Abstract
Nod1 and Nod2 are members of a family of intracellular innate sensors that participate in innate immune responses to pathogens and molecules released during the course of tissue injury, including injury induced by ischemia. Ischemic injury to the kidney is characterized by renal tubular epithelial apoptosis and inflammation. Among the best studied intracellular innate immune receptors known to contribute to apoptosis and inflammation are Nod1 and Nod2. Our study compared and contrasted the effects of renal ischemia in wild-type mice and mice deficient in Nod1, Nod2, Nod (1×2) and in their downstream signaling molecule Rip2. We found that Nod1 and Nod2 were present in renal tubular epithelial cells in both mouse and human kidney epithelial cells and that the absence of these receptors in mice resulted in protection from kidney ischemia reperfusion injury. Significant protection from kidney injury was seen with a deficiency of Nod2 and Rip2, and the simultaneous deficiency of Nod1 and Nod2 provided even greater protection. We conclude that the intracellular sensors Nod1 and Nod2 play an important role in the pathogenesis of acute ischemic injury of the kidney, although possibly through different mechanisms.
Introduction
The innate immune system is responsible for an organism's initial response to potentially dangerous stressors, such as pathogens or tissue injury, and thus plays an essential role in the pathogenesis of many inflammatory disease processes. The innate response is antigen independent and uses both membrane-bound (e.g. toll-like receptors) and intracytoplasmic [e.g. the nucleotide oligomerization domain (NOD)-like receptors (NLRs)] pattern recognition receptors (PRRs) to recognize conserved molecular motifs. Intracytoplasmic PRRs, such as the NLRs, are putative cytoplasmic ligands for molecules released by injured cells and as such are important initial participants in cellular injury (1). Ligation of NLRs by molecules released from injured cells has been shown to ignite a cascade of molecular signaling cascades that ultimately lead to cell death and inflammation (2, 3).
One clinically important disease process that is known to involve activation of PRRs is acute kidney injury (AKI); modeled experimentally by kidney ischemia reperfusion (IR) injury. Ischemia reperfusion injury of the kidney is a complex pathophysiological process that involves both cell death and inflammation. Kidney IR injury is known to involve the toll-like (TLR) family of innate immune receptors (4–6), but little is known about the role of NLRs in ischemia reperfusion injury. This study is the first to our knowledge to describe the role of the intracellular NLRs in kidney IR injury.
The NLRs are a family of PRRs expressed in both immune cells and epithelial cells. These molecules are multi-domain proteins that serve as scaffolds for the assembly of signaling platforms, triggering the activation of molecules involved in apoptosis and inflammation. Among the NLR family, Nod1 and Nod2 have been identified as key detectors of intracellular microbes (they sense peptidoglycan, a heterogeneous polymer found in the cell walls of bacteria) (7–10) and possibly danger signals (molecules released from dead and dying cells) (11). Nod1 is widely expressed in many cell types and Nod2 has been found in macrophages (12), dendritic cells (13), Paneth cells (14), keratinocytes (15), epithelial cells of the intestine (16), lung (17), oral cavity (18) and osteoblasts (19).
Nod1 and Nod2 share structural and functional characteristics. Both contain ligand binding regions [C-terminal leucine-rich repeats (LRRs)], a central nucleotide-binding oligomerization domain (NACHT) and N-terminal caspase recruitment domains (CARDs) that bind downstream signaling molecules (2). Binding of Nod ligands results in oligomerization of the NACHT domains, which results in CARD binding of downstream proteins, such as the serine-threonine kinase RICK (RIP-like interacting CLARP kinase [also known as receptor-interacting protein 2 (Rip2)] (2). Rip2 is important for Nod-induced NFkB activation, as well as apoptotic signaling through association with members of the TNFR-associated factor (TRAF) family and members of the inhibitor of apoptosis protein (IAP) family, cIAP-1 and cIAP-2 (3). IAP family proteins play prominent roles in regulating programmed cell death by virtue of their ability to bind caspases, intracellular cysteine proteases responsible for apoptosis. Stimulation of Nod1 or Nod2 also results in the secretion of proinflammatory cytokines and chemokines (IL-6, CXCL8/IL-8, KC, and MIP2) and Nod1 activation induces neutrophil recruitment in vivo (20). There is mounting evidence that Nod1 and Nod2 signals are likely to contribute to a variety of human diseases such as Crohn's disease (21), Blau syndrome (22), sarcoidosis (23) and possibly asthma (24).
To begin to define the role of Nod1 and or Nod2 in kidney disease, we examined human and mouse kidneys for intracellular Nod1 and Nod2 expression. To ascertain a linkage to disease we tested the response to kidney IR injury using a well-established murine model. Using this model, we examined the dependence of kidney injury and cytokine/chemokine secretion on Nod1 and Nod2 as well as on their dependent downstream signaling kinase Rip2. We also determined the contributions of Nods 1 and 2 to tubular epithelial apoptosis and to inflammation associated with kidney IR injury.
Materials and Methods
Mice
All mice used in the experiments were housed in the vivarium at The Scripps Research Institute and approved for use by the Laboratory Animal Care and Use Committee of the Animal Research Center at The Scripps Research Institute. Animals were handled according to the recommendations of the Humanities and Sciences and the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care. C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Nod-deficient mice [Nod-1−/−; Nod2−/− and Nod (1 × 2) −/− mice] and Rip2−/− mice were obtained from R. Ulevitch (The Scripps Research Institute). All Nod- and Rip2- deficient mice used in these experiments were bred onto a C57BL/6 background by more than 10 generations.
Detection of Nod 1 and 2 in kidneys of WT mice
Nod1 and Nod2 DNA were detected in renal tubular epithelium (RTE) by semiquantitative PCR. To detect Nod1 and Nod2 in murine RTE, the same protocol was used as noted below (detection in human renal tubular epithelium), except that collagenase D was used instead of collagenase A for murine RTE. The first strand of cDNA of each sample was synthesized from 1 mcg of total RNA using a QuantitectRT kit (Qiagen) according to the manufacturer's instructions. For reverse transcription (RT)-PCR, 1 mcg of total cellular RNA was reverse transcribed, and cDNA amplified. The expression of Nod1 and Nod 2 mRNA in murine RTE was each detected by semiquantitative PCR - with 5'-AAGCATTTCTGCTACCCGGAG-3' as a forward primer and 5-AAAGACATCGGTCAGGGTCAC-3' as a reverse primer for Nod1 mRNA and with 5'-CATCTGGTCACCAACATTCG-3' as a forward primer and 5'-GAAGGGGAGAAGCCAATTTC-3' as a reverse primer for Nod2 mRNA. The level of glyceraldehyde 3-phosphate dehydrogenase (GADPH) mRNA in each murine sample was also determined by PCR using 5'-ACCACAGTCCATGCCATCAC-3' as a forward primer and 5'-TCCACCACCCTGTTGCTGTA-3' as a reverse primer. For detection of Nod1 in human RTE the forward primer was 5'-TCCAAAGCCAAACAGAAACTC-3' and reverse was 5'-CAGCATCCAGATGAACGTG-3'. For detection of Nod2 in human RTE the forward primer was 5'-GAAGTACATCCGCACCGAG-3' and reverse -5'-GACACCATCCATGAGAAGACAG-3'. The GADPH housekeeping gene was detected in the human cells by forward primer 5'-CACACCATCTTCCAGGAGC-3' and reverse - 5'CATGAGTCCTTCCACGATACC-3'. The PCR products were separated on a 1% agarose gel and digital photographs taken on a transilluminator.
