Ryanodine receptor-2 upregulation and nicotine-mediated plasticity
This paper reveals molecular insight into nicotine-induced behavioural plasticity. Long-term nicotine exposure results in maintained ryanodine-receptor expression via a newly discovered positive-feedback regulation that involves calcium signals and CREB activation.
Keywords: calcium, CREB, gene expression, neuronal plasticity, sensitization to locomotor activity
Abstract
Nicotine, the major psychoactive component of cigarette smoke, modulates neuronal activity to produce Ca2+-dependent changes in gene transcription. However, the downstream targets that underlie the long-term effects of nicotine on neuronal function, and hence behaviour, remain to be elucidated. Here, we demonstrate that nicotine administration to mice upregulates levels of the type 2 ryanodine receptor (RyR2), a Ca2+-release channel present on the endoplasmic reticulum, in a number of brain areas associated with cognition and addiction, notably the cortex and ventral midbrain. Nicotine-mediated RyR2 upregulation was driven by CREB, and caused a long-lasting reinforcement of Ca2+ signalling via the process of Ca2+-induced Ca2+ release. RyR2 upregulation was itself required for long-term phosphorylation of CREB in a positive-feedback signalling loop. We further demonstrate that inhibition of RyR-activation in vivo abolishes sensitization to nicotine-induced habituated locomotion, a well-characterised model for onset of drug dependence. Our findings, therefore, indicate that gene-dependent reprogramming of Ca2+ signalling is involved in nicotine-induced behavioural changes.
Introduction
In the central nervous system, nicotine has a multitude of effects ranging from enhanced cognitive function to neuroprotection and addiction (Gotti and Clementi, 2004; Gould, 2006; Levin et al, 2006; Rose, 2007; Picciotto and Zoli, 2008). Most of these effects are mediated by nicotinic acetylcholine receptors (nAChRs), a family of ligand-gated plasma membrane ion channels that are located in both pre- and post-synaptic compartments (Dajas-Bailador and Wonnacott, 2004; Gotti and Clementi, 2004). Nicotine activation of nAChRs regulates neuronal function by producing an increase in Ca2+ influx and inducing neuronal depolarisation (Radcliffe and Dani, 1998; Ji et al, 2001). The magnitude of the intracellular Ca2+ signal produced may be enhanced via recruitment of voltage-operated Ca2+ channels or mobilization of Ca2+ from the endoplasmic reticulum (ER) store through the process of Ca2+-induced Ca2+ release (CICR) (Dajas-Bailador and Wonnacott, 2004). Ca2+ signals triggered by nicotine can also modulate gene transcription and hence produce downstream, longer-term modifications of neuronal activity (Chang and Berg, 2001; Hu et al, 2002; Dajas-Bailador and Wonnacott, 2004; Wang et al, 2008).
The initial changes in Ca2+ signalling involved in plasticity, such as those underlying long-term potentiation, are largely post-translational and involve insertion of new Ca2+ channels into post-synaptic membranes (Malinow and Malenka, 2002) and local amplification by Ca2+ release from ER stores (Fitzjohn and Collingridge, 2002). Post-synaptic stimulation can also activate a programme of gene expression that leads to extensive dendritic and synaptic remodelling (Engert and Bonhoeffer, 1999; Greer and Greenberg, 2008). In conjunction with gene-dependent dendritic and synaptic remodelling, receptor/channels involved in Ca2+ signalling are also upregulated. For example, nicotine upregulates ionotropic glutamate receptor subtypes (Dani and De Biasi, 2001; Ferrari et al, 2001, Wang et al, 2007) in the mesolimbic dopamine pathway, which has been linked to the development of nicotine addiction. Thus, the effects of initial Ca2+ signals are considered to be largely independent on new gene expression, whereas more permanent amplification of post-synaptic Ca2+ signals is linked to gene-dependent remodelling.
Here, we show that nicotine signalling in vitro and in vivo stimulates upregulation of the ER Ca2+ receptor/channel RyR2, which results in transcriptional reprogramming of CICR and a long-term amplification of neuronal Ca2+ signals (Supplementary Figure S1). Studies in primary neuronal networks uncovered a novel nicotine-elicited positive-feedback loop involving RyR2 upregulation, the enhancement of Ca2+ signals and the maintained activation of the transcription factor CREB. In addition, we provide evidence that the signalling switch towards CICR is involved in sensitization to nicotine-induced habituated locomotion, a hallmark of nicotine-dependent neuroplasticity (Vezina et al, 2007). Finally, the finding that RyR2 levels were also upregulated upon cocaine administration suggests that reprogramming of intracellular Ca2+ signalling may be a common molecular pathway for neuronal plastic adaptation.
Results
Nicotine selectively upregulates RyR2 via enhanced network Ca2+ activity
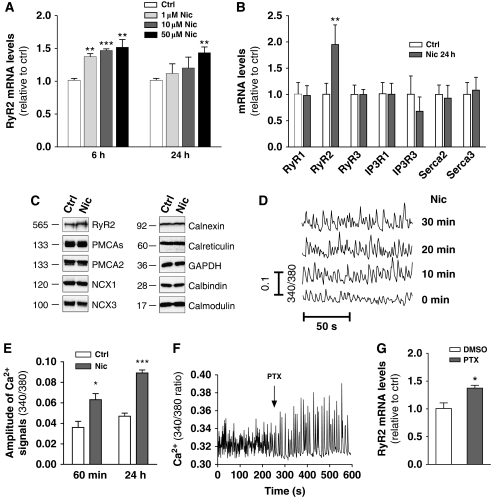
Primary cultures of rat cortical neurons (RCNs) were initially chosen to examine the effects of nicotine exposure on activity-dependent gene expression. These cultures demonstrate spontaneous glutamatergic-network activity resulting in synchronized neuronal Ca2+ transients (Murphy et al, 1992). Treatment of RCNs with nicotine (1–50 μM) (Chang and Berg, 2001) stimulated an increase in the mRNA levels of the type 2 ryanodine receptor Ca2+-release channel (RyR2) (Figure 1A). The magnitude of enhancement in response to a 24-h treatment with 50 μM nicotine ranged from increases of 43±9% (three experiments, P<0.01) (Figure 1A) to 95±24% (four experiments, P<0.01) (Figure 1B) The effect appeared selective over other RyR subtypes, IP3 receptors or indeed all other proteins associated with Ca2+ signalling or Ca2+ homeostasis examined (Figure 1B and C). Upregulation of RyR2 mRNA was inhibited by pre-treatment with the non-competitive neuronal nAChR antagonist mecamylamine (10 μM, 15 min) (Supplementary Figure S2A), and resulted in an 89±16% increase in RyR2 protein levels (P<0.001 using paired t-test) (Figure 1C). While RyR2 upregulation at 6 h could be stimulated by all concentrations of nicotine tested, only 50 μM nicotine appeared capable of maintaining this level of upregulation up to 24 h (Figure 1A). In comparison, repetitive additions of 1 μM nicotine also produced RyR2 upregulation up to 24 h (Supplementary Figure S2B).
Figure 1.
