Trans-activation between 7TM domains: implication in heterodimeric GABAB receptor activation
G-protein-coupled receptors are seven-transmembrane domain proteins that can assemble into dimers or higher oligomers. This study reveals that agonist binding to one subunit of the GABA B receptor dimer results in the activation of the other subunit via direct trans-activation between the 7TM domains.
Keywords: allosteric modulators, anxiety, class C GPCRs, drug addiction, trans-activation
Abstract
Seven-transmembrane domain (7TM) receptors have important functions in cell–cell communication and can assemble into dimers or oligomers. Such complexes may allow specific functional cross-talk through trans-activation of interacting 7TMs, but this hypothesis requires further validation. Herein, we used the GABAB receptor, which is composed of two distinct subunits, GABAB1, which binds the agonist, and GABAB2, which activates G proteins, as a model system. By using a novel orthogonal-labelling approach compatible with time-resolved FRET and based on ACP- and SNAP-tag technologies to verify the heterodimerization of wild-type and mutated GABAB subunits, we demonstrate the existence of a direct allosteric coupling between the 7TMs of GABAB heterodimers. Indeed, a GABAB receptor, in which the GABAB2 extracellular domain was deleted, was still capable of activating G proteins. Furthermore, synthetic ligands for the GABAB2 7TM could increase agonist affinity at the GABAB1 subunit in this mutated receptor. In addition to bringing new information on GABAB receptor activation, these data clearly demonstrate the existence of direct trans-activation between the 7TM of two interacting proteins.
Introduction
G-protein-coupled receptors (GPCR) represent the largest family of cell-surface receptors in mammals. They have evolved to recognize a wide variety of ligands to achieve different functions in cell–cell communication (Lagerström and Schiöth, 2008). In artificial systems such as heterologous cell lines, these seven-transmembrane domain (7TM) proteins have the propensity to associate into dimers or oligomers (Terrillon and Bouvier, 2004; Ferre et al, 2009). Moreover, the potential existence of such complexes also in native tissues (Albizu et al, 2010) could constitute a new level of complexity and influence the development of future drugs (Ambrosio and Lohse, 2010).
A key element for understanding the functional consequences of GPCR oligomerization is to elucidate the possible trans-conformational changes that may occur within receptor complexes (Springael et al, 2007; Han et al, 2009). However, despite intense research, direct trans-activation between 7TMs remains uncertain (Ji et al, 2002; Urizar et al, 2005; Neri et al, 2010), whereas several studies have suggested the existence of trans-inhibition, that is the activation of one partner leads to the inhibition of the other (Kostenis et al, 2005; Sohy et al, 2007; Vilardaga et al, 2008). However, what has been previously interpreted as trans-inhibition may reflect a simple steric hindrance by the second 7TM, which is unnecessary for G-protein coupling (Bayburt et al, 2007; White et al, 2007; Banerjee et al, 2008).
GABA, the main inhibitory neurotransmitter, mediates part of its effects through the metabotropic receptor GABAB. This GPCR is a clear example of trans-activation between two associated subunits, whereby the GABAB1 (GB1) subunit binds agonists, and the GABAB2 (GB2) subunit is responsible for G-protein activation (Galvez et al, 2001). However, studies aimed at identifying the structural basis of this trans-activation mechanism suggest that the extracellular domains of these subunits are responsible for this process (Pin et al, 2005), rather than direct coupling between their 7TMs.
In the light of the structural homology between GABAB and the metabotropic glutamate receptors (mGlu), the current model for GABAB receptor activation is based on the structure of mGlu extracellular Venus Flytrap (VFT) domain dimers, which have been solved in the inactive and active conformations (Kunishima et al, 2000; Muto et al, 2007). Specifically, it is hypothesized that a change in the relative position of the GABAB receptor VFT domains subsequent to the closure of the GB1 VFT should lead to activation of the 7TM of GB2. Consistent with this hypothesis, preventing the positional change of the VFT domain with a ‘glycan wedge' suppresses GABAB receptor activation by orthosteric agonists (Rondard et al, 2008). However, the role of direct allosteric coupling between the 7TM of GB1 and GB2 in GABAB receptor activation has never been evaluated.
In this study, we examined whether direct coupling between the 7TMs of the two subunits of the GABAB receptor occurs during receptor activation. To verify the correct assembly of the GABAB subunits at the cell surface, we have developed a new orthogonal-labelling approach to covalently label each subunit with time-resolved (TR) FRET donors and acceptors. This allowed us to examine the functional properties of a GABAB receptor heterodimer in which the VFT of GB2 had been removed. Surprisingly, we found that this truncated heterodimer could be activated by GABA and obtained evidence of direct coupling between the 7TM of GB1 and GB2. Our study thus provides a clear demonstration that trans-activation between 7TM proteins is possible.
Results
GB2 VFT is not required for the formation of GB1–GB2 heterodimers
Although the 7TM of GB2 is recognized to be the G-protein-activating domain, a GABAB receptor dimer in which both 7TMs are of the GB2 type displays much lower coupling efficacy than the wild-type receptor, indicating that also the 7TM of GB1 is required for efficient G-protein coupling (Galvez et al, 2001; Margeta-Mitrovic et al, 2001). These observations led us to question whether the GB1 7TM could stabilize the active form of GB2 7TM. We thus examined whether the GB1 subunit could directly activate GB2 7TM by co-expressing in HEK-293 cells the wild-type GB1 subunit and a mutated GB2 subunit in which the VFT domain had been deleted (ΔVGB2).