Detection of Nod1 and Nod2 in human renal tubular epithelium
Human kidney tissue was obtained from discarded nephrectomies for renal cell carcinoma (IRB:08-5054, Scripps Clinic and Green Hospital). Immediately after the nephrectomy, the renal pathologist removed a wedge section of normal tissue (distal from the site of tumor). After transport to the laboratory in sterile media, the renal capsule was removed and cortex dissected from the medulla. The cortex was then finely minced and placed in collagenase A (Sigma-Aldrich, St. Louis, MO) and incubated at 37°C for 30 min with frequent shaking. After incubation, the digested mixture was differentially sieved (200-50 um) and washed three times with fresh media. The sieved contents were then centrifuged, pelleted and layered over 30% Percoll (Sigma). After spinning the Percoll mixture at 21,500g for 30 minutes, four distinct bands were apparent as previously described (25, 26). The cells were collected from band 3 and washed three times with Hank's Buffered Salt Solution. The cells were then cultured in specialized tubular epithelial cell growth media [DMEM-Ham's-F-12 (Fischer Scientific, Pittsburgh, PA); Insulin (5 mcg/ml), Transferrin (5 mcg/ml), Selenium (50 nmol/l) supplements, hydrocortisone (Sigma, (0.05 uM), epidermal growth factor (Sigma 10gn/ml) Tri-iodo thyronine (32 ng/ml) Penicillin/streptomycin 1 ml, HEPES (15 mmol/L), to promote growth of tubular epithelial cells but not other cell types. Within a few days a cobblestone morphology characteristic of cultured renal tubular epithelium was evident. Confirmation of renal tubular epithelium was made by positive staining for Lotus Tetragonolobus Lectin (27) (Vector Labs, Burlingame, CA).
Induction of in vivo ischemia/reperfusion injury
All experimental mice were matched for age (8–12 weeks); only male mice were used. As previously published (4, 28), the following methods were used to induce non-lethal IR injury. The mice were anesthetized with isofluorane and injected intraperitoneally with ketamine (100mg/kg)/xylazine (8mg/kg) in saline. Core body temperatures were maintained between 36° C and 37.5° C during surgery by continuous monitoring with a rectal thermometer and automatic heating blanket. Both kidneys were exposed with bilateral flank incisions and ischemia induced by clamping both renal arteries with nontraumatic microvessel clamps (S&T, Neuhausen, Switzerland) for 25 minutes. Renal veins remained unoccluded. Cessation of blood flow was documented by visual inspection. After 25 minutes of ischemia, the clamps were released and reflow verified by visual inspection of the kidneys. All mice received 200 microliters of saline dripped over the open flanks during surgery to keep the tissue moist and 30 microliters of saline per gram body weight injected subcutaneously after surgery to replenish for fluid loss.
Measurement of renal function
Measurement of renal function was conducted at indicated times following reperfusion. In all cases, the mice were anesthetized prior to sacrifice and blood was collected from the inferior vena cava into a syringe preloaded with 3.8% sodium citrate. Sham controls were also anesthetized prior to sacrifice and blood collected in an identical manner. Plasma was isolated by centrifugation at 4000×g for 10 min at 4 °C. Renal function was assessed using the Sigma Diagnostics creatinine kit (Sigma-Aldrich, St. Louis, MO), running all samples in triplicates, and repeating measurements three times for each sample. Baseline (2 weeks before laparotomy) and terminal (at the time of sacrifice) serum creatinines were measured in all animals.
Histological assessments
Histological injury and inflammation
To assess renal histology, kidneys were harvested 24 hrs after kidney IR injury or sham surgery, fixed in a solution of zinc formalin and paraffin embedded. Sections (4 micron) were stained with Hematoxylin and Eosin or Periodic Acid Schiff stain. The tissue slides were blind labeled and reviewed by the renal pathologist who had no knowledge of the experimental groups. A histologic score system, adapted from Kelly et al was used (29). The percentage of tubules in the outer medulla that showed epithelial cell necrosis was estimated and a numerical score assigned to represent degree of injury: 0, no injury; 1 (0–10%); 2 (11–20%); 3 (21–40%); 4 (41–60%); 5 (61–75%); 6 (>75%) tubules injured. The scores represented the pathologist's impression of the severity of tubular injury (including loss of proximal tubule brush border, cell blebbing or vacuolization, and/or cell necrosis): the score ranges of 1–2 represented mild injury, 3–4 represented moderate injury and 5–6 represented severe injury. Infiltrating neutrophils were counted at a magnification of 400×. In the regions containing neutrophils, 10 fields were counted to derive the extent of neutrophil infiltration per field. Pictures of representative fields were recorded.
Apoptosis
To evaluate for evidence of apoptosis in renal cortical tissue after renal IR injury, tissue cryosections (6 micron) were assayed with the TUNEL reaction, using the TdT-mediated dUTP nick-end labeling (TUNEL) assay as provided in the fluorescein FragEL™ DNA Fragmentation detection kit (Calbiochem, San Diego). Cryosections were fixed in 4% formaldehyde and permeabilized in proteinase K (in 10 mM Tris, pH 8). The sections were then exposed to the TdT equilibration buffer and the free 3'- hydroxyl groups of DNA labeled with the fluorescein-conjugated deoxynucleotides. Nuclear staining was identified in cell nuclei with 4',6-diamidino-2-phenylindole (DAPI) and DNA breakage imaged by fluorescence microscopy. Eight random non-overlapping sections were viewed and counted under a grid at 200× magnification. All tissue sections were blind labeled and viewed without knowledge of experimental groups.
Detection of cytokines and chemokines
For cytokine/chemokine measurements, animals were sacrificed at indicated times after ischemic injury, a sample of blood was taken from the inferior vena cava and then kidneys harvested and snap-frozen in liquid nitrogen. The kidneys were homogenized in extraction buffer (10 mM Na-phosphate, pH 7.5; 0.1 Mm-amino caproic acid; 10 U/ml Heparin Na-salt; complete protease inhibitor tablet) for 30 seconds and incubated overnight at 4°C on a rotator. Supernatants were collected after centrifugation at 10,000g for 10 minutes at 4°C. Cytokine and chemokine levels were measured in tissue homogenates using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to manufacturer instructions. The detection limits were 16 pg/ml for IL-6, KC, IL-beta and MIP-2, and 31 pg/ml for TNF-alpha and IFÑgamma. Plasma concentrations of mouse IL-6 were also measured using ELISA kits [limited to 16 pg/ml]. To control for the ability to produce the indicated cytokines and chemokines, WT and Nod (1×2)−/− mice were injected intraperitoneally with lipopolysaccaride (LPS) from Salmonella minnesota R595 (Alexis Biochemicals, Enzo Life Sciences International, Inc, Plymouth Meeting, PA) at a concentration of 10mg/kg body weight. WT and Nod (1×2)−/− serum and kidneys were harvested at serial time points. Nonmanipulated controls were also sacrificed to compare baseline cytokine/chemokine levels.
