Abstract
Objectives. We investigated whether the intention to have children varied according to HIV status and use of highly active antiretroviral therapy (HAART) among women in Soweto, South Africa.
Methods. We used survey data from 674 women aged 18 to 44 years recruited from the Perinatal HIV Research Unit in Soweto (May through December 2007); 217 were HIV-positive HAART users (median duration of use = 31 months; interquartile range = 28, 33), 215 were HIV-positive and HAART–naive, and 242 were HIV negative. Logistic regression models examined associations between HIV status, HAART use, and intention to have children.
Results. Overall, 44% of women reported intent to have children, with significant variation by HIV status: 31% of HAART users, 29% of HAART-naive women, and 68% of HIV-negative women (P < .001). In adjusted models, HIV-positive women were nearly 60% less likely to report childbearing intentions compared with HIV-negative women (for HAART users, adjusted odds ratio [AOR] = 0.40; 95% confidence interval [CI] = 0.23, 0.69; for HAART-naive women, AOR = 0.35; 95% CI = 0.21, 0.60), with minimal differences according to use or duration of HAART.
Conclusions. Integrated HIV, HAART, and reproductive health services must be provided to support the rights of all women to safely achieve their fertility goals.
In sub-Saharan Africa, women of childbearing age comprise 61% of people living with HIV, accounting for over 12 million women.1 In many regions, HIV incidence is increasing most dramatically among young women aged 18 to 30 years,1,2 which coincides with their peak reproductive years.3 Globally, a plethora of evidence indicates that many women living with HIV continue to desire children,4–8 become pregnant,5,6,9 and give birth5,6,10 after knowing their HIV-positive status.
Childbearing decision making can be complex regardless of HIV seropositivity11; among HIV-infected women, however, reproduction introduces additional personal, public health, and clinical care issues.12 The vast majority of conceptions occur without the use of reproductive technologies such as sperm washing and artificial insemination.13 Thus, the unprotected sexual activity required for conception carries a risk of HIV transmission to uninfected sexual partners.14 Reproduction among HIV-positive women also carries a risk of vertical transmission during pregnancy and labor and through breastfeeding.15,16 Moreover, HIV-positive women have a lower life expectancy than HIV-negative women,17 increasing the risk of maternal orphanhood.18 In light of these concerns, early reproductive guidelines for people living with HIV were dissuasive,19 and HIV-positive women who express a desire to have children continue to encounter the disapproval of the community and of health care workers.4,20
Nonetheless, although the potential health risks may have dampened the fertility intentions of some HIV-positive women, stigma associated with childlessness in many societies21 and the strong personal desires for biological parenthood4 remain potent drivers of childbearing intentions, despite an HIV-positive status. Indeed, in some cultural contexts, remaining childless can be a violation of societal norms more stigmatizing than the HIV infection itself.4,22
Expanding access to highly active antiretroviral therapy (HAART) is changing the landscape of childbearing decision making for people living with HIV.23 HAART increases life expectancy,24–26 decreases morbidity,25,27 and dramatically reduces the risks of vertical28 and horizontal29,30 transmission. In this era of expanding access to HAART, the significant reduction in health risks and barriers to reproduction among people living with HIV has coincided with increased calls for a rights- and evidenced-based approach to reproduction.31,32 Since childbearing intentions are among the strongest predictors of eventual childbearing,33 creating effective and responsive sexual and reproductive health services for HIV-positive women in the context of expanding access to HAART requires a clear understanding of expressed childbearing intentions.
Existing evidence concerning the influence of expanding access to HAART on childbearing intentions is largely incomplete. Although recent regional studies have shown that HAART use is associated with higher childbearing intentions, these studies neglected to consider the duration of HAART use6,7 and tended only to compare the childbearing intentions of HIV-positive women without conducting a comparison with HIV-negative women from the same community.6–8 Moreover, the lack of an HIV-negative control group precludes the opportunity to assess whether HAART users begin to resemble HIV-negative women in their childbearing intentions, particularly as HIV is increasingly recognized as a manageable chronic disease.
