Abstract
Background
Hydrophobins are a family of small secreted proteins with a characteristic pattern of eight cysteine residues found exclusively in filamentous fungi. They have originally been divided into two classes based on their physical properties and hydropathy patterns, and are involved in the attachment of hyphae to hydrophobic structures, the formation of aerial structures and appear to be involved in pathogenicity.
Findings
Analysis of nine genome sequences from seven Aspergilli revealed fifty hydrophobins, where each species displayed between two to eight hydrophobins. Twenty of the identified hydrophobins have not previously been described from these species. Apart from the cysteines, very little amino acid sequence homology was observed. Twenty-three of the identified hydrophobins could be classified as class I hydrophobins based on their conserved cysteine spacing pattern and hydropathy pattern. However twenty-six of the identified hydrophobins were intermediate forms. Notably, a single hydrophobin, ATEG_04730, from Aspergillus terreus displayed class II cysteine spacing and had a class II hydropathy pattern.
Conclusion
Fifty hydrophobins were identified in Aspergillus, all containing the characteristic eight cysteine pattern. Aspergillus terreus exhibited both class I and class II hydrophobins. This is the first report of an Aspergillus species with the potential to express both class I and class II hydrophobins. Many of the identified hydrophobins could not directly be allocated to either class I or class II.
Background
Hydrophobins are a family of small proteins found uniquely in filamentous fungi [1]. The currently characterised hydrophobins are approximately 100 AA in size and have little amino acid sequence homology except from eight conserved cysteines in a characteristic pattern [2,3]. The eight cysteines form four disulfide bonds in the pattern Cys1-Cys6, Cys2-Cys5, Cys3-Cys4, Cys7-Cys8 and especially the Cys3-Cys4 loop can vary considerably in length [4]. Based on their distinct hydropathy patterns and physical properties, hydrophobins are traditionally divided into two classes [3]. Class I hydrophobins form highly insoluble membranes in water, organic solvents and 2% SDS, while the membranes formed by class II hydrophobins easily can be dissolved in aqueous ethanol (60%) or 2% SDS [2]. Class I hydrophobins have been identified in Ascomycetes and Basiodiomycetes, while class II hydrophobins have only been identified in Ascomycetes [1]. Typically, a single fungal species only expresses either class I or class II hydrophobins, however previous studies have shown that few species have the ability to express both class I and class II hydrophobins [5,6]. In class I hydrophobins the cysteine doublets are followed by hydrophilic amino acids, while hydrophobic amino acids are observed after the cysteine doublets in class II hydrophobins [2]. Furthermore, considerable variation is seen in the cysteine spacing of class I hydrophobins, while less variation is seen for class II hydrophobins [7]. In this study, we examine nine full genome sequenced Aspergilli for new hydrophobins.
Results and Discussion
Identification of hydrophobins
Nine full genome sequenced Aspergillus species were used to search for new hydrophobins. A total of 50 potential hydrophobins were identified (Table 1) based on the criteria of minimum eight cysteines, two cysteine pairs, a size of app. 100 AA and the cysteine pattern. On species level twenty of the identified hydrophobins have not previously been mentioned in other studies, while the number increases to thirty-one on strain level. The number of identified hydrophobins within the species varied from two to eight between the nine species. All identified hydrophobins had theoretical signal sequences and therefore have the possibility of being secreted. They contain approximately 100 - 200 amino acids and are 8 - 30 kDa in size. Furthermore, they contain eight to ten cysteines, where excess cysteines (above eight) are located before or after the conserved cysteine spacing pattern. Beauvais et al. (2007) have classified AFUA_8G05890 and AFUA_5G01490 as hydrophobins. As AFUA_8G05890 has 11 cysteines, no signal sequence and both proteins lack the conserved cysteine pattern, we disregard these proteins as hydrophobins as they do not fulfil our criteria. Other Aspergillus hydrophobins previously identified fulfilled the criteria [8-16] and have likewise been found and included in this study.
Table 1.
