Abstract
Guanylyl cyclase-A (NPR-A; GC-A) is the major and possibly the only receptor for atrial natriuretic peptide (ANP) or B-type natriuretic peptide. Although mice deficient in GC-A display an elevated blood pressure, the resultant cardiac hypertrophy is much greater than in other mouse models of hypertension. Here we overproduce GC-A in the cardiac myocytes of wild-type or GC-A null animals. Introduction of the GC-A transgene did not alter blood pressure or heart rate as a function of genotype. Cardiac myocyte size was larger (approximately 20%) in GC-A null than in wild-type animals. However, introduction of the GC-A transgene reduced cardiac myocyte size in both wild-type and null mice. Coincident with the reduction in myocyte size, both ANP mRNA and ANP content were significantly reduced by overexpression of GC-A, and this reduction was independent of genotype. This genetic model, therefore, separates a regulation of cardiac myocyte size by blood pressure from local regulation by a GC-mediated pathway.
Atrial natriuretic peptide (ANP) is a circulating hormone synthesized in and secreted from the heart (1–3). Biological actions of administered ANP include a stimulation of natriuresis, diuresis, and vasorelaxation, and an inhibition of renin and aldosterone secretion (4–7). Under normal conditions, ANP is synthesized, stored in granules, and secreted mainly by atrial myocytes. However, during various cardiac pathologies, ventricular myocytes undergo important phenotypic modifications that result in the expression of several fetal genes (e.g., ANP) and hypertrophy (8).
Guanylyl cyclase-A (NPR-A; GC-A), a membrane enzyme that contains a peptide-binding, protein kinase homology and GC catalytic homology domain, is thought to be the principal and possibly the only receptor for ANP. The peptide binds to GC-A with high affinity and fails to cause relaxation of precontracted aortic rings or a natriuretic/diuretic response in GC-A null mice (9–13).
The inhibition of specific signaling pathways has represented a classical means by which to define the function of such pathways. Gene disruption is one method by which to interrupt signaling pathways, and we and others have disrupted the genes for ANP, B-type natriuretic peptide (BNP), or GC-A (14–17). Disruption of the GC-A gene results in mice that display a salt-resistant elevation of blood pressure, and cardiac fibrosis and hypertrophy (16, 17). The cardiac hypertrophy is greater than that seen in other mouse models of hypertension (18), suggesting that GC-A could be directly involved in the regulation of myocyte size. It has been shown, in fact, that ANP inhibits cardiomyocyte hypertrophy under in vitro culture conditions (19–22).
In these studies, we design a genetic mouse model where GC-A null mice are crossed with mice expressing GC-A as a transgene specifically in cardiac myocytes. Based on studies of these mice, we suggest that cardiac myocyte GC-A can act as a negative regulator of myocyte size independently of blood pressure.
Materials and Methods
Production of GC-A Null Mice.
Mice deficient for GC-A were produced and maintained on a C57BL/6 background as previously described (16).
Production of GC-A Transgenic Mice.
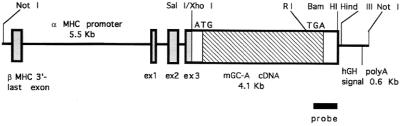
The cDNA insert for the mouse GC-A gene was cloned into a SalI-digested α-myosin heavy chain promoter construct (a generous gift from J. Robbins, Dept. of Pharmacology and Cell Biophysics, University of Cincinnati) (23). The vector contains a 5.8-kb BamHI–MaeIII fragment of the murine α-myosin heavy chain gene that includes the promoter and exons 1–3 from the 5′ untranslated region of the gene and a human growth hormone polyadenylation site (Fig. 1). The vector sequence was removed by digestion with NotI. The linear fragment was gel-purified, eluted with the use of Qiaquick columns (Qiagen, Chatsworth, CA), and injected into the male pronucleus of fertilized, single-cell B6C3F1 strain embryos. Stable founder lines were identified initially by Southern analysis and subsequently by PCR with the use of primers specific for the transgene. Two founders transmitted the transgene to offspring. Similar results were obtained for the two lines of mice. All aspects of animal care and experimentation performed in this study were approved by the Animal Resources Center of the Southwestern Medical Center at Dallas.