Nicotine selectively upregulates RyR2 and enhances cortical network activity. (A) RCNs were treated with 1, 10 and 50 μM nicotine for 6 or 24 h and RyR2 mRNA levels measured relative to controls (**P<0.01; ***P<0.001 using paired t-test). Data are the mean±s.e.m. for 3–4 independent experiments. (B, C) Twenty-four hours nicotine treatment (50 μM) increased RyR2 mRNA levels (n=5–7, **P=0.009, paired t-test) and protein levels selectively over the other proteins examined (***P<0.001, paired t-test). The approximate molecular weights in kDa are indicated. (D, E) Nicotine (50 μM) increased the magnitude of the Ca2+ transients within the cortical network at the time points indicated (*P=0.013, ***P<0.001, unpaired t-test). (F) Treatment of RCNs with PTX (50 μM) enhanced network Ca2+ activity and (G) produced an increase in RyR2 mRNA levels at 24 h (*P=0.032, unpaired t-test compared with 0.1% DMSO control, n=3–7).
In fura-2 loaded RCNs, nicotine treatment produced a rapid increase in the magnitude of the spontaneous Ca2+ transients (Figure 1D), and the effects were maintained during continuous nicotine exposure (after 60 min and 24 h nicotine treatment, Ca2+ signals were increased by 75±34% and 89±16%, respectively. Data from five experiments, P=0.013, and P<0.001, respectively, using unpaired t-test) (Figure 1E). The enhanced expression of RyR2 resulted in a functional increase in RyR-mediated Ca2+-release responses from the ER as determined by stimulation of RCNs with the RyR agonist caffeine (360±62 nM rise in cytosolic Ca2+ in control, and 594±92 nM rise after 24 h nicotine treatment; data are from 175 cells from seven independent experiments, P=0.003 using paired t-test). To determine whether transcriptional upregulation of RyR2, and hence reprogramming of CICR, was a mechanism shared by Ca2+-network enhancing stimuli, RCNs were treated with the GABAA-receptor antagonist picrotoxin (Murphy et al, 1992). Picrotoxin (50 μM) produced a rapid enhancement of network Ca2+ activity (Figure 1F), and similar to nicotine, 24 h treatment with picrotoxin increased RyR2 mRNA levels by 37±5% (three experiments, P=0.032 on unpaired t-test) (Figure 1G).
Nicotine upregulates RyR2 in vivo
To investigate whether nicotine altered RyR2 expression levels in vivo, C57BL6 mice were exposed to either acute or chronic systemic nicotine treatments and RyR2 mRNA levels measured in different brain regions (see Figure 2A for brain regions dissected). In the cortex (Cx), a single nicotine intraperitoneal (1 mg/kg i.p.) injection produced significant increases in RyR2 mRNA at 4, 8 and 24 h post-injection (28±5%, 79±18% and 40±2%, respectively, from 4–5 animals) (Figure 2B). Chronic nicotine exposure (single daily i.p. injections for 5 consecutive days) produced a greater response in the Cx than acute nicotine treatment (72±16% increase at 4 h after last chronic injection, P=0.0007) and also gave rise to a significant RyR2 increase in the subcortical limbic forebrain (SLF), in the striatum (containing the caudate nucleus and putamen, CPu) and in the ventral midbrain (VMB containing the VTA) (Figure 2C). In contrast, RyR2 expression levels in the hippocampus (Hip) did not change with either acute or chronic treatments (Figure 2B and C). Similar to the effect on primary cultures, the effect of chronic nicotine exposure in vivo appeared selective for RyR2, as no increases in mRNA were detectable for other RyR subtypes, InsP3 receptors or Serca pumps (Supplementary Figure S2C).
Figure 2.
Nicotine upregulates RyR2 in vivo. (A) Schematic representation of cholinergic pathways (arrows) and regions dissected in (B–F). (B) A single intraperitoneal nicotine injection (1 mg/kg) induced RyR2 mRNA upregulation in the Cx at 4, 8 and 24 h (**P=0.006, *P=0.012 and **P=0.001, respectively, from 4–5 animals). (C) Chronic nicotine treatment (once a day for 5 days) had greater effect on RyR2 mRNA levels measured in the Cx 4 h after the last injection (chronic nicotine versus control: ***P=0.0007; versus acute nicotine: *P=0.030), and in SLF, CPu and VMB (**P=0.006, ***P=0.0008 and *P=0.017, respectively, from 5–10 animals). (D) Adult rats were trained to self-administer nicotine and the levels of RyR2 were assessed in the Cx (*P=0.002). (E) RyR2 mRNA levels were reduced in the Cx (*P=0.017) and in the VMB (+P=0.059) of β2 nAChR subunit knockout mice. Data are mean±s.e.m. from three animals for each condition. (F) A chronic cocaine exposure (15 mg/kg cocaine daily for 8 days) enhanced RyR2 mRNA levels (**P<0.01). In all panels, data represent the mean±s.e.m. from 5–14 animals/group and comparisons were performed using unpaired t-test.
The time course of RyR2 mRNA upregulation suggested that during chronic nicotine exposure, each of the daily nicotine treatments would be acting upon a newly adapted baseline of raised RyR2 levels. This was confirmed in mice treated with chronic nicotine exposure, where the RyR2 mRNA levels on day 5, prior to the nicotine injection (i.e. 24 h after nicotine injection on day 4), was 38±1% greater than saline controls (3–4 animals, P=0.001 using unpaired t-test) (Supplementary Figure S2D). To investigate RyR2 upregulation in models which more faithfully reproduce the patterns of nicotine administration in humans (Corrigall, 1999), adult rats were allowed to self-administer nicotine (0.03 mg/kg nicotine base/infusion, 15.5±1.4 infusions/h, 1 h/day). Under these conditions, we observed a 49±9% increase in RyR2 mRNA levels in the Cx (data from two animals for each condition) (Figure 2D).
To determine whether in vivo RyR2 expression levels were influenced by basal nAChR tone, transcriptional levels of RyR2 were examined in a mouse model lacking the prevalent isoform (α4β2) of nAChRs in the brain (Picciotto et al, 1995). The β2 subunit knockout mice show impairments in specific learning and memory tasks particularly during aging and do not show the reinforcing effects of nicotine (Picciotto et al, 1998; Zoli et al, 1999). In β2 subunit knockout mice, RyR2 mRNA levels were significantly decreased in the Cx, while a trend for a significant decrease was present in the VMB and to a lesser extent in the CPu (Figure 2E). Chronic nicotine treatment in knockout mice failed to induce the upregulation of RyR2 mRNA (Supplementary Figure S2E), suggesting that β2 subunit-containing receptors were essential for the stimulation of the transcriptional machinery.
Cocaine has addictive properties, and shares with nicotine a number of cellular (the mesolimbic dopamine system), and molecular, targets (Pich et al, 1997). To determine whether cocaine also induced reprogramming of CICR, C57BL6 mice were subjected to chronic cocaine administration (15 mg/kg of cocaine–HCl, once a day for 8 days) and brain regions examined for altered RyR2 expression. Similar to nicotine, chronic cocaine treatment increased RyR2 mRNA levels in the VMB (Figure 2F). In addition, cocaine treatment also enhanced RyR2 mRNA in the Hip but not Cx, SLF or CPu (Figure 2F).
