Smaug assembles an ATP-dependent stable complex repressing nanos mRNA translation at multiple levels
Translational control plays an important role during development of many organisms. Here, a derived cell-free system provides molecular insight into the role of the Smaug complex in translational repression of nanos mRNA and how this is relieved by Oskar at the posterior pole.
Keywords: masking, maternal RNA, Smaug response element, translational regulation
Abstract
The nanos (nos) mRNA encodes the posterior determinant of the Drosophila embryo. Translation of the RNA is repressed throughout most of the embryo by the protein Smaug binding to Smaug recognition elements (SREs) in the 3′ UTR. Translation is locally activated at the posterior pole by Oskar. This paper reports that the SREs govern the time- and ATP-dependent assembly of an exceedingly stable repressed ribonucleoprotein particle (RNP) in embryo extract. Repression can be virtually complete. Smaug and its co-repressor Cup as well as Trailer hitch and the DEAD box protein Me31B are part of the repressed RNP. The initiation factor eIF4G is specifically displaced, and 48S pre-initiation complex formation is inhibited. However, later steps in translation initiation are also sensitive to SRE-dependent inhibition. These data confirm several previously untested predictions of a current model for Cup-dependent repression but also suggest that the Cup model by itself is insufficient to explain translational repression of the nos RNA. In the embryo extract, recombinant Oskar relieves translational repression and deadenylation by preventing Smaug's binding to the SREs.
Introduction
Maturing oocytes, eggs, and early embryos are transcriptionally silent, and gene expression depends on mRNA provided maternally during oocyte development. Many maternal mRNAs are stored in a translationally inert form until they are activated at specific stages of development (Colegrove-Otero et al, 2005; Tadros and Lipshitz, 2005; Vardy and Orr-Waver, 2007). The term ‘masking' describes the translational silencing and storage of such RNAs in the form of ribonucleoprotein particles (RNPs) (Spirin, 1966). Masked mRNPs have been isolated from oocytes and eggs (Ilan and Ilan, 1978; Jenkins et al, 1978; Grainger and Winkler, 1987; Standart et al, 1990). Resistance of the repressed state to the time-consuming purification procedures and the dilutions involved implies a high stability of the masked RNP. This stability may reflect the need for very tight control of translation: As the developmental stages at which masking is required can last for very long periods of time, and masked mRNAs encode important regulators like cyclins or c-mos (Sheets et al, 1995; Gebauer and Richter, 1997; Groisman et al, 2002), even a low level of translation would probably be deleterious.
Translational regulation of maternal mRNAs can be coupled with their localization. The RNA is translationally repressed while it is transported to a particular localization, and then translation is activated locally (Johnstone and Lasko, 2001; Gavis et al, 2007). One RNA regulated in this manner is the nanos (nos) mRNA, which has a key function in the determination of the anteroposterior body axis of the Drosophila embryo. The nos mRNA is localized to the germ plasm at the posterior pole of the embryo and translated only at this position. A gradient of Nos protein developing by locally restricted translation and diffusion is essential for the formation of the anteroposterior body axis (Wang and Lehmann, 1991; Gavis and Lehmann, 1992, 1994). However, ∼95% of the nos RNA remains distributed throughout the embryo (Bergsten and Gavis, 1999). This RNA is translationally silenced and decays over the first 2½ h of development (Gavis and Lehmann, 1994; Dahanukar and Wharton, 1996; Bashirullah et al, 1999). Whereas repression of non-localized nos mRNA in the oocyte depends on Glorund (Kalifa et al, 2006), the protein Smaug is responsible for repression of non-localized nos mRNA during embryonic development (Smibert et al, 1996, 1999; Dahanukar et al, 1999). Two Smaug recognition elements (SREs) in the nos 3′ UTR are necessary and sufficient for repression (Dahanukar and Wharton, 1996; Gavis et al, 1996; Smibert et al, 1996). The SREs also direct the degradation of the nos RNA (Dahanukar and Wharton, 1996; Smibert et al, 1996; Bashirullah et al, 1999), and Smaug is known to recruit the CCR4–NOT complex, which catalyses deadenylation of nos and other mRNAs (Aviv et al, 2003; Temme et al, 2004; Morris et al, 2005; Semotok et al, 2005; Zaessinger et al, 2006; Kadyrova et al, 2007; Tadros et al, 2007). At the posterior pole, localized Oskar protein mediates the local accumulation of nos RNA and prevents both its deadenylation and translational repression (Ephrussi et al, 1991; Ephrussi and Lehmann, 1992; Smith et al, 1992; Dahanukar and Wharton, 1996; Zaessinger et al, 2006), presumably via a direct interaction with Smaug (Dahanukar et al, 1999).
Deadenylation is not only the first step in mRNA degradation but also strongly reduces the translational efficiency of nos mRNA. However, additional mechanisms of repression must exist that operate independently of deadenylation (Jeske et al, 2006). Two such mechanisms have been proposed. First, the protein Cup associates both with Smaug and the translation initiation factor eIF4E; by displacing eIF4G from 4E, Cup may prevent the initiation of nos translation (Nelson et al, 2004). While defects in nos regulation in cup mutant embryos support a role of Cup, key predictions of the model, like an inhibition of 48S pre-initiation complex formation, have not been tested. Second, sucrose gradient centrifugation showed a large fraction of nos mRNA to be associated with rapidly sedimenting particles identified as polysomes by a puromycin run-off experiment. Thus, translational repression was suggested to act during elongation (Clark et al, 2000). This agrees with the observation that the bicaudal mutation, which affects a subunit of the complex interacting with nascent polypeptides at the exit tunnel of the ribosome, allows ectopic translation of nos mRNA (Markesich et al, 2000). However, arguing against repression during elongation, a different study found only ∼5% of nos RNA associated with polysomes, in agreement with the localized fraction (Qin et al, 2007).
We have used extracts prepared from Drosophila embryos to examine the mechanism of translational repression of nos mRNA. Repression is based on the slow and ATP-dependent formation of a stable repressor complex that can shut off translation almost completely. Displacement of eIF4G from the repressed RNP and inhibition of 48S pre-initiation complex formation support the proposed mechanism of Cup action. However, other features of SRE-dependent translation inhibition are not explained by this model. Oskar protein prevents both deadenylation and translation inhibition of nos mRNA in extracts by preventing the association with the SREs.
Results
An SRE-dependent repressor complex assembles slowly
An in vitro system derived from early Drosophila embryos reproduces two key aspects of the regulation of nos mRNA, rapid deadenylation and translational repression. Both reactions depend on SREs in the 3′ UTR (Jeske et al, 2006). For the following studies, firefly luciferase mRNAs were used that contained an ‘internal' poly(A) tail followed by 40 additional nucleotides of mixed sequence. Such an internal tail stimulates translation as efficiently as a ‘terminal' poly(A) tail, but cannot be removed by SRE-dependent deadenylation (Jeske et al, 2006) (Supplementary Table I). Translation of the luciferase RNA fused to the SRE-containing segment of the nos 3′ UTR was compared with the translation of a similar RNA carrying an inactivating point mutation (Aviv et al, 2003, 2006; Johnson and Donaldson, 2006; Oberstrass et al, 2006) in each of the two SRE loop regions (luciferase nos versus luciferase nos SRE− RNA). Translation of the same set of RNAs in rabbit reticulocyte lysate, which does not respond to SREs (Smibert et al, 1999; Clark et al, 2000; Jeske et al, 2006), was routinely used as a control.
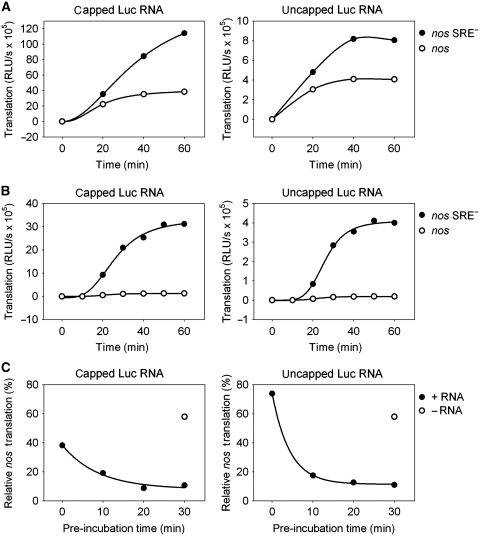
With most batches of extract, SRE-dependent repression after a 1-h translation reaction was approximately three-fold (Table I). However, in time course experiments, translation of the control RNA proceeded linearly, whereas translation of the SRE-containing RNA decreased with time and came almost to a halt after 40 min (Figure 1A). This suggested a slow step in the repression mechanism. A simple two-step incubation procedure confirmed this and improved SRE-dependent repression. The reporter RNAs were pre-incubated for 30 min together with extract and buffer lacking an ATP-regenerating system. Omission of the ATP-regenerating system was sufficient to prevent translation. Then translation was started by the addition of ATP, creatine phosphate, and creatine kinase. With the pre-incubation, translation of nos mRNA was repressed from the very beginning of the translation reaction, and repression factors of 10-fold and higher were routinely obtained (Figure 1B; Table I). With a slight change in buffer conditions, almost 50-fold repression was achieved; in other words, nos RNA was essentially not translated at all (Table I). Thus, the translation repression observed in vitro was at least as efficient as that obtained when luciferase reporter RNAs were injected into Drosophila embryos (∼13-fold repression (Nelson et al, 2004)).