Given the high HIV prevalence among women of reproductive age in Soweto, South Africa,1 we aimed to assess the prevalence of childbearing intentions and to determine whether they varied according to HIV status and HAART use among women. We hypothesized that HIV-positive women would have lower childbearing intentions than would HIV-negative women. In addition, we hypothesized that HIV-positive women receiving HAART would have higher childbearing intentions than would HIV-positive HAART-naive women, with increasing duration of HAART treatment associated with incrementally higher childbearing intentions. Overall, we hypothesized that HAART use would narrow the measurable differences in childbearing intentions between HIV-positive and HIV-negative women.23
METHODS
Our analysis was based on cross-sectional survey data of HIV-positive women (receiving HAART and HAART-naive) and HIV-negative women seeking services at the Perinatal HIV Research Unit (PHRU) in Soweto, South Africa. A medical chart review was also conducted to confirm HIV serology and history of HAART use among HIV-positive women.
Study Setting
The PHRU, one of Africa's largest HIV research and clinical service centers, is housed within the Chris Hani Baragwanath Hospital in Soweto, an urban South African township located 15 km (9.4 miles) outside of Johannesburg. The PHRU clinic sees over 5000 patient visits monthly and provides free antiretroviral therapy and clinical care to medically eligible HIV-positive individuals and ongoing wellness care for those not medically eligible for antiretroviral treatment. The PHRU also operates a prevention studies area that includes a voluntary counseling and testing center.
Eligibility criteria.
To be eligible to participate in the study, women were required to be aged 18 to 49 years, attending a PHRU clinic, residing in Soweto, competent to give informed consent, and willing to allow review of her medical records to confirm HIV status and HAART history. We considered women to be HAART users if they had been taking HAART medications for at least 1 month. We considered women to be HAART–naive if they had never taken HAART, except for vertical transmission prophylaxis.
Study sample.
We enrolled 751 women into the study, including 253 HIV-positive women receiving HAART, 249 HIV-positive women not receiving HAART, and 249 HIV-negative women. This sampling strategy provided 1 case group (HAART users) and 2 comparison groups (HIV-positive HAART-naive women and HIV-negative women).
HAART users were sampled from the PHRU's President's Emergency Plan for AIDS Relief (PEPFAR) Clinic, which has provided free antiretroviral therapy to medically eligible patients since July 2004. Currently, the PEPFAR Clinic has over 1000 patients receiving HAART, 75% of whom are female. PEPFAR patients are followed up every 3 months and generally receive 1 of 2 standard HAART regimens: regimen 1 consists of stavudine (d4T), lamivudine (3TC), and efavirenz (EFV) or nevirapine (NVP); regimen 2 consists of zidovudine (AZT), didanosine (ddI), and lopinavir/ritonavir (LPV/r).34
HIV-positive HAART-naive women were sampled from the PHRU's Wellness Clinic, which opened in January 2003 with the goal of providing preventive care to HIV-positive individuals. Wellness Clinic patients are followed up approximately every 6 months. When patients are medically eligible for HAART, they are referred to the PEPFAR Clinic or to one of the nearby government antiretroviral treatment clinics. There are approximately 3000 active patients in the Wellness Clinic.
HIV-negative women were sampled from the voluntary counseling and testing clinic, which was initiated in mid-2002 and sees approximately 400 people per month. Testing is conducted on-site during visits that last an average of 2 hours. Approximately 65% of attendees are women and approximately 30% of all attendees are HIV positive.
For this analysis of childbearing intentions, we restricted the study sample to women aged 18–44 years who were not sterilized (i.e., did not report a hysterectomy or female sterilization). This yielded an analytic sample of 674 women, including 217 HAART users, 215 HAART-naive women, and 242 HIV-negative women.
Data Collection
Every female patient attending the PEPFAR Clinic and the voluntary counseling and testing clinic was consecutively approached by a research assistant to assess eligibility and interest in participating in the study. Since the Wellness Clinic sees many more women daily than the PEPFAR or voluntary counseling and testing clinics, each morning a research assistant compiled a list of clinical chart numbers of women due to attend the clinic that day. A random sample of chart numbers (40% of the total number of charts present) was then drawn and the corresponding women were approached to assess eligibility and interest in participating in the study.
After confirming eligibility and seeking informed consent, we asked all participants to complete a 15- to 25-minute interviewer-administered questionnaire in English. The study interviewers were multilingual and trained to ensure accurate and consistent translation of the questionnaire if required or requested by the participant. Pilot testing of 45 women revealed that women were able to understand and answer the questionnaire.