Figure 1.
Schematic diagram showing the construct used for production of GC-A transgenic mouse lines. The line labeled “probe” represents the portion of the mouse GC-A cDNA used in Southern or Northern analyses. MHC, myosin heavy chain.
The α-myosin heavy chain promoter drives transgenes exclusively in cardiac myocytes and has been used extensively in previous transgenic studies (24, 25). In the atrium, this promoter is expressed in both embryonic and adult myocytes. However, its expression in ventricular myocytes is observed mainly after birth (26, 27).
GC-A null mice and GC-A transgenic mice were crossed to produce GC-A null mice with cardiac-specific expression of GC-A. Male and female mice 3–6 months of age (24–34 g body weight) were studied, and no apparent gender differences in ratios of heart weight to body weight were seen.
Blood Pressure and Heart Rate.
Blood pressures and heart rates of mice were determined with a BP-98A (Softron, Tokyo, Japan) as previously described (16).
Isolation of Cardiomyocytes.
The cardiac myocytes were enzymatically dissociated from the mouse heart as in previous published protocols, with minor modifications (28). Briefly, 4-month-old mice were heparinized (5 units/g body weight) with an intraperitoneal injection. After 30 min, mice were anesthetized with metofane inhalation, and immediately after excision of the heart, it was placed in PBS. After cannulation of the aortic root, the heart was retrogradely perfused with a solution containing type I collagenase (0.17%) and 10 mM 2,3-butanedione monoxamine in PBS preequilibrated with 100% O2. The tissue was then removed from the cannula, and ventricles were minced and triturated in PBS. The temperature was kept at 37°C throughout the isolation procedure. Freshly isolated myocytes were fixed with 10% formalin in PBS. That the preparation represented cardiac myocytes was verified by fixing the cells, permeabilizing them, staining their nuclei with 4′,6-diamidino-2′-phenylindole dihydrochloride (1 μg/ml), and visualizing them by fluorescence microscopy. Nuclei of cardiac myocytes have a characteristic elongated shape that distinguishes them from those of fibroblasts or endothelial cells.
Cardiomyocyte Area.
The average planar area of a two-dimensional silhouette of the cardiomyocyte was determined by tracing approximately 100 randomly selected myocytes per heart from ventricular fractions. The outer borders of the myocytes were traced, and the myocyte areas were calculated. The averaged value was used in subsequent analysis.
Determination of Ventricular ANP and mRNA Content.
The hearts were removed after cervical dislocation and were immediately frozen in liquid nitrogen. The lysates were obtained by homogenization in ice-cold buffer (1% Nonidet P-40/10% glycerol/137 mM NaCl/20 mM Tris⋅HCl, pH 7.4/4 μg/ml aprotinin/4 μg/ml leupeptin/1 mM PMSF/4 μg/ml pepstatin/20 mM NaF/1 mM sodium pyrophosphate/1 mM orthovanadate). The lysates were kept on ice for 15 min and cleared by centrifugation at 15,000 × g for 20 min at 4°C. Protein concentration was determined by the Bradford method (Bio-Rad). ANP contents of the cardiac ventricles were determined by a commercially available specific RIA (Peninsula Laboratories) according to the manufacturer's instructions. Total RNA was extracted from flash-frozen ventricles with the use of RNA-STAT (Tel-Test, Friendship, TX), and ANP mRNA levels of the ventricles were determined by Northern blot analysis with a specific probe for mouse ANP cDNA.
Data Analysis.
Data are reported as means ± SEMs of more than triplicate separate experiments. Statistical significance was determined by Student's unpaired t test.