Table 1. Impact of 5′ and 3′ end modifications on SRE-dependent repressiona.
| Structure of reporter RNA | − Pre-incubation | +Pre-incubation |
|---|---|---|
| Capped, polyadenylated | 2.8±0.4 (n=4) | 16.0±6.8 (n=10)b |
| Uncapped, polyadenylated | 1.7±0.3 (n=4) | 15.0±6.8 (n=5) |
| Capped, no poly(A) tail | n.d.c | 5.4±0.6 (n=2) |
| Uncapped, no poly(A) tail | n.d. | 4.2±2.2 (n=2) |
| aRepression factors (nos SRE−/nos) based on translation for 1 h in embryo extract. ‘n' indicates the number of batches of extract tested. | ||
| bTranslation in the presence of 27% protein buffer instead of potassium acetate (see Materials and methods) resulted in a repression factor of 44.5±16.5 (n=4). | ||
| cNot done here. In a previous paper, the number was ∼2 (Jeske et al, 2006). | ||
Figure 1.
Efficient repression depends on the slow assembly of a repressor complex. Capped (left panels) or uncapped (right panels) luciferase RNAs containing an internal poly(A) tract were incubated in embryo extract under translation conditions. At the time points indicated, the translation yield was determined by a luciferase assay. (A) Luciferase RNAs containing the nos 3′ UTR fragment (circles) or the fragment with point mutations in the SRE sequences (nos SRE−, filled circles) were mixed with extract, and translation was started immediately. (B) The same experiment as in (A) was carried out, but luciferase RNAs were pre-incubated with extract for 30 min before translation was started by the addition of the ATP-regenerating system (see Materials and methods) at t=0. (C) Luciferase RNAs containing the nos or the nos SRE− 3′ UTR fragment were pre-incubated with extract for the times indicated before translation was started. Luciferase activity obtained from the SRE− RNA after 1 h of translation was set to 100%. The circle indicates the relative translation level obtained from a reaction where luciferase RNAs were added after the pre-incubation step.
The reporter RNA had to be present during the pre-incubation; pre-incubation of the extract in the absence of the mRNA did not improve the efficiency of repression. Repression also increased with the time of pre-incubation (Figure 1C). These observations suggest that a repressive protein complex assembles on the nos RNA during the pre-incubation. Alternative explanations, such as selective degradation, deadenylation or decapping of the nos RNA during pre-incubation, were excluded (Supplementary data; Supplementary Figure S1; see also below).
The nos RNA 3′ UTR segment used here consists of 155 nucleotides and contains two SREs plus a binding site for the repressor Glorund, which controls nos translation in oocytes (Kalifa et al, 2006). When this RNA segment was replaced with three SRE motifs, translation of the reporter RNA was repressed as efficiently (∼15-fold) as the one containing the larger nos segment (data not shown). Thus, sequences outside the SREs are not essential for repression.
Assembly of the repressor complex is ATP-dependent
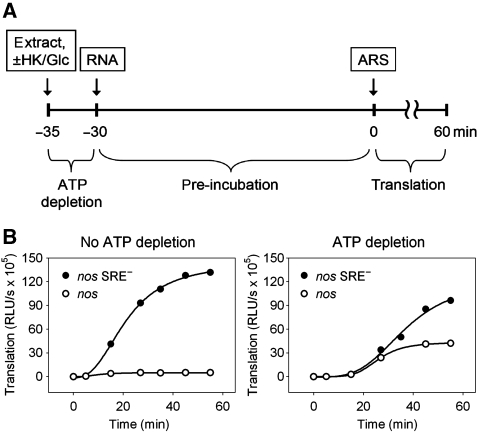
SRE-mediated deadenylation depends on an ATP-regenerating system (Jeske et al, 2006). Therefore, ATP dependence of SRE-mediated translation repression was tested. The average ATP concentration in five batches of embryo extract, measured with a luminescence assay, was 680 μM and ATP did not drop below 200 μM under pre-incubation conditions (data not shown). To investigate a possible role for the remaining ATP in repressor complex formation, ATP was depleted from the pre-incubation mix by incubation with hexokinase and glucose for 5 min before reporter mRNA was added and pre-incubation was carried out. A control reaction received no hexokinase and glucose. At the end of the pre-incubation period, ATP was restored by the addition of ATP, creatine kinase, and creatine phosphate in excess over glucose. In the control reaction without ATP depletion, the anticipated degree of translation repression was obtained. With the ATP depletion/restoration procedure, translation of the control RNA proceeded at a similar rate as without ATP depletion, albeit after a longer lag phase. In contrast, translation of the nos RNA was strongly increased by ATP depletion, and SRE-dependent repression was as low as was typically observed in reactions without any pre-incubation (Figure 2). We conclude that the relatively low level of ATP present in the extract is essential for the formation of the repressor complex.
Figure 2.
Translation repression is ATP dependent. (A) Scheme of the assay: embryo extract was incubated with hexokinase and glucose for 5 min for ATP depletion. Capped luciferase RNAs containing an internal poly(A) tract were then added and pre-incubated for 30 min. Subsequently, translation was started by addition of the ATP-regenerating system (ARS). The control reaction was performed similarly, but the extract was initially incubated for 5 min without addition of hexokinase and glucose. (B) Luciferase RNAs containing the nos (circles) or the nos SRE− (filled circles) 3′ UTR fragment were incubated as described in (A). For a better comparison, the ordinates of the two graphs are drawn in the same scale. The time scale refers to the start of translation.
Pre-incubation does not accelerate SRE-dependent deadenylation
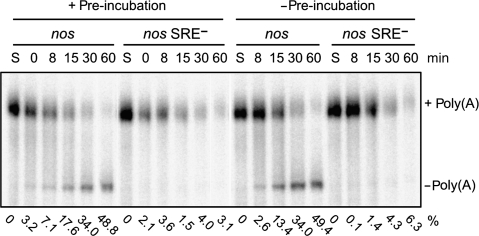
In contrast to the slow assembly of the complex repressing translation, SRE-dependent deadenylation in Drosophila embryo extract proceeds without a pronounced lag phase. However, deadenylation is also ATP dependent: It takes place in the presence of an ATP-regenerating system and is prevented by ATP depletion (Jeske et al, 2006). Deadenylation substrates were subjected to the same pre-incubation protocol as used for the translation experiments. Under these conditions of low ATP concentration, there was almost no deadenylated product at the end of the pre-incubation (Figure 3); thus, the low ATP concentration was insufficient to drive deadenylation. When deadenylation was then started by the addition of the ATP-regenerating system, it proceeded at the same rate as without any pre-incubation. Thus, the slow step in the formation of the translation repression complex is irrelevant for SRE-dependent deadenylation, suggesting that the protein complexes responsible for translation repression and deadenylation are not identical.
Figure 3.
SRE-dependent deadenylation is not accelerated by a pre-incubation step. Polyadenylated, uncapped, and 32P-labelled RNA substrates containing the nos or the nos SRE− 3′ UTR fragment were subjected to a 25-min pre-incubation with Drosophila embryo extract, then deadenylation was started by addition of the ATP-regenerating system (0 min; left half of the panel). After the time points indicated, RNA was isolated and separated on a denaturing polyacrylamide gel. The control reaction (right half of the panel) was performed in the same way but the pre-incubation step was omitted. S, unreacted substrate RNA. The percentage of fully deadenylated product (Jeske et al, 2006) is shown below each lane.
SRE-dependent repression of translation is independent of the cap but partially dependent on the poly(A) tail
When uncapped RNAs were used for the translation assays, luciferase yield dropped by a factor of about 10 (Figure 1; Supplementary Table I), as anticipated from a cap-dependent initiation mechanism. However, SRE-dependent repression was functional with uncapped RNA (Figure 1; Table I). As reported before, translation of RNA lacking a poly(A) tail was 5- to 10-fold less efficient in the embryo lysate (Jeske et al, 2006) (Supplementary Table I). Translation of such RNAs was also repressed by the SREs, but repression was less efficient (Jeske et al, 2006). Even with a pre-incubation step, repression was approximately five-fold less efficient as compared with RNAs with the ‘internal' poly(A) tail (Table I). Thus, while the repression mechanism is fundamentally poly(A) independent, the poly(A) tail appears to assist the formation of the repressor complex; the 5′ cap is dispensable.