Approximately 12 women were interviewed daily by 3 trained research assistants between May and December 2007. Participants were reimbursed 20 South African rand (about US $3) for their transportation costs to and from the PHRU. Research assistants were women from the local community who had previous research experience and were recent social sciences graduates of a local university.
Data Collection Instruments
The questionnaire assessed sociodemographic characteristics (e.g., age, education, employment status, marital status, parity), HIV serostatus and date of HIV-positive diagnosis, clinical stage of disease (CD4 cell count and viral load), HAART history, childbearing intentions, fertility history (number and timing of pregnancies, terminations, stillbirths, miscarriages, and live births), contraceptive practices, and sexual history.
For all HIV-positive women, we reviewed medical records to confirm HIV status and HAART history and to obtain clinical data, including World Health Organization (WHO) Stage of Disease35 and CD4 cell count. Viral load assessments were conducted only on women from the PEPFAR Clinic (i.e., HAART users). The medical record was considered the referent measure for inconsistencies between self-reported and medical record data.
Measures
The primary outcome was self-reported childbearing intentions, which was determined by the answer to the question, “Are you planning to have [any more] children in the future?” Women were free to respond “yes,” “no,” or “don't know.” Given recent findings suggesting that women who answer “don't know” tend to have childbearing intentions similar to those of the majority with stated intentions,36 the small proportion of women who responded “don't know” (5%) were included in the “no” category. There was little difference in the proportion of women reporting “don't know” by HIV and HAART use status.
The primary explanatory variable was current HAART use. The secondary explanatory variable was HIV status. Variables known to be associated with childbearing intentions were included in the analysis to provide an adjusted estimate of the association. Covariates included age, education, employment, monthly household income, current sexual partnership status, number of living children, and HIV clinical variables, including most recent CD4, nadir CD4, and WHO Stage of Disease.
Statistical Analysis
We computed the prevalence of childbearing intentions among each of the 3 groups of women in our study. We conducted 2 separate models to measure the presence and strength of the association between HAART use and the likelihood of reporting childbearing intentions, while controlling for covariates. The first model compared HAART users and HAART–naive women with HIV-negative women. The second model compared HAART users with HAART–naive women and allowed adjustment for HIV-associated clinical characteristics.
In both models, univariate analyses were used to assess the relationship between childbearing intentions and HAART use and covariates. We report differences in childbearing intentions between groups using the Pearson χ2 test (for categorical covariates), analysis of variance (ANOVA), or the independent t test (for continuous variables). We report the association between childbearing intentions and HAART use using a crude odds ratio (OR) with a 95% confidence interval (CI). After testing for collinearity (using Spearman's ρ)37 and interaction,38 we included all covariates with significant associations (P < .10) in the univariate analysis in the final multivariate logistic regression model to obtain adjusted odds ratios (AORs) and 95% CIs. All statistical tests were 2-sided and were considered significant at α = .05. The data were analyzed with SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
In a subanalysis, we conducted the same analyses described in this section but restricted our sample to women aged 18–30 years, the peak childbearing years among women in South Africa.3
RESULTS
A total of 801 women were approached for participation, of whom 751 consented, completed the questionnaire, and underwent a medical record review (response rate = 94%). The analysis of childbearing intentions was restricted to women aged 18–44 years who were not sterilized, yielding a study sample of 674 women.
Baseline Characteristics
There were important differences in baseline covariates by HIV and HAART use status (Table 1). Overall mean age was 30 years (SD = 6.7), with HIV-negative women aged significantly younger than were HIV-positive women. Overall, 51% of women had less than a grade 12 education, 61% were unemployed, and 71% had a monthly household income less than 3000 rand (US $350). Only a small proportion of women were married (8%), but the vast majority were currently in a sexual relationship (78%), with a mean of 0.90 (SD = 0.68) sexual partners in the previous 6 months. One quarter reported that their primary sexual partner was HIV positive, one third reported that their partner was HIV negative, and the remaining 42% did not know the HIV status of their sexual partner. The mean number of lifetime sexual partners was 5.3 (SD = 5.6). Overall, mean parity was 1.4 (SD = 1.1). Of women with at least 1 live birth, 14% had lost a child. Overall, 41% of women had 2 or more living children.