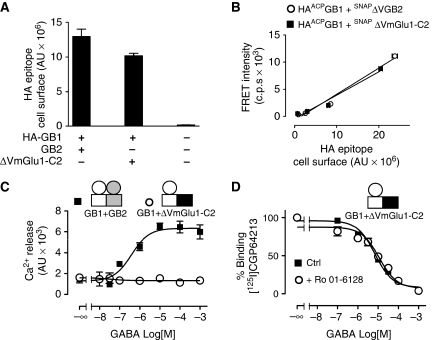
To verify that ΔVGB2 still formed a complex with GB1 at the surface of living cells, we have developed a new method for orthogonal labelling of protein complexes by fluorophores compatible with TR FRET, using the SNAP-tag (Maurel et al, 2008) and ACP-tag technologies (George et al, 2004) (Figure 1A). The SNAP tag (20 kDa) can be covalently labelled with fluorescent non-cell permeable benzyl-guanine derivatives and the smaller ACP tag (8.7 kDa) is covalently labelled with fluorescent coenzyme A derivatives by a phosphopantetheinyl transferase (Supplementary Figure 1). With our new cell-surface-labelling approach, we first showed that SNAPΔVGB2 and wild-type SNAPGB2 could similarly target ACPGB1 to the plasma membrane through measurement of the fluorescence produced by CoA-DY647-labelled ACPGB1 (Figure 1B). Moreover, the different GABAB receptor heterodimers could be detected at the cell surface due to a strong TR-FRET signal caused by the interaction of ACPGB1 with SNAPGB2 or SNAPΔVGB2 (Figure 1B), but not with SNAPmGlu3. The intensity of the TR-FRET signal was proportional to the amount of receptors at the cell surface (Figure 1C) and was similar for both GB1+GB2 and GB1+ΔVGB2 heterodimers. Interestingly, while the SNAP tag could only be tolerated at the terminal ends of the subunits (data not shown), the ACP tag provided similar FRET signals when fused to the N-terminus or inserted into loops of GB1 VFT (Supplementary Figure 1).
Figure 1.
GB2 VFT is not required for the formation of GB1+GB2 heterodimers at the cell surface. (A) Schematic representation of the orthogonal labelling using the SNAP- and ACP-tag technologies to monitor heterodimer formation at the cell surface with donors and acceptors of fluorescence compatible with TR-FRET. (B) CoA-DY647 labelling (open bars) and TR-FRET signal (closed bars) of cells that express ACP-tagged GB1 alone or with the indicated SNAP-tagged constructs labelled with Lumi4-Tb, a fluorescence donor. (C) FRET signal intensity as a function of the amount of HA-tagged ACPGB1 subunit targeted to the cell surface by SNAPGB2 or SNAPΔVGB2. Higher HA-GB1 expression at the cell surface was obtained by transfecting increasing concentrations of plasmids. Data are the mean±s.e.m. of triplicates from a typical experiment.
Functional properties of GABAB heterodimers in which GB2 VFT has been deleted
GABAB receptor-mediated activation of G proteins was assessed by co-expression of the different GABAB subunits with the chimeric G-protein Gαqi9 (a Gαq protein in which the last nine C-terminal residues have been replaced by those from Gαi2), which facilitates the coupling of Gi-coupled receptors to the phospholipase C signalling pathway (Galvez et al, 2001) (Figure 2A). In cells that expressed the truncated GABAB receptor (GB1+ΔVGB2), GABA could produce a Ca2+-mobilization response; however, the potency was 100-fold lower and the efficacy slightly lower than in cells that expressed the wild-type receptor (GB1+GB2). This lower potency was not due to a difference in the cell-surface expression level of the truncated receptor heterodimer compared with the wild-type receptor (Supplementary Figure 2).
Figure 2.
GB2 VFT is dispensable for GABAB receptor activation. (A) Intracellular Ca2+ response following activation by GABA of the HA-tagged GB1 subunit co-expressed with the indicated GB2 constructs. Data are the mean±s.e.m. of triplicates from a typical experiment. (B) Schematic description of the intramolecular rearrangement of the Epac BRET sensor upon cAMP binding. (C, D) Time course of the cAMP response measured with the BRET sensor in cells that express the indicated receptor combination stimulated or not (control) with GABA and/or isoproterenol. The BRET signals obtained after stimulation with isoproterenol (1 μM iso) alone or with GABA (iso+1 mM GABA) were statistically different both in cells expressing wild-type (C) or truncated receptors (D). Inset, values measured at 200 s for each condition (Student's t-test; ***P<0.0001 and **P=0.0014).
The GABA-mediated response in cells expressing the truncated GABAB receptor was clearly due to G-protein activation by ΔVGB2, given the introduction of a point mutation known to prevent G-protein activation (L686P), in the i3 loop of ΔVGB2 (Duthey et al, 2002) suppressed this response (Figure 2A), even though the mutated heterodimer (GB1+ΔVGB2-L686P) was expressed at the cell surface (Supplementary Figure 2). In addition, the activity of both GB1+GB2 and GB1+ΔVGB2 receptor heterodimers was completely abolished by incubation with CGP54626, a competitive antagonist for the GABA-binding site on GB1 VFT, indicating that the response was due to GABA binding to GB1 (Supplementary Figure 2).
Coupling of the GB1+ΔVGB2 receptor heterodimer to its natural and endogenous Gi protein was validated by demonstrating that it could inhibit isoproterenol-induced cAMP formation by use of an intracellular cAMP sensor based on exchange protein directly activated by cAMP (Epac) and bioluminescence resonance energy transfer (BRET) (Jiang et al, 2007) (Figure 2B). Indeed, upon activation of the endogenous β-adrenergic receptors by isoproterenol, the BRET signal decreased, as expected, due to increased cAMP formation. The effect of isoproterenol was significantly diminished following co-incubation with GABA in cells expressing the wild-type receptor heterodimer (GB1+GB2) (Figure 2C) and, to a lesser extent, also in cells expressing the GB1+ΔVGB2 heterodimer (Figure 2D). In both cases, similar receptor expression was detected at the cell surface.