The repressor complex is stable and cap-independent
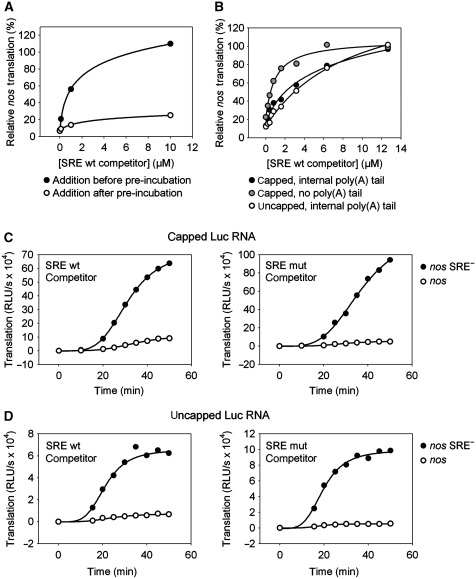
A short SRE-containing RNA mixed with the luciferase reporter was able to block repression of translation: 1 μM competitor RNA was required to reduce repression to two-fold, and ∼10 μM completely eliminated repression. In contrast, addition of excess competitor after pre-incubation had only a very weak effect on SRE-mediated repression (Figure 4A), indicating that the repressor complex formed during pre-incubation is stable. Under these conditions, a slow increase in the rate of luciferase production from the nos RNA was detected (Figure 4C). Assuming equal stability of nos and mutant RNA during incubation, the data could be fitted to a first-order decay of the repressor complex with half-lives of ∼200 and ∼230 min in two independent experiments (data not shown). That the repression is reversible, albeit slowly, provides additional evidence that the mechanism is not based on an irreversible SRE-dependent modification of the mRNA (e.g. degradation).
Figure 4.
The assembled repressor complex is stable. (A) Capped luciferase RNAs containing an internal poly(A) tract and the nos or the nos SRE− 3′ UTR fragment were pre-incubated for 25 min and subsequently translated for 60 min. Increasing amounts of a short-competitor SRE RNA were added to the reaction either before (filled circles) or after (circles) the pre-incubation step. The percentage of translation of the SRE-containing RNA relative to the mutant RNA is plotted against the competitor concentration. (B) Luciferase RNAs containing an internal poly(A) tract with the indicated 5′ and 3′ end modifications were mixed with increasing amounts of the SRE competitor. Subsequently, the RNA mixture was pre-incubated for 25 min with embryo extract followed by a 60-min translation reaction. The percentage of translation of the SRE-containing RNA relative to the mutant RNA is plotted against the competitor concentration. (C) Capped luciferase RNAs containing an internal poly(A) tract and the nos (circles) or the nos SRE− (filled circles) 3′ UTR fragment were pre-incubated for 25 min with embryo extract to allow repressor complex formation on nos RNA. After pre-incubation, 12 μM of wild-type (left) or mutant (right) SRE competitor was added to the mixture together with the ATP-regenerating system (t=0 min), and a time course of translation was measured. (D) The same experiment as described in (C) was performed using uncapped luciferase RNAs.
The chain of interactions postulated by the Cup model, SRE–Smaug–Cup–eIF4E–cap (Wilhelm and Smibert, 2005; Vardy and Orr-Waver, 2007) predicts that the interaction between the cap and eIF4E should contribute to the formation of the repressed RNP. However, the complex was kinetically stable even when it was formed on an RNA lacking a cap (Figure 4D). Irrelevance of the cap for formation of the repressor complex was confirmed by addition of increasing amounts of competitor RNA at the beginning of the pre-incubation. Very similar amounts were required to compete with the repression of capped and uncapped RNA (Figure 4B). In contrast, reporter RNA lacking a poly(A) tail was approximately five-fold more sensitive to the competitor, in agreement with less efficient repression of poly(A)− RNAs (Table I). We conclude that the 5′ cap does not have an important function in the formation of a stable repressed RNP, but the poly(A) tail contributes.
The repressor complex blocks formation of the 48S pre-initiation complex
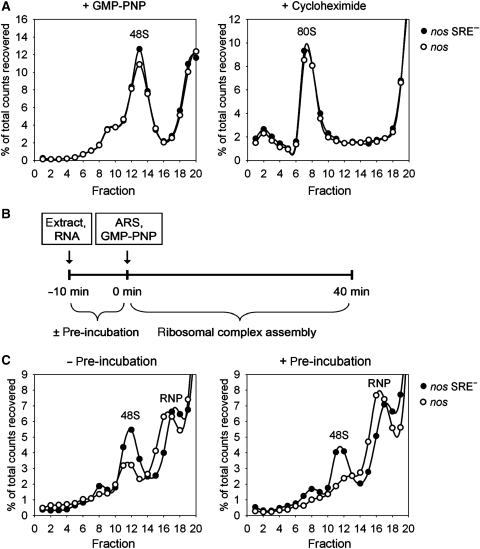
The step at which translation of the nos RNA was blocked was investigated by sucrose gradient centrifugation of initiation complexes. RNAs with a short open reading frame of 27 amino acids and an ‘internal' poly(A) tail were used that were otherwise similar to the luciferase reporter RNAs. In rabbit reticulocyte lysate, both nos RNA and nos SRE− RNA formed 80S complexes in the presence of cycloheximide and 48S pre-initiation complexes in the presence of the non-hydrolyzable GTP analog GMPPNP with indistinguishable efficiencies (Figure 5A). When the same RNAs were incubated for 40 min in Drosophila embryo extract in the presence of GMPPNP and an ATP-regenerating system, 48S complexes were obtained, but their formation was less efficient with the nos RNA (Figure 5B). When the RNAs were pre-incubated for 10 min before GMPPNP and the ATP-regenerating system were added, almost no 48S complexes were formed on the nos RNA, whereas only a slight reduction was visible with the control RNA (Figure 5C). It is important to note that, on the control RNA, stable 48S complexes were formed only during the second phase of the incubation, that is upon the addition of GMPPNP and an ATP-regenerating system; no stable complexes were detectable by gradient centrifugation at the end of the pre-incubation phase (data not shown). Thus, the SRE-dependent repressor complex assembled during the pre-incubation prevented pre-initiation complex formation during the second incubation.
Figure 5.
Repressed nos RNA does not form 48S complexes. (A) 32P-cap-labelled RNAs containing an internal poly(A) tract and the indicated 3′ UTR were incubated with rabbit reticulocyte lysate in the presence of cycloheximide (left panel) or GMP-PNP (right panel) under translation conditions. Resulting ribosomal complexes were resolved by centrifugation in 5–25% linear sucrose gradients (25% sucrose on left). After fractionation from the bottom to the top of the gradient, the radioactivity was monitored, expressed as percentage of total counts recovered and plotted against the fraction number. (B) Scheme of ribosomal complex assembly reaction using Drosophila embryo extract. 32P-cap-labelled RNAs containing an internal poly(A) tract were incubated under translation conditions in the presence of GMP-PNP either with or without a preceding pre-incubation step for 10 min. ARS, ATP-regenerating system. (C) Substrate RNAs with the indicated 3′ UTR were allowed to assemble ribosomal complexes as described in (B) and analysed as described in (A).
Reduction of the 48S peak was accompanied by an increase of radioactivity in the RNP fractions of the gradient, possibly reflecting the formation of the repressed nos RNP. This is supported by the observation that the nos RNP sedimented slightly faster than the RNP formed on the control RNA (Figure 5B and C). In contrast, the slight reduction of the 48S peak formed with the control RNA was accompanied by increased radioactivity in the very top fractions of the gradient presumably containing degraded material.
The repressor complex contains Smaug, Cup, and other repressor proteins and excludes eIF4G
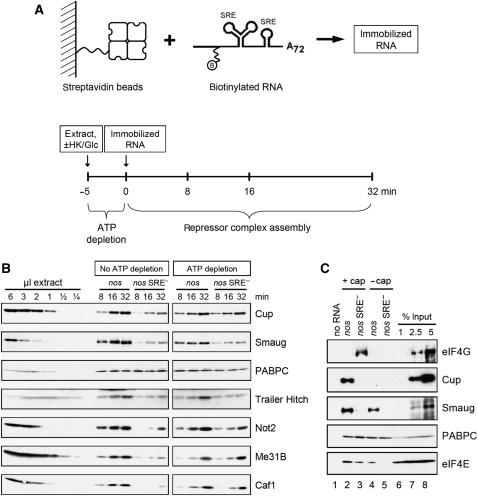
For an analysis of proteins participating in repressor complex formation, short biotinylated RNAs similar to those used for sucrose gradient analysis were immobilized on streptavidin beads and incubated in extract under pre-incubation conditions either with or without ATP depletion (Figure 6A). After pull down and washing, the protein complexes formed on the RNA were analysed by western blotting. As expected, Smaug was enriched on the nos RNA as compared with the mutant control. More Smaug was recovered in the presence of ATP, and recovery also improved with the time of incubation. This indicates an ATP- and time-dependent increase either in the amount of Smaug–RNA complex or in complex stability (Figure 6B). As a control, the cytoplasmic poly(A)-binding protein (PABPC) bound equally well to both RNAs in a time- and ATP-independent manner. Other proteins accumulated on the nos RNA in a similar manner as Smaug: recovery improved with the time of incubation, and the presence of ATP either increased the yield or improved the specificity for the nos RNA. These proteins included Not2 and Caf1, two subunits of the CCR4–NOT complex responsible for nos deadenylation; the DEAD box protein Me31B, a known translational repressor (see Discussion); Trailer Hitch (Tral), one of several proteins associating with Me31B; and Cup, which interacts not only with Smaug (Nelson et al, 2004), but also with Me31B or the Tral–Me31B complex (Nakamura et al, 2004; Wilhelm et al, 2005; Barbee et al, 2006; Tritschler et al, 2008). We conclude that Tral, Me31B, and the CCR4–NOT complex are components, together with Smaug and Cup, of the SRE-dependent repressor complex.