Bone Marrow Transplantation
To examine the role of inflammation in kidney IR injury, Nod (1×2)−/− vs. WT bone marrow was transplanted into WT mice. Male WT mice were lethally irradiated with 2 doses of 5Gy, separated by 3 h, using a 137Cs Gammacell 40 Extractor irradiator (MDS Nordion, Ottawa, ON, Canada). The next day, bone marrow was collected from WT or Nod (1 × 2)−/− mice by flushing femurs and tibia with sterile 10K media [RPMI containing L-glutamine (2 mM), fetal bovine serum (10%), penicillin (100U/ml), streptomycin (100ug/ml), 2-mercaptoethanol (0.05 mM), HEPES (10 mM)]. The bone marrow cells were then washed with sterile PBS and 5 × 106 WT or Nod (1 × 2)−/− bone marrow cells in sterile PBS were injected into the tail vein of recipient irradiated mice. The mice were kept in microisolator cages for 6 weeks to complete engraftment with donor bone marrow and were given trimethoprim-sulfa enriched water until induction of IR injury. Confirmation of Nod (1×2)−/− engraftment after bone marrow transplantation was performed on bone marrow cells of chimeric WT mice engrafted with either Nod (1×2)−/− bone marrow or WT bone marrow. To detect the Nod1 mutation, the WT forward and reverse primers were 5'- GCTTGGCTCCTTTGTCATTG-3' and 5'- ACTGCTGCTTGGCTTTATTCTC-3' respectively. The Nod1 mutant forward and reverse primers were 5'- TTGGTGGTCGAATGGGCAGGTA-3' and 5'- CGCGCTGTTCTCCTCTTCCTCA-3'. The Nod2 WT forward and reverse primers were 5'-ACAGAGATGCCGACACCATACTG-3' and 5'- TGGAGAAGGTTGAAGAGCAGAGTC-3'. The Nod2 mutant forward and reverse primers were 5'-TGACTGTGGCTAATGTCCTTTGTG-3' and 5'- TTCTATCGCCTTCTTGACGAGTTC-3'.
Results
NOD expression within mouse and human kidney
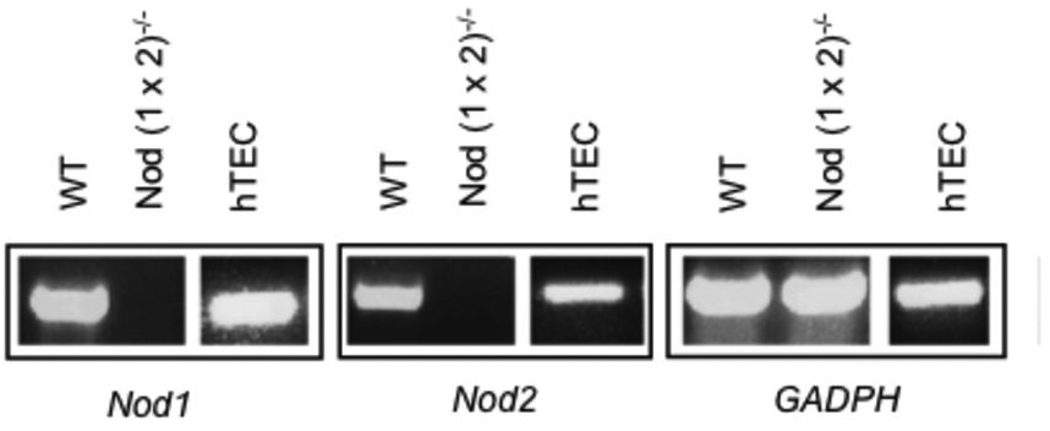
Nod proteins are activated by endogenous ligands and are thought to be sentinel intracellular receptors for molecules released by dead and dying cells. In order to evaluate the role of these innate immune receptors in renal ischemia-reperfusion injury, we first determined whether Nod1 and Nod2 genes were expressed in renal tubular epithelial cells of human and mouse kidneys. As seen in Figure 1, using semiquantitative PCR, we found that Nod1 and Nod2 were highly expressed in renal tubular epithelial cells from both species.
Figure 1. Nod1 and Nod2 in renal tubular epithelium of mice and humans.
Figure 1 shows three representative panels depicting Nod1 and Nod2 genes as well as a housekeeping gene (GADPH) control (listed as Nod1, Nod2, GADPH below each panel). Each panel illustrates gene expression, detected by semiquantitative PCR in renal tubular epithelium of either WT (C57BL/6) mice, Nod (1×2)−/− mice or human tubular epithelial cells (hTEC) extracted from normal human kidney tissue. The results are representative of 4 mice per group and 3 different human kidneys extracted for renal tubular cells with identical results.
Nod signaling and renal IR injury
Since Nod 1 and Nod 2 were expressed in renal tubular epithelium, we next investigated whether these intracellular innate immune sensors contributed to the syndrome of kidney IR injury in a murine model. Using WT and mutant mice bred for more than 10 generations to the same genetic background (C57BL/6), we induced non-lethal IR injury in WT, Nod1−/−, Nod2−/− and Nod (1× 2)−/− mice, using our previously described model (4).
Once activated, Nod1 and Nod2 recruit a serine-threonine kinase called Rip2 (also known as RICK or CARDIAK) through homotypic CARD-CARD interactions (2). Since Nod1 and Nod2 were highly expressed in renal epithelium we also asked whether the absence of Rip2, their putative downstream signaling protein, influenced renal IRI. Rip2 is essential for not only NFkB activation but also for the activation of JNK pathways and induction of cell death through apoptosis (3, 30).