Results
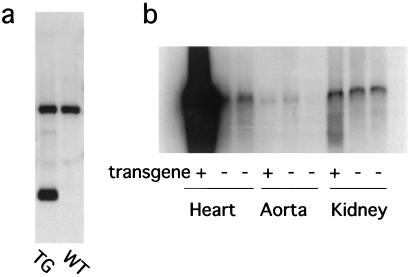
The average ratio of heart weight (mg) to body weight (g) of GC-A null mice at 2–6 months of age was increased to 131% of that of wild-type littermates (n = 14) (not shown). To determine whether myocardial GC-A could alter myocyte size in the potential absence of hemodynamic changes, transgenic animals overexpressing mouse GC-A under the control of the mouse α-myosin heavy chain promoter were generated (Fig. 1). Southern blot analysis revealed that genomic DNA from transgenic mice contains multiple copies of the construct (Fig. 2a). The cardiac GC-A mRNA content of transgenic mice was much higher than that of nontransgenic littermates, whereas there was no apparent genotypic change in GC-A mRNA levels in the aorta or in the kidney (Fig. 2b).
Figure 2.
Successful introduction of the GC-A transgene and its specific expression in the heart. (a) Southern blot analysis of transgenic (TG) and wild-type (WT) mice. Mouse genomic DNA prepared from tails was digested with EcoRI and ApaI and subjected to Southern blot analysis with the use of the probe shown in Fig. 1. The upper band corresponds to the endogenous GC-A gene, and the lower band corresponds to the transgene. (b) Northern blot analysis of RNA from transgenic and wild-type mice. Total RNA was prepared from the heart, aorta, or kidney of transgenic mice (transgene + ) or wild-type mice (transgene −).
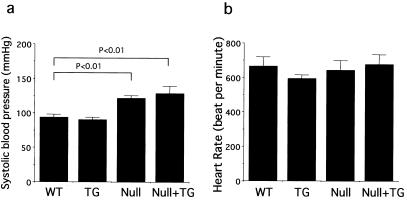
As we have previously reported, the systolic blood pressure of GC-A null mice is higher than that of wild-type mice (122 ± 3.4 mmHg and 94 ± 4.5 mmHg, respectively; P < 0.01). Systolic blood pressure did not change significantly in the presence of the transgene in either wild-type or GC-A null genetic backgrounds (Fig. 3a). There also was no significant change in heart rate between the different genotypes (Fig. 3b). Thus, no hemodynamic change appears to have occurred in the cardiac-specific GC-A transgenic mice.
Figure 3.
GC-A overexpression in the heart does not alter blood pressure in either wild-type or GC-A null backgrounds. Systolic blood pressures (a) and heart rates (b) of wild-type (WT), transgenic (TG), GC-A null (Null), or GC-A null mice with cardiac expression of the GC-A gene (Null + TG) are shown. Systolic blood pressure of the GC-A null mice was significantly greater than that of wild-type animals. There were no significant genotypic differences in heart rates.
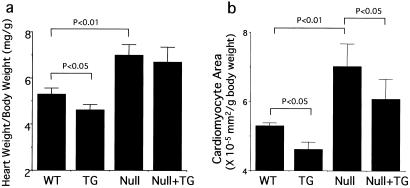
There was a significant increase in the ratio of heart weight (mg) to body weight (g) of the GC-A null mice compared with that of wild-type mice (P = 0.006) (Fig. 4a). The ratio of heart weight to body weight of transgenic mice was significantly less than that of the nontransgenic littermates (4.62 ± 0.23 and 5.33 ± 0.26, respectively; P < 0.03). On the GC-A null background, however, no significant difference between the ratios of heart weight to body weight was seen in the absence or presence of the transgene (7.01 ± 0.45 and 6.71 ± 0.63, respectively; P < 0.4).
Figure 4.
Effect of the GC-A transgene on the ratios of heart weight to body weight and cardiomyocyte area to body weight. Heart-to-body weight ratios (a) and cardiomyocyte areas (b) of wild-type (WT), transgenic (TG), GC-A null (Null), and GC-A null mice with cardiac expression of the GC-A gene (Null + TG) are shown. The heart-to-body weight ratio of GC-A null mice was significantly increased compared with that of wild-type mice. Cardiac overexpression of GC-A also significantly decreased the ratio of heart weight to body weight in the wild-type background. The average areas of isolated cardiomyocytes from GC-A null hearts were significantly increased compared with those of wild-type hearts. Transgenic overexpression of GC-A in cardiomyocytes decreased the area in both wild-type and GC-A null genetic backgrounds.
