Abstract
Aims
Baicalin is the major component found in Scutellaria baicalensis root, a widely used herb in traditional Chinese medicine. Although it has been used for thousands of years to treat stroke, the mechanisms of action of S. baicalensis have not been clearly elucidated. In this report, we studied the modulation of angiogenesis as one possible mechanism by investigating the effects of these agents on expression of vascular endothelial growth factor (VEGF), a critical factor for angiogenesis.
Methods and results
The effects of baicalin and an extract of S. baicalensis on VEGF expression were tested in several cell lines. Both agents induced VEGF expression in all cells without increasing expression of hypoxia-inducible factor-1α (HIF-1α). The expression of reporter genes was also activated under the control of the VEGF promoter containing either a functional or a defective HIF response element (HRE). Only minimal effects were observed on reporter activation under the HRE promoter. Instead, both agents significantly induced oestrogen-related receptor (ERRα) expression as well as the activity of reporter genes under the control of ERRα-binding element. Their ability to induce VEGF expression was suppressed once ERRα expression was knocked down by siRNA or ERRα-binding sites were deleted in the VEGF promoter. We also found that both agents stimulated cell migration and vessel sprout formation from the aorta.
Conclusion
Our results implicate baicalin and S. baicalensis in angiogenesis by inducing VEGF expression through the activation of the ERRα pathway. These data may facilitate a better understanding of the potential health benefits of these agents in the treatment of cardiovascular diseases.
Keywords: Baicalin, VEGF, Angiogenesis, HIF-1α, ERRα
1. Introduction
Angiogenesis, the formation of new blood vessels from pre-existing vessels, plays a critical role in many physiological and pathological conditions, including embryonic development, wound healing, tumour growth, and metastasis.1–3 In a normal healthy body, angiogenesis is tightly controlled by a balance of pro-angiogenic and anti-angiogenic factors.4
Dysregulated angiogenesis contributes to many diseases, including heart and brain ischaemia, neurodegeneration, delayed wound healing, hypertension, and others. In these conditions, insufficient production of angiogenic factors leads to insufficient blood vessel formation and subsequent tissue death. Therapeutic angiogenesis is currently being developed to treat patients with ischaemic vascular conditions by stimulating new blood vessel growth.1,3
Among the many angiogenic factors, vascular endothelial growth factor (VEGF) is one of the most critical and specific factors that stimulate both physiological and pathological angiogenesis. Most VEGF-induced responses in endothelial cells are mediated through binding to receptor tyrosine kinases, VEGF receptor 1 (flt1) and VEGF receptor 2 (KDR/flk1).5 VEGF expression is induced by growth factors, oncogenes, and hypoxia.6 The key regulator of VEGF expression in response to hypoxia is hypoxia-inducible factor-1 (HIF-1), a heterodimeric transcription factor consisting of HIF-1β, which is constitutively expressed, and HIF-1α, which is highly regulated. Levels of HIF-1α expression are determined by the rates of protein synthesis (regulated via an oxygen-independent mechanism) and protein degradation (regulated via an oxygen-dependent mechanism).6 HIF-1 activates VEGF transcription by binding to the hypoxia response element (HRE) in the VEGF promoter. In addition to HRE, multiple transcription factor-binding sites, including ERR, AP-1, Sp-1, Stat3, and CREB, have been identified within the VEGF promoter.7–9
Oestrogen-related receptors (ERRs) are a family of orphan nuclear hormone receptors initially identified based on their homology to the oestrogen receptor ERα. ERRs are generally considered to be constitutively active receptors that interact with coactivators in the absence of exogenous ligands.10 Recent studies have found that peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a potent ligand-independent coactivator that interacts with ERRα and also a major regulator of mitochondrial function in response to exercise and other stimuli.11,12 Through the activation of ERR-α, PGC-1α has been shown to powerfully induce VEGF expression and angiogenesis in cultured muscle cells and skeletal muscle in vivo, as well as in breast cancer cells, in an HIF-1α independent pathway.13,14
VEGF protein and genes have been administered to treat ischaemic disorders. After encouraging results in human Phase I trials,15 large randomized placebo-controlled Phase II/III clinical trials have, however, yielded somewhat disappointing results, partly due to inadequate delivery strategies.16 Alternatively, the use of small molecules for therapeutic angiogenesis has been suggested to provide an advantage over therapies using protein or gene therapy.17,18
We are interested in identifying natural product-derived molecules that can regulate VEGF expression and angiogenesis. By using a VEGF reporter cell line, we found that Scutellaria baicalensis root and its major component, baicalin, stimulated VEGF expression efficiently and induced vessel sprout formation from the aorta in an ex vivo model. The induced VEGF expression was mediated, at least in part, by the activation of the ERR pathway.
2. Methods
2.1. Reagents and antibodies
Deferoxamine mesylate (DFX), baicalin, and antibody for β-actin were from Sigma. Antibody against HIF-1α was from BD Bioscience. Antibody against caspase 3 and GAPDH were from Cell Signaling. Antibody against β-tubulin was from Thermal Scientific. Antibodies against ERRα and PGC-1α were from NOVUS. siRNA for ERRα, PGC-1α, and control siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Preparation of S. baicalensis extract
The root of S. baicalensis (also called Huang Qin) (obtained from E-Fong Herbs, CA, USA) was dissolved in hot water (70°C, 1 h). The solution was centrifuged (18 000 g, 10 min) to remove the insoluble part. The supernatant was then transferred to a new tube and dried under vacuum to full dryness. The dry pellet was weighted, resuspended in water, and characterized for the presence of baicalin using HPLC/UV detection based on a previously published method.19 The extract was found to contain 0.55% (w/w) baicalin. A Shimadzu HPLC system (Columbia, MD, USA) consisting of two LC-10AS solvent delivery pumps, an SIL-10A autoinjector, and an SPD-10A UV–VIS detector was used for quantitative analysis. Chromatographic conditions were as follows: the analytical column was a Phenomenex (Torrance, CA, USA) IB-SIL C18 150 × 4.6 mm ID, mobile phase (A) was 0.05% trifluoroacetic acid (v/v), and mobile phase (B) was acetonitrile. A linear gradient ran over 0–30 min from 20 to 100% B. The flow rate was 1.0 mL/min at ambient temperature and the injection volume was 10 μL. The detector was set to a wavelength of 277 nm. An external calibration method was used for the quantitative analysis. A calibration curve was obtained by plots of the peak area vs. the concentration of the pure calibration standards. All experiments were conducted in duplicate. Standards and samples were prepared by diluting 1:2 with acetonitrile/0.05% trifluoroacetic acid (1/4, v/v) prior to injection.
