8865–8873. doi: 10.1021/jo049798l. [DOI] [PubMed] [Google Scholar]
- 13.Kevill DN, Kamil WA, Anderson SW. Nucleophilic participation in the solvolysis of the t-butyldimethylsulfonium ion. Tetrahedron Lett. 1982;23:4635–4638. [Google Scholar]
- 14.Bentley TW, Garley MS. Correlations and predictions of solvent effects on reactivity: some limitations of multi-parameter equations and comparisons with similarity models based on one solvent parameter. J Phys Org Chem. 2006;19:341–349. [Google Scholar]
- 15.Wells PR. Linear free energy relationships. Academic Press; New York: 1968. p. 67. [Google Scholar]
- 16.Fainberg AH, Winstein S. Correlation of solvolysis rates. III. t-Butyl chloride in a wide range of solvent mixtures. J Am Chem Soc. 1956;78:2770–2777. [Google Scholar]
- 17.Streitwieser A. Solvolytic displacement reactions. McGraw – Hill; New York: 1964. [Google Scholar]
- 18.Winstein S, Grunwald E, Jones HW. The correlation of solvolyses rates and the classification of solvolysis reactions into mechanistic categories. J Am Chem Soc. 1951;73:2700–2707. [Google Scholar]
- 19.Kevill DN. Development and uses of scales of solvent nucleophilicity. In: Charton M, editor. Advances in quantitative structure-property relationships. Vol. 1. JAI Press; Greenwich, CT: 1996. pp. 81–115. [Google Scholar]
- 20.Bentley TW, Schadt FL, Schleyer PvR. Correlation of solvolysis rates with three- and four-parameter relations. Scale of solvent nucleophilicities. J Am Chem Soc. 1972;94:992–995. [Google Scholar]
- 21.Schadt FL, Bentley TW, Schleyer PvR. The SN2-SN1 spectrum. 2. Quantitative treatments of nucleophilic solvent assistance. A scale of solvent nucleophilicities. J Am Chem Soc. 1976;98:7667–7674. [Google Scholar]
- 22.Kevill DN, Anderson SW. An improved scale of solvent nucleophilicity based on the solvolysis of the S-methyldibenzothiophenium ion. J Org Chem. 1991;56:1845–1850. [Google Scholar]
- 23.Fainberg AH, Winstein S. Correlation of solvolysis rates. VII. Neophyl chloride and bromide. J Am Chem Soc. 1957;79:1608–1612. [Google Scholar]
- 24.Winstein S, Fainberg AH, Grunwald E. Correlation of solvolysis rates. VIII. Benzhydryl chloride and bromide. Comparison of mY and Swain’s correlations. J Am Chem Soc. 1957;79:4146–4155. [Google Scholar]
- 25.Liu KT. Nucleophilic solvent intervention in benzylic solvolyses. The use of YBnX scales in Grunwald-Winstein type correlation analysis. J Chin Chem Soc. 1995;42:607–615. [Google Scholar]
- 26.Kevill DN, D’Souza MJ. Concerning the development of scales of solvent ionizing power based on solvolyses of benzylic substrates. J Phys Org Chem. 1992;5:287–294. [Google Scholar]
- 27.Kevill DN, Ismail NHJ, D’Souza MJ. Solvolysis of the (p-Methoxybenzyl)dimethyl sulfonium ion. Development and use of a scale to correct for dispersion in Grunwald-Winstein plots. J Org Chem. 1994;59:6303–6312. [Google Scholar]
- 28.Minegishi S, Kobayashi S, Mayr H. Solvent nucleophilicity. J Am Chem Soc. 2004;126:5174–5181. doi: 10.1021/ja031828z. [DOI] [PubMed] [Google Scholar]
- 29.Kevill DN, D’Souza MJ. Application of the aromatic ring parameter (I) to solvolyses of β-arylalkyl toluene-p-sulfonates. J Chem Soc Perkin II. 1997:257–263. [Google Scholar]
- 30.Fujio M, Goto M, Funatsu K, Yoshino T, Saeki Y, Yatsugi K, Tsuno Y. Solvent effects on the solvolysis of neophyl tosylates. Bull, Chem Soc Jpn. 1992;65:46–54. [Google Scholar]
- 31.Fujio M, Saeki Y, Nakamoto K, Yatsugi K, Goto M, Kim SH, Tsuji Y, Rappoport Z, Tsuno Y. The solvent effects on anchimerically assisted solvolyses. 2. Solvent effects in solvolyses of threo-2-aryl-1-methylpropyl p-toluenesulfonates. Bull Chem, Soc Jpn. 1995;68:2603–2617. [Google Scholar]