CREB-dependent upregulation of RyR2
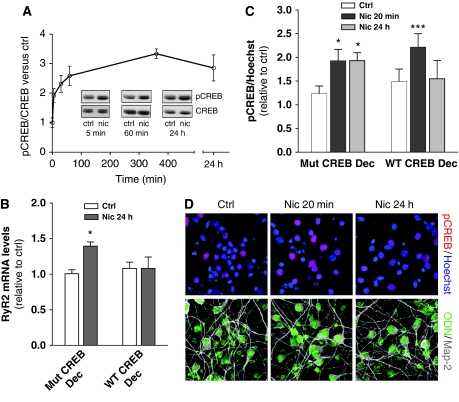
Activity-dependent Ca2+ signals trigger gene transcription mostly via the transcription factor CREB (Hardingham et al, 2001; West et al, 2002). In primary cultures of RCNs, nicotine produced an increase in CREB phosphorylation (pCREB) within 5 min (Figure 3A), which was maintained up to 24 h in the continued presence of nicotine (Figure 3A). In contrast, in the presence of mecamylamine (10 μM, 15 min pre-incubation), nicotine treatment did not alter pCREB levels (3±1% increase and 5±12% decrease respectively, compared with mecamylamine alone, n=3), indicating a requirement for nAChR activation. The enhancement required endogenous glutamatergic Ca2+ activity (both the NMDA-receptor antagonist AP5, and the voltage-operated Ca2+ channel inhibitor nifedipine, inhibited pCREB) (Supplementary Figure S3A and B). Nicotine-mediated pCREB was blocked by KN-93, an inhibitor of Ca2+-calmodulin-dependent protein kinase II (CAMK II), at all time points (Supplementary Figure S3C). However, pCREB at 1 and 24 h also required the additional activation of MAP kinases as demonstrated by PD98039 inhibition of the nicotine-mediated pCREB signal (Supplementary Figure S3D) thereby indicating that longer-term nAChR stimulation by nicotine could recruit downstream kinases distinct from those involved in short-term pCREB (Chang and Berg, 2001; Hu et al, 2002).
Figure 3.
Nicotinic upregulation of RyR2 is pCREB dependent. (A) pCREB/CREB ratio in RCNs treated with nicotine (50 μM) for the indicated times (mean±s.e.m. n=7–13). Inset, representative western blots. (B, C) Transfection of wild-type ODN CREB decoy prevented nicotine-mediated RyR2 upregulation at 24 h and inhibited CREB phosphorylation (measured using immunocytochemistry) in response to 24 h, but not 20 min nicotine treatment (***P<0.001). A mutated CREB decoy was used as a control (*P=0.020 in (B), and *P=0.012 and *P=0.042 for 20 min and 24 h in (C)). Data represent the mean±s.e.m. (n=4). (D) Representative images demonstrating efficiency of transfection of ODN CREB decoy (green).
To determine whether CREB was a transcriptional regulator of RyR2 expression, CREB activity was inhibited by transfection of RCNs with a biotinylated CREB decoy (Mann and Dzau, 2000; Gamble et al, 2007). As demonstrated in Figure 3B, the CREB decoy, but not the mutated control blocked RyR2 upregulation in response to 24 h nicotine treatment (n=4). Interestingly, transfection with the CREB decoy did not alter the short-term (20 min) pCREB signal triggered by nicotine, but blocked nicotine-induced longer-term (24 h) pCREB (n=4) (Figure 3C and D). The effect of the CREB decoy was not due to direct inhibition of neuronal Ca2+ signals (Supplementary Figure S3E). To investigate the presence of potential CREB responsive elements (CREs) in the RyR2 promoter region, we next performed 5′-RACE to identify the transcription start site of the rat RyR2 and then sequenced the immediate 2 kb of the promoter region. In silico analysis identified four putative CREs in this sequence (Supplementary Figure S4A). Electrophoretic mobility shift assays (EMSA) were then performed on oligonucleotides sequences corresponding to these four regions. As identified via supershift using anti-CREB antibody, two of these regions (termed CRE1 and CRE2) were bona fide CREB-binding sites (Supplementary Figure S4B). Consistent with the EMSA results, chromatin immunoprecipitation (ChIP) assay using a pCREB antibody confirmed that CRE1 and CRE2 were binding elements for CREB. Interestingly in RCNs, CRE1 appeared to be constitutively bound by pCREB, whereas binding of pCREB to CRE2 required nicotine pre-treatment (Supplementary Figure S4C).
Long-term enhancement of cortical network Ca2+ activity by nicotine requires RyR activation
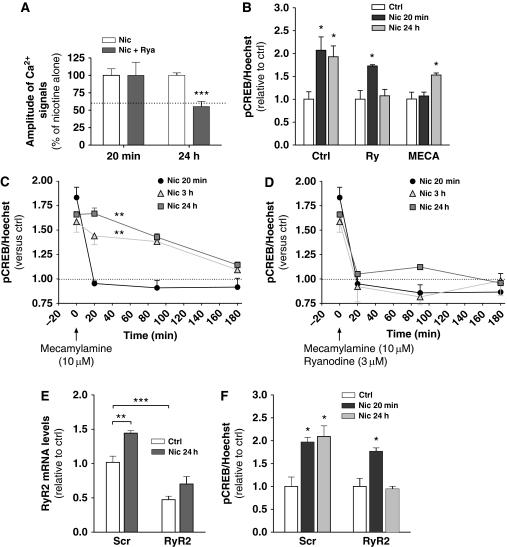
We then went on to determine the role of RyR2 upregulation in the long-term enhancement of network Ca2+ signalling by nicotine. RCNs were pre-treated with the RyR inhibitor ryanodine (Taylor and Broad, 1998) (3 μM, 30 min) and then incubated with nicotine (50 μM) for 20 min or 24 h in the continued presence of ryanodine. Ryanodine alone did not alter the magnitude of basal Ca2+ transients in RCNs (Supplementary Figure S3E), suggesting that RyR-mediated CICR has a negligible role during basal network activity. Similarly, ryanodine treatment did not alter the initial nicotine-mediated enhancement of network Ca2+ signals (Figure 4A) again indicating a lack of involvement of CICR. However, ryanodine reduced to basal levels nicotine-stimulated Ca2+ activity at 24 h (P<0.001 using unpaired t-test, data from three experiments for each condition) (Figure 4A), which suggested that reprogramming of CICR is required to sustain enhanced Ca2+ network activity by nicotine.
Figure 4.