GB2 VFT is necessary for high-affinity agonist binding and high G-protein-coupling efficacy
The lower maximal response and higher agonist EC50 observed with the truncated receptor complex suggested that deletion of the VFT of GB2 could decrease agonist affinity and/or G-protein-coupling efficacy. The potential decrease in GABA affinity observed with the GB1+ΔVGB2 heterodimer compared with the wild-type GB1+GB2 receptor was confirmed by binding studies, in which the GB1+ΔVGB2 heterodimer and GB1 alone showed similar agonist affinity (Figure 3A). These binding experiments were performed in intact cells and with a GB1 subunit, in which the endoplasmic reticulum (ER) retention signal sequence had been mutated to ASA (GB1-ASA) to allow its efficient transport to the cell surface (Pagano et al, 2001). This result confirmed that GB2 VFT is needed to increase GB1 agonist affinity (Liu et al, 2004).
Figure 3.
GB2 VFT is required for high agonist affinity and G-protein-coupling efficacy. (A) Displacement of the non-permeant antagonist [125I]CGP64213 by GABA from the indicated GABAB subunits, expressed alone or in combination. Data are the mean±s.e.m. of triplicates from a typical experiment. The displacement curve for the wild-type GABAB heterodimer (GB1+GB2) was statistically different from the curve for the truncated receptor heterodimer (GB1+ΔVGB2) or for the GB1-ASA subunit alone (Student's t-test; P<0.0001 and P=0.0041, respectively). (B) Inositol-phosphate (IP) accumulation in cells that express the indicated GABAB subunits without (basal) or after stimulation with 1 mM GABA, in the presence of the chimeric Gqi9 protein. (C, D) Effect of CGP35348, a GABAB receptor partial agonist, on Ca2+ release in cells expressing GB1+GB2 (C) or GB1+ΔVGB2 (D).
However, the difference in affinity (five-fold) was less robust than the shift in agonist potency (100-fold), suggesting that in addition to lower agonist affinity at the GB1+ΔVGB2 heterodimer, the truncated heterodimer may also display decreased G-protein-coupling efficacy. This is consistent with the lower maximal effect that was observed when cells that express the GB1+ΔVGB2 receptor heterodimer were used in Ca2+ mobilization (Figure 2A) and in cAMP inhibition assays (Figure 2C and D). This hypothesis was further supported by the lower constitutive activity of the truncated GB1+ΔVGB2 heterodimer, compared with the wild-type GB1+GB2 GABAB receptor, determined by measuring GABA-induced accumulation of inositol phosphate (IP) (Figure 3B). Moreover, the differences in activity were not due to variations in the amount of the different receptor complexes at the cell surface (Supplementary Figure 3). Finally, the lower G-protein-coupling efficacy of the GB1+ΔVGB2 heterodimer was also supported by the absence of effect of CGP35348, a partial agonist of the wild-type receptor (Olpe et al, 1990), on the GB1–ΔVGB2 complex (Figure 3D), at least at the concentration range which is effective for the GB1+GB2 receptor heterodimer (Figure 3C). The lack of effect of CGP35348 on the truncated receptor complex was not due to a binding defect, as CGP35348 could bind to the GB1 domain of wild-type and truncated receptor complexes with similar affinities (Supplementary Figure 4).
GB2 VFT largely limits the agonist activity of positive allosteric modulators
The GABAB receptor positive allosteric modulators (PAMs) CGP7930 and GS39783, which interact with the 7TM of GB2 (Urwyler et al, 2001, 2003; Binet et al, 2004), increased agonist potency, but lacked (GS39783) or had minimal (CGP7930) agonist efficacy at the wild-type GB1+GB2 receptor complex in the absence of GABA (Figure 4A). In contrast, both compounds showed more robust agonist efficacy in the absence of GABA at the truncated GB1+ΔVGB2 receptor heterodimer (Figure 4B), demonstrating that GB2 VFT is needed to limit direct activation of the GB2 7TM by these PAMs.
Figure 4.
Effect of positive allosteric modulators on the GABAB heterodimer in which GB2 VFT has been deleted. Effect of two positive allosteric modulators (PAMs), CGP7930 and GS39783 (100 μM each), on Ca2+ release in cells expressing wild-type (A) or truncated (B) GABAB receptors, and on the displacement of the antagonist [125I]CGP64213 by GABA from the wild-type (C) or truncated (D) GABAB receptor heterodimers. The displacement curve for the wild-type heterodimer (GB1+GB2) or the truncated receptor (GB1+ΔVGB2) was statistically different in the presence of CGP7930 (Student's t-test, P<0.0001 and P=0.005 for the wild-type or the truncated receptor, respectively) or GS39783 (Student's t-test, P=0.0005 and P=0.0067 for the wild-type or the truncated receptor, respectively).
These PAMs are known to increase the affinity of orthosteric agonists at the wild-type receptor (Figure 4C). This effect was still observed, although to a lower extent, in the presence of truncated receptor heterodimers, further demonstrating that allosteric coupling between the 7TM domain of GB2 and the VFT of GB1 could still occur in the absence of GB2 VFT (Figure 4D).
Selective coupling between GB1 and GB2 7TM
We then analysed whether allosteric coupling could occur between GB1 7TM and another 7TM protein, especially one from the class C GPCRs with which GB1 7TM share high sequence homology. To this aim, we co-expressed the GB1 subunit with ΔVmGlu1-C2, in which the VFT domain of mGlu1 was deleted and replaced by an SNAP tag and the C-terminal tail was replaced by the tail of GB2 to mask the GB1 ER retention signal after formation of the heterodimer (Goudet et al, 2005) and to stabilize the formed heterodimers at the cell surface. The presence of the SNAP tag in ΔVmGlu1-C2 and of the ACP tag fused to GB1 allowed us to demonstrate that truncated mGlu1 targeted GB1 to the cell surface (Figure 5A) and that these two proteins remained associated at the cell surface as revealed by the comparable TR-FRET signals obtained in cells that expressed GB1+ΔVGB2 or GB1+ΔVmGlu1-C2 (Figure 5B). This indicates that ΔVmGlu1-C2 can form heterodimers with GB1 at the cell surface similarly to ΔVGB2.
Figure 5.