Aspergillus hydrophobins
| Species | Gene | Size (Da) | n(AA) | n(cys) | Eight cysteine pattern | Theoretical class | Common name |
|---|---|---|---|---|---|---|---|
| A. oryzae RIB40 | AO090012000143 | 14304 | 145 | 8 | CN{8}CCN{38}CN{10}CN{5}CCN{21}C | I | RolAa |
| AO090020000588 | 15231 | 151 | 8 | CN{7}CCN{39}CN{17}CN{5}CCN{17}C | I | New | |
| A. niger CBS 513.88 | An03g02360b | 12486 | 122 | 8 | CN{6}CCN{32}CN{25}CN{5}CCN{4}C | I | |
| An03g02400b | 13063 | 131 | 8 | CN{6}CCN{31}CN{23}CN{5}CCN{6}C | Intermediate | ||
| An04g08500b | 14397 | 146 | 8 | CN{7}CCN{39}CN{20}CN{5}CCN{17}C | I | ||
| An15g03800b | 13225 | 130 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | ||
| An01g10940b | 10693 | 100 | 8 | CN{14}CCN{17}CN{11}CN{7}CCN{8}C | Intermediate | ||
| An07g03340b | 16207 | 162 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | ||
| An09g05530b | 20465 | 202 | 9 | CN{8}CCN{33}CN{11}CN{5}CCN{16}C | Intermediate | ||
| An08g09880b | 9169 | 91 | 9 | CN{7}CCN{16}CN{6}CN{5}CCN{10}C | Intermediate | ||
| A. niger ATCC 1015 | JGI128530 | 10803 | 105 | 7 | Fragment (similar to An07g03340) | Intermediate | (New) |
| JGI35683 | 10693 | 100 | 8 | CN{14}CCN{17}CN{11}CN{7}CCN{8}C | Intermediate | (New) | |
| JGI45683 | 13063 | 131 | 8 | CN{6}CCN{31}CN{23}CN{5}CCN{6}C | Intermediate | (New) | |
| JGI45685 | 13716 | 132 | 8 | CN{6}CCN{32}CN{25}CN{5}CCN{14}C | I | (New) | |
| JGI53462 | 13224 | 130 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | (New) | |
| JGI194815 | 14397 | 146 | 8 | CN{7}CCN{39}CN{20}CN{5}CCN{17}C | I | (New) | |
| JGI43184 | 20381 | 201 | 9 | CN{8}CCN{33}CN{11}CN{5}CCN{16}C | Intermediate | (New) | |
| E. nidulans FGSC A4 | AN7539.2c | 10798 | 109 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | |
| AN8803.2c | 15625 | 157 | 8 | CN{7}CCN{39}CN{18}CN{5}CCN{17}C | I | RodAd | |
| AN6401.2c | 16131 | 162 | 8 | CN{6}CCN{38}CN{22}CN{5}CCN{35}C | Intermediate | ||
| AN8006.2c | 13183 | 135 | 8 | CN{6}CCN{31}CN{23}CN{5}CCN{6}C | I | DewAe | |
| AN1837.2c | 13397 | 135 | 8 | CN{7}CCN{39}CN{18}CN{5}CCN{17}C | I | ||
| AN0940.2c | 10594 | 101 | 8 | CN{13}CCN{17}CN{12}CN{7}CCN{8}C | Intermediate | ||
| A. fumigatus AF293 | AFUA_8G07060 | 15996 | 155 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | RodCf |
| AFUA_5G09580 | 16153 | 159 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | RodAf/g | |
| AFUA_2G14661 | 12928 | 125 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | New | |
| AFUA_1G17250 | 14299 | 140 | 8 | CN{7}CCN{36}CN{18}CN{5}CCN{18}C | I | RodBf/h | |
| AFUA_5G03280 | 19825 | 190 | 9 | CN{7}CCN{33}CN{11}CN{5}CCN{14}C | I | RodFf | |
| A. fumigatus A1163 | AFUB_016640 | 14300 | 140 | 8 | CN{7}CCN{36}CN{18}CN{5}CCN{18}C | I | (RodB New) |
| AFUB_057130 | 16153 | 159 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | (RodA New) | |
| AFUB_080740 | 15996 | 155 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | (RodC New) | |
| AFUB_051810 | 19825 | 190 | 9 | CN{7}CCN{33}CN{11}CN{5}CCN{14}C | Intermediate | (RodF New) | |
| A. terreus NIH 2624 | ATEG_10285 | 13978 | 129 | 8 | CN{5}CCN{28}CN{14}CN{8}CCN{13}C | Intermediate | New |
| ATEG_08089 | 18936 | 177 | 8 | CN{8}CCN{33}CN{11}CN{5}CCN{14}C | Intermediate | New | |
| ATEG_07808 | 11677 | 115 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | New | |