Figure 6.
RNA pull-down assays reveal components of the repressed RNP: exclusion of eIF4G. (A) Scheme of the RNA pull-down assay. Biotinylated and 32P-labelled RNA substrates containing an external poly(A) tail were immobilized on streptavidin beads (upper panel). Embryo extract was incubated for 5 min in the presence of hexokinase and glucose for ATP depletion or without these reagents as a control. Immobilized RNA was added and incubated with the extract for the times indicated (lower panel). The beads were washed and bound proteins eluted by boiling in SDS sample buffer. Proteins were separated on an SDS–polyacrylamide gel and analysed by western blotting. (B) Proteins were detected in an RNA pull-down assay as described in (A) using capped nos and nos SRE− RNA substrates. In all, 36 μl of embryo extract were used for each time point of the pull down. For comparison, various amounts of extract were analysed in the same western blot (shown in the first six lanes). (C) eIF4G is excluded from the repressed RNP. An RNA pull-down assay was carried out as described in (A) except that no ATP depletion was carried out. Capped and uncapped substrate RNAs carrying the nos or the nos SRE− sequences were compared as indicated. Immobilized RNAs were incubated with embryo extract for 30 min. Beads without immobilized RNA were used as a background control.
The postulated chain of interactions, cap–eIF4E–Cup–Smaug–SRE, predicts that binding of all three proteins should be affected by both the cap and the SRE, and that eIF4G should be competed off eIF4E by Cup. The predictions were confirmed in a pull-down experiment (Figure 6C). Whereas the control protein PABPC bound equally well to all RNAs, Smaug was strongly enriched on the nos RNAs as compared with the SRE mutant RNAs (compare lanes 2 and 4 with 3 and 5), and also bound slightly better to the capped as compared with the uncapped RNA (lanes 2 and 4). Cup was enriched on the capped nos RNA (lane 2). Binding to the uncapped nos RNA was below the detection limit in this experiment (lane 4), but a low level of binding was visible in others. No binding was visible with the SRE mutant RNAs (lanes 3 and 5). eIF4E was not detectable on the RNA lacking both a cap and an SRE (lane 5), the levels were intermediate on RNAs containing either a cap or an SRE (lanes 3 and 4), and highest on the RNA containing both (lane 2). In agreement with the model, this suggests that eIF4E can associate with the RNA either via the cap or via Smaug and Cup. Most importantly, eIF4G binding was seen only on the RNA that contained a cap but lacked an SRE and, thus, did not bind Smaug and Cup (lane 3). This strongly supports displacement of eIF4G by Smaug.
SRE-dependent repression also affects steps subsequent to eIF4G binding and 48S complex formation
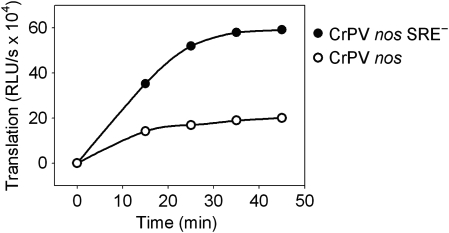
The results obtained so far confirm the inhibition of 48S pre-initiation complex formation predicted by the Cup model of SRE-dependent repression. In order to test whether subsequent steps are also inhibited in an SRE-dependent manner, the internal ribosome entry site from the cricket paralysis virus intergenic region (CrPV IRES) was fused to the reporter RNAs. CrPV IRES-dependent initiation operates by 80S ribosome formation directly on the IRES, independently of initiation factors (Wilson et al, 2000; Pestova and Hellen, 2003); eIF4G and its interaction with eIF4E are not required for CrPV IRES-dependent translation (Deniz et al, 2009; Garrey et al, 2010). Thus, if a displacement of eIF4G were the only mechanism of SRE-dependent repression, CrPV IRES-dependent translation should be resistant. At elevated salt concentrations, CrPV IRES-dependent translation in embryo extract was 180-fold more efficient than cap-dependent translation and insensitive to cap competition, as expected (Supplementary Figure S2). CrPV IRES-dependent translation was repressed approximately three-fold by the SREs (Figure 7). At the salt concentrations used, cap-dependent translation was inhibited to the same low extent. Repression was only observed with a pre-incubation (data not shown). Thus, the SRE-dependent repressor complex inhibits not only eIF4G binding and 48S complex formation but also at least one later step in translation.
Figure 7.
CrPV IRES-dependent translation is repressed in an SRE-dependent manner. Uncapped luciferase reporter RNAs containing the CrPV-IRES, the indicated 3′ UTR and internal poly(A) tails were pre-incubated for 30 min with embryo extract under the conditions described in Materials and methods. Subsequently, translation was started by the addition of an ATP-regenerating system, and luciferase activity was measured at the time points indicated.
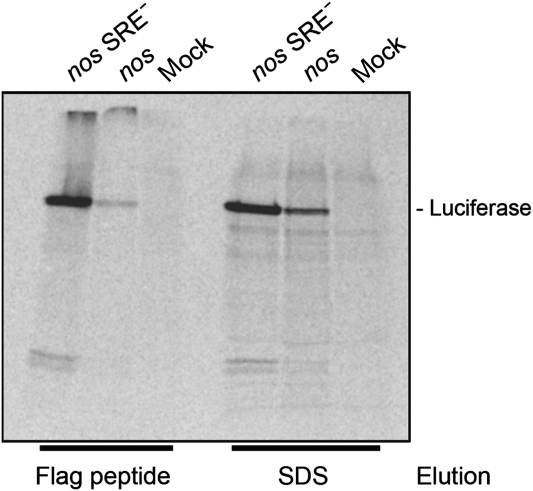
One possibility to explain the inhibition of CrPV IRES-dependent initiation would be that the SREs affect nascent peptide chains (see Introduction). To test this hypothesis, capped mRNAs encoding flag-tagged luciferase were translated in embryo extract in the presence of 35S-methionine. In addition to the serine protease inhibitor used routinely during extract preparation, the proteasome inhibitor MG132 was included. Products derived from the reporter RNAs were separated from the background of endogenous translation products by selection on anti-flag beads and then analysed by gel electrophoresis. Both flag-luciferase nos and flag-luciferase nos SRE− RNAs yielded mostly full-length products; no significant increase in partial translation products was detectable with the repressed RNA (Figure 8). Quantitative assays showed that the incorporation of 35S was inhibited to the same extent (7.1±0.7-fold) as luciferase activity (6.8±0.3-fold). This confirms that the proportion of partial translation products, which contribute to 35S incorporation but not to luciferase activity, was not different between nos and nos SRE− RNAs (data not shown). Thus, an effect of the SREs on nascent peptide chains was not detectable, in agreement with earlier data (Clark et al, 2000).
Figure 8.
SRE-dependent repression has no effect on nascent peptide chains. Capped luciferase reporter RNAs with an N-terminal flag tag and an internal poly(A) tract were translated as in Figure 1B. 35S-Methionine was added at 2 μCi/reaction. Translation reactions were stopped with 5 μl 50 mM EDTA and 1 mg/ml RNase A, incubated for 20 min at 30°C and mixed with 85 μl IP buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.0, 0.5% Triton X-100, 1 mg/ml yeast total RNA). The mixture was transferred to 20 μl anti-FLAG agarose (Sigma) and incubated for 40 min at 8°C with shaking. The agarose was washed five times with 200 μl IP buffer. Proteins were sequentially eluted with FLAG peptide (1 mg/ml in IP buffer) and SDS-loading buffer and separated on a 10% SDS–PAGE. The picture shows the autoradiogram of the gel. ‘Mock' is a translation reaction without added reporter RNA.
Oskar overcomes deadenylation and translation repression
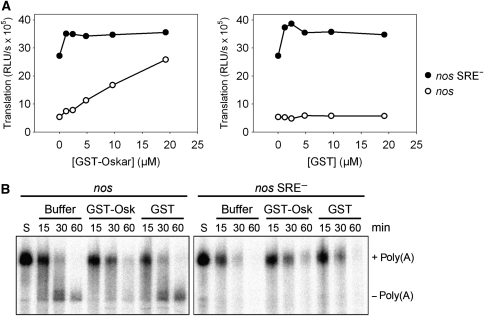
In the embryo, translational repression of the nos RNA is overcome at the posterior pole by a mechanism involving Oskar. In the embryo extract, the addition of Short Oskar (Markussen et al, 1995) fused to GST (Supplementary Figure S3) was sufficient to prevent SRE-dependent translation inhibition (Figure 9A). Translation of the control RNA was not affected, and the addition of GST had no effect on either RNA. GST-Oskar was unable to relieve repression when it was added after the pre-incubation during which the repressor complex was formed (data not shown). In agreement with in vivo observations (Zaessinger et al, 2006), GST-Oskar also prevented SRE-dependent deadenylation (Figure 9B). Higher Oskar concentrations were necessary for the inhibition of deadenylation compared with the relief of translation repression. Note that the luciferase reporter RNAs used for the translation assays carried an internal poly(A) tail resistant to deadenylation; thus, Oskar affects translation independently of the inhibition of deadenylation.