TABLE 1.
Baseline Characteristics of HIV-Positive Women Receiving Highly Active Antiretroviral Therapy (HAART), HIV-Positive HAART-Naive Women, and HIV-Negative Women: Soweto, South Africa, 2007
| Variable | HAART Users (n = 217), No. (%) or Mean (SD) | HAART–naive Women (n = 215), No. (%) or Mean (SD) | HIV Negative (n = 242), No. (%) or Mean (SD) | Overall (n = 674), No. (%) or Mean (SD) | Pa |
| Sociodemographic characteristics | |||||
| Mean age, y | 33.5 (5.0) | 32.0 (5.7) | 25.0 (6.0) | 30.0 (6.7) | <.001 |
| Age group, y | <.001 | ||||
| 18–24 | 6 (3) | 22 (10) | 138 (57) | 166 (25) | |
| 25–29 | 38 (18) | 47 (22) | 47 (19) | 132 (20) | |
| 30–34 | 83 (38) | 75 (35) | 41 (17) | 199 (30) | |
| 35–39 | 59 (27) | 45 (21) | 7 (3) | 111 (17) | |
| 40–44 | 30 (14) | 25 (12) | 9 (4) | 64 (10) | |
| Education | <.001 | ||||
| Less than grade 12 | 138 (64) | 128 (60) | 75 (31) | 341 (51) | |
| Grade 12 or higher | 78 (36) | 87 (40) | 167 (69) | 332 (49) | |
| Employment status | .072 | ||||
| Employed | 89 (41) | 91 (43) | 80 (33) | 260 (39) | |
| Unemployed | 127 (59) | 122 (57) | 162 (67) | 411 (61) | |
| Household income per month, rand | <.001 | ||||
| < 3000 | 182 (84) | 169 (79) | 125 (52) | 476 (71) | |
| ≥ 3000 | 21 (10) | 35 (16) | 75 (31) | 131 (19) | |
| Do not know or refused | 14 (6) | 11 (5) | 42 (17) | 67 (10) | |
| Currently in a sexual relationship | <.001 | ||||
| No | 62 (29) | 51 (24) | 33 (14) | 146 (22) | |
| Yes | 155 (71) | 164 (76) | 209 (86) | 528 (78) | |
| Mean no. of sexual partners in previous 6 mo | 0.77 (0.48) | 0.82 (0.53) | 1.09 (0.88) | 0.90 (0.68) | <.001 |
| HIV status of regular sexual partner or husbandb | <.001 | ||||
| Do not know | 57 (38) | 72 (49) | 78 (41) | 207 (42) | |
| HIV negative | 28 (19) | 24 (16) | 109 (58) | 161 (33) | |
| HIV positive | 66 (44) | 52 (35) | 2 (1) | 120 (25) | |
| Mean parity | 1.8 (1.1) | 1.8 (1.1) | 0.80 (0.9) | 1.4 (1.1) | <.001 |
| Ever lost a childc | <.001 | ||||
| No | 152 (78) | 168 (87) | 132 (99) | 452 (86) | |
| Yes | 44 (22) | 25 (13) | 2 (1) | 71 (14) | |
| Living children | <.001 | ||||
| 0 | 29 (13) | 24 (11) | 109 (45) | 162 (24) | |
| 1 | 80 (37) | 77 (36) | 82 (34) | 239 (35) | |
| ≥ 2 | 108 (50) | 114 (53) | 51 (21) | 273 (41) | |
| HIV history and clinical characteristics | |||||
| Mean no. of months since HIV diagnosis | 67.6 (35.8) | 49.8 (33.1) | NA | 58.7 (35.6) | <.001 |
| Recent CD4 count, cells/μL | .009 | ||||
| < 200 | 37 (17) | 39 (18) | NA | 76 (18) | |
| 200–349 | 52 (24) | 79 (37) | NA | 131 (31) | |
| ≥ 350 | 124 (58) | 95 (45) | NA | 219 (51) | |
| Nadir CD4 count, cells/μL | <.001 | ||||
| < 50 | 62 (29) | 3 (1) | NA | 65 (15) | |
| 50–199 | 139 (65) | 44 (21) | NA | 183 (43) | |
| 200–349 | 5 (2) | 83 (39) | NA | 88 (21) | |
| ≥ 350 | 7 (3) | 83 (39) | NA | 90 (21) | |
| WHO Stage of Disease | .587 | ||||
| Stage I or II | 207 (97) | 205 (96) | NA | 412 (97) | |
| Stage III or IV | 6 (3) | 8 (4) | NA | 14 (3) | |
| Disclosed HIV status to anybody | .003 | ||||
| No | 5 (2) | 19 (9) | NA | 24 (6) | |
| Yes | 211 (98) | 196 (91) | NA | 407 (94) | |
Note. NA = not applicable; WHO = World Health Organization. All women (n = 674) were aged 18 to 44 years and nonsterilized.