Reprogramming of CICR occurs between initial- and longer-term nicotine treatments. (A) Pre-treatment with ryanodine (3 μM, 30 min) inhibited Ca2+ signals after 24 h (***P<0.001), but not 20 min nicotine (50 μM) treatment. The dotted line indicates the approximate basal amplitude of Ca2+ signals. Data are the mean±s.e.m. (n=3). (B) RCNs were treated with nicotine for 20 min or 24 h (Ctrl), with either the addition or ryanodine or mecamylamine (10 μM) for the last 60 min of the treatment and pCREB/Hoechst ratio were measured. Data are the mean±s.e.m. (n=3). (C, D) RCNs were treated with nicotine for 20 min, 3 or 24 h before the addition of either C, mecamylamine alone or D, mecamylamine plus ryanodine and the rate of decay in pCREB signal measured (***P<0.001, two-way ANOVA followed by Bonferroni's post hoc) (n=3). (E) Downregulation of RyR2 via siRNA transfection (***P=0.0008) prevented nicotine-induced enhanced RyR2 mRNA levels and (F) CREB activation at 24 h, while activation at 20 min was unaffected (*P=0.016). Scr siRNA was used as a control (**P=0.006 in (E), and *P=0.034 and *P=0.013, respectively, in (F)). Data, when not stated differently, represent the mean±s.e.m., n=3–5, and comparisons were performed using unpaired t-test.
Nicotine-mediated long-term pCREB is RyR2 dependent
We then investigated whether reprogramming of Ca2+ signalling towards RyR activation was required for nicotine-mediated long-term pCREB. Pre-treatment with ryanodine (3 μM, 40 min) did not block initial nicotine-mediated pCREB (Figure 4B). However, addition of 3 μM ryanodine for the last 1 h of a 24-h nicotine treatment markedly inhibited the ability of nicotine to maintain the pCREB signal (n=3) (Figure 4B). The notion that nicotine-stimulated activity-dependent changes would progressively depend on reprogramming of intracellular Ca2+ signalling implies that long-term pCREB would become, at least in part, independent of nAChR activation. To this end, we used mecamylamine to block nicotinic stimulation at different times after nicotine addition. Initial nicotine-mediated pCREB was fully sensitive to 10 μM mecamylamine (Figure 4B), whereas addition of mecamylamine for the last 1 h of a 24-h nicotine treatment reduced the number of pCREB-positive neurons only partially (n=3) (Figure 4B). To investigate this phenomenon in greater detail, cortical networks were treated with nicotine for 20 min, 3 or 24 h before the addition of mecamylamine alone, or mecamylamine plus ryanodine, and the ability of the cultures to maintain CREB signalling was measured. Addition of mecamylamine to 20 min nicotine-treated neurons reduced pCREB signals at the earliest time point measured (n=3) (20 min, Figure 4C). In contrast, pCREB levels in cultures treated with nicotine for 3 and 24 h did not return to basal levels until 3 h post-mecamylamine treatment (n=3) (Figure 4C), suggesting that the CICR component of the pCREB signal required at least 3 h of nicotine exposure to become established. Addition of ryanodine concurrent with mecamylamine reduced pCREB to basal levels for all treatment conditions (n=3) (Figure 4D), indicating that RyR activation was indeed responsible for the maintenance of CREB signalling in the absence of continued nAChR stimulation (Figure 4D).
These data are consistent with a positive-feedback loop whereby in response to initial periods of nicotine stimulation CREB-dependent RyR upregulation was required to maintain longer-term nicotine-mediated CREB signalling. To investigate whether RyR2 upregulation was indeed critical for this process, RyR2 expression was inhibited via RyR2-targeted RNA interference oligonucleotides (siRNA). RCNs treated with siRNA maintained their ability to produce spontaneous network Ca2+ signals (Supplementary Figure S3E). In these cultures, RyR2-siRNA reduced RyR2 mRNA by 53±5% compared with scrambled controls (P<0.001 using unpaired t-test, n=4–5) (Figure 4E). Under these conditions, nicotine-treatment produced a partial recovery in RyR2 expression levels (Figure 4E); however. this was not sufficient to support a maintained pCREB signal in response to longer-term nicotine treatment (50 μM, 24 h) (Figure 4F). In contrast, RyR2 downregulation did not prevent initial nicotine-mediated pCREB (P=0.034 using unpaired t-test, n=3–5) (Figure 4F).
RyR inhibition prevents sensitization to nicotine-induced habituated locomotion
Similar to other addictive drugs, repeated exposure to nicotine induces a form of plasticity called sensitization. Sensitization consists in increased neurochemical (e.g. release of dopamine in ventral striatum) or behavioural (e.g. locomotion) response to a constant dose of nicotine, and may be relevant to the processes leading from initial drug use to drug dependence (Vezina et al, 2007). Nicotine sensitization is thought to result from plastic changes in the VTA (Kita et al, 1992; Panagis et al, 1996; Vezina et al, 2007). In order to examine the contribution of RyRs on nicotine-induced behavioural changes in vivo, we performed an analysis of habituated locomotor activity after acute or repeated (5–8 days) administration of systemic nicotine (1 mg/kg nicotine bitartrate i.p.). Rats received bilateral intra-VTA perfusion of ryanodine (50 μM) or artificial cerebrospinal fluid (aCSF) starting 30 min before each nicotine injection and maintained throughout the 60-min post-nicotine observation period. As previously shown (Ferrari et al, 2001; Addy et al, 2007), a peak increase of locomotion was observed within 10 min of acute nicotine administration (Figure 5A). No differencein nicotine-elicited locomotion was observed between rats receiving intra-VTA ryanodine or aCSF (P=0.896 on unpaired t-test), indicating that nicotine acute locomotor effects were not altered by intra-VTA ryanodine perfusion. In aCSF-treated rats, a progressive increase in locomotor response to daily nicotine administration was observed such that, on the 5th day of the repeated nicotine treatment, a clear sensitization to nicotine injection was present (Figure 5A and B). In contrast, in rats treated with intra-VTA ryanodine perfusion, sensitization to nicotine-induced locomotor activity was markedly inhibited (P=0.011 on unpaired t-test on 5th day, between rats receiving intra-VTA ryanodine versus aCSF) (Figure 5A and B). Importantly, repeated ryanodine treatment did not alter locomotion during the 75-min habituation period (Supplementary Figure S5). Finally, after 2 days of washout, rats were again treated to a nicotine challenge. The habituated locomotion response was maintained in the aCSF group, and similar to day 5, rats receiving intra-VTA ryanodine had a significantly reduced response to nicotine (P=0.030 on unpaired t-test) (Figure 5B, 8th day of nicotine treatment).
Figure 5.
RyR activation is required for nicotine-mediated sensitization. (A, B) Effects of intra-VTA perfusion of aCSF or ryanodine (50 μM, 30 min before nicotine administration) on habituated locomotion elicited by nicotine (1 mg/kg, i.p.). (A) Time course of habituated locomotion after acute (day 1) or repeated (day 5) nicotine administration in rats receiving ryanodine or aCSF intra-VTA perfusion. (B) Development of nicotine sensitization in rats treated daily with nicotine while receiving ryanodine or aCSF intra-VTA perfusion. Nicotine treatment was repeated on day 8, after 2 days in which no treatments were performed (*P=0.011 and *P=0.030 for 5 and 8 days, respectively). Data represent the mean±s.e.m. of 14 animals/group and comparisons were performed using unpaired t-test.