Selective coupling between the GB1 and GB2 7TM domains. (A) ELISA measurement of the amount of HA-tagged GB1 at the cell surface when co-expressed with GB2 or ΔVmGlu1-C2. (B) FRET signal intensity following interaction of DY647-labelled ACPGB1 with Lumi4-Tb™-labelled SNAPΔVGB2 or SNAPΔVmGlu1-C2 subunits. Increasing HA epitope cell-surface signals were obtained by transfection of increasing DNA concentrations. (C) Intracellular Ca2+ response mediated by wild-type (GB1+GB2) or GB1+ΔVmGlu1-C2 receptor heterodimers. (D) Effect of Ro 01-6128, a mGlu1 positive allosteric modulator, on the displacement of [125I]CGP64213 by GABA from the GB1+ΔVmGlu1-C2 heterodimer (ns, Student's t-test, P=0.4287).
However, there was a lack of allosteric coupling between the VFT of GB1 and the 7TM of ΔVmGlu1-C2, which was revealed by the absence of Gq activation induced by GABA (Figure 5C) and by the inability of the PAM Ro 01-6128 (Knoflach et al, 2001) that directly activates mGlu1 7TM (Supplementary Figure 5) to increase the agonist affinity at the GB1 subunit (Figure 5D). This clearly illustrates an absence of allosteric coupling between the GB1 agonist-binding site and the G-protein-coupling site of mGlu1.
Direct trans-activation between the 7TMs of GB1 and GB2
Our results indicate that allosteric coupling between GB1 VFT and GB2 7TM can occur in the absence of the VFT of GB2. This may be the result of either a direct interaction of GB1 VFT with GB2 7TM, or an indirect effect involving the 7TM of GB1. The latter implies that the closed form of GB1 VFT could induce a conformational change in GB1 7TM that would lead to activation of the 7TM domain of GB2.
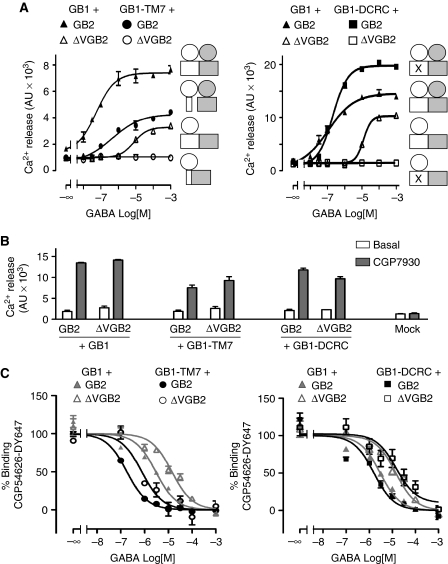
To discriminate between these two hypotheses, we performed three different types of experiments. First, we tested whether GB1 VFT could directly activate the 7TM of GB2 by measuring the intracellular calcium response of a chimeric protein made of GB1 VFT and the 7TM of GB2 (GB1/2 chimera) (Galvez et al, 2001). The lack of calcium response in cells that expressed the GB1/2 chimera alone or in combination with GB2 7TM (ΔVGB2) indicates that GB1 VFT does not directly activate the 7TM of GB2 and implies that GB1 7TM is necessary for the activation of the GB1+ΔVGB2 truncated receptor (Figure 6A; Supplementary Figure 7A and 8). Furthermore, co-expression of ΔVGB2 and of a truncated GB1 subunit, in which the VFT domain was anchored to the cell surface via the transmembrane helix TM7 and the C-terminal region of GB1 (GB1-TM7), was not functional (Figure 7A, left panel), despite there being sufficient cell-surface expression and association of the two subunits, as demonstrated by ELISA and TR-FRET, respectively (Supplementary Figure 7B).
Figure 6.
(A) The GB1 7TM domain is important for the activation of GB2. Intracellular Ca2+ response mediated by the wild-type (GB1+GB2) or the chimeric GB1/2 subunit (GB1 VFT fused to GB2 7TM) expressed alone or in combination with the wild-type or truncated GB2 subunit. (B) Steric hindrance on top of truncated GB2 does not prevent signal transmission. Intracellular Ca2+ response mediated by GB1 co-expressed with truncated GB2, fused or not to the SNAP tag or the ACP tag at the N-terminus or in the second extracellular loop (e2).
Figure 7.
Direct trans-activation between the 7TM domains of GB1 and GB2. Intracellular Ca2+ response mediated by wild-type GB1 or the indicated GB1 mutants (GB1-TM7, in which GB1 VFT is anchored to the membrane through the seventh TM of GB1; or GB1-DCRC, in which D649 and R665, two residues of GB1 7TM, were mutated to cysteines) co-expressed with full-length or truncated GB2, after stimulation with increasing concentrations of GABA (A) or 100 μM CGP7930 (B). Data are the mean±s.e.m. of three independent experiments performed in triplicates. (C) Displacement of CGP54626-DY647 by GABA at the cell surface. The difference between the binding curves obtained with cells that express wild-type GB1 and cells that express the GB1-DCRC mutant was not significant (NS, Student's t-test).
Second, to prevent the potential action of GB1 VFT directly on GB2 7TM, we created a region of steric hindrance on the extracellular surface of ΔVGB2 by introducing an SNAP tag or an ACP tag at the N-terminus or in the second extracellular loop (e2). All these ΔVGB2 variants formed complexes with GB1 and were activated by GABA in a similar manner to the untagged ΔVGB2 (Figure 6B; Supplementary Figure 6). This suggests that GB1 VFT could still activate GB2 7TM despite the steric hindrance on the GB2 7TM extracellular surface.
Third, in order to perturb or prevent potential conformational changes in GB1 7TM, which might lead to GB2 7TM activation according to our second hypothesis, we introduced a pair of cysteine residues at various positions of GB1 7TM that are expected to form disulfide bridges according to a 3D homology model of this domain based on the rhodopsin structure. We found that one mutant, in which D649 and R665 were replaced by cysteines (GB1-DCRC), possibly linking the extracellular parts of TM2 and of TM3, was able to generate a functional receptor, but only when assembled with wild-type GB2 but not with ΔVGB2 (Figure 7A, right panel). The lack of activity of the GB1-DCRC+ΔVGB2 combination was not due to the absence of properly assembled subunits at the cell surface, as shown by cell-surface labelling and TR-FRET data (Supplementary Figure 7C), nor to modification of the GB1-DCRC-binding properties (Figure 7C), nor to inability of ΔVGB2 to activate G proteins in these conditions, as demonstrated through the maintenance of agonist activity of the PAM CGP7930 (Figure 7B).