| ATEG_06492 | 17374 | 175 | 8 | CN{7}CCN{40}CN{16}CN{5}CCN{17}C | I | New | |
| ATEG_04730 | 11797 | 121 | 8 | CN{10}CCN{11}CN{16}CN{8}CCN{10}C | II | New | |
| A. flavus NRRL 3357 | AFLA_094600 | 8377 | 83 | 8 | CN{7}CCN{16}CN{6}CN{5}CCN{9}C | Intermediate | New |
| AFLA_131460 | 10867 | 106 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | New | |
| AFLA_060780 | 27807 | 251 | 8 | CN{6}CCN{30}CN{23}CN{5}CCN{4}C | I | New | |
| AFLA_014260 | 14304 | 145 | 8 | CN{8}CCN{38}CN{10}CN{5}CCN{21}C | I | New | |
| AFLA_063080 | 9362 | 87 | 9 | CN{5}CCN{17}CN{7}CN{7}CCN{12}C | Intermediate | New | |
| AFLA_098380 | 23415 | 217 | 10 | CN{7}CCN{39}CN{17}CN{5}CCN{44}C | I | New | |
| AFLA_064900 | 9147 | 91 | 10 | CN{7}CCN{15}CN{6}CN{5}CCN{8}C | Intermediate | New | |
| A. clavatus NRRL 1 | ACLA_001890 | 10214 | 100 | 8 | CN{7}CCN{16}CN{6}CN{5}CCN{26}C | Intermediate | New |
| ACLA_048810 | 18458 | 182 | 8 | CN{7}CCN{33}CN{11}CN{5}CCN{15}C | Intermediate | New | |
| ACLA_010960 | 14671 | 145 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | New | |
| ACLA_072820 | 16127 | 158 | 8 | CN{7}CCN{39}CN{21}CN{5}CCN{17}C | I | New | |
| ACLA_018290 | 12820 | 126 | 8 | CN{5}CCN{32}CN{6}CN{5}CCN{13}C | Intermediate | New | |
| ACLA_007980 | 14558 | 144 | 8 | CN{7}CCN{36}CN{18}CN{5}CCN{17}C | Intermediate | New |
Forty-five of the identified proteins contained domains classifying them as hydrophobins by Pfam. The remaining five hydrophobins could not be classified. Four of these (An01g10940, JGI35683, AN0940.2, AFLA_063080) can be differentiated from the rest in displaying a distinctive cysteine pattern. They have a similar cysteine pattern of CN{5-13}CCN{17}CN{7-12}CN{7}CCN{8-12}C (where N signifies any other amino acid than cysteine) and group together in the phylogenetic tree (Additional file 1), but still with other hydrophobins. They also have hydropathy patterns that differ from both class I and class II hydrophobins and can therefore theoretically not be placed in either class. Furthermore, their hydropathy patterns differ from each other, so they do not form a new class either. The fifth hydrophobin (ATEG_10285) differs in having a different cysteine spacing compared to all other identified hydrophobins, but still clusters with other hydrophobins in the phylogenetic tree (Additional file 1). Forty-four of the identified hydrophobins displayed class I cysteine spacing pattern, but only twenty-four had a characteristic class I hydropathy plot resulting in only twenty-three identified class I hydrophobins (see Additional file 2 and Table 1). Only one identified hydrophobin displayed a characteristic class II cysteine spacing pattern and had a class II hydropathy pattern, while the rest (twenty-six) were intermediate forms. However, as the majority of the identified hydrophobins have not physically been isolated and characterised, a differentiation into type of class is only provisional. As many of the identified hydrophobins displayed intermediate forms, they may also exhibit solubility characteristics between the two known classes. As these intermediate forms blur the original classification, it could be speculated, whether an extension of the classical two class system would be in place as more fungal genomes become available.