2.3. Cell culture
Human fibroblast MRC-5 cells (ATCC) were cultured in Eagle's MEM with 10% FBS. Human umbilical vascular endothelial cells (HUVEC, Clonetics, Lonza) were cultured in the endothelial growth medium (EGM-2, from Lonza for HUVEC) containing 10% FCS. U251 human glioma cells a were cultured in DMEM medium, as described previously.20 For cell culture under hypoxia, cells were grown in a chamber containing 1% O2, 5% CO2, and 94% N2 at 37°C or induced by a hypoxia mimetic agent DFX (250 μM, Sigma).
2.4. Construction of plasmids
To construct the luciferase reporter plasmid (pGL4/VEGF-Luc), a 2135 bp fragment of the human VEGF gene promoter (−2080 to +54) containing an HIF-1α-binding site at positions −985 and −939 was cloned into the upstream of the luciferase gene of the pGL4.14/[luc2/Hygro] plasmid (Promega, Madison, WI, USA), as described previously.20 A reporter construct with all three ERR-binding sites deleted in the VEGF promoter was generated using a mutagenesis kit from Stratagene (La Jolla, CA, USA). Reporter constructs containing a wild-type VEGF promoter (−1155 to +597) (VEGF-pGL3)―either with a functional HIF-1-binding site (VEGF-HRE) or with a mutated HIF-1-binding site (VEGF-mHRE)―were kindly provided by Dr Marina Schorpp-Kistner (DKFZ, Germany).21 A reporter construct containing 5× HIF-1-binding sites (HRE) was kindly provided by Andrew Kung (Dana-Farber Cancer Institute, Boston, MA, USA).22
2.5. Transient transfection
Fugene 6 (Roche) was used to transfect report constructs; RNAiMAX (Invitrogen) was used to transfect siRNA, according to the manufacturer's instructions.
2.6. Reporter cell line and luciferase reporter assay
U251/VEGF-luc reporter cell line was established as reported previously.20 Transfected U251 cells and reporter cell line U251/VEGF-Luc were seeded in 48-well plates (2.5 × 104/well) or in 96-well plates (1.5 × 104/well) the day before treatment. Cells were then treated with baicalin and S. baicalensis in a serum-free medium for indicated dose and times. Luciferase activity was determined, as described previously.20
2.7. Quantitative real-time PCR
Total RNA was extracted from cell lines using Qiagen RNeasy Min Kit (Qiagen, CA, USA). Quantitative real-time PCR (qRT-PCR) was determined in ABI Prism 7900HT Sequence Detection System using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) as described previously.20 The following primers were used with annealing temperature 60°C:
- VEGF
- Forward: AGCCTTGCCTTGCTGCTCTAC
- Reverse: TGATGATTCTGCCCTCCTCCTT
- HIF-1α
- Forward: TGAGGAAATGAGAGAAATGCTTACA
- Reverse: ACACTGAGGTTGGTTACTGTTGGT
- β-Actin
- Forward: AGAGATGGCCACGGCTGCTT
- Reverse: GCCACAGGACTCCATGCCCA
- ERRα
- Forward: AAAGCCTTCTTCAAGAGGACCAT
- Reverse: TGGTGATCTCACACTCGTTGGA
- PGC-1α
- Forward: AGTCCCACACACAGTCGCAGTC
- Reverse: CTGGGGTCAGAGGAAGAGATAAAG
2.8. Quantification of VEGF
Quantikine human VEGF Elisa kit from R&D system was used to measure human VEGF levels in the conditioned medium, collected as described previously.20
2.9. Immunoblot
Total cell extract was prepared in Laemmli sample buffer, electrophoresed on SDS gels, and immunoblotted as described previously.20
2.10. Migration assay
Endothelial cell migration was assessed as described previously.20 Briefly, HUVEC (1 × 105) were plated in EBM-2 medium containing 0.05% FCS in the upper chamber of the transwell (8 μm pore, Costar). Serum-free DMEM, containing VEGF (50 ng/mL) or various conditioned media, was added to the lower chamber of the transwell. After 5 h, non-migrated cells were removed by cotton swab and migrated cells were stained and examined under a microscope. The number of migrated cells was quantified by counting the cells at ×40 objectives.
2.11. Chick aortic ring assay
The aortic arch was dissected from day 12–14 chick embryos, cut into rings, and embedded into Matrigel (BD Biosciences) in four-well plates (NUNC), as described previously.23,24 Aortic rings were fed with an MCDB-131 serum-free medium (GIBCO, Invitrogen) containing baicalin (25 μM) and S. baicalensis (100 μg/mL). Growing sprouts were photographed with an Olympus inverted IX81 at ×40 magnification.