Taken together, these data validate the hypothesis of a direct trans-activation between the 7TMs of the GABAB heterodimer.
Discussion
Several years ago, the hypothesis of possible direct trans-activation between 7TMs was proposed, but never firmly demonstrated. In this study, we show that such trans-activation occurs in the GABAB receptor. The best evidence comes from a GABAB receptor lacking GB2 VFT in which the GB2 7TM is directly activated by the 7TM of GB1.
Allosteric coupling between the VFT and the 7TM domains in the GABAB receptor is expected to result from a change in the relative position of the VFTs, according to the solved structures of the dimeric mGlu1 VFT domains in their inactive and active forms (Kunishima et al, 2000; Muto et al, 2007). This was confirmed by the observation in our laboratory that agonist activation of one of the two VFTs in an mGlu dimer is equally capable of activating either of the 7TM domains (Brock et al, 2007). In the case of the GABAB receptor, introduction of an N-glycan wedge at the interface between the GB1 and GB2 VFTs, at a position that should prevent the relative movement associated with dimer activation, resulted in a non-functional receptor (Rondard et al, 2008). Herein, we show that although required for the activation of the full-length GABAB receptor, the relative movement of the two VFTs is not the only conformational change required for G-protein activation. Indeed, our data with ΔVGB2 revealed that the closed state of GB1 can activate GB2 7TM, an allosteric coupling that can only occur when associated with the change in the relative position of the VFTs in the full-length receptor. These data suggest for the first time that the activation mechanisms of mGlu and GABAB receptors might be different. Indeed, the direct allosteric coupling observed here between the VFT and the 7TM domains of GB1 does not seem to occur in mGlu receptors, possibly due to the presence of a 45 Å long cysteine-rich domain between the VFT and the 7TM of the mGlu receptors.
How does the ligand-bound closed form of GB1 VFT activate the 7TM domain of GB2 in the absence of GB2 VFT? One possibility is that the active, closed form of GB1 VFT directly interacts with and activates the 7TM domain of GB2. Such a process has been shown for the glycoprotein receptor, whereby the agonist bound to the extracellular domain of one protomer acts directly on the second protomer (Ji et al, 2002; Urizar et al, 2005; Rivero-Müller et al, 2010). However, this is unlikely to be the case for the GABAB receptor as (i) the chimeric subunit made of the VFT of GB1 and the 7TM of GB2 is non-functional, (ii) steric hindrance on the external surface of ΔVGB2 does not prevent its activation by GB1 and (iii) no trans-activation could be measured between the GB1-TM7 subunit (that is the isolated GB1 VFT domain, anchored to the plasma membrane via TM7) and ΔVGB2 despite their correct assembly at the cell surface. We, therefore, propose that closure of GB1 VFT results in a conformational change of GB1 7TM that is sufficient to activate the associated GB2 7TM.
In support of this model, the insertion in the 7TM of GB1 of two cysteines expected to cross-link the extracellular part of TM2 and TM3 (GB1-DCRC mutant) prevented the activation of ΔVGB2 by GB1, further indicating that a conformational change in the 7TM domain of GB1 is required to activate ΔVGB2. We also hoped that the GB1-DCRC mutant would allow us to estimate the relative importance of 7TM trans-activation in the activity of the full-length receptor. Surprisingly, these mutations did not decrease the full-length GABAB receptor-coupling efficacy, but rather increased it by 25%. This finding could suggest that trans-activation between 7TM domains does not have a function in the activation process of the wild-type receptor. We believe that this is unlikely for several reasons. First, such allosteric coupling between these two 7TM proteins is expected to require a tight and very precise association between both proteins, conserved during evolution. Accordingly, no trans-activation between the 7TMs of GB1 and mGlu1 or between two GB2 7TMs was observed. The existence of such a precise allosteric coupling leads us to believe that this mechanism is not exclusive to the GABAB receptor with GB2 VFT deleted, but also to the full-length receptor. In agreement with this hypothesis, the replacement of the 7TM of GB1 by the 7TM of GB2 (as in the GB1/2-GB2 combination; Galvez et al, 2001; Margeta-Mitrovic et al, 2001), or the deletion of the 7TM of GB1 largely decreased coupling efficacy. These results are consistent with GB1 7TM having a positive function in the activation process, possibly through trans-activation of GB2 7TM as this domain is sufficient for G-protein activation (Binet et al, 2004). But then, how can the GB1-DCRC+GB2 receptor heterodimer be still perfectly active when the two substitutions in GB1 7TM should perturb the 7TM conformational change? Based on our results and on all the previous data on the crucial importance of the relative movement between VFTs for GABAB receptor activation, we suggest that the VFTs change in position might lead to GB2 7TM activation through two allosteric pathways: (1) one direct from the GB2 VFT to the GB2 7TM and (2) a second one that interconnects GB1 VFT to GB1 7TM, which, in turn, trans-activates GB2 7TM (Figure 8). Our results indicate that coupling between GB1 VFT and GB1 7TM can result from GB1 VFT closure (which is likely to occur in the GB1-ΔVGB2 combination) and from the relative movement between VFTs. Accordingly, only one allosteric pathway leading to the activation of GB1 7TM remains in the GB1-ΔVGB2 combination (from GB1 VFT to GB1 7TM and finally to GB2 7TM), explaining why this receptor heterodimer displays lower coupling efficacy than the wild-type GB1+GB2 receptor. This reduction in coupling options also explains why the transduction process is blocked in the GB1-DCRC+ΔVGB2 heterodimer. In contrast, in the wild-type full-length receptor, the GB1 7TM domain is stabilized in its active form by (1) the closed GB1 VFT, (2) the new position of GB1 VFT and (3) the active form of GB2 7TM. These different pathways should be sufficient to overcome the energy barrier brought about by the mutations in GB1-DCRC and, therefore, they explain how the GB1-DCRC+GB2 heterodimer can still be fully activated by agonists. Moreover, the gain in coupling efficacy in this receptor heterodimer in comparison with the wild-type receptor may result from more stable active GB1 due to the mutations. We think that this hypothesis about the presence of two allosteric pathways can explain our results; however, other explanations may exist and further work is necessary to clarify this issue.