Discussion
Persistent changes in synaptic responses such as those involved in memory, learning or addiction involve cooperation between increased synaptic activity and protein synthesis (Malinow and Malenka, 2002; Greer and Greenberg, 2008). In this study, we show that nicotine enhances synaptic signalling via genetic reprogramming of CICR with upregulation of the RyR2 and persistent amplification of neuronal Ca2+ transients (Supplementary Figure S1). This signalling switch makes long-term pCREB partly self-sustaining. RyR2 upregulation was also observed in response to chronic cocaine exposure, and both nicotine and cocaine treatments produced upregulation in the ventral midbrain (containing the VTA). Nicotine also produced significant upregulation in several cortical and subcortical telencephalic regions, but not in the hippocampus, which was instead targeted by cocaine administration, thereby demonstrating brain region specificity in response to drug exposure. In primary cultures, enhancing neuronal Ca2+ activity using either nicotine or picrotoxin increased RyR2 mRNA levels, demonstrating that the RyR2, and by association CICR, is subject to activity-dependent gene regulation. Previous work has shown that RyR2 mRNA and protein levels in the hippocampus are upregulated in response to spatial learning tasks (Cavallaro et al, 1997; Zhao et al, 2000). Significantly, a single training experience only produced a transient increase in RyR2 levels, which peaked around 12 h and returned to control levels by 24 h (Cavallaro et al, 1997). In contrast, repetitive training experiences (every day for 4 days) produced a RyR2 upregulation that was now maintained at least up to 24 h (Zhao et al, 2000), thereby linking RyR2 expression levels with intensity of training and the onset of stable long-term memory. Combined with our data, these results suggest that RyR2 may be a key molecular target for genetic reinforcement of neuronal activity.
Several lines of evidence support a positive-feedback loop whereby longer-term effects of nicotine are mediated by transcriptional reinforcement of synaptic Ca2+ signalling: (1) reinforcement of synaptic glutamatergic network activity by short-term nicotine signalling was independent of gene transcription and did not involve a significant RyR component, whereas dependency on RyR developed during longer-term signalling; (2) suppression of nicotine-induced RyR2 upregulation by CREB decoys selectively suppressed longer-term pCREB; (3) suppression of RyR2 expression levels using siRNA oligonucleotides inhibited the long-term (24 h), but not the short-term pCREB elicited by nicotine. Notably, the partial recovery (to the original basal levels) of RyR2 in siRNA-treated neurons, upon nicotine treatment, was not sufficient to support long-term CREB signalling, indicating that basal RyR function alone is not sufficient to sustain long-term signalling and (4) enhanced network Ca2+ activity and long-term pCREB signalling were partially independent of continued nAChR stimulation and, as shown by the mecamylamine experiments, pCREB signal became to some extent self-sustaining. Finally, as Ca2+ mobilisation from the ER after RyR2 upregulation drives activation of MEK1-dependent pCREB, our data may also provide a plausible explanation for the finding that different kinase families are involved in the short- and long-term pCREB formation after nicotine stimulation (Hu et al, 2002).
Administration of nicotine in vivo upregulates RyR2 levels in areas of the brain that constitute crucial components of cognitive and motivational systems. To assess the physiological relevance of these findings, we investigated the phenomenon of sensitization to nicotine-elicited habituated locomotion. Nicotine sensitization, mediated by the nAChRs expressed in the VTA, is a well-recognised phenomenon that requires neuronal plasticity and appears relevant for drug dependence in humans (Kita et al, 1992; Panagis et al, 1996; Addy et al, 2007; Vezina et al, 2007). By using microdialysis of ryanodine into the VTA, we were able to titrate a concentration that did not alter either basal locomotor activity, or the locomotor activity stimulated by a single systemic administration of nicotine. Next we were able to demonstrate that local VTA perfusion with ryanodine, to inhibit RyRs, prevented the development of sensitization to habituated locomotion induced by repeated nicotine administration. The protocol employed in this series of experiments meant that on each day, the VTA was perfused with ryanodine 30 min before the addition of nicotine and then stopped after a further 60 min. The immediate gene-independent nicotine stimulation of a locomotor response was unaffected by this treatment, whereas the plastic, sensitized, response to repeated nicotine treatment was abolished. These data suggest that reprogramming of CICR is required for nicotine-mediated sensitization, a well-recognized example of neuroadaptation to prolonged nicotine exposure in vivo.
Data from β2 knockout mice indicate that RyR2 upregulation requires activation of the α4β2-nAChR subtype. These receptors have relatively low Ca2+ permeability, and instead modulate neuronal function via Na+-dependent depolarisation (Dajas-Bailador and Wonnacott, 2004; Gotti and Clementi, 2004). α4β2-nAChRs are found in both pre- and post-synaptic compartments in the cortex (Alkondon et al, 2000) and midbrain (Champtiaux et al, 2003) where they can modulate circuit activity. In the VTA, β2-containing nAChRs, located on intrinsic neurons, are required for nicotine stimulation of dopamine release (Zhou et al, 2001) and nicotine reinforcing properties (Maskos et al, 2005). The data reported in this paper present a model in which a newly adapted RyR2 baseline is a key initial step in the consolidation of the mesocorticolimbic circuit and therefore in the sensitization to nicotine-elicited habituated locomotion, a model for the onset of addiction-related behaviours. Two distinct mechanisms, both sensitive to ryanodine perfusion in the VTA, could be involved in the long-term enhancement of circuit activity. First, upregulation of RyR2 in the glutamatergic pre-synaptic terminals that form synapses with VTA dopaminergic neurons may potentiate neurotransmitter release, thereby leading to enhanced excitation of the post-synaptic dopaminergic neurons. Second, the genetic reprogramming of CICR in the dopaminergic neurons themselves may amplify the propagation of dendritic Ca2+ waves to the nucleus (Berridge, 1998) and result in a prolonged transcriptionally active state. The persistent activation of Ca2+-dependent transcription factors is likely to result in the induction of secondary waves of gene expression leading to the strengthening and consolidation of synaptic connections (McClung and Nestler, 2008). Our studies demonstrate that gene-dependent switching of Ca2+ signalling from plasma membrane receptor signals to CICR is relevant to nicotine-mediated plasticity and may be a general mechanism enforcing behavioural effects via long-term transcriptional changes in the brain.
Materials and methods
In vivo nicotine treatments
Male C57BL6 (Charles River) mice were kept under controlled temperature and humidity conditions with standardised lighting and had free access to food and water. For acute nicotine treatment, 2-month-old mice (4–5 per group) were treated with a single intraperitoneal (i.p.) injection of 1 mg/kg nicotine ((−) nicotine hydrogen (+) tartrate dissolved in physiological solution (NaCl 0.9%, buffered to pH 7.4 with NaOH)) (Matta et al, 2007) or saline, and killed via rapid decapitation at 2, 4, 8 or 24 h after the injection. Chronic nicotine treatment involved a total of five injections of 1 mg/kg nicotine salt on consecutive days. Animals were then killed 4 h after the last injection. For experiments involving cocaine, mice were treated with a single i.p. injection (15 mg/kg of cocaine–HCl) every day for 8 days and then were killed 4 h after the last injection. Brains were dissected on ice and frozen in liquid nitrogen before storage at −80°C until RNA extraction. Total RNA was extracted from animal tissues using the RNeasy kit (Qiagen), and tested for purity (A260/280 ratios) and integrity (denaturating gel electrophoresis). Reverse transcription and real-time PCR amplification was performed using specific primers for mouse RyR2 (for TGCTTCATCTGTGGGATTGG, rev CCTGTGTGTTCTGTTTCATCC). A GAPDH Taqman probe (for TCACCACCATGGAGAAGGC, rev GCTAAGCAGTTGGTGGTGCA, probe 5′-[6-FAM]ATGCCCCCATGTTTGTGATGGGTGT[BHQ-6-FAM]-3′) was used as a reference gene.