2.12. Statistic analysis
Data were presented as mean ± SD. Student's t-test was used to compare the means of two groups. ANOVA test was used to compare the means of multiple groups. All the experiments were repeated two to four times. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Effect of baicalin and S. baicalensis on VEGF expression
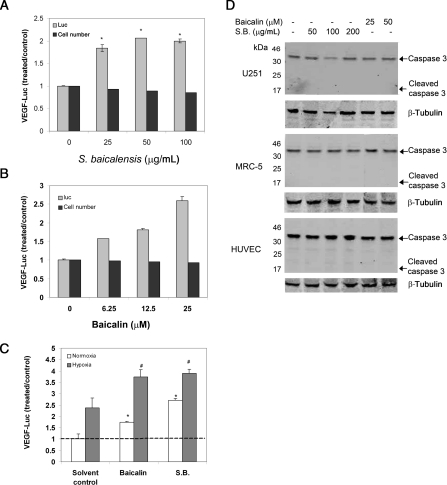
VEGF is a critical factor in the regulation of angiogenesis. To identify new agents that regulate VEGF expression among plant extracts, a reporter cell line, U251/VEGF-Luc, was generated that produced luciferase activity under the human VEGF promoter.20 An extract from the root of S. baicalensis was found to stimulate VEGF reporter activity effectively in U251/VEGF-Luc cells under normoxia (Figure 1A). Baicalin, a major component in S. baicalensis root, was similarly found to induce VEGF reporter activity (Figure 1B). Both agents further induced reporter activity under hypoxia (Figure 1C). At the tested concentrations, no toxicity was observed as these agents had little effect on the cell number (Figure 1A and B, black bar) or cleavage of caspase 3 (Figure 1D). A 25 μM baicalin or 50 μg/mL S. baicalensis extract was used for most subsequent experiments, as at these concentrations, baicalin or S. baicalensis could strongly stimulate VEGF expression with a minimal effect on the cell number. These results suggested that S. baicalensis, as well as baicalin, could activate VEGF gene transcription.
Figure 1.
Baicalin and S. baicalensis (S.B.) stimulate VEGF reporter activity. (A and B) U251 cells expressing luciferase reporter containing the human VEGF gene (U251/VEGF-luc) were incubated (24 h, under normoxia) with various concentrations of (A) S. baicalensis and (B) baicalin. (C) Baicalin (25 μM) and S. baicalensis (50 μg/mL) were incubated with U251/VEGF-luc (24 h) under both normoxia and hypoxia conditions. Samples were assessed for luciferase activity. Data are represented as the ratio to solvent control (water) under normoxia and are mean ± SD (n = 3). *P < 0.05 vs. control under normoxia; #P < 0.05 vs. control under hypoxia. (D) Scutellaria baicalensis and baicalin had little effect on the cleavage of caspase 3. Cell lysates from U251 cells, MRC-5 cells, and HUVEC treated with S. baicalensis or baicalin were analysed by immunoblotting with an antibody against caspase 3. β-Tubulin was used as a loading control.
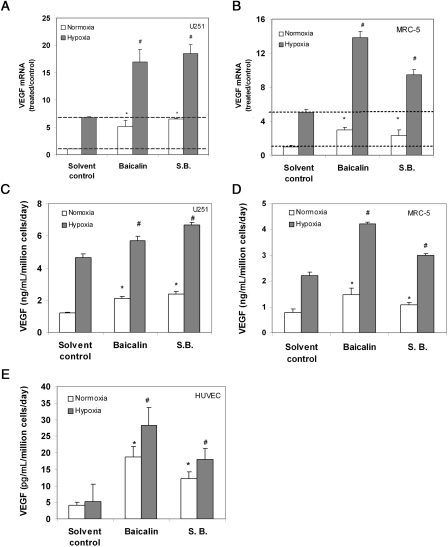
We next asked whether VEGF mRNA expression was induced by baicalin and S. baicalensis treatment. qRT-PCR data indicated that both agents significantly stimulated VEGF mRNA expression in U251 glioma cells. They also further increased VEGF mRNA levels under hypoxia (1% oxygen) (Figure 2A). The treatment of U251 cells with baicalin or S. baicalensis also led to an increase in VEGF protein expression under normoxia and a further increase under hypoxia by enzyme-linked immunosorbent assay (ELISA) (Figure 2C).
Figure 2.
Baicalin and S. baicalensis (S.B.) induce VEGF expression. (A and B) Baicalin induces VEGF mRNA expression in (A) U251 and (B) MRC-5 cells. Cells were incubated (24 h) with baicalin (25 μM) and S. baicalensis (50 μg/mL) under both normoxia and hypoxia, in the presence of 1% oxygen; qRT-PCR was used to determine the effect of these agents on VEGF mRNA expression. Data are expressed as a ratio to solvent control (water) under normoxia and are mean ± SD. (n = 3). *P < 0.05 vs. control under normoxia; #P < 0.05 vs. control under hypoxia. (C–E) Baicalin stimulates VEGF protein expression in (C) U251, (D) MRC-5, and (E) HUVEC. Cells were incubated (24 h) with baicalin (25 μM), and S. baicalensis (50 μg/mL) in a serum-free medium under normoxia or hypoxia conditions; conditioned media were then collected and analysed for the presence of VEGF by ELISA. Data were normalized to cell numbers and incubation time. *P < 0.05 vs. solvent control under normoxia; #P < 0.05 vs. solvent control under hypoxia.
To address whether these agents could also induce VEGF expression in other normal cells, VEGF expression was measured in HUVEC and MRC-5 human fibroblasts. Our results showed the induction of VEGF mRNA expression in MRC-5 cells (Figure 2B) and VEGF protein expression in both MRC-5 and HUVEC (Figure 2D and E). Under these concentrations, both reagents had little effect on the cleavage of caspase 3, suggesting these reagents had little toxic effect on these two cell lines (Figure 1D). Taken together, these results suggested baicalin- and S. baicalensis-induced VEGF expression by stimulating VEGF transcription.
