Abstract
Aims
Hypoxia is a common stress to the foetus and results in increased cardiac vulnerability to adult ischaemic injury. This study tested the hypothesis that foetal hypoxia causes programming of increased AT2 receptor (AT2R) expression in the heart, resulting in the heightened cardiac susceptibility to adult ischaemic injury.
Methods and results
Time-dated pregnant rats were divided between normoxic and hypoxic (10.5% O2 from days 15 to 21 of gestation) groups. Hypoxia resulted in significantly increased AT2R in the heart of adult offspring. Multiple glucocorticoid response elements (GREs) were identified at the AT2R promoter, deletion of which increased the promoter activity. Consistently, ex vivo treatment of isolated foetal hearts with dexamethasone for 48 h decreased AT2R expression, which was inhibited by RU 486. Hypoxia decreased glucocorticoid receptors (GRs) in the hearts of foetal, 3-week-old and 3-month-old offspring, resulting in decreased GR binding to the GREs at the AT2R promoter. The inhibition of AT2R improved postischaemic recovery of left ventricular function and rescued the foetal hypoxia-induced cardiac ischaemic vulnerability in male adult animals. In contrast, the inhibition of AT1 receptors decreased the postischaemic recovery.
Conclusion
The results demonstrate that in utero hypoxia causes programming of increased AT2R gene expression in the heart by downregulating GR, which contributes to the increased cardiac vulnerability to adult ischaemic injury caused by prenatal hypoxic exposure.
Keywords: Foetal programming, Hypoxia, Heart, Angiotensin II receptors, Glucocorticoids
1. Introduction
Epidemiological and animal studies have shown a clear association of adverse intrauterine environment with an increased risk of hypertension and ischaemic heart disease in the adulthood.1–4 Hypoxia is one of the most important and clinically relevant stresses that can adversely affect foetal development. There is clear evidence of a link between hypoxia and foetal intrauterine growth restriction and an increased risk of cardiovascular disease in offspring.5–13 Animal studies have demonstrated that foetal hypoxia causes a significant increase in the number and size of binucleated myocytes in the foetal heart,14 resulting in the heightened heart susceptibility to acute ischaemia and reperfusion injury in adult male offspring in a sex-dependent manner.10,15–17
Angiontensin II (Ang II) plays a fundamental role in the regulation of cardiovascular homeostasis, and it has been implicated in programming of cardiovascular disease induced by adverse in utero environment during the foetal development.18–22 Recent studies have demonstrated a link between foetal insults to differential epigenetic modifications of type 1 (AT1R) and type 2 (AT2R) Ang II receptor genes in the adrenal and kidney and the resultant alteration of their expression patterns in adult life, which may ultimately lead to the development of hypertension.22–24 However, the effect of foetal hypoxia on the ontogeny of Ang II receptors in the heart has not been determined. Both AT1R and AT2R are expressed in cardiac myocytes and have significant pathophysiological roles in heart diseases.25–29 Yet the effect of AT1R and AT2R on ischaemia and reperfusion injury in the heart remains controversial, depending on systemic vs. local blockade, as well as chronic vs. acute blockade of AT1R and AT2R. Although long-term systemic administration of AT1R antagonists reduced ischaemic injury,30 studies of the acute effects of AT1R and AT2R antagonists on the recovery of left ventricular function in the setting of ischaemia and reperfusion injury in isolated working rat hearts suggested a cardioprotective effect of AT2R blockade, but not AT1R blockade.31,32 The expression of both AT1R and AT2R is regulated by glucocorticoids.25 It has been suggested that, in rats, glucocorticoids play an important role in foetal programming of AT1R and AT2R expression patterns in offspring.33 The present study tests the hypothesis that foetal hypoxia causes programming of increased AT2R gene expression in the heart by down-regulating glucocorticoid receptors (GRs), which contributes to the increased ischaemic vulnerability of the heart in adult rats, resulting from foetal hypoxia.
2. Methods
An expanded Methods section is available in the Supplementary material online.