Differences between groups are reported with the Pearson χ2 test (for categorical variables) and the independent t test or analysis of variance (ANOVA; for continuous variables).
Restricted to those who reported having a regular sexual partner or husband and who responded to the question about partner's HIV status (n = 488).
Restricted to those who reported having had a live birth (n = 523).
Among HIV-positive women, mean time since HIV diagnosis was 59 months (SD = 36). Fifty-eight percent of HAART users had recent CD4 counts of 350 cells/μL or higher, compared with 45% of HAART–naive women. Overall, 15% had nadir CD4 counts of less than 50 cells/μL. Nearly all (97%) were in WHO Stage of Disease I or II, and 94% had disclosed their HIV status to someone. Among HAART users, median duration of HAART use was 31 months (interquartile range = 28, 33), with a range of 1 month to 89 months. Most (81%) had undetectable viral loads (< 50 copies/mL).
Prevalence of and Factors Associated With Childbearing Intentions in the Overall Sample
Overall, 44% of women reported that they intended to have (more) children. This varied significantly by HIV status, with 31% of HAART users, 29% of HIV-positive HAART–naive women, and 68% of HIV-negative women reporting childbearing intentions (P < .001).
In the unadjusted analyses, many of the measured covariates were significantly associated with childbearing intentions (Table 2). Compared with HIV-negative women, HIV-positive women were significantly less likely to report childbearing intentions (for HAART users, OR = 0.21, 95% CI = 0.14, 0.32; for HAART–naive women, OR = 0.20, 95% CI = 0.13, 0.29).
TABLE 2.
Univariate and Adjusted Analyses of Variables Associated With Childbearing Intentions Among Nonsterilized Women Aged 18 to 44 Years: Soweto, South Africa, 2007
| Intend to Have Children (n = 380), No. (%) | Do Not Intend to Have Children (n = 294), No. (%) | OR (95% CI) | AOR (95% CI) | |
| HIV and HAART use status | ||||
| HIV negative (Ref) | 78 (21) | 164 (56) | 1.00 | 1.00 |
| HIV positive, HAART-naive | 152 (40) | 63 (21) | 0.20 (0.13, 0.29) | 0.35 (0.21, 0.60) |
| HIV positive, receiving HAART | 150 (39) | 67 (23) | 0.21 (0.14, 0.32) | 0.40 (0.23, 0.69) |
| Age, y | ||||
| 18–24 (Ref) | 52 (14) | 114 (39) | 1.00 | 1.00 |
| 25–29 | 68 (18) | 64 (22) | 0.43 (0.27, 0.69) | 1.88 (1.00, 3.52) |
| 30–34 | 124 (33) | 75 (26) | 0.28 (0.18, 0.43) | 1.44 (0.78, 2.64) |
| 35–39 | 83 (22) | 28 (10) | 0.15 (0.09, 0.26) | 1.29 (0.62, 2.71) |
| 40–44 | 52 (14) | 12 (4) | 0.11 (0.05, 0.21) | 0.84 (0.34, 2.06) |
| Education | ||||
| Less than grade 12 (Ref) | 222 (59) | 119 (40) | 1.00 | 1.00 |
| Grade 12 or higher | 157 (41) | 175 (60) | 2.08 (1.53, 2.84) | 0.92 (0.61, 1.39) |
| Employment status | ||||
| Unemployed (Ref) | 230 (61) | 181 (62) | 1.00 | – |
| Employed | 147 (39) | 113 (38) | 0.98 (0.71, 1.34) | |
| Household income per month, rand | ||||
| < 3000 (Ref) | 305 (80) | 171 (58) | 1.00 | 1.00 |
| ≥ 3000 | 50 (13) | 81 (28) | 2.89 (1.94, 4.31) | 1.40 (0.84, 2.33) |
| Do not know or refused | 25 (7) | 42 (14) | 3.00 (1.77, 5.09) | 1.73 (0.90, 3.33) |
| Currently in a sexual relationship | ||||
| No (Ref) | 111 (29) | 35 (12) | 1.00 | 1.00 |
| Yes | 269 (71) | 259 (88) | 3.05 (2.01, 4.63) | 3.07 (1.86, 5.05) |
| Living children | ||||
| 0 (Ref) | 33 (9) | 129 (44) | 1.00 | 1.00 |
| 1 | 117 (31) | 122 (42) | 0.27 (0.17, 0.42) | 0.30 (0.18, 0.50) |
| ≥ 2 | 230 (61) | 43 (15) | 0.05 (0.03, 0.08) | 0.06 (0.03, 0.11) |
Note. AOR = adjusted odds ratio; CI = confidence interval; HAART = highly active antiretroviral therapy; OR = odds ratio. The total sample size was N = 674.