3.2. Effect of baicalin and S. baicalensis on HIF-1α pathway
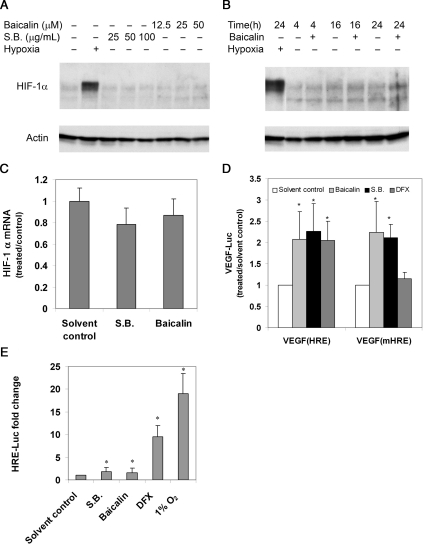
To explore the underlying mechanism of the stimulation of VEGF production, we asked whether baicalin and S. baicalensis modulated the levels of HIF-1α, a major regulator of VEGF expression. Both protein and mRNA levels of HIF-1α were examined in U251/VEGF-Luc cells treated with baicalin and S. baicalensis by western blot and qRT-PCR, respectively. We found little effect of baicalin and S. baicalensis on HIF-1α protein (Figure 3A and B) and RNA (Figure 3C) expression. As a control, HIF-1α was significantly elevated under hypoxia (Figure 3A and B). These data suggested that the activation of VEGF expression by baicalin and S. baicalensis was independent of HIF-1α expression.
Figure 3.
The effect of baicalin and S. baicalensis (S.B.) on the activation of HIF-1α. (A and B) Baicalin has little effect on HIF-1α production. U251 cells were incubated under normoxia or hypoxia (16 h) in the absence or presence of increasing concentrations of baicalin and S. baicalensis (A) or incubated for various times (B). Whole-cell lysates were analysed by immunoblotting with antibody against HIF-1α. β-Actin was used as a loading control. (C) Scutellaria baicalensis and baicalin do not enhance HIF-1α mRNA expression. U251/VEGF-luc cells were incubated (24 h) with S. baicalensis (50 μg/mL) and baicalin (25 μM), then analysed for HIF-1α mRNA expression by qRT-PCR. Data were normalized to β-actin and were represented as a ratio to solvent control (water for S.B. or ethanol for baicalin). (D and E) Baicalin did not act through binding to HRE. U251 cells were transfected with plasmids expressing luciferase reporter genes driven by the VEGF promoter (VEGF) containing either a wild-type HIF-1α-binding site (VEGF-HRE) or VEGF promoter containing a defective HRE site (VEGF-mHRE) (D), or a HRE reporter plasmid containing five copies of the HRE site (5× HRE) (E). Transfected cells were then incubated with baicalin (25 μM), S. baicalensis (50 μg/mL), or DFX (250 μM), a known agent that induces HIF-1α activity and assayed for luciferase activity. Data are expressed as a ratio to solvent control (water for S.B. and DFX or ethanol for baicalin) under normoxia. *P < 0.05 vs. solvent control.
We further tested the effect of baicalin and S. baicalensis on luciferase activity driven by a mutant VEGF promoter containing a defective HIF-1α-binding site (HRE). U251 cells were first transfected with the reporter or control plasmid prior to treatment. Baicalin and S. baicalensis induced luciferase activity driven by the VEGF promoter, regardless of whether the promoter contained a functional or defective HRE (Figure 3D). In contrast, DFX, an agent known to induce HRE activity, was able to induce the wild-type reporter, but had little effect on the mutant reporter. In a separate experiment, we looked at the effect on a luciferase reporter driven by a promoter containing five consecutive HREs. Compared with the strong activation of luciferase activity by both DFX and hypoxia (1% O2), baicalin and S. baicalensis had weak stimulation on the same reporter construct (Figure 3E). Together, these results suggested that the increased VEGF expression induced by baicalin and S. baicalensis was not likely mediated through binding to the HRE site in the VEGF promoter.
3.3. Involvement of ERRα pathway in baicalin and S. baicalensis-induced VEGF expression
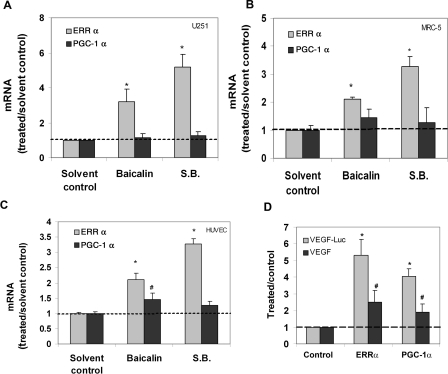
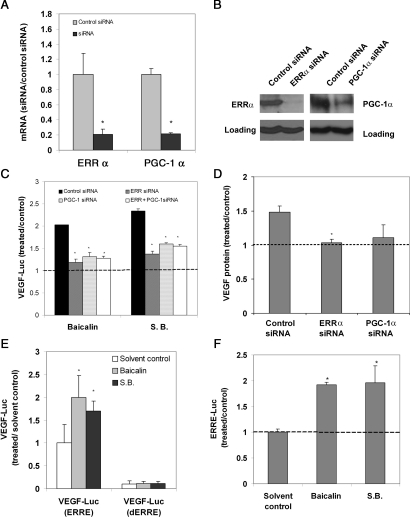
In addition to HRE, many other transcriptional-binding sites, including ERRE (ERR response element), have also been found within the VEGF promoter.8,12,13 Recent studies have found that ERRα interacts with PGC-1α and significantly stimulates VEGF expression and angiogenesis in an HIF-1α-independent pathway.13,14 qRT-PCR was used to investigate whether the PGC-1α/ERRα pathway was involved in VEGF expression induced by baicalin. We found that the expression of ERRα, but not PGC-1α, was significantly induced by baicalin and S. baicalensis treatment under normoxia in all three cell lines (Figure 4). We also observed that overexpression of ERRα or PGC-1α could lead to the induction of VEGF expression in the cells transfected with cDNA encoding ERRα or PGC-1α (Figure 4D), in agreement with the previous report.13
Figure 4.