2.1. Experimental animals
Pregnant rats were randomly divided into two groups: (i) normoxic control (n = 12), and (ii) hypoxic treatment of 10.5% O2 from days 15 to 21 of gestation (n = 12), as described previously.17 Half of the normoxic and hypoxic animals were killed at day 21 of gestation and the foetuses were removed for the studies. The other half of pregnant rats were allowed to give birth and offspring were studied at 3 weeks and 3 months old. Each experimental group had five animals from different dams. Hearts were isolated from the 21-day foetuses, 3-week-old and 3-month-old offspring. For western immunoblots and DNA isolation, hearts were flash frozen in liquid N2 and stored at −80°C until analysis. RNA was extracted immediately and stored at −80°C. For ex vivo studies, separate pregnant rats were used and hearts were isolated from day 17 foetal rats and cultured in M199 media at 37°C in 95% air/5% CO2.34 Previous work has demonstrated that the intact heart can survive and beat for at least 6 days in M199 media and up to 3 weeks within ideal conditions.34,35 These hearts in organ culture did not suffer from a loss of contractile function or any dedifferentiation.35 After 24 h of recovery, hearts were treated with dexamethasone and/or RU 486 (GR antagonist) for 48 h in seven experimental groups: control, 0.01 μM, 0.1 μM, 1 μM dexamethasone treatments, 1 μM dexamethasone plus 0.1 μM or 1 μM RU 486 treatments, and 1 μM RU 486 alone. Each experimental group had six animals from different dams. All procedures and protocols were approved by the Institutional Animal Care and Use Committee, and followed the guidelines by US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Western blot analysis
Protein was isolated from the hearts, and AT1R, AT2R, and GR protein abundance were measured with western blot analysis using the primary antibodies against AT1R (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100 dilution), AT2R (Santa Cruz; 1:200 dilution), and GR (Santa Cruz; 1:2000 dilution). Each experimental group had samples from five animals. To assure equal loading and minimize any confounding influence of variability among gels, an internal standard sample was loaded to each gel and band intensities were normalized to actin and the internal control.
2.3. Real-time RT–PCR
RNA was extracted from the hearts and mRNA abundance of AT1aR, AT1bR, and AT2R was determined by real-time RT–PCR.
2.4. Site-directed mutagenesis and reporter gene assay
Rat AT2R promoter sequence was obtained from rat genome data base (http://www.ncbi.nlm.nih.gov/mapview). Genomic DNA isolated from rat hearts was used as PCR template for DNA amplification. A 2130 bp fragment spanning −2080 bp to +49 bp relative to the transcriptional start site was amplified and cloned into pCR4-TOPO vector. The KpnI/XhoI fragment flanking the AT2R promoter region was then inserted into the luciferase reporter gene plasmid, pGL3 to yield the full-length promoter–reporter plasmid. Promoter analyses identified the presence of multiple glucocorticoid response elements (GREs). Site-specific deletions of GREs were constructed, respectively, and the reporter gene assay was performed using a rat embryonic heart-derived myogenic cell line H9c2, as described previously.34
2.5. Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were collected from the hearts, and electrophoretic mobility shift assay (EMSA) was performed using the oligonucleotide probes of GREs at rat AT2R promoter region, as described previously.34
2.6. Hearts subjected to ischaemia and reperfusion
Hearts of 3-month-old offspring were isolated and retrogradely perfused via the aorta in a modified Langendorff apparatus, as previously described.17 After the baseline recording, hearts were perfused for 5 min in the absence or presence of losartan (1 μM, a selective AT1R antagonist), or PD 123,319 (0.3 μM, a selective AT2R antagonist), or losartan plus PD 123,319, followed by subjection to 20 min of global ischaemia and 30 min of reperfusion, an approach used in many previous studies in a Langendorff preparation.36–38 Previous studies with prolonged reperfusion from 60 to 180 min showed that myocardial infarction and left ventricular recovery reached a plateau at approximately 30 min of reperfusion.10,38,39 Each experimental group had hearts from five animals. Left ventricular developed pressure (LVDP), heart rate (HR), dP/dtmax, dP/dtmin, and LV end-diastolic pressure (LVEDP) were continuously recorded. Myocardial infarct size was measured at the end of reperfusion with 1% triphenyltetrazolium chloride, and was expressed as a percentage of the total left ventricular weight. Lactate dehydrogenase (LDH) activity was measured in coronary effluent collected at 30 s before the onset of ischaemia, and at 0, 1, 2, 3, 4, 5, 10, 15, 20, and 30 min of reperfusion, using TOX 7 assay kit from Sigma following the manufacture's instructions. The data were expressed as area under curve (AUC).
2.7. Statistical analysis
Data are expressed as mean ± SEM. Statistical significance (P < 0.05) was determined by analysis of variance (ANOVA) followed by Neuman–Keuls post hoc testing or Student's t-test, where appropriate.