Figure 9.
Recombinant Oskar prevents both SRE-dependent translation repression and deadenylation. (A) Translation reaction mixtures lacking RNA and the ATP-regenerating system were incubated with increasing amounts of recombinant GST-Oskar (left panel) or GST (right panel) for 15 min. Capped luciferase RNAs containing an internal poly(A) tract and with the indicated 3′ UTR fragments were then added, and the incubation continued for another 25 min before translation was started by the addition of the ATP-regenerating system and allowed to proceed for 60 min. Luciferase activity is plotted against concentration of the protein indicated. When no protein was added (0 μM), protein buffer was used instead. (B) Deadenylation reaction mixtures were incubated for 15 min with GST-Oskar, GST, or protein buffer in the absence of RNA and an ATP-regenerating system. Deadenylation was started by addition of polyadenylated, 32P-labelled substrate RNAs with the 3′ UTR fragments indicated and buffer containing the ATP-regenerating system. At the time points indicated, RNA was isolated and separated on a denaturing polyacrylamide gel. S, unreacted substrate RNA.
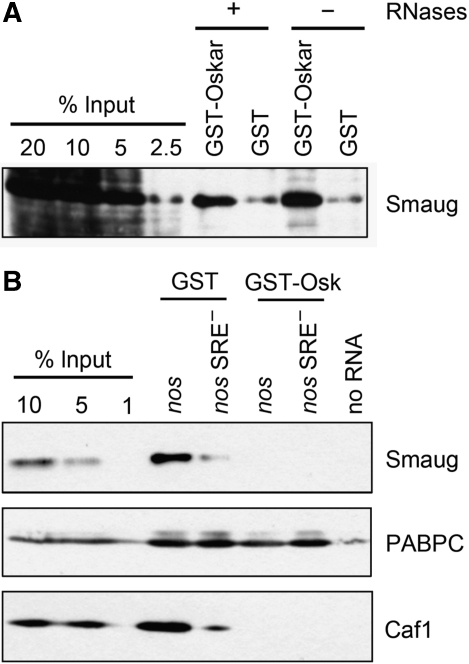
In a GST pull-down assay, GST-Oskar added to embryo extract bound endogenous Smaug, in agreement with a previously reported interaction of an Oskar fragment with Smaug prepared by in vitro translation (Dahanukar et al, 1999). The Oskar–Smaug association was resistant to RNase (Figure 10A). Pull down of the SRE-containing RNA showed that GST-Oskar inhibited the association of Smaug with the RNA, confirming a previous observation (Zaessinger et al, 2006) (Figure 10B). CAF1 was also not associated with the immobilized RNA when Smaug binding was prevented by GST-Oskar (Figure 10B), in agreement with the inhibition of deadenylation by GST-Oskar and with a recruitment of the CCR4–NOT complex by Smaug. As a control, binding of PABPC was not affected. Whether the effect of Oskar on deadenylation and translational repression reflects a simple interaction with Smaug or depends on additional proteins remains to be seen.
Figure 10.
Recombinant Oskar associates with Smaug and prevents its binding to the SRE. (A) Oskar binds to Smaug independently of RNA. Embryo extract was incubated with GST-Oskar or GST in the presence or absence of RNases (see Materials and methods). After capture of the proteins on glutathione beads and washing, bound proteins were eluted by boiling in SDS sample buffer. Samples were separated on an SDS–polyacrylamide gel and analysed by western blotting. Different amounts of the input extract were loaded for comparison. (B) Oskar prevents binding of Smaug to SREs. An RNA pull-down assay was carried out as described in Figure 6 (A) using capped RNAs with the 3′ UTR fragments indicated. Embryo extract was incubated with GST-Oskar or GST for 15 min, immobilized RNAs were added and incubated with the mixture for another 30 min. Beads without RNA were used as background control. Bound proteins were analysed by western blotting with antibodies against Smaug, CAF1, and PABPC as indicated. Different amounts of the input extract were loaded for comparison.
Discussion
We have examined the mechanism of SRE-dependent translational repression of the nos RNA. Three results are unusual: Formation of the repressor complex is slow and ATP dependent; once formed, the repressor complex is very stable; and repression is almost complete, ∼50-fold under favourable conditions. In agreement with a previously proposed model (Nelson et al, 2004), the protein Cup is incorporated into the repressor complex, and eIF4G is displaced. Consequently, translation is inhibited at the step of 48S pre-initiation complex formation. However, a late step in translation initiation is also inhibited. This, together with the ATP dependence of repressor complex formation and the presence of Me31B and Tral in the repressor complex indicates that the Cup model is not sufficient to explain SRE-dependent translation repression.
The unusual stability of the repressor complex and its potency in translation repression match the properties expected for a ‘masked' RNP tightly repressing the synthesis of important regulatory proteins over extended periods of time. One feature linking the SRE-dependent repressor complex and previous analyses of masked RNPs is the presence of Me31B, a conserved translational repressor. Its Xenopus orthologue, Xp54, is a component of masked RNPs and is able to inhibit translation (Ladomery et al, 1997; Minshall et al, 2001, 2009). In flies, Me31B has a function in the translational repression of oskar and bicaudal D (Nakamura et al, 2001) and of mRNAs in neuronal cells (Barbee et al, 2006). Me31B and mammalian p54/RCK/DDX6 are involved in miRNA-dependent repression (Chu and Rana, 2006; Eulalio et al, 2007b). In yeast, dhh1Δ/pat1Δ double mutants have a general defect in translational repression, and Dhh1p (the yeast orthologue of Me31B) prevented 48S pre-initiation complex formation in vitro, independently of specific RNA sequences. CrPV IRES-driven translation was also inhibited (Coller and Parker, 2005). Interestingly, the time- and ATP-dependent formation of a repressed mRNP with a protein fraction from Xenopus oocytes has been described (Yurkova and Murray, 1997). The protein fraction was later reported to contain Xp54 (personal communication cited in Minshall et al (2001)). While this reaction was independent of specific RNA sequences, the phenomenon is strongly reminiscent of our observations and suggests that we are dealing with a conserved mechanism. The association of Me31B with Tral and Cup is also conserved. For example, RAP55 and eIF4E-T, the Xenopus orthologues of Tral and Cup, are found, together with Xp54, in protein complexes and mRNPs in oocytes, and both RAP55 and eIF4E-T can repress translation in tethering assays or upon overproduction (Ferraiuolo et al, 2005; Tanaka et al, 2006; Minshall et al, 2007). The function of Me31B, Tral, and Cup is reflected in their localization in P bodies (Parker and Sheth, 2007; Eulalio et al, 2007a) and other RNP granules (Standart and Minshall, 2008). A role for Me31B and Tral in SRE-dependent repression is likely but remains to be proven.
While the kinetic stability of the SRE–Smaug complex has not yet been examined, the long time required for the formation of the repressor complex, its ATP dependence and its kinetic stability suggest the possibility that the repressor complex may be formed by an energy-consuming, catalysed assembly process and is not governed by simple association–dissociation equilibria. Both efficient repression of translation and specific protein binding in pull-down assays were dependent on the presence of ATP. Interestingly, ATP not only promoted binding of proteins to the SRE-containing RNA but also appeared to reduce binding of the same proteins to the control RNA. Thus, ATP might be consumed in RNP remodelling reactions (Jankowsky and Bowers, 2006) that disassemble complexes formed on the ‘wrong' RNA. One might speculate that this kind of proofreading is necessary because of the stability and potent repressor activity of the complex once it has formed. A pre-incubation comparable to the one used here also functions in cell-free systems for miRNA-dependent repression, although an ATP requirement has so far only been found for deadenylation (Mathonnet et al, 2007; Thermann and Hentze, 2007; Iwasaki et al, 2009; Zdanowicz et al, 2009).
According to the ‘closed loop' model of translation initiation, eIF4G, which is essential for the recruitment of the small ribosomal subunit, is bound cooperatively by simultaneous interactions with PABPC occupying the poly(A) tail and with eIF4E associated with the cap (Tarun and Sachs, 1996; Jackson et al, 2010). Alternative 5′–3′ protein assemblies can be used for translational repression. Repressor proteins bound to sites in the 3′ UTR engage the cap in a manner that excludes eIF4G (Stebbins-Boaz et al, 1999; Cao and Richter, 2002; Cho et al, 2005; Minshall et al, 2007; Standart and Minshall, 2008). SRE-dependent repression of nos translation has been proposed to rely on a similar mechanism. According to this model, SRE-bound Smaug associates with Cup, which in turn binds eIF4E and evicts eIF4G (Nelson et al, 2004). The oskar RNA is believed to be regulated in a similar manner, except that Cup is recruited by the RNA-binding protein Bruno (Wilhelm et al, 2003; Nakamura et al, 2004). This model is based on the well-established interaction between eIF4E and Cup and on genetic evidence that Cup is required for nos and oskar repression (Wilhelm et al, 2003; Nakamura et al, 2004; Nelson et al, 2004; Zappavigna et al, 2004).