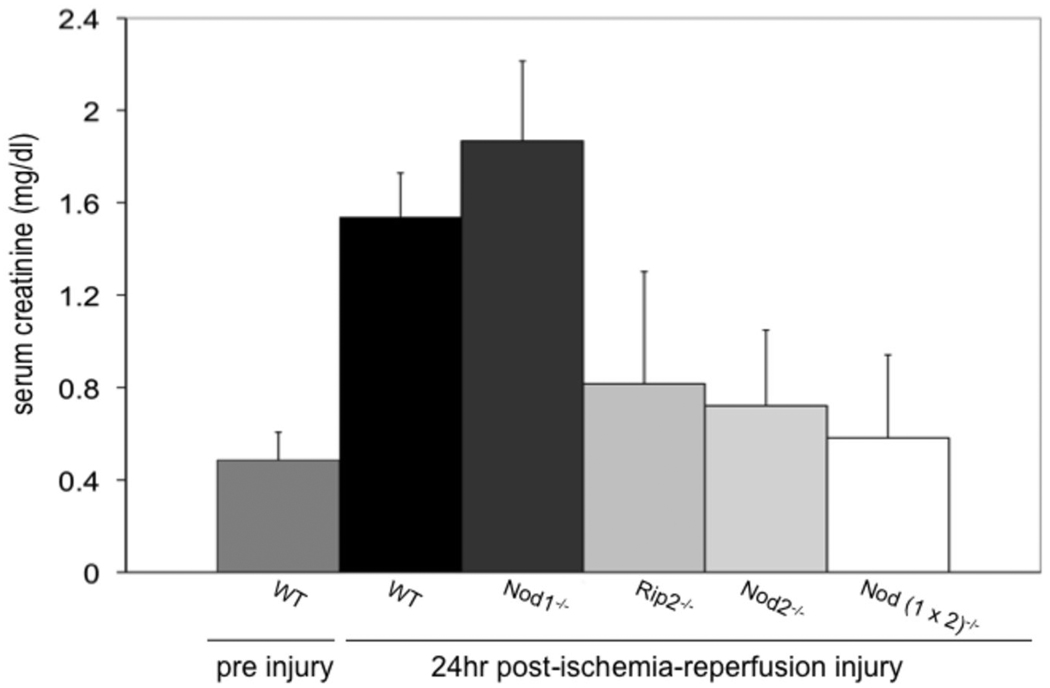
Figure 2 shows serum creatinine levels two weeks before injury for WT mice and 24 hours after injury for each group of mice. Pre injury creatinines are listed in the figure legend for Nod1−/−, Rip2−/−, Nod2−/− and Nod (1×2)−/− mice; none of the preinjury creatinines of the mutant mice differed statistically from those of the WTs. As seen in figure 2, there was no significant difference in renal injury induced by this kidney IR injury model between WT and Nod1−/− (p=0.14) deficient mice. There was however a significant difference in injury between the WT and Rip2−/− (p=0.01), Nod2−/− (p=0.0008), Nod (1×2)−/− (p=0.0007) mice. Sham operated mice showed no difference in serum creatinines from baseline, as expected (data not shown). Interestingly the functional data show that a deficiency in Nod2 and Rip2 provided more protection from injury than a deficiency in Nod1, despite the presence of Nod1 in the murine kidney.
Figure 2. Kidney injury following ischemia/reperfusion injury.
WT, Nod deficient and Rip2-deficient mice were subjected to 25 min ischemia/24hr reperfusion and serum obtained at 24 h to detect creatinines from WT (n=11), Nod1−/− (n=11), RIP2−/−, (n=6) Nod2−/− (n=11) and Nod (1 × 2) −/− mice (n=11). Pre injury creatinines were obtained 2 weeks earlier for each group of mice (WT pre injury values are shown in the figure). WT pre injury creatinines did not differ significantly from Nod1−/− (0.3±0.032), for Rip2−/− (0.5±0.059), Nod2−/− (0.35±0.12) and Nod (1×2)−/− (0.43±0.057). Shams had no significant change from baseline pre injury levels (data not shown). Error bars represent SDs of creatinines and statistical significance was determined with two-tailed Student’s t test.
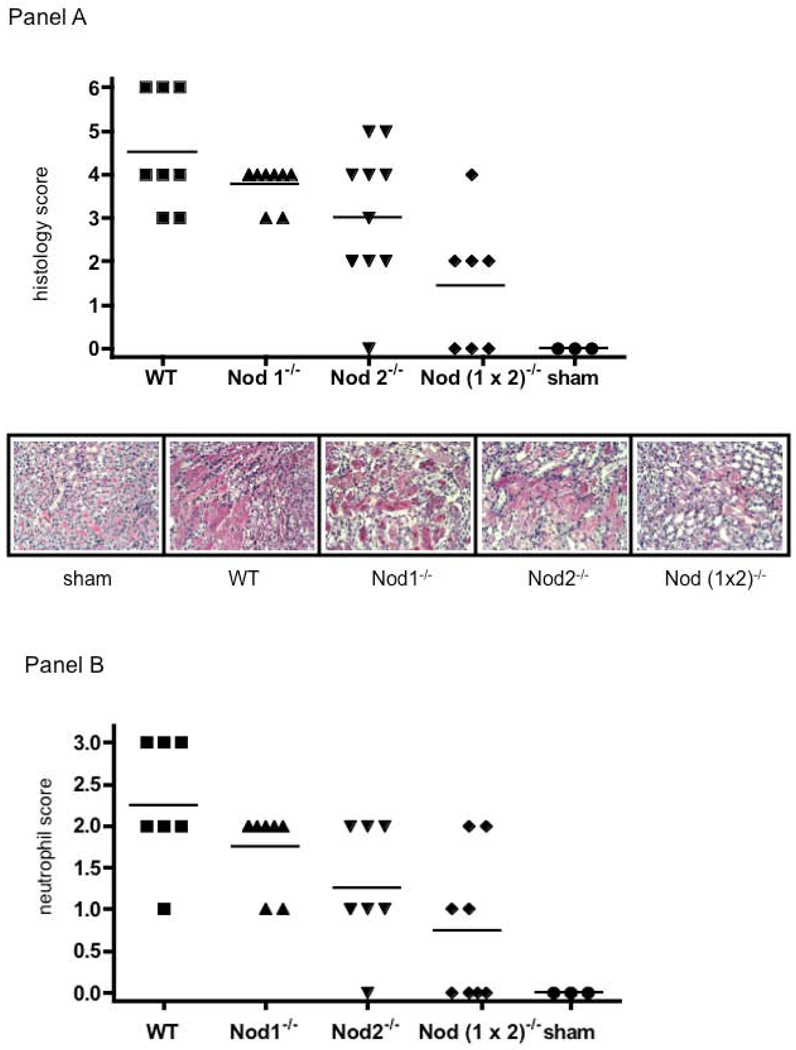
Histological injury was assessed in each group by a pathologist who was blinded to the experimental groups (Figure 3). Using a modified standard histologic scoring system (29), tissue injury was scored 24 hr after the initial ischemic insult (Figure 3, Panel A); the means of each group are shown in the figure legend. WT and Nod1−/− kidneys experienced abundant tubular injury; characterized as severe injury by the renal pathologist. Moderate injury characterized Nod 2−/− kidneys and the least amount of injury was seen in the Nod (1×2)−/− kidneys. Sham operated kidneys showed no injury.
Figure 3.
Panel A. Histological evaluation of renal injury after bilateral renal artery clamping. Panel A shows blinded histological scoring of renal injury from WT (n=8), Nod1−/− (n=8), Nod2−/− (n=10-, Nod (1×2)−/− (n=7), and sham (n=3), mice that were subjected to 25min ischemia/24hr reperfusion. The top panel shows the blinded histologic score (see materials and methods for details of scoring) and the bottom panel shows a representative micrograph of each group of mice (PAS, 200×). Means of histological score are as follows; WT (m=4.5); Nod1−/− (m= 3.8, p=0.14); Nod2−/− (m=3.0, p=0.17); Nod (1 × 2)−/− (m=1.4, p=0.04); Sham (m=0). Panel B. Neutrophil infiltration into kidneys of WT versus Nod deficient mice after bilateral renal artery clamping. This figure shows neutrophil infiltration following 25min ischemia/24hr reperfusion in WT (n=8), Nod1−/− (n=8, p=00.11), Nod2−/− (n=8, p=0.013), Nod (1×2)−/− (n=8, p=0.002) and sham (n=3) mice. The top panel shows the blinded score (see materials and methods for details of scoring) of neutrophil infiltration. Statistical significance was determined with a two-tailed Student's t-test.