3. Results
3.1. Effect of development and foetal hypoxia on AT1R and AT2R protein and mRNA abundance in the heart
Protein and mRNA abundance of both AT1R and AT2R showed a development-dependent reduction in the heart, and no sex difference was observed (Figure 1A and B). Whereas the expression levels in both 3-week-old and 3-month-old offspring were significantly decreased, when compared with those in the foetus, there were no significant differences in the expression levels between 3-week-old and 3-month-old offspring. In 3-month-old offspring, AT2R mRNA decreased to less abundance than that of AT1aR and AT1bR, resulting in a significant decrease in the AT2R/AT1R receptor ratio in the adult heart. Maternal hypoxia had no significant effect on the litter size (11.2 ± 0.9 vs. 12.0 ± 0.8). Previous studies in the same rat model showed that maternal hypoxia resulted in a decrease in birth weight, but had no significant effect on body weight of 3-month-old offspring.10,17 There was a significant decrease in protein abundance of AT1R, but not AT2R, resulting in an increased AT2R to AT1R ratio in hypoxic vs. control foetal hearts (Figure 1C). This was associated with a decrease in AT1bR mRNA (Figure 1D). The same expression pattern persisted in the hearts of 3-week-old male offspring, whereas no significant differences in AT1R and AT2R were observed in females. In 3-month-old offspring, prenatal hypoxia increased protein (Figure 1C) and mRNA (Figure 1D) abundance of AT2R, but not AT1R, in the male heart. In females, both AT1R and AT2R were increased and the AT2R to AT1R ratio was not significantly changed (Figure 1C). Consistently, AT1bR and AT2R mRNA abundance were significantly increased in the female heart (Figure 1D).
Figure 1.
Effect of development and foetal hypoxia on AT1R and AT2R protein and mRNA abundance. Hearts were isolated from near-term (21 days) foetuses, 3-week-old (3W) and 3-month (3M)-old male (M) and female (F) offspring in the control (C) and hypoxic (H) groups. Effect of development on receptor protein (A) and mRNA (B) abundance and the effect of hypoxia on receptor protein (C) and mRNA (D) levels are shown. Data are means ± SEM. Data in (A) were analysed by two-way ANOVA. Data in (B)–(D) were analysed by one-way ANOVA. *P < 0.05, offspring vs. foetus; †P < 0.05, hypoxia vs. control. n = 5.
3.2. Inhibitory effect of GREs on the AT2R promoter activity
Rat AT2R promoter has a TATAA element at −46 from the transcription start site (see Supplementary material online, Figure S1). Deletion of the TATAA element significantly decreased the promoter activity (Figure 2A). Multiple GREs were identified at rat AT2R promoter. These include GRE1 (−1853), GRE2 (−1674), GRE3 (−1526), GRE4 (−1159), GRE5 (−945), GRE6 (−676), GRE7 (−107), and GRE8 (+13) (see Supplementary material online, Figure S1). Site-specific deletion of each GRE independently caused a significant increase in the promoter activity (Figure 2A). While the GRE4 deletion stimulated the luciferse activity by 2.43-fold, deletion of other GREs increased the promoter activity by 1.5- to 1.9-fold.
Figure 2.
Effect of GREs on the AT2R promoter activity. (A) AT2R promoter reporter gene constructs of wild-type (WTAT2R) and site-specific deletion of GREs and TATA were transiently co-transfected with pRL-SV40 driven R. reniformis luciferase in a rat embryonic heart-derived myogenic cell line H9c2. After 48 h, firefly and R. reniformis luciferase activities in cell extracts were measured using a dual-luciferase reporter assay system. The promoter activities were then calculated by normalizing the firefly luciferase activities to R. reniformis luciferase activity. Data are means ± SEM. *P < 0.05 (one-way ANOVA), deletions vs. wild-type WTAT2R. n = 6. (B) GR in nuclear extracts (NE) was demonstrated by western blot. Supershift with GR antibody (Ab) was used to identify protein shift at GRE6 binding site. r-hGR, human recombinant GR.
3.3. Binding of GRs to the GREs at the AT2R promoter
Binding of nuclear proteins to the putative GREs at the AT2R promoter was evaluated by EMSA. Sequences of GRE oligos used in EMSA were presented in Supplementary material online, Figure S2. Immunoblot analysis confirmed the presence of GRs in the nuclear extracts used in EMSA (Figure 2B, left panel). We first defined our criteria by analysing GRE6, as it was originally suggested at the AT2R promoter.40 As shown in Figure 2B (right panel), incubation of nuclear extracts from rat hearts with double-stranded oligonucleotide probes of GRE6 resulted in a shift of DNA–protein complex, which was further supershifted by an anti-GR antibody. Extended studies showed identical electrophoretic mobility of protein–DNA complexes for GREs 1, 2, 3, 4, 5, 7, and 8 with that of GRE6 in EMSA, which were blocked by the cold competitions with homologous, heterologous (AT1aR GRE), and the consensus GRE (CGRE) oligos (see Supplementary material online, Figure S2).