To determine whether GC-A overexpression reduces cardiomyocyte size, we next measured areas of freshly isolated cardiomyocytes. The average cardiomyocyte area of GC-A null mice was significantly increased compared with that of wild-type mice (6.05 ± 0.26 and 5.15 ± 0.14 × 10−5 mm2/g body weight, respectively) as shown in Fig. 4b. With cardiac overexpression of GC-A, the average cardiomyocyte area was decreased either in the wild-type background (4.78 ± 0.18 × 10−5 mm2/g body weight) or the GC-A null background (5.5 ± 0.5 × 10−5 mm2/g body weight) relative to mice not carrying the GC-A transgene.
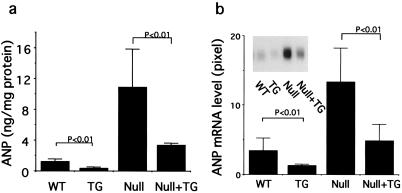
Because cardiac hypertrophy is accompanied by an activation of the cardiac fetal gene program in the ventricle and reexpression of ANP is one of the best markers of this change (29, 30), we next examined ventricular expression of ANP and of ANP mRNA. Ventricular ANP concentrations in GC-A null mice were 8-fold higher than that of wild-type mice (10.9 ± 4.9 and 1.26 ± 0.29 ng/mg protein, respectively) (Fig 5a). Cardiac overexpression of GC-A reduced ventricular ANP concentrations to approximately 30% in both wild-type and GC-A null mice. A similar effect was seen with respect to ventricular ANP mRNA levels (Fig. 5b), also compatible with the constitutive secretory pathway of ventricular ANP (31).
Figure 5.
Ventricular ANP (a) and ANP mRNA (b) content of wild-type (WT), transgenic (TG), GC-A null (Null), and GC-A null mice with cardiac expression of GC-A (Null + TG). ANP or ANP mRNA was extracted from ventricles and measured with a RIA or Northern blot analysis, respectively. The ventricle of GC-A null mice contained higher amounts of ANP as well as ANP mRNA. Cardiac-specific expression of GC-A significantly decreased both ANP and ANP mRNA content in wild-type and in GC-A null backgrounds.
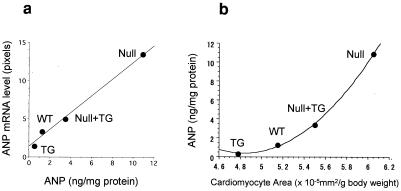
There were strong correlations between ANP content and ANP mRNA levels (Fig. 6a) and between ANP content and cardiomyocyte size (Fig. 6b). Whereas there appeared to be a linear relationship between ANP mRNA and ANP levels, the relationship between ANP and cardiomyocyte area to body weight was exponential in nature. Thus, as cardiomyocyte area increased, a disproportionate increase in ANP occurred, reflecting the activation of a fetal gene expression program. Ventricular BNP was also reduced somewhat in GC-A transgenic animals, but the effects were much less dramatic than with ANP (data not shown).
Figure 6.
Correlation of ANP mRNA and ANP content of ventricular cardiomyocytes (a) or ANP content and cardiomyocyte area as a function of genotype (b). The genotypes were wild-type (WT), GC-A transgene expression in wild-type background (TG), GC-A null (Null), or GC-A transgene expression in GC-A null mice (Null + TG).
Discussion
GC-A-deficient mice display a marked cardiac hypertrophy, which in general is much greater than that seen in other mouse models of hypertension (17, 18). The phenotype has suggested the possibility that the GC-A signaling pathway is an important component in the regulation of cardiac myocyte size independent of blood pressure.