Our data confirm several key predictions of the Cup model: Cup is present in the repressed RNP. This is specific, as it is SRE dependent and, therefore, presumably Smaug dependent. Cup binding is also cap dependent, consistent with its association with the cap-binding protein eIF4E. eIF4G is specifically displaced, whereas eIF4E remains part of the repressed RNP; thus, the eIF4F complex is disrupted, consistent with the proposed role of Cup. Finally, 48S pre-initiation complex formation is prevented, as would be expected from the displacement of eIF4G.
However, a number of observations either contradict the Cup model or are not explained by it. First, the 5′ cap is dispensable for repression. RNAs lacking a cap are efficiently repressed, and the stability of the repressor complex is independent of the cap. Second, inhibition of translation is not restricted to the displacement of eIF4G and prevention of 48S complex formation; a late step in translation is also affected, as shown by the inhibition of CrPV IRES-dependent translation. Third, the Cup model does not explain the ATP requirement in the formation of the repressor complex. Fourth, the repressed RNP contains proteins not accommodated by the model of Cup interfering with the eIF4E–eIF4G interaction, most of all Me31B. We are not aware of genetic evidence for a Me31B requirement in nos regulation, but such evidence does exist for oskar (Nakamura et al, 2001). Cup might have a second role in repression of nos mRNA by recruiting Me31B to the SRE via its interaction with Tral (Wilhelm et al, 2005; Tritschler et al, 2008). In summary, while our data suggest that key aspects of the Cup model are correct, the model is not sufficient to explain the repression of nos mRNA translation. Experiments on oskar regulation support the view that the Cup model only covers one aspect of translational repression of the oskar and nos mRNAs (Chekulaeva et al, 2006). It has recently been suggested that subunits of the CCR4–NOT complex may repress translation independently of the deadenylation process (Cooke et al, 2010); thus, the CCR4–NOT complex might also have a function in translational repression of nos.
The inhibition of multiple steps in translation initiation can be explained in two ways. It is possible that components of the repressor complex target multiple individual steps through specific protein–protein interactions, similar to the inhibition of the eIF4E–eIF4G interaction by Cup. Alternatively, the repressor complex might sequester the mRNA in a manner that makes it generally inaccessible. This hypothetical mechanism has been termed ‘structural masking' and compared to the condensation of chromatin (Spirin, 1994). ‘Structural masking' of the mRNA might most easily account for the effect of the repressor complex on CrPV IRES-dependent initiation, which provides few if any targets for specific protein–protein interactions. In vitro, ribosome assembly on the IRES can proceed from separate subunits in the absence of any initiation factor (Wilson et al, 2000; Pestova and Hellen, 2003; Spahn et al, 2004). In vivo, preformed 80S ribosomes might associate with the IRES or separate subunits might assemble with the help of eIF5B (Pestova et al, 2004). An SRE-dependent inhibition acting on nascent chains, which might also explain repression of CrPV IRES-dependent translation, was not detectable in vitro.
The SREs direct both translational repression and deadenylation of the nos mRNA (Dahanukar and Wharton, 1996; Gavis et al, 1996; Smibert et al, 1996; Bashirullah et al, 1999). A role for Smaug in both processes has been shown by genetic evidence (Dahanukar et al, 1999; Nelson et al, 2004; Zaessinger et al, 2006). However, the protein complexes responsible for the two processes differ. Deadenylation proceeds without a pronounced lag phase, is not accelerated by a pre-incubation step, and requires a high concentration of ATP. In contrast, formation of the translation repressor complex is slow and can proceed at a lower ATP concentration. Furthermore, deadenylation proceeds efficiently with up to 50 nM RNA, whereas translational repression works well only at 1–10 nM. Finally, we have repeatedly had batches of extract that were active in SRE-dependent deadenylation but deficient in translational repression and vice versa. Thus, it appears that Smaug recruits different effector proteins that affect either deadenylation or translation; factors required for the latter may be less abundant in the extract.
Materials and methods
DNA templates for in vitro transcription
Plasmids coding for luciferase RNAs, deadenylation substrates, and SRE-competitor (‘SRE only') RNAs have been described (Jeske et al, 2006). Construction of templates for RNAs used for sucrose gradients and pull downs is described in the Supplementary data (Supplementary Text; Supplementary Table II). These RNAs (1-AUG nos, 1-AUG nos SRE−) contained a short open reading frame coding for 27 amino acids. The construction of templates for the flag tag-luciferase RNAs and for those containing the IRES of the cricket paralysis virus intergenic region (a gift of Nicola Gray via Antje Ostareck) is also described in the Supplementary data. All DNA constructs contained a poly(A) sequence of 72 nucleotides downstream of the 3′ UTR. Cutting the plasmids with BamHI, HindIII, or KpnI prior to in vitro transcription resulted in RNAs that carried no poly(A) tail, an external poly(A) tail, or an ‘internal' poly(A) tail followed by 40 nucleotides of a mixed sequence, respectively.
In vitro transcription
RNA synthesis was carried out as described (Jeske et al, 2006). 3′-O-methyl-m7GpppG cap dinucleotide (New England Biolabs) was incorporated instead of m7GpppG. The SRE-competitor RNAs were neither capped nor polyadenylated. RNA substrates for sucrose gradient analyses were produced by in vitro transcription of KpnI-digested 1-AUG templates in the absence of a cap dinucleotide and radioactive nucleotides. In all, 6 pmol of the purified RNA was used in an in vitro capping reaction according to the ‘alternate cap 0 capping protocol' supplied with the ScriptCap™ m7G Capping System (Epicentre), except that the GTP concentration was 4 μM, 1 μM [α-32P] GTP (3000 Ci/mmol, Hartmann Analytics, Braunschweig, Germany) was added, and the enzyme concentration was doubled. The resulting cap-labelled RNA was purified as described (Jeske et al, 2006). The capping efficiency was at least 55%. RNA substrates for RNA pull-down assays were synthesized by in vitro transcription of HindIII-digested 1-AUG templates in the presence of [α-32P] UTP, 80 μM Biotin-16-UTP (Roche) and 1 mM UTP.
Drosophila embryo extracts and in vitro assays
Extracts were prepared as described (Jeske and Wahle, 2008) except that centrifugation was for 30 min at 20 800 × g instead of 20 min at 14 500 × g. Extracts from 0.5- to 2.5-h-old embryos were mainly used.
Translation assays using Drosophila embryo extract have been described (Jeske et al, 2006). Luciferase RNAs were used at 1 nM, and magnesium acetate was 1 mM. GTP and spermidine were omitted, and incubation was at 22°C. When GST-Osk or GST was added, only 20% extract was used. ATP depletion was carried out and confirmed as described (Jeske et al, 2006) except that 5 mM glucose was used. The ATP-regenerating system consisted of creatine kinase, creatine phosphate, and ATP (Jeske et al, 2006). For translation of CrPV-IRES containing luciferase RNAs, magnesium acetate was increased to 1.5 mM and potassium acetate was used at 150 mM. MG132 (Calbiochem) was used at 1 μM. Luciferase activities (RLU/s) reported in the figures correspond to 10 μl of translation reaction.
Deadenylation assays were performed as described but spermidine was omitted (Jeske and Wahle, 2008). In all, 20% Drosophila embryo extract and 10 nM substrate RNAs were used.
Sucrose gradient analysis
In all, 13.5 nM of 32P-cap-labelled 1-AUG RNA substrates were incubated with embryo extract under translation conditions in a volume of 10 μl for the time indicated. GMPPNP was used at 5 mM. After incubation, the reactions were diluted with 50 μl gradient buffer (24 mM Hepes-KOH, 100 mM potassium acetate, 4 mM magnesium acetate, and 1 mM dithiothreitol, pH 7.4) and loaded on top of an 11-ml 5–25% (w/v) sucrose gradient in gradient buffer. Gradients were centrifuged in an SW40Ti rotor (Beckman) at 38 000 r.p.m. and 4°C for 2 h followed by an ∼40 min deceleration without braking. Fractions of ∼500 μl were collected from bottom to top of the gradient with continuous monitoring of the optical density at 280 nm, and the radioactivity was determined by Čerenkov counting.
Sucrose gradient analysis using rabbit reticulocyte lysate (untreated; Promega) was performed similarly with the following modifications. In the presence of 5 mM GMP-PNP, 10 nM RNA was incubated in a volume of 25 μl, and the reaction was diluted with 75 μl of gradient buffer. In the presence of 1 mM cycloheximide, 13.5 nM RNA and a volume of 10 μl were used and diluted with 50 μl buffer. RNA substrates were incubated for 30 min at 30°C using the same buffer mixture as for translation in Drosophila embryo extract.