3.4. Dexamethasone inhibits AT2R expression in the heart
Dexamethasone treatment for 48 h produced a dose-dependent decrease in mRNA and protein abundance of AT2R in the intact foetal rat hearts (see Supplementary material online, Figure S3). RU 486 alone had no effect on AT2R mRNA but blocked the dexamethasone-induced reduction of AT2R mRNA abundance (see Supplementary material online, Figure S3).
3.5. Effect of development and foetal hypoxia on GR abundance and GR binding to GREs at the AT2R promoter
GR abundance in the heart was significantly increased in offspring when compared with the foetus (Figure 3A). However, the expression levels were not significantly different between 3-week-old and 3-month-old offspring. Additionally, no sex difference was observed (Figure 3A). Hypoxia decreased GR expression in the foetal heart, which was sustained in the hearts of both 3-week-old and 3-month-old offspring in a sex-independent manner (Figure 3B). Nuclear GR protein abundance was also decreased in 3-week-old and 3-month-old offspring (Figure 3B). In accordance, there were similar extent decreases in GR bindings to GREs 4, 6, 7, and 8 at the AT2R promoter in hearts of 3-month-old offspring (Figure 4A). The binding affinity of GR to GREs was determined in competition studies performed in pooled nuclear extracts from the hearts of adult offspring with the increasing ratio of unlabelled/labelled oligonucleotides encompassing the GRE4 at −1159 in the AT2R promoter. Foetal hypoxia had no significant effect on the binding affinity of nuclear extracts to the GRE in the hearts of both male and female adult rats (Figure 4B).
Figure 3.
Effect of development (A) and foetal hypoxia (B) on GR protein abundance. Hearts were isolated from near-term (21 days) foetuses, 3-week-old (3W) and 3-month (3M)-old male (M) and female (F) offspring in the control (C) and hypoxic (H) groups. Total cellular (T) and nuclear (NE) GR abundance was determined. Data are means ± SEM. Data in (A) were analysed by two-way ANOVA. *P < 0.05, offspring vs. foetus. n = 5. Data in (B) were analysed by t-test, *P < 0.05, hypoxia vs. control. n = 5.
Figure 4.
Effect of foetal hypoxia on GRE binding at the AT2R promoter. (A) Binding of the GR to GREs was determined by EMSA in nuclear extracts from the hearts of 3-month-old male and female offspring in the control (C) and hypoxic (H) groups. Data are means ± SEM. *P < 0.05 (t-test), hypoxia vs. control. n = 5. (B) The binding affinity of GR to GREs was determined in competition studies performed in pooled nuclear extracts from the hearts of adult offspring with the increasing ratio of unlabelled/labelled oligonucleotides encompassing the GRE4 at −1159 in the AT2R promoter.
3.6. Functional role of AT2R in acute ischaemia and reperfusion injury
The functional significance of AT2R in modulating the postischaemic recovery of left ventricle (LV) function after acute ischaemia was determined in a Langendorff preparation using a selective AT2R inhibitor, PD 123,319. As shown in Supplementary material online, Table S1, there were no significant differences in LVDP, HR, dP/dtmax, dP/dtmin, and coronary flow rate at the baseline among all groups. PD 123,319 significantly improved the postischaemic recovery of LVDP (Figure 5A and B), as well as dP/dtmax and dP/dtmin (see Supplementary material online, Figure S4) in both male and female hearts. Consistently, PD 123,319 decreased LVEDP (see Supplementary material online, Figure S4), myocardial infarct size, and LDH release (Figure 5C and D) after myocardial ischaemia in both male and female animals. In contrast, an AT1R selective inhibitor losartan impaired the postischaemic recovery of LVDP (Figure 5A and B) and dP/dtmax and dP/dtmin (see Supplementary material online, Figure S4), and significantly increased LVEDP (see Supplementary material online, Figure S4), myocardial infarct size, and LDH release (Figure 5C and D). In the presence of both PD 123,319 and losartan, there were no significant differences in the postischaemic recovery of LV function and myocardial infarction (Figure 5; see Supplementary material online, Figure S4). The recovery of HR and coronary flow rate was not significantly different among all groups (data not shown).