Nicotine self-administration experiments were performed in operant chambers (Coulbourn Instruments, Leigh Valley) as previously described (Chiamulera et al, 2010). Adult male Sprague-Dawley rats (Charles River, Italy) were initially trained to lever press to obtain food reward. Then, rats were implanted with a silastic catheter in the right jugular vein, and assigned to separate food (control), or nicotine self-administration groups. Nicotine self-administration was initiated under a fixed-ratio-1 (FR1) schedule of reinforcement. Each active lever press led to an infusion of nicotine (0.03 mg/kg/infusion) delivered over a 1-s period. Each session lasted until rats had received 25 infusions of nicotine or 3 h had elapsed, whichever event occurred first. When the animals met the criterion of 25 infusions within the end of the daily session, the FR value was increased to FR2 and then to FR3 with session duration lasting up to 1 h. Rats were considered to reach a stable nicotine self-administration under a FR3 schedule of reinforcement when the value of reinforcers/session did not vary >20% between three consecutive sessions.
Habituated locomotion
Adult male pathogen-free Sprague-Dawley rats (Charles-River, Calco, Italy) were used. All animal experimentation was conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Animals were deeply anaesthetized with halothane (Merial Italia, Milan, Italy) and placed in a flat-skull position in a Kopf stereotaxic apparatus. Two microdialysis probe guide cannulae (CMA/12, CMA Microdialysis, Stockholm, Sweden) were implanted into the right and left VTA (coordinates from Bregma: A=−5.3 mm, L=3.0 mm, D=−6.3 mm from dura; inserted at an angle of 16°) and secured to the skull with dental cement. After 1–3 days, a microdialysis probe (CMA/12, 2 mm dialysis membrane, 20 000 Da cutoff; CMA Microdialysis) was inserted into each guide cannula. The probes were connected to a microinfusion pump (CMA/100, CMA Microdialysis) and continuously perfused at 1.1 μl/min with aCSF (KCl 2.5 mM, NaCl 125 mM, CaCl2 1.26 mM, MgCl2 1.18 mM, Na2HPO4 2 mM, pH 7.4).
Locomotor activity was measured in motility cages (Macrolon III, 38 × 20 × 16 cm3) equipped with four infrared beams for detection of horizontal movements and four infrared beams for vertical movements (Med-Associates Inc., St Albans). Inter-beam distance was 8 cm horizontally and 6 cm vertically, and activity was recorded for 135 min in 5 min intervals (MED-PC Software). The animals were placed into the cages for a 75-min habituation period (Ferrari et al, 2001). After 45 min, they were perfused through the two dialysis cannulae with ryanodine (50 μM) or aCSF. After a further 30 min, rats were injected with nicotine (1.0 mg/kg, nicotine bitartrate, i.p.) or vehicle. Habituated locomotion was measured for 60 min following this treatment. The procedure was repeated for 5 consecutive days (development of sensitization) and, after 2 days in which no treatment was performed, the procedure was repeated on day 8. At the end of locomotion experiments, the animal was killed with a lethal injection of pentobarbital (65 mg/kg i.p.), the brain was removed and the position of the microdialysis probes was verified by visual inspection. The probe track was visible as a small hemorrhagic line. Data from brains with hemorrhagic lines >2 mm in diameter, or incorrectly inserted probes were discarded.
Primary cell culture
Cortical neurons were isolated from embryos from 17 days pregnant rats as described previously (Gendron et al, 2001). Dissociated neurons were plated on poly-lysine-coated dishes or glass coverslips and cultured in Neurobasal medium supplemented with 2 mM Glutamax, B27 supplement (50X serum-free supplement) and 1% penicillin–streptomycin (Invitrogen–Gibco). Half the medium was replaced with fresh medium supplemented with cytosine arabinoside (final concentration 10 μM) 7 days after plating in order to block the division of mitotic cells. Neurons were used between 8 and 10 DIV.
Immunoblotting
Western blotting was performed using the isoform-specific antibodies followed by incubation with an HRP-conjugated secondary antibody (Chemicon). Immunoreactivity was visualized with ECL development performed using X-ray film or with a luminescence image analyser (Kodak Image Station 440 and the Software Kodak 3.5). For experiments measuring pCREB, control and nicotine-treated samples were terminated removing the culture medium and addition of loading buffer (10% glycerol, 2% SDS, bromophenol blue 15 μM, 1% 2-mercaptoethanol, 62.5 mM Tris–Cl buffer pH 6.8) and the proteins were separated using SDS–PAGE. Protein bands were then quantified via densitometric analysis using Image J software (National Institutes of Health, Bethesda). The pCREB signal was then normalised to total CREB in the sample.
Total RNA extraction and reverse transcription
Total RNA was extracted from animal tissue and primary RCNs using RNeasy Mini Kit (Qiagen) or Trizol reagent (Invitrogen), following the manufacturer's instruction. Total RNA was eluted by adding 30 μl of RNase-free water onto the silica-gel membrane. Total RNA (0.5 μg) was reverse transcribed by using a random decamer primer (RETROscript kit, Ambion) following the manufacturer's instructions and cDNA used for real-time PCR.
Real-time PCR
Real-time PCR was performed with SYBR Green on an ABI Prism 7700 Sequence Detection System (Applied Biosystem). Reactions were performed in a mixture containing 12.5 μl Master Mix, 50–900 nM primers, 1 μl DNA sample from reverse-transcription reaction and nuclease-free water (total vol. 25 μl). The PCR protocol used consisted of a 15-s denaturation at 95°C followed by 1 min annealing and extension at 60°C for 40 cycles. GAPDH was used as reference gene. PCR primers for ryanodine receptor (RyR) isoforms 1, 2 and 3, SERCA isoforms 2 and 3 and IP3R isoforms 1 and 3 were designed using the Primer Express 2.0 Software program (Perkin-Elmer). The primers used were:
GAPDH: GGATAAACCTGCCAACTATGATGAC, GTAGCCCAGGATGCCCTTTAGT.
RyR1: GAGTCTAAGCGCCAGTTCATCTTC, GCTCACGAACAGCTCCATCTTC.
RyR2: CAATAAAAGCGAGGATGGTGACA, CATACATGTGGAACATGTAACACGTT.
RyR3: CTTCTTGGTGGAACAGCCTTTG, GCCGCTTTCCGTTGCTTT.
IP3R1: GGCAACTGGAAGACCATAAAAGG, CCTGAAGGTCTGATGTTTCCATAGTAA.