An examination of the multiple alignment (Additional file 3) of the putative hydrophobins revealed very low similarity between the hydrophobins. Apart from the eight cysteines a proline was observed in the majority of the sequences (82%) situated in close proximity to the theoretical signal sequence cleavage site. This proline may be involved in the correct cleavage of the signal sequence and thereby influence the eventual secretion of the hydrophobins. Tryptophan is rarely seen in hydrophobins [2], and only twelve of the identified hydrophobins from Aspergilli contained between 1-5 tryptophan residues.
Several groups are revealed in the phylogenetic tree (Additional file 1) and it seems that hydrophobins cluster according to their cysteine spacing pattern. A common feature in 44 of the 50 hydrophobins is a conserved spacing of five amino acids between the fifth and sixth cysteines, while the remaining six hydrophobins contain either seven or eight amino acids. This spacing of five cysteines is also observed in other known class I hydrophobins (eg. SC3, EAS and MPG1) [7] and may be a common feature in class I hydrophobins.
Previously Yang et al. (2006) [17] used primary structure analysis to identify new members of the hydrophobin family. By searching the Uniprot Knowledgebase using the key word hydrophobin followed by a BLAST against the NCBI database, Yang et al. retrieved several sequences. However, by using the above mentioned method putative hydrophobin sequences may be missed as hydrophobins have high sequence diversity, and may not resemble known hydrophobins sufficiently to be picked up by a BLAST. In our search we found five hydrophobins (An01g10940, JGI35683, AN0940.2, AFLA_063080, ATEG_10285), which do not resemble the other identified hydrophobins. If these hydrophobins are used to conduct a BLAST, no known hydrophobins appear in the results. So if the method described by Yang et al. was used, these putative hydrophobins would likely have been missed. Furthermore, Yang et al. uses their identified sequences to create motifs, and thereby identify nine new hydrophobins including five E. nidulans (A. nidulans) hydrophobins. In our approach we only sort our putative hydrophobins by the criteria of size, number of cysteines and the eight cysteine pattern, thereby not eliminating any hydrophobins even if they do not contain any common motifs.
Class I and class II hydrophobins of Aspergillus terreus
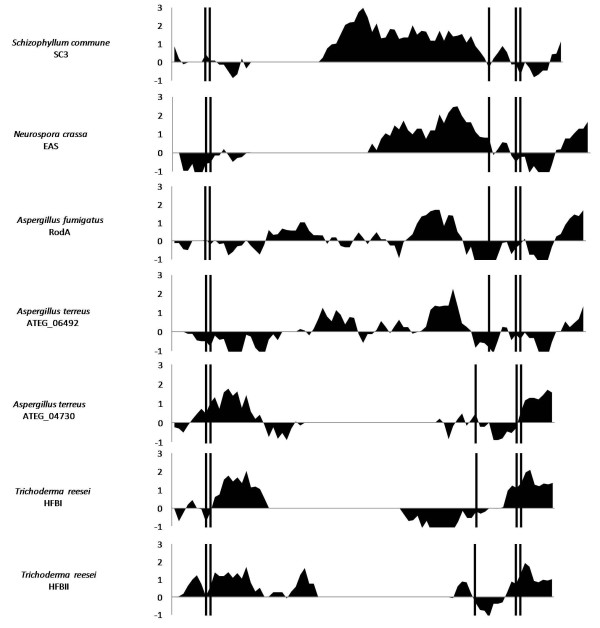
In Aspergillus terreus five different hydrophobins were identified. ATEG_06492 displayed a characteristic class I hydrophobin cysteine spacing pattern (CN{7}CCN{40}CN{16}CN{5}CCN{17}C), whereas a class II hydrophobin spacing pattern was observed for ATEG_04730 (CN{10}CCN{11}CN{16}CN{8}CCN{10}C). Comparison of ATEG_06492 and ATEG_04730 to hydropathy patterns of known class I and class II hydrophobins indicates that A. terreus has genes for both class I and class II hydrophobins (Figure 1). The hydrophobins SC3 (Schizophyllum commune), EAS (Neurospora crassa) and RodA (Aspergillus fumigatus) are known class I hydrophobins, where the cysteine doublets are followed by a stretch of hydrophilic amino acids. Likewise, the cysteine doublets in ATEG_06492 are followed by a stretch of hydrophilic amino acids contrasting ATEG_04730, where hydrophobic amino acids follow the cysteine doublets. Similarly, the