- 32.Fujio M, Saeki Y, Nakamoto K, Kim SH, Rappoport Z, Tsuno Y. Solvent effects on anchimerically assisted solvolyses. III. Solvent effects on the solvolyses of (1-arylcycloalkyl) methyl p-toluenesulfonates. Bull Chem Soc Jpn. 1996;69:751–764. [Google Scholar]
- 33.Liu KT, Hou SJ, Tsao ML. B-strain and solvolytic reactivity revisited. Nucleophilic solvent participation and abnormal rate ratios for tertiary chloroalkanes. J Org Chem. 1998;63:1360–1362. [Google Scholar]
- 34.da Roza DA, Andrews LJ, Keefer RM. Solvent system ethanol-2,2,2-trifluoroethanol as a medium for solvolytic displacement. J Am Chem Soc. 1973;95:7003–7009. [Google Scholar]
- 35.Takeuchi K, Takasuka M, Shiba E, Tokunaga H, Endo T, Ushino T, Tokunaga K, Okazaki T, Kinoshita T, Ohga Y. The Grunwald-Winstein relationship in the solvolysis of crowded tertiary alkyl chlorides. Hindered hydration and hydrophobic effect. J Phys Org Chem. 2001;14:229–238. [Google Scholar]
- 36.Bentley TW, Dau-Schmidt JP, Llewellyn G, Mayr H. Solvation effects adjacent to the reaction site. Differences in solvation between alkyl, alkenyl or alkynyl, and aryl groups in binary aqueous mixtures. J Org Chem. 1992;57:2387–2392. [Google Scholar]
- 37.Kevill DN, D’Souza MJ. Additional YCl values and correlation of the specific rates of solvolysis of tert-butyl chloride in terms of NT and YCl scales. J Chem Res Synop. 1993:174–175. [Google Scholar]
- 38.Reis MC, Elvas-Leitão R, Martins F. The influence of carbon-carbon multiple bonds on the solvolyses of tertiary alkyl halides: A Grunwald-Winstein analysis. Int J Mol Sci. 2008;9:1704–1716. doi: 10.3390/ijms9091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi K, Ohga Y, Ushino T, Takasuka M. Structural effects of the Grunwald-Winstein correlations in the solvolysis of some simple tertiary alkyl chlorides. J Phys Org Chem. 1997;10:717–724. [Google Scholar]
- 40.Takeuchi K, Ohga Y, Ushino T, Takasuka M. Grunwald Winstein relations in the solvolyses of highly congested simple secondary and tertiary alkyl systems. Evidence for the Brønsted base-type solvation in the standard 1- and 2-adamantyl systems. J Org Chem. 1997;62:4904–4905. [Google Scholar]
- 41.Brown HC, Fletcher RS. Chemical effects of steric strains. I. The effect of structure upon the hydrolysis of tertiary aliphatic chlorides. J Am Chem Soc. 1949;71:1845–1854. [Google Scholar]
- 42.Liu KT, Hou SJ, Tsao ML. Nucleophilic solvent participation in the solvolysis of tertiary bromoalkanes. J Chin Chem Soc. 2009;56:425–430. [Google Scholar]
- 43.Fainberg AH, Winstein S. Correlation of solvolysis rates. VI. t-Butyl and α-phenylethyl bromides. J Am Chem Soc. 1957;79:1602–1608. [Google Scholar]
- 44.Harris JM, Mount DL, Smith MR, Neal WC, Jr, Dukes MD, Raber DJ. Application of the ethanol-trifluoroethanol method to solvolyses for which nucleophilic involvement is questioned. J Am Chem Soc. 1978;100:8147–8156. [Google Scholar]
- 45.Bentley TW, Bowen CT, Parker W, Watt CIF. SN2 character of solvolyses of tert-butyl halides and of trifluoroacetolyses of secondary alkyl sulfonates. J Am Chem Soc. 1979;101:2486–2488. [Google Scholar]
- 46.Kevill DN, D’Souza MJ. Application of the aromatic ring parameter (I) to solvolyses of extremely crowded alkyl derivatives. Tetrahedron Lett. 1998;39:3973–3976. [Google Scholar]
- 47.Allard B, Casadevall E. Comparative study of the electrophilic properties of some protic solvents characterized by spectroscopic and kinetic solvent-sensitive standard process. Nouv J Chim. 1985;9:565–568. [Google Scholar]
- 48.Abraham MH, Grellier PL, Abboud J-LM, Doherty RM, Taft RW. Solvent effects in organic chemistry. Recent developments. Can J Chem. 1988;66:2673–2686. [Google Scholar]