After adjustment for covariates shown in Table 1, HIV status remained significantly associated with childbearing intentions. Compared with HIV-negative women, HIV-positive women were significantly less likely to report childbearing intentions (for HAART users, AOR = 0.40; 95% CI = 0.23, 0.69; for HAART–naive women, AOR = 0.35; 95% CI = 0.21, 0.60). Currently being in a sexual relationship and having fewer living children also remained independently associated with childbearing intentions.
Factors Associated With Childbearing Intentions Among HIV-Positive Women
As seen in Table 3, in an analysis restricted to HIV-positive women, HAART users and HAART-naive women were equally likely to report childbearing intentions (OR = 1.08; 95% CI = 0.71, 1.63). There were no significant differences in childbearing intentions by any of the measured clinical characteristics, including duration of HIV diagnosis, recent CD4, nadir CD4, WHO Stage of Disease, and disclosure of HIV status.
TABLE 3.
Univariate and Adjusted Analyses of Variables Associated With Childbearing Intentions Among Nonsterilized, HIV-Positive Women Aged 18 to 44 Years: Soweto, South Africa, 2007
| Intend to Have Children, No. (%) or Mean (SD) | Do Not Intend to Have Children, No. (%) or Mean (SD) | OR (95% CI) | AOR (95% CI) | |
| HAART use | ||||
| HIV positive, HAART-naive (Ref) | 152 (50) | 63 (48) | 1.00 | 1.00 |
| HIV positive, receiving HAART | 150 (50) | 67 (52) | 1.08 (0.71, 1.63) | 1.16 (0.72, 1.86) |
| Mean age,a y | 33.2 (5.6) | 31.7 (4.9) | 0.95 (0.91, 0.99) | 0.99 (0.94, 1.04) |
| Education | ||||
| Less than Grade 12 (Ref) | 110 (37) | 55 (42) | 1.00 | – |
| Grade 12 or higher | 191 (63) | 75 (58) | 1.27 (0.84, 1.94) | |
| Employment status | ||||
| Unemployed (Ref) | 179 (60) | 70 (54) | 1.00 | – |
| Employed | 120 (40) | 60 (46) | 1.28 (0.84, 1.94) | |
| Household income per month, rand | ||||
| < 3000 (Ref) | 256 (85) | 95 (73) | 1.00 | 1.00 |
| ≥ 3000 | 32 (11) | 24 (18) | 2.02 (1.13, 3.61) | 1.64 (0.85, 3.17) |
| Do not know or refused | 14 (5) | 11 (8) | 2.13 (0.93, 4.83) | 1.90 (0.75, 4.80) |
| Currently in a sexual relationship | ||||
| No (Ref) | 94 (31) | 19 (15) | 1.00 | 1.00 |
| Yes | 208 (69) | 111 (85) | 2.64 (1.53, 4.55) | 2.98 (1.63, 5.46) |
| Living children | ||||
| 0 (Ref) | 19 (6) | 34 (26) | 1.00 | 1.00 |
| 1 | 92 (30) | 65 (50) | 0.40 (0.21, 0.75) | 0.36 (0.18, 0.71) |
| ≥ 2 | 191 (63) | 31 (24) | 0.09 (0.05, 0.18) | 0.09 (0.04, 0.17) |
| Mean no. of months since HIV diagnosis | 58.2 (35.2) | 59.9 (36.3) | 1.00 (1.00, 1.01) | – |