Here a GC-A transgene expressed within the cardiac myocyte failed to significantly alter blood pressures of either wild-type or GC-A null mice, and yet cardiomyocyte size was significantly reduced when the transgene was expressed in either genotype. Therefore, the GC-A signaling pathway appears to protect against cardiac myocyte hypertrophy. Whether the protection is facilitated by ANP or BNP stimulation of the enzyme is not known. Previous mouse models where ANP or BNP was introduced as a transgene (32, 33) or where the peptide genes were disrupted (14, 15) have been often confounded with alterations in blood pressure, as is also the case for the GC-A gene disruption (16, 17). Thus changes in cardiac size are likely due to a combination of local and pressure-induced signaling pathways in these genetic models. The experiments here appear to finally separate blood pressure signaling pathways from other pathways that regulate myocyte size, namely that involving GC-A.
That the GC-A or GC-B signaling pathways can inhibit cell proliferation has been reported by numerous groups (34–36), and recently Chrisman and colleagues (37) also have shown a dramatic and rapid desensitization of GC-B by various growth factors or serum. The soluble forms of GC, in contrast, were not acutely affected by the growth factors or serum. It has been suggested, therefore, that a strong adversarial relationship exists between growth factor and particulate GC signaling pathways (37). Given that GC-A null mice display a prominent cardiac hypertrophy and fibrosis (17, 18), it is possible that the elimination of GC-A results in a loss of control over growth factor-induced cardiomyocyte hypertrophy and fibroblast proliferation in the heart.
In addition to cardiomyocyte hypertrophy, the increase in heart weight of GC-A null mice could also be due to an increase in cardiomyocyte number. Because ANP has been suggested to induce apoptosis in rat neonatal cardiomyocytes (38), the absence of ANP/BNP-induced elevations of cGMP in GC-A-deficient mice could lead to decreased numbers of apoptotic cardiac myocytes, resulting in increased total myocyte numbers. This increase in myocytes would likely arise from either a decrease in apoptosis during embryogenesis or additional rounds of replication. This study did not directly address myocyte numbers.
Because cardiac hypertrophy protects against ventricular pressure overload, reversal of hypertrophy with GC-A overexpression in the face of high blood pressure could cause deleterious effects. In fact, histological examination revealed that GC-A transgenic mice, when crossed with GC-A-deficient animals, displayed multiple focal cardiomyocyte degeneration (data not shown). This alteration in cardiac pathology may offer an explanation for the lack of a significant decrease in cardiac weight in response to expression of the GC-A transgene in null animals.
In the present study, ventricular ANP levels are suppressed in the mice with overexpression of the GC-A gene in either wild-type or GC-A null backgrounds. The decrease in ANP levels coincides with a decrease in cardiomyocyte area and supports work suggesting that ventricular ANP represents an excellent marker for cardiomyocyte hypertrophy (7).
In summary, cardiac overexpression of GC-A reduced cardiomyocyte size and ventricular ANP expression in either wild-type or GC-A null background. The genetic models produced here have separated an indirect regulation of cardiomyocyte size by hemodynamic alteration from a direct regulation by GC-A.
Acknowledgments
We thank Drs. Eric Olson and Brian Mercer for their assistance in the production of the transgenic animals.
Abbreviations
- GC-A
guanylyl cyclase-A
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
References
- 1.de Bold A J, Borenstein H B, Veress A T, Sonnenberg H. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Thibault G, Amiri F, Garcia R. Annu Rev Physiol. 1999;61:193–217. doi: 10.1146/annurev.physiol.61.1.193. [DOI] [PubMed] [Google Scholar]
- 3.Ruskoaho H. Pharmacol Rev. 1993;44:479–602. [PubMed] [Google Scholar]