Representative photomicrographs are shown for each of the groups, as noted in the figure legend. Neutrophil infiltration was also blindly quantified at 24 hrs following injury. As seen in Figure 3, Panel B, the pattern of neutrophil infiltration followed the pattern of histological injury, with the least amount of neutrophil infiltration seen in the IR injured Nod (1×2)−/− kidneys.
Since the simultaneous absence of both Nod1 and Nod2 provided the greatest protection from functional and histological kidney IR injury, we focused our subsequent evaluations on the absence of both Nod 1 and 2 by comparing and contrasting WT and Nod (1×2)−/− mice.
Apoptosis in WT vs. Nod deficient kidneys after renal IR injury
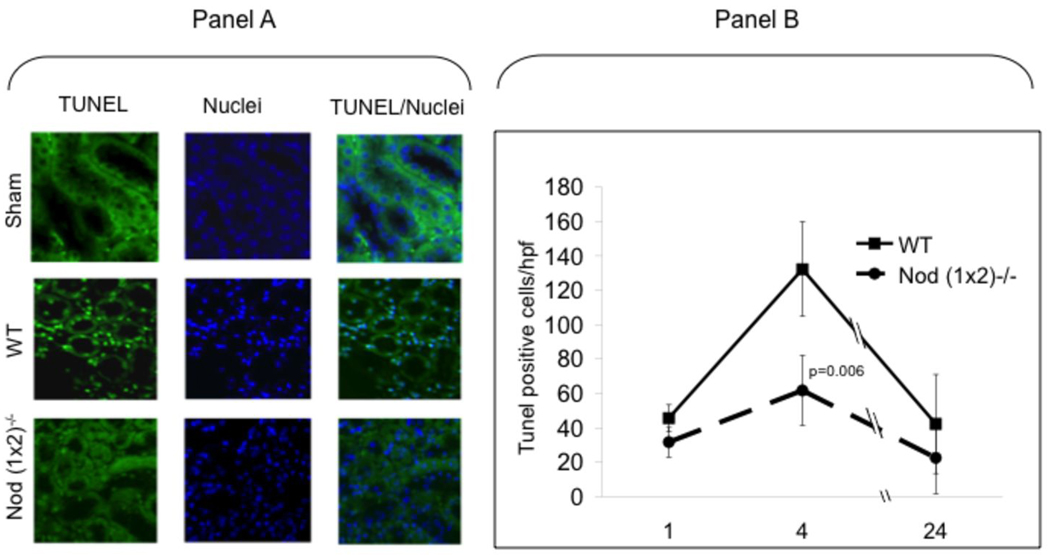
Renal ischemia results in a spectrum of tissue injury determined in part by the hypoxic interval. The ischemic tubular injury ranges from apoptosis to necrosis, depending on the length of anoxic insult. To determine whether signals generated through the Nod receptors contributed directly to renal tubular apoptosis in this model, tissue sections were examined for evidence of apoptosis in situ by the TUNEL assay. In vivo apoptosis is a dynamic process (31) and therefore kidneys were removed from mice subjected to 25 min of ischemia after 1hr, 4hr and 24hr of reperfusion. As shown in Figure 4, apoptosis peaked at four hours in both groups of mice. There was a significant difference in the peaks of apoptosis between WT vs. Nod (1×2)−/− mice (p=0.006), suggesting the absence of Nod proteins protected the kidney from renal tubular apoptosis.
Figure 4. Renal apoptosis in IR injured kidneys.
WT versus Nod (1×2)−/− mice were subjected to ischemia (25min bilateral renal artery clamping) and reperfusion (1hr, 4hr, 24hr). After indicated times of reperfusion, the mice were sacrificed, kidneys removed, fixed and paraffin-embedded. After deparaffinization, the TUNEL assay was performed and images viewed using fluorescent microscopy. Panel A shows representative images of TUNEL and DAPI staining 4hrs after reperfusion. Panel B shows results of blinded counting of TUNEL-positive cells in nine random, non-overlapping, sections (n=4 mice per group). Data are expressed as means ± SD. Statistical significance was determined using a Student’s two-tailed T test.
Cytokine and chemokine expression after murine IRI
In vitro studies have shown that upon ligand recognition, Nod1 and Nod2 activation result in the transcription of a large repertoire of genes, many of which are dependent on NFkB activation (2). In DCs, macrophages and monocytes, activation of Nod1 and Nod2 lead mainly to the production of pro-inflammatory cytokines (IL-6, IL-8/KC, TNFa, IL-1b) and expression of costimulatory molecules and adhesion molecules (2, 11). In epithelial cell lines, triggering of the Nod pathway induces the production of proinflammatory mediators (TNFa, IL-6, IL-8/KC, MIP2, and MCP-1) (2). These cytokines/chemokines help to recruit and activate inflammatory effector cells and establish an appropriate immune response to the IR injured kidney.
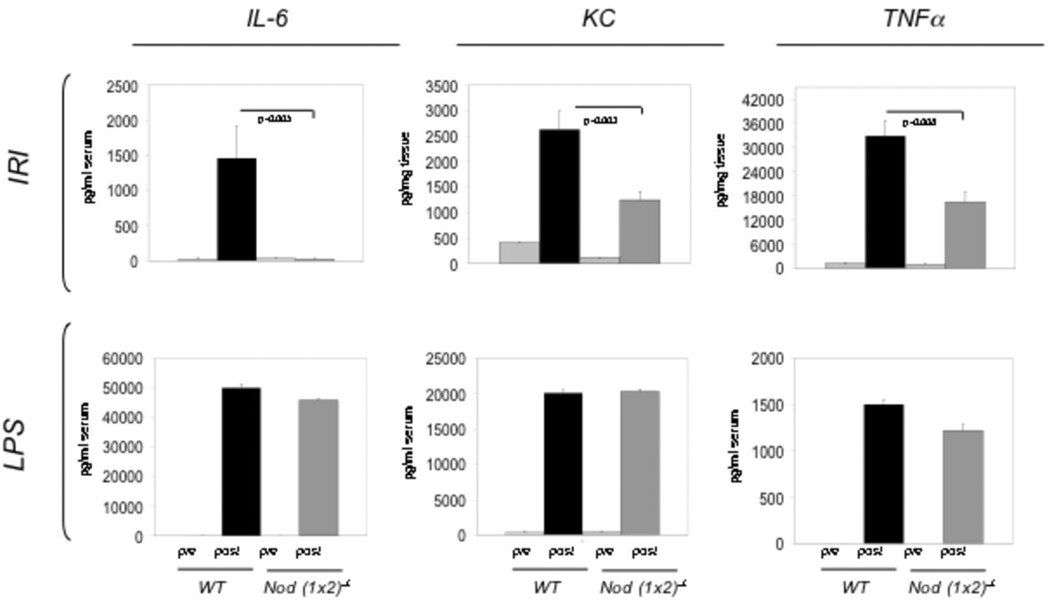
Proinflammatory cytokines and chemokines known to participate in the inflammatory response to kidney IR injury (4, 32) were investigated 24hr after IR injury in WT and Nod (1 × 2)−/− mice (Figure 5). As shown in Figure 5, there was significantly less IL-6, KC and TNFa 24 hr following IR injury in Nod (1×2)−/− mice. KC has been validated as an early biomarker for renal IR injury (32, 33), serum IL-6 levels are highly correlated with severity of tissue injury (34–36) and TNFa plays an important role in KC-induced neutrophil infiltration (37). There was no significant difference in IFNg, IL-1b, MIP2 and MCP-1 levels at 24 hr following renal injury between the two groups of mice.