The effect of baicalin and S. baicalensis on ERRα mRNA expression. (A) U251 cells, (B) MRC-5 cells, and (C) HUVEC were incubated (24 h) with baicalin (25 μM) and S. baicalensis (50 μg/mL) under normoxia. qRT-PCR was used to determine the effect of these agents on ERRα and PGC-1α mRNA expression. Data are expressed as the ratio to solvent control (water for S.B. or ethanol for baicalin) alone. *P < 0.05 vs. solvent control. (D) Overexpression of ERRα or PGC-1α increases VEGF reporter activity and VEGF expression. U251 cells were transfected with plasmids encoding ERRα or PGC-1α. The transfected cells were then assayed for the VEGF luciferase activity or VEGF protein expression by ELISA. Data are expressed as a ratio to control cDNA. *P < 0.05 vs. control cDNA for VEGF-luc activity; #P < 0.05 vs. control cDNA for VEGF expression.
To further address whether ERRα/PGC-1α was involved in VEGF induction, we studied the effect of baicalin and S. baicalensis on VEGF expression when ERRα or PGC-1α was knocked down by siRNA. As shown in Figure 5A and B, the expression of ERRα or PGC-1α was significantly reduced by transfection of ERRα or PGC-1α siRNA, respectively, but not by control siRNA. We further observed that in ERRα- or PGC-1α-depleted cells, baicalin and S. baicalensis failed to stimulate VEGF reporter activity (Figure 5C) or VEGF expression (Figure 5D).
Figure 5.
The effect of baicalin and S. baicalensis on the activation of ERRα/PGC-1α pathway. (A and B) The depletion of ERRα or PGC-1α by siRNA. U251 cells were transfected with ERRα siRNA, PGC-1α siRNA, and control siRNA. Cells were analysed for the expression of ERRα and PGC-1α by (A) qRT-PCR, data are represented as ratio to control siRNA, and (B) western, GAPDH was used as a loading control. (C and D) Baicalin failed to induce VEGF expression in ERRα- or PGC-1α-depleted cells. The siRNA-transfected cells were treated (24 h) with baicalin (25 μM) and S. baicalensis (100 μg/mL) and assayed for luciferase activity under normoxia (C) and VEGF protein expression by ELISA (D). Data are expressed as a ratio to solvent control (water for S.B. or ethanol for baicalin). *P < 0.05 vs. control treated with control siRNA. (E) The deletion of ERRE led to the decreased expression of VEGF reporter activity. U251 cells were transfected with luciferase reporter activity driven by the VEGF promoter either with functional ERREs or deleted ERREs. Cells were then treated with baicalin (25 µM) and S. baicalensis (100 μg/mL) and assayed for luciferase activity. Data are expressed as a ratio to control transfected with wild-type VEGF reporter and treated with solvent control (water for S.B. or ethanol for baicalin). *P < 0.05 vs. solvent control. (F) Baicalin was able to induce luciferase activity under a promoter containing ERR DNA-binding site (ERRE). Cells were transfected with an ERRE reporter plasmid. The transfected cells were then treated (24 h) with baicalin (25 μM) and S. baicalensis (100 μg/mL) under normoxia and assayed for luciferase activity. Data are expressed as the ratio to solvent control (water for S.B. or ethanol for baicalin) and are mean ± SD. (n = 3). *P < 0.05 vs. solvent control.
There are three putative ERR-binding sites (ERREs) found in the human VEGF promoters (−2080 to +54). To address whether these binding sites could mediate the induced VEGF luciferase activity by baicalin, cells were transfected with a luciferase reporter under a VEGF promoter with ERREs either intact or deleted. As shown in Figure 5E, VEGF luciferase activity was significantly reduced and the capability of baicalin and S. baicalensis to induce VEGF reporter activity was also diminished when these ERRE sites were removed. These results suggested that ERRE plays a critical role in regulating VEGF expression.
We next asked whether baicalin and S. baicalensis could directly induce ERRE reporter activity. A luciferase reporter construct containing a binding site for ERRα was transfected into cells, which were then treated with baicalin and S. baicalensis. Our results showed that both agents directly induced reporter gene expression driven by a promoter containing ERRα-binding element (ERRE) (Figure 5F). Taken together, our data implicated the ERRα/PGC-1α pathway in the induction of VEGF expression by baicalin and S. baicalensis.