- 4.Maack T. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- 5.Steinhelper M E, Cochrane K L, Field L J. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 6.Brenner B M, Ballermann B J, Gunning M E, Zeidel M L. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 7.Garbers D L. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 8.Chien R. Basic Res Cardiol. 1992;87, Suppl. 2:49–58. doi: 10.1007/978-3-642-72477-0_5. [DOI] [PubMed] [Google Scholar]
- 9.Chinkers M, Garbers D L, Chang M S, Lowe D G, Chin H M, Goeddel D V, Schulz S. Nature (London) 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 10.Lowe D G, Chang M S, Hellmiss R, Chen E, Singh S, Garbers D L, Goeddel D V. EMBO J. 1989;8:1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez M J, Garbers D L, Kuhn M. J Biol Chem. 1997;272:23064–23068. doi: 10.1074/jbc.272.37.23064. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto I, Dubois S K, Garbers D L. Proc Natl Acad Sci USA. 1996;93:6215–6219. doi: 10.1073/pnas.93.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois S K, Kishimoto I, Lillis T O, Garbers D L. Proc Natl Acad Sci USA. 2000;97:4369–4373. doi: 10.1073/pnas.97.8.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John S W, Krege J H, Oliver P M, Hagaman J R, Hodgin J B, Panag S C, Flynn T G, Smithies O. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 15.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, et al. Proc Natl Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. . (First Published March 28, 2000; 10.1073/pnas.070371497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez M J, Wong S K, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers D L, Beuve A. Nature (London) 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 17.Oliver P M, Fox J E, Kim R, Rockman H A, Kim H S, Reddick R L, Pandy K N, Milgram S L, Smithies O, Maeda N. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbers D L, Dubois S K. Annu Rev Biochem. 1999;68:127–155. doi: 10.1146/annurev.biochem.68.1.127. [DOI] [PubMed] [Google Scholar]
- 19.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Hypertension. 2000;35:19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Clemo H F, Baumgarten C M. Circ Res. 1995;77:741–749. doi: 10.1161/01.res.77.4.741. [DOI] [PubMed] [Google Scholar]
- 21.Clemo H F, Feher J J, Baumgarten C M. J Gen Physiol. 1992;100:89–114. doi: 10.1085/jgp.100.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemo H F, Baumgarten C M. Am J Physiol. 1991;260:C681–C690. doi: 10.1152/ajpcell.1991.260.4.C681. [DOI] [PubMed] [Google Scholar]
- 23.Gulick J, Subramaniam A, Neumann J, Robbins J. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- 24.Robbins J. Annu Rev Physiol. 2000;62:261–287. doi: 10.1146/annurev.physiol.62.1.261. [DOI] [PubMed] [Google Scholar]
- 25.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 26.Ng W A, Grupp I L, Subramaniam A, Robbins J. Circ Res. 1991;68:1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- 27.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Circ Res. 1996;78:504–509. doi: 10.1161/01.res.78.3.504. [DOI] [PubMed] [Google Scholar]
- 28.Wolska B M, Solaro R J. Am J Physiol. 1996;271(3 Part 2):H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 29.de Bold A J, Bruneau B G, Kuroski de Bold M L. Cardiovasc Res. 1996;31:7–18. [PubMed] [Google Scholar]
- 30.Izumo S, Nadal-Ginard B, Mahdavi V. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloch K D, Seidman J G, Naftilan J D, Fallon J T, Seidman C E. Cell. 1986;47:695–702. doi: 10.1016/0092-8674(86)90512-x. [DOI] [PubMed] [Google Scholar]
- 32.Steinhelper M E, Cochrane K L, Field L J. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujisaki H, Ito H, Hirata Y, Tanaka M, Hata M, Lin M, Adachi S, Akimoto H, Marumo F, Hiroe M. J Clin Invest. 1995;96:1059–1065. doi: 10.1172/JCI118092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey K N, Nguyen H T, Li M, Boyle J W. Biochem Biophys Res Commun. 2000;271:374–379. doi: 10.1006/bbrc.2000.2627. [DOI] [PubMed] [Google Scholar]
- 36.Itoh H, Pratt R E, Ohno M, Dzau V J. Hypertension. 1992;19:758–761. doi: 10.1161/01.hyp.19.6.758. [DOI] [PubMed] [Google Scholar]
- 37.Chrisman T D, Garbers D L. J Biol Chem. 1999;274:4293–4299. doi: 10.1074/jbc.274.7.4293. [DOI] [PubMed] [Google Scholar]
- 38.Chusho H, Ogawa Y, Tamura N, Suda M, Yasoda A, Miyazawa T, Kishimoto I, Komatsu Y, Itoh H, Tanaka K, et al. Endocrinology. 2000;141:3807–3813. doi: 10.1210/endo.141.10.7692. [DOI] [PubMed] [Google Scholar]