Figure 5. Cytokine and chemokine expression in WT vs. Nod (1×2)−/− mice.
The top row (labeled IRI) shows peak levels of the indicated cytokines/chemokines (24hrs post IRI). IL-6 levels were detected in serum, whereas KC and TNFα were detected in tissue homogenates. The bottom row (labeled LPS) shows peak levels of the indicated cytokines/chemokines after i.p. injection with LPS (10mg/kg). Peak levels of serum IL-6 and KC were noted at 3hrs post-LPS injection, whereas the serum peak for TNFa was seen at 6 hrs post injection. The pre bars represent unmanipulated controls whereas the post bars represent after either IRI or LPS injection. Statistical significance was determined using a Student’s two-tailed T test. The data represent six mice per group and error bars indicate SDs.
Biochemical and functional studies have revealed that Nod2 deficient mutants exhibit reduced ability to activate NFkB in response to the Nod2-specific ligand muramyldipeptide (MDP), but they maintain a normal response to LPS stimulation (10, 38). To confirm the ability of WT and Nod-deficient mice to produce the tested cytokines/chemokines, mice were injected intraperitoneally with ultrapure lipopolysaccaride (from Salmonella minnesota R595) and serum tested at a range of intervals after the injection of LPS (0, 1hr, 3hr, 6hr, 24hr) to obtain the peak of cytokine/chemokine expression; TNFa peaked at 1hr, IL-6 at 6hrs and KC at 24hrs. There were no significant differences in production of the tested cytokines/chemokines between the LPS-injected WT and Nod (1×2)−/− mice.
WT mice transplanted with Nod (1 × 2)−/− bone marrow are only modestly protected from renal IR injury
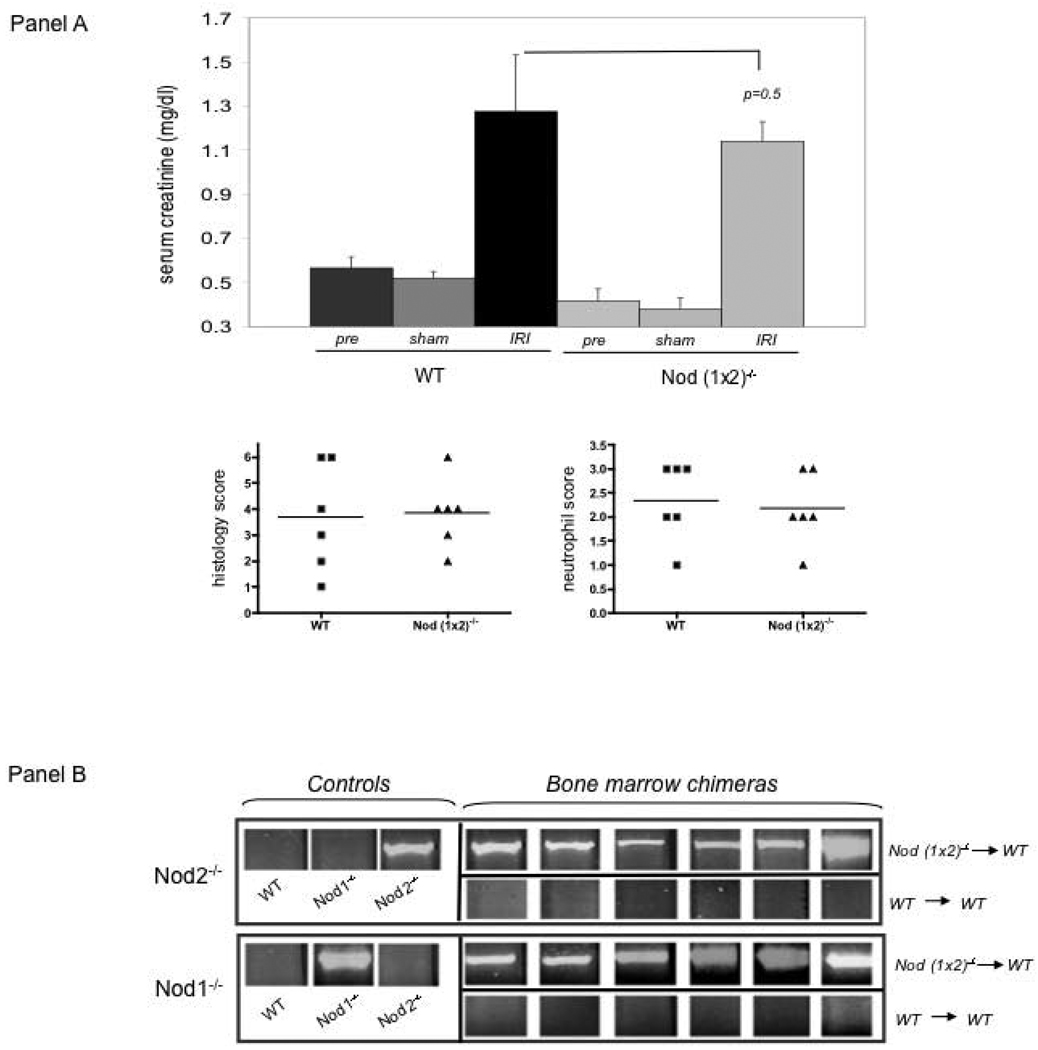
Ischemic renal injury is a complex process that involves not only direct damage to renal tubular epithelium, but also inflammation in response to the tissue injury. To assess the role of Nod 1 and Nod 2 in systemic (inflammation) injury following renal IRI, we replaced the bone marrow of WT mice with Nod (1×2)−/− bone marrow (Figure 6). As seen in Figure 6, Panel A, there was a small difference (p=0.05) between WT mice replaced with synegenic bone marrow vs. WT mice replaced with Nod (1×2)−/− bone marrow, suggesting that inflammation might play a role, albeit small, in the primary protection from kidney IR injury offered by absence of Nod receptors in this model. Blinded analysis of histologic damage and neutrophil infiltration though did not show significant differences between the groups (Figure 6, Panel A, Histology Score and Neutrophil Score). Analysis of proinflammatory cytokine/ chemokine secretion (IL-6, KC and TNFa) also showed no differences between the two groups (data not shown). Panel B confirms that the bone marrow of WT mice was indeed replaced by the indicated mutant marrow. Future studies are ongoing to define the role of the Nod receptors on various inflammatory cells known to participate in the syndrome of kidney IR injury (39–41).
Figure 6. Chimeric mice subjected to ischemia/reperfusion injury.