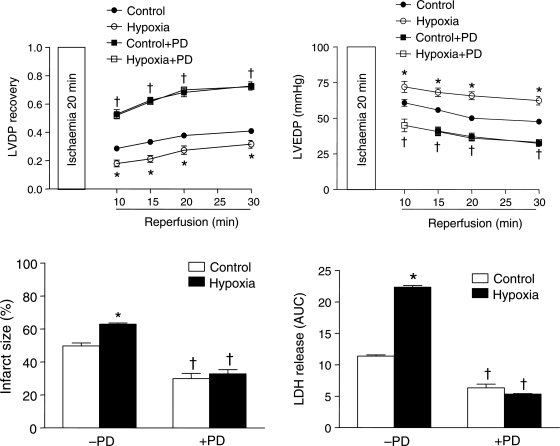
Figure 5.
Effect of AT1R and AT2R inhibitors on cardiac ischaemia and reperfusion injury. Hearts were isolated from 3-month-old male and female rats and were pretreated in the absence or presence of losartan (1 μM), or PD123,319 (PD, 0.3 μM), or losartan + PD for 5 min before subjecting to 20 min of ischaemia and 30 min of reperfusion in a Langendorff preparation. Post-ischaemic recovery of LVDP and infarct size were determined. LDH release over 30 min of reperfusion was measured as AUC. Data are means ± SEM. Data in (A) and (B) were analysed by two-way ANOVA. Data in (C) and (D) were analysed by one-way ANOVA. *P < 0.05, treatment vs. control. n = 5.
3.7. Inhibition of AT2R restores foetal hypoxia-induced cardiac vulnerability to ischaemic injury in offspring
To determine whether increased AT2R in the heart is an important factor in the foetal hypoxia-induced increase in cardiac ischaemic susceptibility in adult male offspring, additional studies were performed in the hearts of adult offspring that had been exposed to hypoxia before birth. In the absence of PD 123,319, foetal hypoxia was associated with a significant decrease in postischaemic recovery of LVDP and increases in LVEDP, infarct size, and LDH release (Figure 6), as well as decreases in the recovery of dP/dtmax and dP/dtmin (see Supplementary material online, Figure S5), as previously reported.17 PD 123,319 increased postischaemic recovery of left ventricular function and abolished the difference in ischaemic injury observed between the control and hypoxic rats (Figure 6 and see Supplementary material online, Figure S5).
Figure 6.
Rescue effect of PD123,319 on hypoxia-mediated ischaemic vulnerability. Hearts were isolated from 3-month-old male offspring that had been exposed to normoxia (control) or hypoxia before birth, and were treated in the absence or presence of PD123,319 (0.3 μM) for 5 min before subjecting to 20 min of ischaemia and 30 min of reperfusion in a Langendorff preparation. Post-ischaemic recovery of LVDP, end-diastolic pressure (LVEDP), and infarct size were determined. LDH release over 30 min of reperfusion was measured as AUC. Data are means ± SEM. Data were analysed by two-way ANOVA. *P < 0.05, hypoxia vs. control; †P < 0.05, +PD123,319 vs. −PD123,319. n = 5.
4. Discussion
The present study demonstrates for the first time that foetal hypoxia results in programming of differential expression patterns of angiotensin II receptors in the heart of offspring. Whereas maternal hypoxia had no effect on the litter size, it decreased the birth weight, which could contribute to the changes observed. The finding that prenatal hypoxia increased AT2R expression in adult hearts is intriguing and suggests epigenetic reprogramming of a foetal gene of pathophysiological significance in the heart in a developmental-dependent manner. Consistent with the previous studies,40–42 we identified the TATAA box region at the AT2R promoter, which plays a critical role in the transcription of AT2R gene in rat hearts. The sequences of multiple GREs identified at rat AT2R gene promoter are imperfect representation (half sites) of the positive regulatory CGRE sequence, 5′-GGTACAnnnTGTTCT-3′.43 The finding of the inhibitory effects of these GREs on the AT2R promoter activity suggests a novel mechanism of glucocorticoids in regulating the ontogeny of cardiac AT2R gene expression patterns during the development. Normal foetal adrenal produces low levels of glucocorticoids. Among other functions, the low foetal glucocorticoid levels may contribute to maintaining the relatively high levels of AT2R in foetal tissues, whereas the substantial increases in circulating glucocorticoid levels and GR abundance in the heart during the postnatal development are likely to play a key role in down-regulating AT2R in the adult in a tissue-specific manner. This is supported by the finding that dexamethasone caused a dose-dependent decrease in AT2R protein and mRNA expression in the heart, which was inhibited by RU486, indicating a GR-mediated response.