IP3R3: GGCTCTGGCGTGGATCAG, CGATGCTCTCCTGGAAGATCT.
SERCA2: TGTATTCGACACGGAGCTGAAG, GCTGTTTTATGACCGAGTTGCA.
SERCA3: TGGCCACCAGGGACACA, GCCTGTCTCGTACTGTACAAACTGA.
Relative quantitation was performed using the comparative CT method (Livak and Schmittgen, 2001). For the initial screen (Figure 1C), triplicate PCR reactions were made from a single condition and the experiment was repeated three to seven times. For the rest of the experiments, each experimental condition was repeated at least in triplicate, and the experiments were repeated at least three times. The ΔCT values were then expressed relative to the first control sample (defined as 1), and the mean±s.e.m. calculated from the separate experiments.
Measurements of intracellular Ca2+ signals
Changes in [Ca2+]i were measured in individual cortical neurons by single cell fluorescent microscopy using the Ca2+ indicators Fura-2-AM (Molecular probes) or the lower affinity probe Fura-4f-AM (Molecular probes). Neuronal cultures grown on 28 mm glass coverslips for 10 days were loaded with 2 μM of the appropriate dye for 45 min at 37°C in artificial cerebral-spinal solution (CSS-5: 120 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 15 mM glucose and 25 mM HEPES, pH 7.4) supplemented with 10 μM glycine. Coverslips were then mounted on the stage of an inverted epifluorescence microscope (Zeiss Axiovert 200) and maintained at 37°C. Cells were sequentially excited at 340 and 380 nm, and emission fluorescence was collected via a 510-nm band pass filter using a cooled CCD camera (Hamamatsu). Data are presented as the 340/380 ratio of emissions, except for experiments examining the effect of caffeine where the 340/380 ratio was converted into approximate intracellular Ca2+ concentrations. For experiments measuring caffeine-induced activation of RyRs, fura-2 loaded cultures were perfused in 0 Ca2+ CSS-5 for 150 s, before the addition of caffeine (30 mM) in order to prevent any contribution from extracellular Ca2+. Measurements of spontaneous network Ca2+ transients were made by calculating the mean peak minus basal Ca2+ response and are expressed as 340/380 ratio units.
CREB decoy
The biotinylated oligodeoxynucleotide (ODN) CREB decoy was purchased from GeneDetect. The sequence of the decoy was: 5′-[Biotin]AGAGATTGCCTTGACGTCAGAGAGCTAG-3′. The mutant decoy sequence was: 5′-AGAGATTGCCTTGTGGTCAGAGAGCTAG-3′. RCNs grown in 12-well plates were transfected with Lipofectamine2000 (1 μl/well) plus decoy (0.1 μM) at day 7 in culture. After 4 h, an equal volume of fresh medium (plus cytosine arabinoside) was added. RCNs were then treated with 50 μM nicotine at 9 or 10 DiV as appropriate. Transfection efficiency was around 80% as defined by immunocytochemical analysis for biotin.
RyR2 downregulation using siRNA oligonucleotides
Pre-designed Accell siRNA pools were purchased from Dharmacon. These were designated Scr for scrambled non-targeting pool, and RyR2 for the pool targeted against rat RyR2. The RyR2 pool consisted of oligonucleotides targeted against four distinct sequences; CUUUAAUUGAUCAGUAUUU, UUAGUGAUUUCUAUUGGUA, GAAUUGAGGUGCAAGAUUU and GCUUCAUACACAUCGAUAU. The total concentration of Accell siRNA used was 1 μM, and efficiency of knockdown measured via real-time PCR after 48 h.
5′-RACE and sequencing of promoter region
5′-RACE was performed according to the manufacturer's instructions (Invitrogen). Primers used were:
GSP1: CCGTACTGACAGGGACTGCT. GSP2: TGGAAGTAGACTCCAAAAGCA. GSP3: CTGTTGCCAAATCCTTCTGC.
Bands obtained were cloned into a pCR2.1 vector (TA Cloning, Invitrogen) and sequenced. To obtain the sequence of the immediate 2 kb of the RyR2 promoter region genomic rat DNA (Qiagen) was subject to PCR amplification using a high fidelity Taq polymerase (Roche). Primers used were:
L1: AAATCCTGAAAGCCCTCTTCCAG. R1: TCCCTGGTCAATTCTGGCTC.
The band was then gel extracted (Qiagen) and sequenced. Additional primers spanning inside the fragment were used to overcame sequencing limits:
L2: GTGTACGAGGCTCTGCATGG. L3: GTAAGAACAGTGGGTGTTTT.
Electrophoretic mobility supershift assay
RCNs were harvested by scraping, washed in ice-cold phosphate-buffered saline, and then resuspended in hypotonic lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.51 mM DTT, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), pH 7.9). After 15 min incubation on ice, the cells were vortexed for 30 s. Nuclei were pelleted by centrifugation at 16 000 g for 15 s and then lysed in hypertonic lysis buffer (20 mM HEPES, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 10% glycerol, pH 7.9). Insoluble debris was removed by centrifugation at 16 000 g for 5 min. The supernatants containing nuclear proteins were collected and normalized for protein content by Bradford assay (Bio-Rad). To perform the electrophoretic mobility supershift assay, double-stranded oligonucleotides containing the CRE consensus sequences identified in the RyR2 gene were labelled with γ-[32P]ATP (Amersham) and polynucleotide kinase (NEB) and then purified using a Quick Spin column (Roche). Sequences used were:
CRE1: CCAGTTTGAATGATGTACCAGATGCC.
CRE2: GATTACTTATGATTACGTGGAGTGT.
CRE3: ACGAAATACCGTCAGGTACTGATGG.
CRE4: TGTTGGACTTGAGGTCAGGAAGGGC.
Nuclear extracts (20 μg protein/reaction) were incubated with 30 000 c.p.m. [32P]-labelled CRE probe for 20 min at room temperature in a 20-μl binding reaction (50 mM Tris–HCl, 2 mM EDTA, 100 mM KCl, 0.5 mM DTT, 2.5 μg/ml Salmon sperm, pH 7.5). For autocompetition assays, nuclear extracts were pre-incubated with a 100-fold molar excess of unlabelled CRE oligonucleotide for 10 min before addition of [32P]-labelled CRE oligonucleotide. For antibody supershift assays, nuclear extracts were pre-incubated with 1 μg CREB antibody (Cell Signaling) for 10 min before addition of [32P]-labelled CRE oligonucleotide. Binding reactions were loaded on a 5% non-denaturing polyacrylamide gel in 0.5% Tris-borate-EDTA buffer. After electrophoresis, dried gels were exposed to Kodak XAR film at −80°C with an intensifying screen.