WT mice were irradiated, injected with either WT (n=6) or Nod (1 ×2)−/− (n=6) bone marrow 6 weeks earlier and, after engraftment, subjected to 25min ischemia/24 h reperfusion. Panel A shows serum creatinines of mice treated with 25min ischemia/24hr reperfusion as well as blinded histological scores of renal injury and neutrophil infiltration. Pre creatinine values were obtained 2 weeks prior to injury. Sham creatinines were obtained after laparatomy, without IRI. Six mice were treated per group. Error bars represent SDs. Statistical significance was determined by Student T test. Panel B confirms chimeric bone marrow was indeed engrafted in the indicated experimental groups. The top panels show the Nod2 mutant allele (indicating the Nod2−/−) was detected in Nod2−/− control mice, but not in Wt or Nod1−/− control mice. As shown in the WT mice transplanted with Nod (1×2)−/− bone marrow (titled Bone Marrow Chimeras), the Nod2 mutation was highly expressed. The bottom panels show the Nod1 mutant allele is detected in Nod1−/− mice, but not in WT or Nod2−/− mice. The Nod1−/− mutation is also expressed in the WT mice transplanted with the Nod (1×2) −/− bone marrow (Bone Marrow Chimeras).
Discussion
The pathogenesis of kidney IR is complex and many causative mechanisms converge to produce the pathognomonic findings of tubular epithelial cell apoptosis/necrosis and post-injury inflammatory cell infiltration (42–44). A complex network of cross-talk occurs between injured renal tubular cells, endothelial cells, and inflammatory cells; each cell type generates and responds to a variety of cytokines and chemokines. Despite this complexity, a growing body of evidence highlights a significant role for innate pattern recognition receptors in both the induction of ischemic kidney injury and the inflammatory response to the ischemic tissue. Since renal tubular apoptosis and inflammation are cardinal features of kidney IR injury, the investigations in this study focused on a family of intracellular innate immune receptors known to play a role in apoptosis and inflammation, the nucleotide-binding oligomerization domain (NOD)-like receptor family members Nod1 and Nod2.
Our studies are the first, to our knowledge, to demonstrate Nod1 and Nod2 receptors expressed within renal tubular epithelial cells in both murine and human kidneys. The finding of renal epithelial cell expression is not surprising, as Nod1 has already been described in various human epithelial cells (17) and Nod2 has been found in intestinal epithelium (Paneth cells) (16), in murine and cainine ophthalmic epithelial cells (45), in skin (15), lung epithelium (17), human endometrium (46) and in human dental pulp tissue (47). Nod1 is generally considered to be expressed in all cell types (2), whereas Nod2 expression is restricted to specialized epithelium and inflammatory cells. The expression within renal tubular epithelial cells and inflammatory cells positions these innate intracellular proteins as sentinel participants in responses to endogenous danger signals associated with kidney IR injury.
To further define the role of Nod1 and Nod 2 in the murine kidney, we compared and contrasted kidney IR injury induced in WT mice to injury in Nod-deficient mice. Interestingly, we found that despite the presence of both Nod1 and Nod2 in renal tubular epithelial cells, a deficiency in Nod2 provided greater protection than a deficiency in Nod1 for kidney IR injury. Since much is yet to be learned about the in vivo role of Nod receptor molecules, there are likely many potential reasons for the differential sensitivity observed in this study - including tissue specific ligand processing or even tissue specific expression of Nod dependent regulatory signaling molecules. Nod1 and Nod2 ligands that are produced by bacteria enter the cell via endocytosis and epithelial transporters. Different epithelial transporters for Nod ligands have already been identified in tissue (48, 49) and recent reports suggest differential tissue distributions and polymorphisms in these transporters (50, 51). Future studies will need to address the role of these peptide transporters in Nod1 versus Nod2 specific responses in the kidney. The molecular details of Nod-downstream signaling mediators are not yet completely understood and additional explanations for differential susceptibility to kidney IR injury will likely come from clarification of the downstream Nod1 and Nod2 regulatory proteins (52–57).
Consistent with the finding that Nod2-deficient mice were protected from kidney IR injury, we also found Rip2-deficient mice were also protected. Upon oligomerization of Nod1 or Nod2, the serine/threonine kinase Rip2 is recruited through homophilic CARD-CARD interactions. Rip2 in turn, is cross-activated to transduce downstream signals leading to NFkB activation and to apoptotic signaling pathways (3). Rip2 had previously been shown to contribute to TLR2 signaling (58), although more recently has been found to be a mediator of Nod1 and Nod2 signaling independent of TLRs (59). Notably, we have already shown that TLR2 deficient mice are protected from kidney IR injury (4), suggesting there might be an as yet undefined role for Rip2 in mediating several PRR responses in the kidney.
The greatest protection from kidney IR injury was offered by a simultaneous deficiency of Nod1 and Nod2; even surpassing the protection offered by Nod2 alone. These data suggest that although functional protection was not seen per se in the Nod1 deficient mice, that Nod1-mediated signals might play a less obvious role in renal IR injury. One possible role for Nod1 in our model might be through regulation of inflammation in response to the ischemic tissue. Nod1 has been shown to be important for neutrophil infiltration in vivo (20) and neutrophil accumulation quickly follows ischemia-reperfusion injury in the kidney (60, 61). There were fewer neutrophil infiltrates in the injured Nod1 deficient mice than in the injured WT mice. Since Nod1 activation induces neutrophil recruitment in vivo (20) it is possible that the combined effect of Nod1 and Nod2 deficiency produced more complete protection because mechanisms that were operative in the presence of Nod1 (e.g. neutrophil recruitment) were more completely inhibited in the absence of both Nod proteins. Arguing against this theory though is the finding that there were no significant differences in histologic injury, neutrophil infiltration or proinflammatory cytokine secretion WT mice transplanted with WT bone marrow versus Nod (1×2)−/− bone marrow. Future studies will need to more extensively analyze neutrophil function as well as the role of other inflammatory mediators in the two groups of animals.
The pathophysiology of kidney IR injury is complex and involves both direct renal tubular injury as well as extension of the injury through inflammation. Nod1 and Nod2 are known participants in cellular stress responses and we found that mice with simultaneously deficient in Nod1 and 2 were protected from renal tubular apoptosis and inflammation. The primary protection appeared to be protection from apoptosis, as Nod (1×2)-deficient bone marrow did not offer chimeric WT mice complete protection from kidney IR injury. The protection afforded by a deficiency in Nod1 and Nod2, positions these receptors as potential therapeutic targets for prevention and treatment of ischemic kidney injury. Future studies will likely illuminate an important role of the Nod family of PRRs in normal and stress-related renal tubular cell responses and will better define tissue-specific expression of not only the Nod receptors, but also their downstream signaling molecules.
Acknowledgements
We thank Susan Abel, RN for her assistance with the acquisition of human tissue for extraction of renal tubular epithelium and Terri Thinnes for her technical help with the Nod-deficient mice. We would also like to thank Dr. Charles Surh for his assistance with the bone marrow transplant studies.