The finding that foetal hypoxia caused significant decreases in both total and nuclear GR protein abundance in the heart of offspring provides a mechanism that may contribute to the partial reversal of glucocorticoid-dependent downregulation of AT2R, resulting in the increased AT2R expression in the heart. Indeed, foetal hypoxia resulted in about a 50% decrease in GR binding to the GREs at the AT2R promoter in adult hearts, which were similar to the extent of decreased nuclear GR protein abundance. The finding that the binding affinity of nuclear extracts to the GREs in the hearts was not significantly different between control and foetal hypoxic animals suggests that the decreased GR binding to the GREs at rat AT2R promoter is mainly mediated by the reduced GR density. In the present study, the foetal origin of hypoxia-mediated repression of GR expression was demonstrated in the foetal heart. The sustained downregulation of GR density in adult hearts suggests an epigenetic modification of GR gene repression, albeit the mechanisms are not known at present. The inert response of AT2R expression to hypoxia in the foetal heart is possibly due to the low glucocorticoid levels in the foetus.
To less extent, AT1bR gene is also negatively regulated by glucocorticoids.44 Unlike in rodents, in large mammals including humans there is only one type of AT1R. In contrast, both humans and rodents have one type of AT2R. Because there is no human equivalent to the rodent AT1bR, it may be difficult to translate the present findings directly into the humans. Nonetheless, although the expression of AT1aR and AT1bR genes in rodents are under different promoter controls, they are functionally and pharmacologically indistinguishable at the protein level. In the present study, the increased AT1R protein abundance in the heart of female adult offspring by foetal hypoxia is mainly mediated by the upregulation of AT1bR gene expression. Although the cause of the gender difference in programming of AT1R expression patterns in the heart remains unclear, it has been shown in rats that foetal corticosterone exposure results in increased AT2R in the kidney of both male and female offspring, but increased AT1R only in females.24 In the presents study, the increased AT1R in hearts of the hypoxic females is likely to balance the elevated AT2R and thus protects the female hearts from the heightened ischaemic injury resulting from prenatal hypoxia.
The finding that foetal hypoxia induced changes in AT1R and AT2R expression patterns in the heart of offspring suggest a possible pathophysiological consequence.25,45 Indeed, our previous studies in the same animal model demonstrated that foetal hypoxia caused an increase in heart susceptibility to ischaemia and reperfusion injury in male offspring in a sex-dependent manner.17 It has been well accepted that long-term, systemic administration of AT1R blockers prevents the deleterious consequences of ischaemia and reperfusion injury and reduces cardiac remodelling. The function that AT2R plays in normal or diseased hearts is much less clear and appears controversial.46–48 The effects of long-term and systemic blockade of AT1R or AT2R (as well as receptor knockout studies) on the heart involve multiple mechanisms, whereas the acute and direct effects of AT1R and AT2R on modulating ischaemia and reperfusion injury may be quite different as those seen in the long-term and systemic effects. Indeed, the present study demonstrates a novel direct cardioprotective effect of AT1R and the opposite effect of AT2R in acute ischaemia reperfusion injury in a gender-independent manner. Given the finding that the coronary flow rate was not altered, the effects are mainly mediated by myocardial cell responses. Furthermore, the finding of the lack of effect with the combination of both AT1R and AT2R blockade indicates an interaction between AT1R and AT2R in the heart, and suggests that the ratio of AT2R to AT1R is an especially important consideration in the cardiac susceptibility to acute ischaemia and reperfusion injury. Supporting this notion, it has been proposed that the pathophysiological function of AT2R is context-specific, i.e. the ratio of AT2R to AT1R.49
The increased ratio of AT2R to AT1R in the heart of adult male offspring demonstrated in the present study is likely to play a key role in the heightened heart susceptibility to acute ischaemia and reperfusion injury in adult male offspring that had been exposed to hypoxia before birth. This is supported by the finding that the blockade of AT2R with PD 123,319 rescued the myocardial phenotype of the increased ischaemic susceptibility in male offspring after prenatal hypoxic exposure, providing the cause-and-effect evidence for the role of increased AT2R in foetal programming of enhanced cardiac vulnerability to acute ischaemic injury. Future studies of developmental overexpression of AT2R in the heart are needed to determine whether the increased AT2R mimics and is sufficient for the hypoxic response. Whereas the mechanisms underlying the different effects of AT1R and AT2R in acute cardiac ischaemic injury remain unclear, it has been known that AT1R promotes cell growth and proliferation, yet AT2R mediates antigrowth and apoptosis.25,50 These apparent opposite effects provide a congruent functional basis for understanding the different effects of AT1R and AT2R in modulating acute cardiac ischaemic injury vs. long-term cardiac remodelling. Our previous study suggested that downregulation of PKCɛ in the heart played a role in the increased ischaemic susceptibility in adult male offspring resulting from prenatal hypoxia.17 Given that AT1R stimulation and AT2R blockade activate PKCɛ and mimic ischaemic preconditioning by reducing infarct size,51–53 it is possible that foetal hypoxia-induced programming of increased AT2R/AT1R ratio in the heart of adult males suppresses the PKCɛ activity, leading to the enhanced susceptibility to ischaemic injury.