Figure 8.
Model of the mechanism of GABAB receptor activation. Schematic diagrams representing the heterodimeric GABAB receptor in which the direct allosteric coupling between the four different domains are highlighted with double head arrows. The table summarizes the different combinations of GB1 and GB2 constructs that were co-expressed and the intramolecular signalling pathways that occur in these receptors. In the absence of the VFT of GB2, the allosteric coupling between VFT and 7TM domains of GB1 (2), resulting from the GB1 VFT closure only, is reduced and, therefore, called 2*.
When GPCR dimers/oligomers were first proposed, their evidence was based on binding cooperativity phenomena (Mattera et al, 1985; Hirschberg and Schimerlik, 1994; Wreggett and Wells, 1995; Armstrong and Strange, 2001; Christopoulos and Kenakin, 2002; Albizu et al, 2006; Springael et al, 2007), on photoaffinity labelling (Avissar et al, 1983) and co-immunoprecipitation (Hebert et al, 1996) experiments, and on complementation studies, such as co-expression of two loss-of-function mutants (Ji et al, 2002; Urizar et al, 2005; Rivero-Müller et al, 2010). Their association was suggested to allow specific functional cross-talk. Although few examples of trans-activation have been reported (Ji et al, 2002; Urizar et al, 2005; Haack et al, 2010; Neri et al, 2010), these data were usually interpreted as domain swapping or domain exchange between two non-functional receptors leading to the reconstitution of a functional 7TM protein, rather than as a clear trans-conformational effect (Maggio et al, 1993; Chinault et al, 2004; Rivero-Müller et al, 2010). Co-expression of two receptor-G-protein fusions, one with a loss-of-function receptor mutant and the other with a mutated G protein was also used in an attempt to validate trans-activation between GPCRs (Carrillo et al, 2003). However, the activation of the G protein may also be the result of a direct interaction between wild-type partners rather than of a trans-activation between receptors (Snook et al, 2006).
In contrast, recent experiments are more consistent with the notion that agonist activation of one receptor prevents activation of its associated receptor, as illustrated by the negative agonist-binding cooperativity reported for several homo- and heterodimeric receptors (Albizu et al, 2006, 2010; Springael et al, 2007). In addition, biophysical analysis of the conformational status of one receptor in a complex by intramolecular FRET (Vilardaga et al, 2008) and of purified and reconstituted receptor dimers (Mesnier and Banères, 2004; Damian et al, 2008) revealed that activation of one protomer can induce a conformational change in the associated subunit, but does not promote G-protein activation, which is consistent with the conformational change being different compared with the conformation of the subunit in an agonist-bound state.
Taken together, the present data are the first to demonstrate that agonist occupancy in one protomer of a GPCR dimer might lead to activation of the associated protomer through direct trans-activation between 7TMs. Although such a process could be specific for this obligatory GPCR heterodimer, it may also occur in other 7TM protein pairs including those with an orphan 7TM protein.
Materials and methods
Materials
GABA was obtained from Sigma (St Louis, MO). CGP54626, CGP35348, CGP7930 and GS39783 were purchased from Tocris (Fisher Scientific BioBlock, Illkirch, France). Diphenylacetylcarbamic acid ethyl ester (Ro 01-6128) was synthesized by the in-house facility. [125I]CGP64213 was purchased from Anawa (Zurich, Switzerland). SNAP-Lumi4-Tb™ and CGP54626-DY647 are from Cisbio Bioassays. Foetal bovine serum, culture medium and other solutions used for cell culture were from Invitrogen (Carlsbad, CA).
CoA-DY647 synthesis
A total of 3 μmol of coenzyme A (Fluka, Sigma) in 50 mM PIPES buffer pH 6.5 (400 μl) were mixed with 3 μmol of DY647-maleimide (Dyomics, Jena, Germany) in dry DMSO (300 μl), at room temperature for 2 h. After purification by HPLC using water/0.2% trifluoroacetic acid as eluent with acetonitrile gradient, 1.8 μmol of product were obtained and analysed by electrospray ionization on a Waters Micromass ZQ™ 2000 (m/z=1532.2 (M+H+)).
Plasmids and transfection
pRK5 plasmids encoding wild-type rat GB1a, GB1ASA, GB2 (Rondard et al, 2008), ΔVGB2 (Binet et al, 2004) and rat mGlu3 (Brabet et al, 1998), tagged or un-tagged with a haemagglutinin (HA) epitope at their N-terminal end and under the control of a cytomegalovirus promoter, were described previously. ΔVGB2-L686P was obtained by subcloning the fragment containing the mutation GB2-L686P (Duthey et al, 2002) into ΔVGB2. The ΔVmGlu1-C2 construct was obtained from the mGlu1-C2 chimera previously described (Goudet et al, 2005) by deletion of the VFT and cysteine-rich domain. ACP- and SNAP-tag sequences (New England Biolabs) were subcloned upstream of the cDNA of GABAB or mGlu subunits after the N-terminal HA tag, unless mentioned. The ACP tag was inserted in GB1 VFT after residue Arg455 by creating an NheI restriction site and subcloning of the NheI–NheI fragment that encodes the ACP tag. The resulting construct includes an ASGG linker between Arg455 of GB1 and the N-terminal residue of ACP, and a GGAS linker between the C-terminal residue of ACP and His456 of GB1. The GB1-TM7 construct was generated by removing a fragment between two PshA1 restriction sites, one at the end of the sequence coding for the VFT and the other that have been created just before the seventh TM (TM7) by site-directed mutagenesis. The GB1-DCRC construct was obtained by site-directed mutagenesis that introduced two point mutations, one in TM2 (D649C) and one in TM3 (R665C). The Epac1 cDNA was obtained from Dr Lily Jiang (University of Texas Southwestern, Dallas).