| Recent CD4 | ||||
| < 200 (Ref) | 55 (19) | 21 (16) | 1.00 | |
| 200–349 | 89 (30) | 42 (33) | 1.25 (0.66, 2.30) | |
| ≥ 350 | 153 (52) | 66 (51) | 1.13 (0.63, 2.02) | – |
| Nadir CD4 | ||||
| < 50 (Ref) | 45 (15) | 20 (16) | 1.00 | |
| 50–199 | 124 (42) | 59 (46) | 1.07 (0.58, 1.97) | |
| 200–349 | 64 (22) | 24 (19) | 0.84 (0.42, 1.71) | |
| ≥ 350 | 64 (22) | 26 (20) | 0.91 (0.46, 1.83) | – |
| WHO Stage of Disease | ||||
| Stage I or II (Ref) | 286 (96) | 126 (98) | 1.00 | – |
| Stage III or IV | 11 (4) | 3 (2) | 0.62 (0.17, 2.26) | |
| Disclosed HIV status to anybody | ||||
| No (Ref) | 18 (6) | 6 (5) | 1.00 | – |
| Yes | 283 (94) | 124 (95) | 1.31 (0.51, 3.39) |
Note. AOR = adjusted odds ratio; CI = confidence interval; OR = odds ratio; WHO = World Health Organization.
A continuous variable; AOR represents the change in odds per 1 year of increased age.
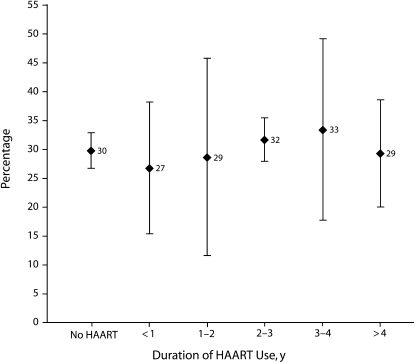
After adjustment for covariates shown in Table 1, HAART users and HAART-naive women remained equally likely to report childbearing intentions (AOR = 1.16; 95% CI = 0.72, 1.86). Currently being in a sexual relationship and having fewer living children remained independently associated with childbearing intentions. As shown in Figure 1, there was no association between length of time on HAART and reported childbearing intentions.
FIGURE 1—
Percentage of HIV-positive women who intend to have (more) children, by length of time on highly active antiretroviral therapy (HAART): Soweto, South Africa, 2007.
Note. Women (n = 432) were aged 18 to 44 years and nonsterilized. Bars represent standard errors.
Subanalyses
Although childbearing intentions among young women (aged 18–30 years) were higher than they were among the total sample, they still varied significantly by HIV status: 38% of HAART users, 34% of HAART–naive women, and 75% of HIV-negative women aged 18–30 years expressed childbearing intentions (P < .001). In multivariate analyses, we found that the same variables that were associated with childbearing intentions in the overall sample were similarly associated with childbearing intentions among young women (data not shown). This was the case for both models (i.e., all women and only HIV-positive women).
DISCUSSION
In contrast with our primary hypothesis, we found that the childbearing intentions of HIV-positive women did not differ by HAART use or duration of HAART use. Consistent with our secondary hypothesis, however, HIV-positive women were significantly less likely than were HIV-negative women to report childbearing intentions.