The present investigation provides novel evidence of foetal programming of upregulation of the AT2R/AT1R ratio in the heart of adult males, linking foetal hypoxia and the increased susceptibility to ischaemia and reperfusion injury in the heart of adult male offspring in a sex-dependent manner. Given that hypoxia is one of the most important and clinically relevant stresses to the foetus, and that large epidemiological studies indicate a link between in utero adverse stimuli during gestation and an increased risk of ischaemic heart disease in the adulthood, the present study provides a mechanistic understanding worthy of investigation in humans.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health [HL82779 (L.Z.), HL83966 (L.Z.), HL89012 (L.Z.), and HD31226 (L.Z.)].
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. doi:10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. doi:10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. doi:10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. doi:10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 5.Heydeck D, Roigas J, Roigas C, Papies B, Lun A. The catecholamine sensitivity of adult rats is enhanced after prenatal hypoxia. Biol Neonate. 1994;66:106–111. doi: 10.1159/000244097. doi:10.1159/000244097. [DOI] [PubMed] [Google Scholar]
- 6.Roigas J, Roigas C, Heydeck D, Papies B. Prenatal hypoxia alters the postnatal development of beta-adrenoceptors in the rat myocardium. Biol Neonate. 1996;69:383–388. doi: 10.1159/000244335. doi:10.1159/000244335. [DOI] [PubMed] [Google Scholar]
- 7.Butler TG, Schwartz J, McMillen IC. Differential effects of the early and late intrauterine environment on corticotrophic cell development. J Clin Invest. 2002;110:783–791. doi: 10.1172/JCI15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, et al. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. doi:10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- 9.Davis DR, Wilson K, Sam MJ, Kennedy SE, Mackman N, Charlesworth JA, et al. The development of cardiac fibrosis in low tissue factor mice is gender-dependent and is associated with differential regulation of urokinase plasminogen activator. J Mol Cell Cardiol. 2007;42:559–571. doi: 10.1016/j.yjmcc.2006.11.017. doi:10.1016/j.yjmcc.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. doi:10.1016/S1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 11.Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 2004;53:11–17. [PubMed] [Google Scholar]
- 12.Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113:1058–1069. [PubMed] [Google Scholar]
- 13.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. doi:10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H1712–H1719. doi: 10.1152/ajpheart.00898.2003. doi:10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. doi:10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 17.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. doi:10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188:435–442. doi: 10.1677/joe.1.06506. doi:10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- 19.Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans. 1999;27:88–93. doi: 10.1042/bst0270088. [DOI] [PubMed] [Google Scholar]
- 20.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. doi:10.1016/0300-9629(94)00177-U. [DOI] [PubMed] [Google Scholar]
- 21.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 22.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. doi:10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 24.Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, et al. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579:503–513. doi: 10.1113/jphysiol.2006.125773. doi:10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 26.Sechi LA, Griffin CA, Grady EF, Kalinyak JE, Schambelan M. Characterization of angiotensin II receptor subtypes in rat heart. Circ Res. 1992;71:1482–1489. doi: 10.1161/01.res.71.6.1482. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 28.Schneider MD, Lorell BH. AT(2), judgment day: which angiotensin receptor is the culprit in cardiac hypertrophy? Circulation. 2001;104:247–248. doi: 10.1161/01.cir.104.3.247. [DOI] [PubMed] [Google Scholar]