HEK-293 and COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and transfected by electroporation as described elsewhere (Liu et al, 2004). Ten millions cells were transfected with 2 μg of each plasmid of interest and completed to a total amount of 10 μg with the plasmid encoding the pRK5 empty vector. To allow efficient coupling of the receptor to the phospholipase C pathway, cells were also transfected with the chimeric G-protein Gαqi9 (2 μg) (Galvez et al, 2001). For cell-surface expression and functional assays of GB1-TM7 or GB1-DCRC co-expressed with full-length or truncated GB2 subunits, experiments were performed after incubation for 24 h (37°C, 5% CO2) or 48 h (24 h at 37°C, 5% CO2 and then 24 h at 30°C, 5% CO2).
Cell-surface quantification by ELISA, ligand-binding assay, intracellular calcium release and IP measurements
Detection of the HA-tagged constructs at the cell surface by ELISA, ligand-binding assay on intact HEK-293 cells using 0.1 nM [125I]CGP64213 and measurements of the calcium signal in HEK-293 cells were performed as previously described (Liu et al, 2004; Rondard et al, 2008). For ligand-binding assay with the non-permeant fluorescent antagonist CGP54626-DY647, HEK-293 cells (100000) were co-transfected by Lipofectamine 2000 (Invitrogen) with pRK5 plasmids encoding wild-type GB1 (50 ng), GB1-TM7 (50 ng) or GB1-DCRC (50 ng) and pRK5 plasmids encoding for GB2 (50 ng) or ΔVGB2 (50 ng) and complete to a total amount of 200 ng of plasmid DNA with the pRK5 empty vector. Forty-eight hours later (24h at 37°C, 5% CO2 and then 24h at 30°C, 5% CO2), intact cells were washed once in Tris-Krebs buffer (20 mM Tris-Cl, pH 7.4, 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl, 1.8 mM CaCl2) and incubated with both 5 nM CGP54626-DY647 (Cisbio Bioassays) and increasing concentrations of GABA for 3h at 4°C. After three washes with Tris-KREBS buffer, fluorescence was measured as the specific DY647 emission spectrum (665 nm) with an Infinite®F500 reader (Tecan, Switzerland). Measurements of IP accumulation were performed using the IP-One Tb kit (Cisbio Bioassays).
Orthogonal labelling of the cell-surface proteins and TR FRET measurements
Twenty hours after transfection, COS-7 cells were first incubated with a mix composed of 10 mM MgCl2, 1 mM DTT, 5 μM CoA-DY647 and 1 μM Sfp synthase (New England Biolabs) in PBS at room temperature for 1 h. After three washes with PBS, the total fluorescence emitted at 682 nm after excitation at 640 nm was measured using the Analyst AD System (Molecular Devices). Then, SNAP-tag labelling was performed with 200 nM Lumi4-Tb™ in DMEM/10% foetal bovine serum at 37°C in 5% CO2 for 1 h, as previously described (Maurel et al, 2008). After three washes with PBS, FRET was measured as the specific DY647 emission spectrum (665 nm) after excitation of Lumi4-Tb™ at 337 nm minus the background signal measured in the absence of DY647, and the fluorescence emission of Lumi4-Tb was monitored at 620 nm with a RUBYstar reader (BMG Labtech, Offenfurg, Germany).
cAMP BRET sensor in living cells
HEK-293 cells (10 millions) were transfected with the Epac1 (1 μg), GB1 (1 μg) and GB2 (1 μg) constructs. Twenty hours later, cells were washed twice and incubated in PBS containing 5 μM coelenterazine with or without 1 mM GABA and 1 μM isoproterenol to stimulate endogenous β2-adrenergic receptors. The BRET signal was calculated as the difference of emission at 535/485 nm detected by a Mithras reader (Berthold, Germany) (Jiang et al, 2007).
Statistical analysis
For each individual experiment, to assess whether the differences between the relevant samples are statistically significant, an unpaired Student's t-test was computed based on the curve fitting of data or on means using Prism 2 (GraphPad Software). P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank L Comps-Agrar and K Johnsson for constructs and Drs M Ayoub, T Durroux, J Kniazeff, G Labesse and F Rassendren for constructive discussions. We thank Dr G Stewart for English editing. J-PP is supported by the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM) and by grants from the Agence Nationale de la Recherche (ANR-BLAN06-3_135092), and by an unrestricted grant from Senomyx (La Jolla, CA). CM is supported by a fellowship from the Fondation pour la Recherche Médicale.