cysteine doublets are followed by hydrophobic amino acids in the known class II hydrophobins HFBI and HFBII from Trichoderma reesei. Therefore ATEG_06492 displays a characteristic class I hydropathy pattern, while ATEG_04730 displays a class II hydropathy pattern. Comparison of ATEG_04730 to class II hydrophobins HFBI and HFBII showed 37% and 35% sequence identity, while comparison to class I hydrophobins RodA, SC3 and EAS showed 21%, 16% and 20% sequence identity. In contrast ATEG_06492 showed 20% and 29% sequence identity to class II hydrophobins HFBI and HFBII, but 51%, 21% and 24% to class I hydrophobins RodA, SC3 and EAS. Furthermore, a phylogenetic analysis (Figure 2) revealed that ATEG_04730 clusters with HFBI and HFBI, while ATEG_06492 clusters with RodA, EAS and SC3, strongly indicating that ATEG_04730 can be classified as a class II hydrophobin, while ATEG_06492 is classified as a class I hydrophobin. As neither ATEG_06492 nor ATEG_04730 have physically been isolated or characterised, these can obviously only tentatively be classified as a class I and a class II hydrophobin respectively. This is the first report of an Aspergillus species with the potential to express both class I and class II hydrophobins.
Figure 1.
Hydropathy patterns. Hydropathy patterns of SC3 from S. commune, EAS from N. crassa, RodA from A. fumigatus, HFBI and HFBII from T. reesei and proteins ATEG_06492 and ATEG_04730 from A. terreus. The amino acids of the hydrophobins are shown along the x-axis, where cysteines are indicated by vertical lines. Hydrophobic amino acids are shown above the x-axis, while hydrophilic amino acids are shown below. Only the part of the sequence from the first to the eighth cysteine was used to create the hydropathy pattern.
Figure 2.

Phylogenetic tree of class I and class II hydrophobins. Sequences of SC3 (S. commune), EAS (N. crassa), RodA (A. fumigatus), HFBI and HFBII (T. reesei) were obtained from the National Center for Biotechnology Information (NCBI). The phylogenetic tree was constructed based on a multiple alignment of identified hydrophobins using Phylogeny.fr [22]. Branches with support values less than 50% were collapsed.
Conclusion
Analysis of nine genome sequences from seven Aspergilli revealed fifty hydrophobins, where each species displayed between two and eight hydrophobins. Twenty of the identified hydrophobins have not previously been described from these species. All identified hydrophobins contained two cysteine pairs, were approximately 100-200 AA in size, and displayed the common eight cysteine pattern. Besides the cysteines, very little amino acid sequence homology was observed. Twenty-three of the identified hydrophobins could be classified as class I hydrophobins based on their conserved cysteine spacing pattern and hydropathy pattern, but the majority seem to be intermediate forms. A single hydrophobin, ATEG_04730, from Aspergillus terreus displayed a clear class II cysteine spacing and had a class II hydropathy pattern. Furthermore, this hydrophobin grouped together with other known class II hydrophobins in a phylogenetic analysis, showing a close phylogenetic relationship to these. As Aspergillus terreus also has the potential to express a class I hydrophobin, this is the first reported case of an Aspergillus species with the potential to express both class I and class II hydrophobins.