Protein purification
The coding region of short Oskar (fly base accession number CG10901-PC) was cloned into the pGEX-6P-1 vector (Promega) as described in the Supplementary data. GST and GST-Oskar were expressed in Escherichia coli and purified on glutathione sepharose 4FF (GE Healthcare). Lysis and washing buffer consisted of 20 mM Tris–HCl, 500 mM potassium acetate, 0.1 mM EDTA, 0.05% (v/v) Tween 20, 1 mM DTT, pH 8.0. Elution buffer was 100 mM Tris, 200 mM potassium acetate, 50 mM glutathione, 1 mM DTT, pH 8.0. Eluted proteins were concentrated, glutathione was removed and the buffer changed to protein buffer (100 mM Tris–acetic acid, 200 mM potassium acetate, pH 8.0) by Centricon filtration. The concentrations of GST and GST-Oskar were determined from the UV spectra based on calculated extinction coefficients. Due to the presence of shorter fragments, the measured concentration overestimates the concentration of full-length Oskar.
RNA pull-down assay
Biotinylated and 32P-labelled RNA substrates were incubated with Dynabeads® M-270 Streptavidin (Invitrogen) in 10 × SSC buffer supplemented with 0.5 μg/μl methylated BSA for 1 h at 15°C with constant agitation. Repressor complex assembly was performed in a reaction containing 10 nM immobilized RNA, 40% Drosophila embryo extract, 250 μg/ml yeast tRNA (Roche), 16 mM Hepes-KOH pH 7.4, 1 mM magnesium acetate, 27% (v/v) protein buffer, and 30 μg/ml heparin at room temperature with mixing at 10 s intervals. (The concentration of heparin used did not affect SRE-dependent deadenylation. Concentrations up to 20 μg/ml did not affect translation repression; higher concentrations could not be tested as translation was inhibited.) The tube was transferred onto ice, and the beads were washed twice with a buffer consisting of 16 mM Hepes-KOH pH 7.4, 150 mM potassium chloride, 1 mM magnesium acetate, 27% (v/v) protein buffer, 30 μg/ml heparin, and 1 mM dithiothreitol. During the second washing step, the beads were transferred into a fresh tube. This reduced the background probably caused by proteins bound to the tube wall. When GST-Osk or GST was tested in the RNA pull-down assay, only 20% of extract were used and extra protein buffer was omitted.
GST pull-down assay
A measure of 90 μl reactions were assembled on ice and contained 20% Drosophila embryo extract, 250 μg/ml yeast tRNA, 1 mM magnesium acetate, 16 mM Hepes-KOH pH 7.4, 30 μg/ml heparin, and 26.2 μM GST-Oskar or GST protein. After incubation for 50 min at 22°C, reactions were diluted with 200 μl protein buffer and supplemented with 750 μM CaCl2. Each reaction was split; one half was supplemented with 1500 U micrococcal nuclease (Fermentas) and 1 μg RNase A, the second half was left without RNases. GST-Oskar or GST were captured on 20 μl packed volume of MagneGST™ Glutathione Particles (Promega) equilibrated in protein buffer by incubation in the cold room for 5 h with head-over-tail-rotation. Beads were washed three times with 300 μl protein buffer. During the last washing step, the mixture was transferred to a fresh tube. Proteins were eluted by boiling in 30 μl of SDS sample buffer. In all, 40% of the eluted proteins were separated in a 4–12% gradient SDS–polyacrylamide gel and analysed by western blotting.
Western blotting
The procedure and antibodies to Caf1 and Not2 have been described (Jeske et al, 2006). Other antibodies were kind gifts of the following colleagues: Me31B, Cup, and eIF4E, Akira Nakamura; Smaug, Craig Smibert and Robin Wharton; Tral, Elisa Izaurralde; eIF4G, Kent Duncan and José Sierra; and PABPC, Nahum Sonenberg.
Supplementary Material
Acknowledgments
We are grateful to Kent Duncan, Nicki Gray, Elisa Izaurralde, Akira Nakamura, Antje Ostareck, José Sierra, Craig Smibert, Nahum Sonenberg, and Robin Wharton for generously providing reagents; Andreas Ladurner, Paul Lasko, Jan Medenbach, Mikiko Siomi, Yukihide Tomari, and Gerhard Wagner for reagents used for additional experiments not reported here; Fátima Gebauer for advice on extract preparation; and Juliane Buschmann and Gunter Reuter for support. This work was supported by a grant from the DFG.
Author contributions: MJ, BM, and AA did the experiments; MJ, BM, and EW designed the experiments and interpreted the results; and MJ and EW wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aviv T, Lin Z, Ben-Ari G, Smibert CA, Sicheri F (2006) Sequence-specific recognition of RNA hairpins by the SAM domain of Vts1p. Nat Struct Mol Biol 13: 168–176 [DOI] [PubMed] [Google Scholar]
- Aviv T, Lin Z, Lau S, Rendl LM, Sicheri F, Smibert CA (2003) The RNA binding SAM domain of Smaug defines a new family of post-transcriptional regulators. Nat Struct Biol 10: 614–621 [DOI] [PubMed] [Google Scholar]
- Barbee SA, Estes PS, Cziko A-M, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R, Ramaswami M (2006) Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirullah A, Halsell SR, Cooperstock RL, Kloc M, Karaiskakis A, Fisher WW, Fu W, Hamilton JK, Etkin LD, Lipshitz HD (1999) Joint action of two RNA degradation pathways controls the timing of maternal transcript elimination at the midblastula transition in Drosophila melanogaster. EMBO J 18: 2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten SE, Gavis ER (1999) Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development 126: 659–669 [DOI] [PubMed] [Google Scholar]
- Cao Q, Richter JD (2002) Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J 21: 3852–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Hentze MW, Ephrussi A (2006) Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 124: 521–533 [DOI] [PubMed] [Google Scholar]
- Cho PF, Poulin F, Cho-Park YA, Cho-Park IB, Chicoine JD, Lasko P, Sonenberg N (2005) A new paradigm for translational control: inhibition via 5′-3′ mRNA tethering by bicoid and the eIF4E cognate 4EHP. Cell 121: 411–423 [DOI] [PubMed] [Google Scholar]
- Chu C-Y, Rana TM (2006) Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol 4: 1122–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Wyckoff D, Gavis ER (2000) Synthesis of the posterior determinant nanos is spatially restricted by a novel cotranslational regulatory mechanism. Curr Biol 10: 1311–1314 [DOI] [PubMed] [Google Scholar]
- Colegrove-Otero LJ, Minshall N, Standart N (2005) RNA-binding proteins in early development. Crit Rev Biochem Mol Biol 40: 21–73 [DOI] [PubMed] [Google Scholar]
- Coller J, Parker R (2005) General translational repression by activators of mRNA decapping. Cell 122: 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke A, Prigge A, Wickens M (2010) Translational repression by deadenylases. J Biol Chem 285: 28506–28513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Walker JA, Wharton RP (1999) Smaug, a novel RNA-binding protein that operates a translational switch in Drosophila. Mol Cell 4: 209–218 [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Wharton RP (1996) The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes Dev 10: 2610–2620 [DOI] [PubMed] [Google Scholar]
- Deniz N, Lenarcic EM, Landry DM, Thompson SR (2009) Translation initiation factors are not required for Dicistroviridae IRES function in vivo. RNA 15: 932–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R (1991) oskar Organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66: 37–50 [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Lehmann R (1992) Induction of germ cell formation by oskar. Nature 358: 387–392 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Izaurralde E (2007a) P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol 8: 9–22 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Rehwinkel J, Stricker M, Huntzinger E, Yang S-F, Doerks T, Dorner S, Bork P, Boutros M, Izaurralde E (2007b) Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev 21: 2558–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N (2005) A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol 170: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrey JL, Lee Y-Y, Au HHT, Bushell M, Jan E (2010) Host and viral translational mechanisms during cricket paralysis virus infection. J Virol 84: 1124–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R (1992) Localization of nanos RNA controls embryonic polarity. Cell 71: 301–313 [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R (1994) Translational regulation of nanos by RNA localization. Nature 369: 315–318 [DOI] [PubMed] [Google Scholar]
- Gavis ER, Lunsford L, Bergsten SE, Lehmann R (1996) A conserved 90 nucleotide element mediates translational repression of nanos RNA. Development 122: 2791–2800 [DOI] [PubMed] [Google Scholar]
- Gavis ER, Singer RH, Hüttelmaier S (2007) Localized translation through messenger RNA localization. In Translational Control in Biology and Medicine, Mathews MB, Sonenberg N, Hershey JWB (eds) Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Gebauer F, Richter JD (1997) Synthesis and function of mos: the switch of vertebrate oocyte meiosis. Bioessays 19: 23–28 [DOI] [PubMed] [Google Scholar]
- Grainger JL, Winkler MM (1987) Fertilization triggers unmasking of maternal mRNAs in sea urchin eggs. Mol Cell Biol 7: 3947–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman I, Jung M-Y, Sarkissian M, Cao Q, Richter JD (2002) Translational control of the embryonic cell cycle. Cell 109: 473–483 [DOI] [PubMed] [Google Scholar]