Author contributions: CM executed the experiments and participated in the writing of the paper; HT executed the experiments; EB and LL generated the tools for HTRF experiments; CV participated in the development of the calcium assay; ET supervised the work at Cisbio Bioassays; JPP and PR contributed to the supervision of the project and to the writing of the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, Barberis C, Durroux T (2006) Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol 70: 1783–1791 [DOI] [PubMed] [Google Scholar]
- Albizu L, Cottet M, Kralikova M, Stoev S, Seyer R, Brabet I, Roux T, Bazin H, Bourrier E, Lamarque L, Breton C, Rives ML, Newman A, Javitch J, Trinquet E, Manning M, Pin JP, Mouillac B, Durroux T (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol 6: 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio M, Lohse MJ (2010) Microscopy: GPCR dimers moving closer. Nat Chem Biol 6: 570–571 [DOI] [PubMed] [Google Scholar]
- Armstrong D, Strange PG (2001) Dopamine D2 receptor dimer formation: evidence from ligand binding. J Biol Chem 276: 22621–22629 [DOI] [PubMed] [Google Scholar]
- Avissar S, Amitai G, Sokolovsky M (1983) Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. Proc Natl Acad Sci USA 80: 156–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Huber T, Sakmar TP (2008) Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J Mol Biol 377: 1067–1081 [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem 282: 14875–14881 [DOI] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prézeau L (2004) The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem 279: 29085–29091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabet I, Parmentier ML, De Colle C, Bockaert J, Acher F, Pin JP (1998) Comparative effect of L-CCG-I, DCG-IV and gamma-carboxy-L-glutamate on all cloned metabotropic glutamate receptor subtypes. Neuropharmacology 37: 1043–1051 [DOI] [PubMed] [Google Scholar]
- Brock C, Oueslati N, Soler S, Boudier L, Rondard P, Pin JP (2007) Activation of a dimeric metabotropic glutamate receptor by intersubunit rearrangement. J Biol Chem 282: 33000–33008 [DOI] [PubMed] [Google Scholar]
- Carrillo JJ, Pediani J, Milligan G (2003) Dimers of class A G protein-coupled receptors function via agonist-mediated trans-activation of associated G proteins. J Biol Chem 278: 42578–42587 [DOI] [PubMed] [Google Scholar]
- Chinault SL, Overton MC, Blumer KJ (2004) Subunits of a yeast oligomeric G protein-coupled receptor are activated independently by agonist but function in concert to activate G protein heterotrimers. J Biol Chem 279: 16091–16100 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54: 323–374 [DOI] [PubMed] [Google Scholar]
- Damian M, Mary S, Martin A, Pin JP, Banères JL (2008) G protein activation by the leukotriene B4 receptor dimer. Evidence for an absence of trans-activation. J Biol Chem 283: 21084–21092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin JP, Prézeau L (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J Biol Chem 277: 3236–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R (2009) Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5: 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prézeau L, Pin JP (2001) Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J 20: 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Pick H, Vogel H, Johnsson N, Johnsson K (2004) Specific labeling of cell surface proteins with chemically diverse compounds. J Am Chem Soc 126: 8896–8897 [DOI] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prézeau L, Pin JP (2005) Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem 280: 24380–24385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack KK, Tougas MR, Jones KT, El-Dahr SS, Radhakrishna H, McCarty NA (2010) A novel bioassay for detecting GPCR heterodimerization: transactivation of beta 2 adrenergic receptor by bradykinin receptor. J Biomol Screen 15: 251–260 [DOI] [PubMed] [Google Scholar]
- Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5: 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M (1996) A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem 271: 16384–16392 [DOI] [PubMed] [Google Scholar]
- Hirschberg BT, Schimerlik MI (1994) A kinetic model for oxotremorine M binding to recombinant porcine m2 muscarinic receptors expressed in Chinese hamster ovary cells. J Biol Chem 269: 26127–26135 [PubMed] [Google Scholar]
- Ji I, Lee C, Song Y, Conn PM, Ji TH (2002) Cis- and trans-activation of hormone receptors: the LH receptor. Mol Endocrinol 16: 1299–1308 [DOI] [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, Fraser I, Sternweis PC (2007) Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282: 10576–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA (2001) Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci USA 98: 13402–13407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977 [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Schiöth HB (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7: 339–357 [DOI] [PubMed] [Google Scholar]
- Liu J, Maurel D, Etzol S, Brabet I, Ansanay H, Pin JP, Rondard P (2004) Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J Biol Chem 279: 15824–15830 [DOI] [PubMed] [Google Scholar]
- Maggio R, Vogel Z, Wess J (1993) Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular ‘cross-talk' between G-protein-linked receptors. Proc Natl Acad Sci USA 90: 3103–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY (2001) Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc Natl Acad Sci USA 98: 14649–14654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Pitts BJ, Entman ML, Birnbaumer L (1985) Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. Evidence for homo- and heterotropic cooperativity in ligand binding analyzed by computer-assisted curve fitting. J Biol Chem 260: 7410–7421 [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, Trinquet E, Pin JP (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods 5: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnier D, Banères JL (2004) Cooperative conformational changes in a G-protein-coupled receptor dimer, the leukotriene B(4) receptor BLT1. J Biol Chem 279: 49664–49670 [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H (2007) Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA 104: 3759–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri M, Vanni S, Tavernelli I, Rothlisberger U (2010) Role of aggregation in rhodopsin signal transduction. Biochemistry 49: 4827–4832 [DOI] [PubMed] [Google Scholar]
- Olpe HR, Karlsson G, Pozza MF, Brugger F, Steinmann M, Van Riezen H, Fagg G, Hall RG, Froestl W, Bittiger H (1990) CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol 187: 27–38 [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prézeau L, Blahos J, Pin JP, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B (2001) C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J Neurosci 21: 1189–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, Prézeau L (2005) Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J 272: 2947–2955 [DOI] [PubMed] [Google Scholar]
- Rivero-Müller A, Chou YY, Ji I, Lajic S, Hanyaloglu AC, Jonas K, Rahman N, Ji TH, Huhtaniemi I (2010) Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci USA 107: 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Trinquet E, Labesse G, Pin JP, Liu J (2008) Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J 27: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook LA, Milligan G, Kieffer BL, Massotte D (2006) Mu-delta opioid receptor functional interaction: insight using receptor-G protein fusions. J Pharmacol Exp Ther 318: 683–690 [DOI] [PubMed] [Google Scholar]
- Sohy D, Parmentier M, Springael JY (2007) Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem 282: 30062–30069 [DOI] [PubMed] [Google Scholar]
- Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M (2007) Allosteric properties of G protein-coupled receptor oligomers. Pharmacol Ther 115: 410–418 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Galès C, Bouvier M, Smits G, Vassart G, Costagliola S (2005) Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J 24: 1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K (2001) Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol 60: 963–971 [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K (2003) N,N'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acid B receptor function. J Pharmacol Exp Ther 307: 322–330 [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ (2008) Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol 4: 126–131 [DOI] [PubMed] [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R (2007) Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci USA 104: 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett KA, Wells JW (1995) Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem 270: 22488–22499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.