Methods
Availability of genomic data
The sequences of Aspergillus oryzae RIB40, Aspergillus niger CBS 513.88, Emericella nidulans FGSC A4, Aspergillus fumigatus AF293, Aspergillus fumigatus A1163, Aspergillus terreus NIH 2624, Aspergillus flavus NRRL 3357 and Aspergillus clavatus NRRL 1 were obtained from the Central Aspergillus Data Repository (CADRE) [18], while the sequence of Aspergillus niger ATCC 1015 was obtained from DOE Joint Genome Institute.
Identification of putative hydrophobins
A Perl program was constructed to search the nine Aspergillus genomes for putative hydrophobins by identification of the common C..CC..C..C..CC..C cysteine motif [2,3]. The identified putative hydrophobins were further sorted for size and number of cysteine residues resulting in fifty putative hydrophobins. The identified putative hydrophobin sequences were used to conduct a BLAST search against the NCBI (National Center for Biotechnology Information) non-redundant (nr) database to differentiate between known and newly identified hydrophobins. The sequences were examined for domains using Pfam to verify their function as hydrophobins [19] and the presence of and location of signal peptide cleavage sites using SignalP 3.0 to examine their theoretical ability to be secreted [20].
Protein sequence analysis
A multiple sequence alignment of the identified hydrophobin sequences was conducted using MUSCLE [21] and based on this alignment a phylogenetic tree was constructed [22-25].
Generation of hydropathy plots
Hydropathy patterns were determined using the hydropathy scale set by Kyte and Doolittle [26]. A nine amino acid window was used and data was extracted using Protscale on the ExPASy Proteomics Server [27]. The hydropathy patterns were aligned around the cysteine pairs placing gaps in the sequences where the hydrophobic and hydrophilic regions alternate. Only the part of the sequence from the first cysteine to the eight was used for examining the hydropathy pattern.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BGJ carried out the sequence analysis, blast searches, domain searches, phylogenetic analysis, created hydropathy patterns and drafted the manuscript. MRA wrote the Perl program used for searching the Aspergillus sequences and participated in data analysis. MHP, JCF and IS gave general direction and manuscript revisions. All authors read and approved the final manuscript.
Supplementary Material
Phylogenetic tree of identified hydrophobins in Aspergilli. The phylogenetic tree was constructed based on a multiple alignment of identified hydrophobins using Phylogeny.fr (Dereeper et al., 2008). Branches with support values less than 50% were collapsed. N signifies any other amino acid than cysteine.
Theoretical class of identified hydrophobins based on cysteine spacing and hydropathy plot. The hydropathy plots were created using ProtScale (Gasteiger et al. 2005).
Multiple alignment of putative hydrophobins in Aspergilli. Comparison of hydrophobins identified in full genome sequenced Aspergilli. Amino acid residues are colored by conservation (> 80%). Figure created using Jalview [28].
Contributor Information
Britt G Jensen, Email: brgj@bio.dtu.dk.
Mikael R Andersen, Email: mr@bio.dtu.dk.
Mona H Pedersen, Email: mohp@bio.dtu.dk.
Jens C Frisvad, Email: jcf@bio.dtu.dk.
Ib Søndergaard, Email: ibs@bio.dtu.dk.
Acknowledgements
We acknowledge The Danish Research Council for Technology and Production Sciences for support (project 09-064225).