3.4. Angiogenesis potential of baicalin and S. baicalensis
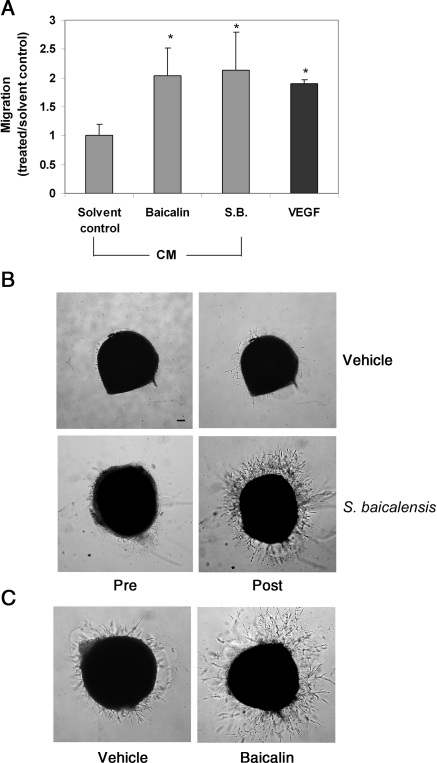
VEGF is a critical factor in angiogenesis induction. To address whether baicalin and S. baicalensis could induce angiogenesis, we used an endothelial cell migration assay to examine the angiogenesis potential of conditioned media of U251 cells, which have been treated with S. baicalensis, baicalin, or vehicle. Conditioned medium from cells treated with baicalin or S. baicalensis was able to induce endothelial cell migration more effectively (Figure 6A), consistent with our observation that a higher level of VEGF expression was produced in the medium of cells treated with baicalin and S. baicalensis.
Figure 6.
Angiogenesis potential of S. baicalensis and baicalin. (A) The effect of U251 conditioned medium on endothelial cell migration. HUVEC were placed in the top chamber of a transwell, whereas a serum-free medium, containing either VEGF (50 ng/mL) positive control or conditioned medium (12.5%) derived from U251 cells treated with S. baicalensis, baicalin, or vehicle, was placed in the bottom chamber. Migrated cells were quantified 5 h after incubation. Data are expressed as the ratio to solvent control (water for S.B. or ethanol for baicalin) and are mean ± SD. (n = 3). (B) Chick aortic rings were placed in Matrigel and fed with an MCDB-131 serum-free medium. After 24 h, S. baicalensis (200 μg/mL) was added to the culture. Sprout formation from aorta samples were examined both before and 2 days after S. baicalensis treatment. (C) The effects of baicalin (25 μM) on endothelial cell sprout formation from aorta samples were examined 2 days after baicalin or vehicle treatment. Scale bar, 100 μm.
We further examined the effect of S. baicalensis and baicalin on the vessel sprout formation using the chick aortic arch assay, a widely used ex vivo angiogenesis model.24 In this assay, chick aortic rings were embedded in Matrigel and fed with a medium containing S. baicalensis or baicalin. Sprout formation was examined by microscopy. We found that a greater number of sprouts were formed in the presence of S. baicalensis or baicalin (Figure 6B and C), indicating the treatment-induced microvessel sprouting and angiogenesis.
4. Discussion
The root of S. baicalensis has been widely used in traditional Chinese medicine for thousands of years in the treatment of various diseases, including stroke.25,26 Scutellaria baicalensis and its constituent, baicalin, have also been investigated for their ability to prevent injury caused by ischaemia, which displays similar clinical manifestations to stroke and myocardial infarction.27–30 However, the protective effects of baicalin and S. baicalensis in these cases could not be explained solely by their antioxidant property.27 In this study, we found S. baicalensis- and baicalin-induced VEGF protein and mRNA expression in the culture.
The ability of S. baicalensis to induce VEGF production provides an additional potential mechanism to explain why S. baicalensis prevents cell damage by ischaemia. First, VEGF is one of the most potent angiogenesis factors. Elevated levels of VEGF may enhance myocardial angiogenesis, a desirable response to prevent cardiomyocyte death.31 In fact, many efforts are being made to develop agents that can promote neovascularization in ischaemic tissues.32 In addition to its ability to induce vessel growth, a growing body of evidence has shown that VEGF is a survival factor that can enhance cardiomyocytes and neuronal cell survival and reduce infarct size in the brain and heart.33 Therefore, it is conceivable that S. baicalensis could be used to treat conditions that require angiogenesis, including wound healing, cardiovascular, and ischaemic disease tissue repair.2,3,34,35 The potential of using natural product-derived molecules as candidates for therapeutic neovascularization has been documented previously and includes resveratrol, ginseng, curcumin, and sokotrasterol sulfate,17,18,36–38 among others. Scutellaria baicalensis has been associated with the prevention and treatment of cardiovascular diseases26 and treatment of foot ulcers in diabetic patients.34 In addition, it has also been used in treating gastrointestinal ulcers. Its clinical benefits may be attributed to the stimulatory effect of S. baicalensis on VEGF expression, as VEGF has been shown to promote the healing of gastrointestinal ulcers, including inflammatory bowel disease.39
Although our study suggested the modulation of angiogenesis as one mechanism to explain the benefiting effects of baicalin and S. baicalensis, such as in treating condition caused by ischaemic stroke, further studies are needed to better explore the therapeutic potential of baicalin and S. baicalensis. First, little is known about the metabolism of baicalin and S. baicalensis in the human body. Furthermore, baicalin could undergo some modifications by liver enzymes, such as glucuronidation, sulfation, and etc. These modifications are highly variable depending on each individual.40 How baicalin is metabolized and modified and how these metabolites affect its biological activity needs to be further investigated. Finally, although S. baicalensis has been used in traditional Chinese medicine for the treatment of ischaemic stroke, no comprehensive clinical trial has been conducted to demonstrate the efficacy of this substance. Further study is needed to understand whether these substances can improve pathological conditions caused by ischaemic stroke by inducing VEGF in vivo and whether patients with stroke can benefit from this activity.