- Ilan J, Ilan J (1978) Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol 66: 375–385 [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kawamata T, Tomari Y (2009) Drosophila argonaute 1 and argonaute 2 employ distinct mechanisms for translational repression. Mol Cell 34: 58–67 [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CUT, Pestova TV (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 10: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky E, Bowers H (2006) Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases. Nucleic Acids Res 34: 4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NA, Kaumeyer JF, Young E, Raff RA (1978) A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchin eggs. Dev Biol 63: 279–298 [DOI] [PubMed] [Google Scholar]
- Jeske M, Meyer S, Temme C, Freudenreich D, Wahle E (2006) Rapid ATP-dependent deadenylation of nanos mRNA in a cell-free system from Drosophila embryos. J Biol Chem 281: 25124–25133 [DOI] [PubMed] [Google Scholar]
- Jeske M, Wahle E (2008) Cell-free deadenylation assays using Drosophila embryo extracts. Meth Enzymol 448: 107–118 [DOI] [PubMed] [Google Scholar]
- Johnson PE, Donaldson LW (2006) RNA recognition by the Vts1p SAM domain. Nat Struct Mol Biol 13: 177–178 [DOI] [PubMed] [Google Scholar]
- Johnstone O, Lasko P (2001) Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet 35: 365–406 [DOI] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH, Wharton RP (2007) Translational control of maternal cyclin B mRNA by Nanos in the Drosophila germline. Development 134: 1519–1527 [DOI] [PubMed] [Google Scholar]
- Kalifa Y, Huang T, Rosen LN, Chatterjee S, Gavis ER (2006) Glorund, a Drosophila hnRNP F/H homolog, is an ovarian repressor of nanos translation. Dev Cell 10: 291–301 [DOI] [PubMed] [Google Scholar]
- Ladomery M, Wade E, Sommerville J (1997) Xp54, the Xenopus homologue of human RNA helicase p54, is an integral component of stored mRNP particles in oocytes. Nucleic Acids Res 25: 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesich DC, Gajewski KM, Nazimiec ME, Beckingham K (2000) bicaudal encodes the Drosophila beta NAC homolog, a component of the ribosomal translational machinery. Development 127: 559–572 [DOI] [PubMed] [Google Scholar]
- Markussen F-H, Michon A-M, Breitwieser W, Ephrussi A (1995) Translational control of oskar generates Short OSK, the isoform that induces pole plasm assembly. Development 121: 3723–3732 [DOI] [PubMed] [Google Scholar]
- Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, Filipowicz W, Duchaine TF, Sonenberg N (2007) MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 317: 1764–1767 [DOI] [PubMed] [Google Scholar]
- Minshall N, Kress M, Weil D, Standart N (2009) Role of p54 RNA helicase activity and its C-terminal domain in translational repression, P-body localization and assembly. Mol Biol Cell 20: 2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall N, Reiter MH, Weil D, Standart N (2007) CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes. J Biol Chem 282: 37389–37401 [DOI] [PubMed] [Google Scholar]
- Minshall N, Thom G, Standart N (2001) A conserved role of a DEAD box helicase in mRNA masking. RNA 7: 1728–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Hong A, Lilly MA, Lehmann R (2005) twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development 132: 1165–1174 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Amikura R, Hanyu K, Kobayashi S (2001) Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development 128: 3233–3242 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K (2004) Drosophila Cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell 6: 69–78 [DOI] [PubMed] [Google Scholar]
- Nelson MR, Leidal AM, Smibert CA (2004) Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J 23: 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstrass FC, Lee A, Stefl R, Janis M, Chanfreau G, Allain FH-T (2006) Shape-specific recognition in the structure of the Vts1p SAM domain with RNA. Nat Struct Mol Biol 13: 160–167 [DOI] [PubMed] [Google Scholar]
- Parker R, Sheth U (2007) P bodies and the control of mRNA translation and degradation. Mol Cell 25: 635–646 [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CUT (2003) Translation elongation after assembly of ribosomeson the cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev 17: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Lomakin IB, Hellen CUT (2004) Position of the CrPV IRES on the 40S subunit and factor dependence of IRES/80S ribosome assembly. EMBO Rep 5: 906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Ahn S, Speed TP, Rubin GM (2007) Global analyses of mRNA translational control during early Drosophila embryogenesis. Genome Biol 8: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD, Smibert CA (2005) Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila embryo. Curr Biol 15: 284–294 [DOI] [PubMed] [Google Scholar]
- Sheets MD, Wu M, Wickens M (1995) Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature 374: 511–516 [DOI] [PubMed] [Google Scholar]
- Smibert CA, Lie YS, Shillinglaw W, Henzel WJ, Macdonald PM (1999) Smaug, a novel and conserved protein, contributes to repression of nanos mRNA translation in vitro. RNA 5: 1535–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert CA, Wilson JE, Kerr K, Macdonald PM (1996) Smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes Dev 10: 2600–2609 [DOI] [PubMed] [Google Scholar]
- Smith JL, Wilson JE, Macdonald PM (1992) Overexpression of oskar directs ectopic activation of nanos and presumptive pole cell formation in Drosophila embryos. Cell 70: 849–859 [DOI] [PubMed] [Google Scholar]
- Spahn CMT, Jan E, Mulder A, Grassucci RA, Sarnow P, Frank J (2004) Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Cell 118: 465–475 [DOI] [PubMed] [Google Scholar]
- Spirin AS (1966) On ‘masked' forms of messenger RNA in early embryogenesis and in other differentiating systems. Curr Top Dev Biol 1: 1–38 [PubMed] [Google Scholar]
- Spirin AS (1994) Storage of messenger RNA in eukaryotes: envelopment with protein, translational barrier at 5′ side, or conformational masking by 3′ side? Mol Rep Dev 38: 107–117 [DOI] [PubMed] [Google Scholar]
- Standart N, Dale M, Stewart E, Hunt T (1990) Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3′-untranslated region. Genes Dev 4: 2157–2168 [DOI] [PubMed] [Google Scholar]
- Standart N, Minshall N (2008) Translational control in early development. Biochem Soc Trans 36: 671–676 [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B, Cao Q, de Moor CH, Mendez R, Richter JD (1999) Maskin is a CPEB-associated factor that transiently interacts with eIF-4E. Mol Cell 4: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Waver T, Hughes TR, Westwood JT, Smibert CA, Lipshitz HD (2007) SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev Cell 12: 143–155 [DOI] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD (2005) Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila. Dev Dyn 232: 593–608 [DOI] [PubMed] [Google Scholar]
- Tanaka KJ, Ogawa K, Takagi M, Imamoto N, Matsumoto K, Tsujimoto M (2006) RAP55, a cytoplasmic mRNP component, represses translation in Xenopus oocytes. J Biol Chem 281: 40096–40106 [DOI] [PubMed] [Google Scholar]
- Tarun SZ, Sachs AB (1996) Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J 15: 7168–7177 [PMC free article] [PubMed] [Google Scholar]
- Temme C, Zaessinger S, Simonelig M, Wahle E (2004) A complex containing the CCR4 and CAF1 proteins is involved in mRNA deadenylation in Drosophila. EMBO J 23: 2862–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermann R, Hentze MW (2007) Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447: 875–878 [DOI] [PubMed] [Google Scholar]
- Tritschler F, Eulalio A, Helms S, Schmidt S, Coles M, Weichenrieder O, Izaurralde E, Truffault V (2008) Similar modes of interaction enable Trailer Hitch and EDC3 to associate with DCP1 and Me31B in distinct protein complexes. Mol Cell Biol 28: 6695–6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy L, Orr-Waver TL (2007) Regulating translation of maternal messages: multiple repression mechanisms. Trends Cell Biol 17: 547–554 [DOI] [PubMed] [Google Scholar]
- Wang C, Lehmann R (1991) Nanos is the localized posterior determinant in Drosophila. Cell 66: 637–647 [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Buszczak M, Sayles S (2005) Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev Cell 9: 675–685 [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Hilton M, Amos Q, Henzel WJ (2003) Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J Cell Biol 163: 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Smibert CA (2005) Mechanisms of translational regulation in Drosophila. Biol Cell 97: 235–252 [DOI] [PubMed] [Google Scholar]
- Wilson JE, Pestova TV, Hellen CUT, Sarnow P (2000) Initiation of protein synthesis from the A site of the ribosome. Cell 102: 511–520 [DOI] [PubMed] [Google Scholar]
- Yurkova MS, Murray MT (1997) A translation regulatory particle containing the Xenopus oocyte Y box protein mRNP3+4. J Biol Chem 272: 10870–10876 [DOI] [PubMed] [Google Scholar]
- Zaessinger S, Busseau I, Simonelig M (2006) Oskar allows nanos mRNA translation in Drosophila embryos by preventing its deadenylation by Smaug/CCR4. Development 133: 4573–4583 [DOI] [PubMed] [Google Scholar]
- Zappavigna V, Piccioni F, Villaescusa JC, Verrotti AC (2004) Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc Natl Acad Sci USA 101: 14800–14805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdanowicz A, Thermann R, Kowalska J, Jemielity J, Duncan K, Preiss T, Darzynkiewicz E, Hentze M (2009) Drosophila miR2 primarily targets the m7GpppN cap structure for translational repression. Mol Cell 35: 881–888 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.