Footnotes
This work was supported by a National Institutes of Health Grant (1R01DK60151) and a Scripps Translational Science Institute Pilot Grant (1UL1RR025774) award to DBM and a Renal Research Collaborative/Price Charities Grant awarded to A.J.K.
References
- 1.Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreig A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huan Z, Knoefel WT, Reed JC. XIAP mediates NOD signaling via interaction with RIP2. PNAS e pub. 2009 doi: 10.1073/pnas.0907131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 5.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 6.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamaillard M, Hashimoto M, Horie Y, Junya M, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nuñez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 8.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zathringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 9.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 10.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 11.Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH. Nod-like proteins in inflammation and disease. J Pathol. 2008;214:136–148. doi: 10.1002/path.2271. [DOI] [PubMed] [Google Scholar]
- 12.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 13.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73:7967–7976. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, Greenson JK, Keshav S, Nunez G. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 16.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 17.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Uehara A, Sugawara Y, Kurata S, Fujimoto Y, Fukase K, Kusumoto S, Satta Y, Sasano T, Sugawara S, Takada H. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005;7:675–686. doi: 10.1111/j.1462-5822.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 19.Marriott I, Rati DM, McCall SH, Tranguch SL. Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun. 2005;73:2967–2973. doi: 10.1128/IAI.73.5.2967-2973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, Mak TW, Nunez G, Chinnaiyan AM, Fukase K, Inohara N. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 22.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara I, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 24.Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, Boardman B, von Mutius E, Weiland SK, Leupold W, Fritzsch C, Klopp N, Musk AW, James A, Nunez G, Inohara N, Cookson WO. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005;14:935–941. doi: 10.1093/hmg/ddi087. [DOI] [PubMed] [Google Scholar]
- 25.Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984;25:383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- 26.McLaren J, Whiting P, Simpson J, Hawksworth G. Isolation and characterisation of human proximal tubular cells derived from kidney cortical segments. Hum Exp Toxicol. 1995;14:916–922. doi: 10.1177/096032719501401110. [DOI] [PubMed] [Google Scholar]
- 27.Nadasdy T, Laszik Z, Blick KE, Johnson DL, Burst-Singer K, Nast C, Cohen AH, Ormos J, Silva FG. Human acute tubular necrosis: a lectin and immunohistochemical study. Hum Pathol. 1995;26:230–239. doi: 10.1016/0046-8177(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 28.Frank RD, Schabbauer G, Holscher T, Sato Y, Tencati M, Pawlinski R, Mackman N. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia-reperfusion injury. J Thromb Haemost. 2005;3:531–540. doi: 10.1111/j.1538-7836.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva Correia J, Miranda Y, Leonard N, Hsu J, Ulevitch RJ. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 2007;14:830–839. doi: 10.1038/sj.cdd.4402070. [DOI] [PubMed] [Google Scholar]
- 31.Perl M, Chung CS, Ayala A. Apoptosis. Crit Care Med. 2005;33:S526–S529. doi: 10.1097/01.ccm.0000185499.28006.4c. [DOI] [PubMed] [Google Scholar]
- 32.Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, King LS, Rabb H. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290:F1187–F1193. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- 33.Safirstein R, Megyesi J, Saggi SJ, Price PM, Poon M, Rollins BJ, Taubman MB. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol. 1991;261:F1095–F1101. doi: 10.1152/ajprenal.1991.261.6.F1095. [DOI] [PubMed] [Google Scholar]
- 34.Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. The Swiss-Dutch J5 Immunoglobulin Study Group. Am J Med. 1991;91:23–29. doi: 10.1016/0002-9343(91)90069-a. [DOI] [PubMed] [Google Scholar]
- 35.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 37.Vieira S, Lemos H, Grespan R, Napimoga M, Dal-Secco D, Freitas A, Cunha T, Verri W, Jr, Souza-Junior D, Jamur M, Fernandes K, Oliver C, Silva J, Teixeira M, Cunha F. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogura Y, Saab L, Chen FF, Benito A, Inohara N, Nunez G. Genetic variation and activity of mouse Nod2, a susceptibility gene for Crohn's disease. Genomics. 2003;81:369–377. doi: 10.1016/s0888-7543(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 39.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, Tang WW. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 40.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 41.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004;66:500–506. doi: 10.1111/j.1523-1755.2004.761_6.x. [DOI] [PubMed] [Google Scholar]
- 44.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 45.Scurrell E, Stanley R, Schoniger S. Immunohistochemical detection of NOD1 and NOD2 in the healthy murine and canine eye. Vet Ophthalmol. 2009;12:269–275. doi: 10.1111/j.1463-5224.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 46.King AE, Horne AW, Hombach-Klonisch S, Mason JI, Critchley HO. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 2009;15:311–319. doi: 10.1093/molehr/gap020. [DOI] [PubMed] [Google Scholar]
- 47.Lin ZM, Song Z, Qin W, Li J, Li WJ, Zhu HY, Zhang L. Expression of nucleotide-binding oligomerization domain 2 in normal human dental pulp cells and dental pulp tissues. J Endod. 2009;35:838–842. doi: 10.1016/j.joen.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 48.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 49.Biegel A, Knutter I, Hartrodt B, Gebauer S, Theis S, Luckner P, Kottra G, Rastetter M, Zebisch K, Thondorf I, Daniel H, Neubert K, Brandsch M. The renal type H+/peptide symporter PEPT2: structure-affinity relationships. Amino Acids. 2006;31:137–156. doi: 10.1007/s00726-006-0331-0. [DOI] [PubMed] [Google Scholar]
- 50.Swaan PW, Bensman T, Bahadduri PM, Hall MW, Sarkar A, Bao S, Khantwal CM, Ekins S, Knoell DL. Bacterial peptide recognition and immune activation facilitated by human peptide transporter PEPT2. Am J Respir Cell Mol Biol. 2008;39:536–542. doi: 10.1165/rcmb.2008-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.20963. [DOI] [PubMed] [Google Scholar]
- 52.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenstiel P, Huse K, Till A, Hampe J, Hellmig S, Sina C, Billmann S, von Kampen O, Waetzig GH, Platzer M, Seegert D, Schreiber S. A short isoform of NOD2/CARD15, NOD2-S, is an endogenous inhibitor of NOD2/receptor-interacting protein kinase 2-induced signaling pathways. Proc Natl Acad Sci U S A. 2006;103:3280–3285. doi: 10.1073/pnas.0505423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeBlanc PM, Yeretssian G, Rutherford N, Doiron K, Nadiri A, Zhu L, Green DR, Gruenheid S, Saleh M. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe. 2008;3:146–157. doi: 10.1016/j.chom.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Clark NM, Marinis JM, Cobb BA, Abbott DW. MEKK4 sequesters RIP2 to dictate NOD2 signal specificity. Curr Biol. 2008;18:1402–1408. doi: 10.1016/j.cub.2008.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kufer TA, Kremmer E, Banks DJ, Philpott DJ. Role for erbin in bacterial activation of Nod2. Infect Immun. 2006;74:3115–3124. doi: 10.1128/IAI.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lecine P, Borg JP, Nunez G. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem. 2005;280:40301–40309. doi: 10.1074/jbc.M508538200. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 59.Park JH, Kim TGM, Kanneganti CTD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]