HIF-1α/HRE is one of the most important pathways regulating VEGF expression; however, it is unlikely the major pathway that mediates VEGF expression induced by baicalin or S. baicalensis, given that baicalin and S. baicalensis treatment: (i) had little effect on cellular production of HIF-1α; (ii) activated reporter genes under a VEGF promoter containing a defective HRE; (iii) further enhanced VEGF expression under hypoxia, contrary to the effects of DFX; and (iv) had minimal effects on reporter gene expression under the HRE promoter. Our results collectively suggested that the activation of the ERRα and PGC-1α pathway was most likely involved in VEGF induction by baicalin and S. baicalensis. PGC-1α and ERRα, key regulators of mitochondrial biogenesis, have been shown to effectively regulate VEGF expression independent of HIF-1α.13 ERRα is constitutively active in the absence of exogenous ligand.10 A recent study has shown that ERR activity can be further increased by several isoflavones and one flavone via binding to ERR.41 Here, we found that baicalin and S. baicalensis could induce ERRα activity through a novel mechanism by up-regulating ERRα expression. Although the expression of PGC-1α was not induced by baicalin, down-regulation of PGC-1α by PGC-1α siRNA would lead to the decreased interaction between PGC-1α and ERRα and reduced expression of VEGF. Elevated level of ERRα by baicalin would promote the interaction between ERRα and PGC-1α and stimulate VEGF expression. Further research is required to elucidate the mechanism underlying the activation.
Although both baicalin and S. baicalensis can induce VEGF expression, we observed that they differed in potency. It is possible that the S. baicalensis extract contained additional components other than baicalin that could also affect VEGF expression in either a positive or a negative manner.
Baicalein, another major component in the root of S. baicalensis, also induced VEGF expression (data not shown), which was consistent with a previous report showing induction of VEGF expression independent of HIF-1α expression.42 Our preliminary results showed that a different mechanism might be involved in the activation of VEGF, since baicalein alone did not induce reporter expression driven by ERRE (data not shown).
Although we find that baicalin can induce angiogenesis by stimulating VEGF expression, it has been previously shown to inhibit angiogenesis by suppressing endothelial cell proliferation, migration, and tube formation in vitro, as well as vessel formation in chicken chorioallantoic membrane.43 Although previous study was focused on the effect of baicalin on endothelial cells, our results were focused on the effect of baicalin on VEGF expression, a different aspect in regulating angiogenesis. It is possible that baicalin has both pro-angiogenesis (via elevating VEGF expression as shown in our study) and anti-angiogenesis (via suppressing endothelial cell function as shown in the previous study) activities. Previous studies have also suggested that S. baicalensis, as well as baicalein, could function as a potent anti-coagulant agent.44 Since many clotting-related proteins have been previously shown to play roles in angiogenesis,45 it is possible that S. baicalensis may also be able to regulate angiogenesis through modulating coagulation pathway. The overall effect of S. baicalensis, baicalin or baicalein, on angiogenesis might rely on its concentration and the context of its action site.
Baicalin belongs to the family of flavonoids, present in fruits, vegetables, and plant-derived beverages, as well as in many dietary supplements.46 As components of dietary supplements, flavonoids have attracted great attention in the prevention and treatment of cardiovascular diseases.46 Flavonoids have been shown to both negatively and positively influence VEGF expression. For example, catechins such as EGCG have been reported to both inhibit and stimulate VEGF expression;47,48 apigenin and chrysin to inhibit VEGF expression,49,50 and quercetin to induce VEGF expression through increasing HIF-1α expression.39 In this study, we found baicalin-induced VEGF expression without increasing HIF-1α. To our knowledge, this is the first example that a flavonoid can increase VEGF expression through the activation of the ERRα/PGC-1α pathway. It remains to be defined how each flavonoid specifically affects VEGF expression.
In summary, our data implicated baicalin and S. baicalensis in the induction of VEGF expression in various cell types, which was, at least in part, mediated through the activation of the ERRα/PGC-1α pathway. Although future research is needed to further elucidate S. baicalensis-induced signalling pathways, our study provides a mechanism by which S. baicalensis and baicalin exert their biological activity. These data may assist in the development of improved approaches to stimulate angiogenesis, as well as facilitate a better understanding of the potential health benefits of these agents in the treatment of cardiovascular diseases.
Funding
This work was supported by Stop Cancer Foundation, Concern Foundation and Markel Friedman Fund (W.W.) and NIH grant RO1 ES08258 (S.C.).
Acknowledgements
We thank Silvia da Costa for critical reading of this manuscript and Richard Jove for his valuable suggestions. We thank Marina Schorpp-Kistner (DKFZ, Germany) for providing VEGF reporter plasmids, VEGF(HRE)-Luc and VEGF(mHRE)-Luc; Andrew Kung (Dana-Farber Cancer Institute, Boston, MA, USA) for providing HRE luciferase reporter plasmid, HRE-Luc.