Chromatin immunoprecipitation
The ChIP assay was performed using the EZ ChIP Chromatin Immunoprecipitation Kit essentially as described in the manufacturer's instructions (Upstate). PCR on the obtained genomic DNA was performed with the following primers:
RyR2 CRE1.1_for GGACAAAGATAGGAAAGGTGACA
RyR2 CRE1.1_rev CAAGGGCCCAACCTATACAT
RyR2 CRE2.1_for GTTCTTTCTTCCCGCCATGT
RyR2 CRE2.1_rev TCCACAAATGCCGTGTCTAA
RyR2 CRE3.1_for TGTGCATGTGCCTACATCAA
RyR2 CRE3.2_rev ACAGGGGTGGGAGATATGGT
TH CRE1.1_for GTGGTCCCGAGTTCTGTCTC
TH CRE1.1_rev AGGTGCCTGTGACAGTGGAT
Amplification conditions were as follows: (1) 95°C for 1 min; (2) 96°C for 20 s; (3) 61°C for 30 s and (4) 68°C for 1 min; repeat 2–4 for 45–53 cycles depending on primer efficiency. PCR reactions were visualized on a 2% agarose gel.
Immunocytochemistry
Immunocytochemistry was performed on RCNs grown on 16 mm coverslips coated with poly-L-lysine. Briefly, RCNs were washed with ice-cold PBS, fixed with 4% PFA for 10 min at room temperature, followed by 5 min with methanol at −20°C. Incubations with primary antibodies were performed in 5% goat serum, 0.3% TritonX and PBS. Anti-pCREB antibody (rabbit monoclonal, Cell Signaling) was used at 1:50 dilution; anti-MAP2 antibody (mouse monoclonal, AbCam) was used at 1:300. Streptavidin-Alexa 488 (Invitrogen) conjugated was used at 1:500.
Statistical analysis
Data were analysed using a two-way ANOVA (with pre-treatments and time as factors) followed by Bonferroni's post hoc, paired or unpaired Student's t-test (GraphPad Prism) as indicated.
Supplementary Material
Acknowledgments
We thank Maria Guerra-Martin for the preparation of cortical neurons, Drs Alessio Zanardi, Alice Fornari and Sabina D'Oro for help with in vivo experiments, Luca Crepaldi and Antonella Riccio for help with the ChIP, Rita Neumann and Sir Alec Jeffreys for help with PCR from genomic DNA. Brain tissues from rats with nicotine self-administration were a kind gift of Dr Christian Chiamulera (Dipartimento di Medicina e Sanità Pubblica, Università di Verona, Verona, Italy). EZ and GL designed and performed experimental work, data analysis and contributed to manuscript writing. DB designed and performed initial experiments and contributed intellectually to paper content. EM performed ChIP and EMSA experiments. MZ designed, performed and analysed in vivo experiments with SG and GL. KWY performed some of the Ca2+ imaging experiments. KWY and PN designed experiments and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Addy NA, Fornasiero EF, Stevens TR, Taylor JR, Picciotto MR (2007) Role of calcineurin in nicotine-mediated locomotor sensitization. J Neurosci 27: 8571–8580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX (2000) Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci 20: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ (1998) Neuronal calcium signalling. Neuron 21: 13–26 [DOI] [PubMed] [Google Scholar]
- Cavallaro S, Meiri N, Yi C-L, Musco S, Ma W, Goldberg J, Alkon DL (1997) Late memory-related genes in the hippocampus revealed by RNA fingerprinting. Proc Natl Acad Sci USA 94: 9669–9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knockout mice. J Neurosci 23: 7820–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KT, Berg DK (2001) Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron 32: 855–865 [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Tedesco V, Zangrandi L, Giuliano C, Fumagalli G (2010) Propranolol transiently inhibits reinstatement of nicotine-seeking behaviour in rats. J Psychopharmacol 24: 389–395 [DOI] [PubMed] [Google Scholar]
- Corrigall WA (1999) Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1: 11–20 [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25: 317–324 [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M (2001) Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav 70: 439–446 [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399: 66–70 [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novère N, Picciotto MR, Changeux JP, Zoli M (2001) Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci 15: 1810–1818 [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Collingridge GL (2002) Calcium stores and synaptic plasticity. Cell Calcium 32: 405–411 [DOI] [PubMed] [Google Scholar]
- Gamble KL, Allen GC, Zhou T, McMahon DG (2007) Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci 27: 12078–12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron TF, Mealing GAR, Paris J, Lou A, Edwards A, Hou ST, MacManus JP, Hakim AM, Morley P (2001) Attenuation of neurotoxicity in cortical cultures and hippocampal slices from E2F1 knockout mice. J Neurochem 78: 316–324 [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74: 363–396 [DOI] [PubMed] [Google Scholar]
- Gould TJ (2006) Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol 34: 93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME (2008) From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59: 846–860 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H (2001) Nuclear calcium signalling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci 4: 261–267 [DOI] [PubMed] [Google Scholar]
- Hu M, Liu Q-S, Chang KT, Berg DK (2002) Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci 21: 616–625 [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA (2001) Timing and location of nicotinic activity enhances or depresses hippocampal synaptic activity. Neuron 31: 131–141 [DOI] [PubMed] [Google Scholar]
- Kita T, Okamoto M, Nakashima T (1992) Nicotine-induced sensitization to ambulatory stimulant effect produced by daily administration into the ventral tegmental area and the nucleus accumbens in rats. Life Sci 50: 583–590 [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioural characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184: 523–539 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA trafficking and synaptic plasticity. Ann Rev Neurosci 25: 103–126 [DOI] [PubMed] [Google Scholar]
- Mann MJ, Dzau VJ (2000) Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest 106: 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436: 103–107 [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190: 269–319 [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ (2008) Neuroplasticity mediated by altered gene expression. Neuropsychopharm 33: 3–17 [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Gil Wier W, Baraban JM (1992) Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J Neurosci 12: 4834–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G, Nisell M, Nomikos GG, Chergui K, Svensson TH (1996) Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunoreactivity in the nucleus accumbens of the rat. Brain Res 730: 133–142 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M (2008) Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Frontiers Biosci 13: 492–504 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Pich EM, Brûlet P, Changeux JP (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in brain. Nature 374: 65–67 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391: 173–177 [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C (1997) Common neural substrates for the addictive properties of nicotine and cocaine. Science 275: 83–86 [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA (1998) Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci 18: 7075–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE (2007) Multiple brain pathways and receptors underlying tobacco addiction. Biochem Pharmacol 74: 1263–1270 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Broad LM (1998) Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci 19: 370–375 [DOI] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN (2007) Exposure to nicotine and sensitization of nicotine-induced behaviours. Prog Neuropsychopharmacol Biol Psychiatry 31: 1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM (2007) Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharm 32: 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gutala R, Hwang YY, Kim JM, Konu O, Ma JZ, Li MD (2008) Strain- and region-specific gene expression profiles in mouse brain in response to chronic nicotine treatment. Genes Brain Behav 7: 78–87 [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME (2002) Regulation of transcription factors by neuronal activity. Nat Rev Neurosci 3: 921–931 [DOI] [PubMed] [Google Scholar]
- Zhao W, Meiri N, Xu H, Cavallaro S, Quattrone A, Zhang L, Alkon DL (2000) Spatial learning induced changes in expression of the ryanodine type II receptor in rat hippocampus. FASEB J 14: 290–300 [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA (2001) Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci 4: 1124–1229 [DOI] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP (1999) Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J 18: 1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.