- 29.Xu F, Mao C, Rui C, Xu Z, Zhang L. Cardiovascular effects of losartan and its relevant clinical application. Curr Med Chem. 2009;16:3841–3857. doi: 10.2174/092986709789178046. doi:10.2174/092986709789178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshiyama M, Kim S, Yamagishi H, Omura T, Tani T, Yanagi S, et al. Cardioprotective effect of the angiotensin II type 1 receptor antagonist TCV-116 on ischemia-reperfusion injury. Am Heart J. 1994;128:1–6. doi: 10.1016/0002-8703(94)90002-7. doi:10.1016/0002-8703(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 31.Ford WR, Clanachan AS, Jugdutt BI. Opposite effects of angiotensin AT1 and AT2 receptor antagonists on recovery of mechanical function after ischemia-reperfusion in isolated working rat hearts. Circulation. 1996;94:3087–3089. doi: 10.1161/01.cir.94.12.3087. [DOI] [PubMed] [Google Scholar]
- 32.Ford WR, Khan MI, Jugdutt BI. Effect of the novel angiotensin II type 1 receptor antagonist L-158,809 on acute infarct expansion and acute anterior myocardial infarction in the dog. Can J Cardiol. 1998;14:73–80. [PubMed] [Google Scholar]
- 33.McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. doi:10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. doi:10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildenthal K. Long-term maintenance of spontaneously beating mouse hearts in organ culture. J Appl Physiol. 1971;30:153–157. doi: 10.1152/jappl.1971.30.1.153. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. doi:10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 37.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. doi:10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 38.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, et al. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73:488–496. doi: 10.1016/j.cardiores.2006.11.003. doi:10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315:1125–1135. doi: 10.1124/jpet.105.090803. doi:10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 40.Ichiki T, Kambayashi Y, Inagami T. Transcription of the rat angiotensin II type 2 receptor gene. Biochem Biophys Res Commun. 1996;222:566–571. doi: 10.1006/bbrc.1996.0784. doi:10.1006/bbrc.1996.0784. [DOI] [PubMed] [Google Scholar]
- 41.Koike G, Winer ES, Horiuchi M, Brown DM, Szpirer C, Dzau VJ, et al. Cloning, characterization, and genetic mapping of the rat type 2 angiotensin II receptor gene. Hypertension. 1995;26:998–1002. doi: 10.1161/01.hyp.26.6.998. [DOI] [PubMed] [Google Scholar]
- 42.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- 43.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. doi:10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 44.Bogdarina IG, King PJ, Clark AJ. Characterization of the angiotensin (AT1b) receptor promoter and its regulation by glucocorticoids. J Mol Endocrinol. 2009;43:73–80. doi: 10.1677/JME-09-0036. doi:10.1677/JME-09-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin system. Circulation. 2004;109:8–13. doi: 10.1161/01.CIR.0000096609.73772.C5. doi:10.1161/01.CIR.0000096609.73772.C5. [DOI] [PubMed] [Google Scholar]
- 46.Akishita M, Iwai M, Wu L, Zhang L, Ouchi Y, Dzau VJ, et al. Inhibitory effect of angiotensin II type 2 receptor on coronary arterial remodeling after aortic banding in mice. Circulation. 2000;102:1684–1689. doi: 10.1161/01.cir.102.14.1684. [DOI] [PubMed] [Google Scholar]
- 47.Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, et al. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. doi: 10.1161/01.HYP.0000100445.80029.8E. doi:10.1161/01.HYP.0000100445.80029.8E. [DOI] [PubMed] [Google Scholar]
- 48.Senbonmatsu T, Ichihara S, Price E, Jr., Gaffney FA, Inagami T. Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J Clin Invest. 2000;106:R1–R5. doi:10.1172/JCI10037. [PubMed] [Google Scholar]
- 49.Booz GW. Cardiac angiotensin AT2 receptor: what exactly does it do? Hypertension. 2004;43:1162–1163. doi: 10.1161/01.HYP.0000128531.39964.c0. doi:10.1161/01.HYP.0000128531.39964.c0. [DOI] [PubMed] [Google Scholar]
- 50.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. doi:10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz RJ, Wilson GJ. Selective blockade of AT1 angiotensin II receptors abolishes ischemic preconditioning in isolated rabbit hearts. J Mol Cell Cardiol. 1997;29:129–139. doi: 10.1006/jmcc.1996.0258. doi:10.1006/jmcc.1996.0258. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Tsuchida A, Cohen MV, Downey JM. Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol. 1995;27:883–892. doi: 10.1016/0022-2828(95)90038-1. doi:10.1016/0022-2828(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Clanachan AS, Jugdutt BI. Enhanced expression of angiotensin II type 2 receptor, inositol 1,4, 5-trisphosphate receptor, and protein kinase cepsilon during cardioprotection induced by angiotensin II type 2 receptor blockade. Hypertension. 2000;36:506–510. doi: 10.1161/01.hyp.36.4.506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.