Conflict of interest: none declared.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting Hif-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13:5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 8.Pages G, Pouyssegur J. Transcriptional regulation of the vascular endothelial growth factor gene—a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Arany Z, Foo S-Y, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1[agr] Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 10.Stein RA, McDonnell DP. Estrogen-related receptor {alpha} as a therapeutic target in cancer. Endocr Relat Cancer. 2006;13:S25–S32. doi: 10.1677/erc.1.01292. [DOI] [PubMed] [Google Scholar]
- 11.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 14.Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, et al. Estrogen-related receptor alpha is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res. 2008;68:8805–8812. doi: 10.1158/0008-5472.CAN-08-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry TD, Abraham JA. Review of preclinical and clinical results with vascular endothelial growth factors for therapeutic angiogenesis. Curr Interv Cardiol Rep. 2000;2:228–241. [PubMed] [Google Scholar]
- 16.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 17.Murphy S, Larrivee B, Pollet I, Craig KS, Williams DE, Huang XH, et al. Identification of sokotrasterol sulfate as a novel proangiogenic steroid. Circ Res. 2006;99:257–265. doi: 10.1161/01.RES.0000233316.17882.33. [DOI] [PubMed] [Google Scholar]
- 18.Sefcik LS, Petrie Aronin CE, Botchwey EA. Engineering vascularized tissues using natural and synthetic small molecules. Organogenesis. 2008;4:215–227. doi: 10.4161/org.4.4.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkoshi E, Nagashima T, Sato H, Fujii Y, Nozawa K, Nagai M. Simple preparation of baicalin from Scutellariae Radix. J Chromatogr A. 2009;1216:2192–2194. doi: 10.1016/j.chroma.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Zhang K, Chen S, Wen W. Grape seed extract inhibits VEGF expression via reducing HIF-1alpha protein expression. Carcinogenesis. 2009;30:636–644. doi: 10.1093/carcin/bgp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt D, Textor B, Pein OT, Licht AH, Andrecht S, Sator-Schmitt M, et al. Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis. EMBO J. 2007;26:710–719. doi: 10.1038/sj.emboj.7601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Wen W, Lu J, Zhang K, Chen S. Grape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signaling pathway. Cancer Prev Res. 2008;1:554–561. doi: 10.1158/1940-6207.CAPR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 25.Kubo M, Kimura Y, Odani T, Tani T, Namba K. Studies on scutellariae radix. Planta Med. 1981;43:194–201. doi: 10.1055/s-2007-971499. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- 27.Woo AYH, Cheng CHK, Waye MMY. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc Res. 2005;65:244–253. doi: 10.1016/j.cardiores.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Shao ZH, Li CQ, Vanden Hoek TL, Becker LB, Schumacker PT, Wu JA, et al. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 1999;31:1885–1895. doi: 10.1006/jmcc.1999.1021. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Sun X, Fang JS, Zhang M, Sucher NJ. Flavonoids from Radix Scutellariae as potential stroke therapeutic agents by targeting the second postsynaptic density 95 (PSD-95)/disc large/zonula occludens-1 (PDZ) domain of PSD-95. Phytomedicine. 2004;11:277–284. doi: 10.1078/0944711041495173. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z-J, Li P, Wang Z, Li P-T, Zhang W-S, Sun Z-H, et al. A comparative study on the individual and combined effects of baicalin and jasminoidin on focal cerebral ischemia-reperfusion injury. Brain Res. 2006;1123:188–195. doi: 10.1016/j.brainres.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, et al. Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol. 2004;36:547–559. doi: 10.1016/j.yjmcc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Renault M-A, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74:159–171. doi: 10.1016/j.mvr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 34.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 35.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 36.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. Journal of Molecular and Cellular Cardiology. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, et al. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- 38.Kiran MS, Kumar VB, Viji RI, Sherin GT, Rajasekharan KN, Sudhakaran PR. Opposing effects of curcuminoids on serum stimulated and unstimulated angiogenic response. J Cell Physiol. 2008;215:251–264. doi: 10.1002/jcp.21307. [DOI] [PubMed] [Google Scholar]
- 39.Jeon H, Kim H, Choi D, Kim D, Park S-Y, Kim Y-J, et al. Quercetin activates an angiogenic pathway, hypoxia inducible factor (HIF)-1-vascular endothelial growth factor, by inhibiting HIF-prolyl hydroxylase: a structural analysis of quercetin for inhibiting HIF-prolyl hydroxylase. Mol Pharmacol. 2007;71:1676–1684. doi: 10.1124/mol.107.034041. [DOI] [PubMed] [Google Scholar]
- 40.Lai MY, Hsiu SL, Chen CC, Hou YC, Chao PD. Urinary pharmacokinetics of baicalein, wogonin and their glycosides after oral administration of Scutellariae Radix in humans. Biol Pharm Bull. 2003;26:79–83. doi: 10.1248/bpb.26.79. [DOI] [PubMed] [Google Scholar]
- 41.Suetsugi M, Su L, Karlsberg K, Yuan Y-C, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- 42.Cho H, Lee H-Y, Ahn D-R, Kim SY, Kim S, Lee KB, et al. Baicalein induces functional hypoxia-inducible factor-1{alpha} and angiogenesis. Mol Pharmacol. 2008;74:70–81. doi: 10.1124/mol.107.040162. [DOI] [PubMed] [Google Scholar]
- 43.Liu JJ, Huang TS, Cheng WF, Lu FJ. Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- 44.Liu XF, Liu ML, Iyanagi T, Legesse K, Lee TD, Chen SA. Inhibition of rat liver NAD(P)H:quinone acceptor oxidoreductase (DT-diaphorase) by flavonoids isolated from the Chinese herb scutellariae radix (Huang Qin) Mol Pharmacol. 1990;37:911–915. [PubMed] [Google Scholar]
- 45.Browder T, Folkman J, Pirie-Shepherd S. The hemostatic system as a regulator of angiogenesis. J Biol Chem. 2000;275:1521–1524. doi: 10.1074/jbc.275.3.1521. [DOI] [PubMed] [Google Scholar]
- 46.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 47.Zhou YD, Kim YP, Li XC, Baerson SR, Agarwal AK, Hodges TW, et al. Hypoxia-inducible factor-1 activation by (-)-epicatechin gallate: potential adverse effects of cancer chemoprevention with high-dose green tea extracts. J Nat Prod. 2004;67:2063–2069. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sartippour MR, Shao Z-M, Heber D, Beatty P, Zhang L, Liu C, et al. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 49.Fang J, Zhou Q, Liu L-Z, Xia C, Hu X, Shi X, et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1{alpha} and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 50.Fu B, Xue J, Li Z, Shi X, Jiang B-H, Fang J. Chrysin inhibits expression of hypoxia-inducible factor-1{alpha} through reducing hypoxia-inducible factor-1{alpha} stability and inhibiting its protein synthesis. Mol Cancer Ther. 2007;6:220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]