References
- Wosten HA. Hydrophobins: multipurpose proteins. Annu Rev Microbiol. 2001;55:625–646. doi: 10.1146/annurev.micro.55.1.625. [DOI] [PubMed] [Google Scholar]
- Wessels JGH. Hydrophobins: Proteins that change the nature of the fungal surface. LONDON: ACADEMIC PRESS LTD; 1997. [DOI] [PubMed] [Google Scholar]
- Wessels JGH. Developmental Regulation of Fungal Cell-Wall Formation. Annual Review of Phytopathology. 1994;32:413–437. [Google Scholar]
- Kwan AH, Winefield RD, Sunde M, Matthews JM, Haverkamp RG, Templeton MD. et al. Structural basis for rodlet assembly in fungal hydrophobins. Proc Natl Acad Sci USA. 2006;103:3621–3626. doi: 10.1073/pnas.0505704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder MB, Szilvay GR, Nakari-Setala T, Penttila ME. Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol Rev. 2005;29:877–896. doi: 10.1016/j.femsre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Segers GC, Hamada W, Oliver RP, Spanu PD. Isolation and characterisation of five different hydrophobin-encoding cDNAs from the fungal tomato pathogen Cladosporium fulvum. Mol Gen Genet. 1999;261:644–652. doi: 10.1007/s004380050007. [DOI] [PubMed] [Google Scholar]
- Kershaw MJ, Talbot NJ. Hydrophobins and repellents: proteins with fundamental roles in fungal morphogenesis. Fungal Genet Biol. 1998;23:18–33. doi: 10.1006/fgbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- Stringer MA, Timberlake WE. dewA encodes a fungal hydrophobin component of the Aspergillus spore wall. Mol Microbiol. 1995;16:33–44. doi: 10.1111/j.1365-2958.1995.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Maeda H, Yoneda S. The fungal hydrophobin RolA recruits polyesterase and laterally moves on hydrophobic surfaces. Molecular Microbiology. 2005;57:1780. doi: 10.1111/j.1365-2958.2005.04803.x. [DOI] [PubMed] [Google Scholar]
- Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ. et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25:221–231. doi: 10.1038/nbt1282. [DOI] [PubMed] [Google Scholar]
- de Groot PW, Brandt BW, Horiuchi H, Ram AF, de Koster CG, Klis FM. Comprehensive genomic analysis of cell wall genes in Aspergillus nidulans. Fungal Genet Biol. 2009;46(Suppl 1):S72–S81. doi: 10.1016/j.fgb.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Stringer MA, Dean RA, Sewall TC, Timberlake WE. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 1991;5:1161–1171. doi: 10.1101/gad.5.7.1161. [DOI] [PubMed] [Google Scholar]
- Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C. et al. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cellular Microbiology. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- Parta M, Chang Y, Rulong S, Pinto-DaSilva P, Kwon-Chung KJ. HYP1, a hydrophobin gene from Aspergillus fumigatus, complements the rodletless phenotype in Aspergillus nidulans. Infect Immun. 1994;62:4389–4395. doi: 10.1128/iai.62.10.4389-4395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thau N, Monod M, Crestani B, Rolland C, Tronchin G, Latge JP. et al. rodletless mutants of Aspergillus fumigatus. Infect Immun. 1994;62:4380–4388. doi: 10.1128/iai.62.10.4380-4388.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, Debeaupuis JP, Crameri R, Carey M, Charles F, Prevost MC. et al. Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol. 2003;69:1581–1588. doi: 10.1128/AEM.69.3.1581-1588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Deng Y, Zhang C, Elasri M. Identification of new members of hydrophobin family using primary structure analysis. BMC Bioinformatics. 2006;7(Suppl 4):S16. doi: 10.1186/1471-2105-7-S4-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabey JE, Anderson MJ, Giles PF, Miller CJ, Attwood TK, Paton NW. et al. CADRE: the Central Aspergillus Data REpository. Nucleic Acids Res. 2004;32:D401–D405. doi: 10.1093/nar/gkh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE. et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von HG, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F. et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online--a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005;33:W557–W559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkens MR, Appel RD, In: The Proteomics Protocols Handbook. Walker JM, editor. Humana Press Inc; 2005. Protein Identification and Analysis Tools on the ExPASy Server; pp. 571–607. full_text. [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of identified hydrophobins in Aspergilli. The phylogenetic tree was constructed based on a multiple alignment of identified hydrophobins using Phylogeny.fr (Dereeper et al., 2008). Branches with support values less than 50% were collapsed. N signifies any other amino acid than cysteine.
Theoretical class of identified hydrophobins based on cysteine spacing and hydropathy plot. The hydropathy plots were created using ProtScale (Gasteiger et al. 2005).
Multiple alignment of putative hydrophobins in Aspergilli. Comparison of hydrophobins identified in full genome sequenced Aspergilli. Amino acid residues are colored by conservation (> 80%). Figure created using Jalview [28].