Our finding that nearly one third of HIV-positive women reported childbearing intentions, with HAART use having a minimal effect on their decision, contrasts with findings from other sub-Saharan African sites that have reported threefold higher childbearing intentions among HAART users6 and higher childbearing intentions associated with increasing duration of HAART use.7 A recent American study showed that HAART use was associated with a lower prevalence of fertility desires.39 Our findings are, however, consistent with those of a recent Canadian study that reported no association between HAART use and childbearing intentions.40
Potential reasons for our findings may relate to the nature of HAART and HIV care services at the PHRU. HAART has been available at the PHRU since July 2004, nearly 3 years longer than at government clinics in South Africa41 and at least 2 years longer than in most sub-Saharan African settings.42 The lack of association between HAART use and childbearing intentions in our study may reflect the fact that HIV-positive women who are not yet receiving HAART can be confident that treatment is available once they are medically eligible, thereby minimizing differences between groups. Moreover, HAART–naive women were sampled from the Wellness Clinic, which provides regular clinical care to HIV-positive women. Thus, any influence that regular contact with health care providers had on childbearing decision making may have been similar in both groups. In this way, our findings from the PHRU may be more comparable to findings from British Columbia,40 where HAART has been available at no charge to medically eligible HIV-infected individuals since 1996.26,40 Consistent with other studies of HIV-positive and HIV-negative women around the world, number of living children and current partnership status were also strongly associated with childbearing intentions.6,7,39,40,43
Although HAART use did not affect childbearing intentions among HIV-positive women, we did find that HIV-positive women were 60% less likely than were HIV-negative women to report an intention to have (more) children. It is difficult to compare these findings to other settings since we could identify no studies that directly measured differences in childbearing intentions between HIV-negative and HIV-positive women. Moreover, although the expressed childbearing intentions of HIV-positive women were lower than were those of HIV-negative women, they remain substantial. We found that nearly one third of HIV-positive women reported childbearing intentions, a proportion that increased significantly with younger age and fewer living children. Our findings are at the upper range of childbearing intentions for HIV-positive women reported from other settings.6–8,40,43
All women, including women living with HIV, should be supported to achieve their reproductive goals in the healthiest and safest possible manner.32 Given the high prevalence of childbearing intentions among HIV-positive women, it is critical that factual and nonstigmatizing information and support be incorporated into HIV treatment services to optimize healthy outcomes for mother, father, and baby. This includes counseling services regarding HAART and pregnancy,44 safer options to conceive (including HAART as prevention45), safer labor options, comprehensive prevention of mother-to-child transmission services, antenatal and postnatal care, and infant feeding options. Currently, no clear guidelines are available regarding the ideal time for pregnancy for an HIV-positive woman (with respect to CD4 level, stage of treatment, treatment regimen, viral load, or HIV and health status of her partner). This information is urgently needed.
Although 30% of HIV-positive women reported intentions to have (more) children in our study, 70% did not. Thus, it is critical to ensure that effective, nonjudgmental family planning services (including access to termination-of-pregnancy services) are available to all women who need them. This is of particular importance since evidence has shown that despite lower childbearing intentions, infected women may be as likely as uninfected women to become pregnant46–48 and to terminate a pregnancy.47 More work must be done on the provision of contraceptive options to close the gap between reported childbearing intentions and actual childbearing.
It must be stressed that the childbearing intentions of HIV-negative women in this HIV hyperendemic setting were very high (68%). In South Africa overall, the prevalence of HIV infection is highest among young women, which corresponds with the peak reproductive years.3 Conception requires unprotected sexual activity, and the HIV status of the sexual partners for many of these women is unknown. Indeed, only 20% of the general adult population of South Africa knows their HIV status.49,50 For HIV-negative women in Soweto, reproduction must therefore be considered an important risk for HIV acquisition.
Limitations
The limitations of this study must be acknowledged. First, the cross-sectional nature of this analysis precluded us from determining causality between the explanatory variable and the outcome, even though childbearing intentions were assessed as a future event, whereas HIV and HAART use status were assessed in the present. Although reverse causality is considered unlikely, longitudinal studies are needed to investigate a potentially time-sensitive relationship between HIV status, HAART use, and childbearing intentions. Second, there is a risk of social desirability bias whereby HIV-positive women may underreport their childbearing intentions because of disapproving views expressed by community health workers and community members.4,20 If underreporting was differential, then our effect estimates are likely somewhat inflated. We took precautions against reporting bias by using standardized questions of childbearing intentions and employing nonclinic staff to conduct the interviews.
Third, recent literature has described the limitations of using dichotomized measures of childbearing intentions. Moreover, others have emphasized the dynamic nature of childbearing intentions, which we were unable to fully capture in this study.4,51,52 Fourth, there were important baseline differences between the HIV-positive and HIV-negative women in our study that cannot be fully adjusted for in the analyses. In particular, HIV-positive women in our study were significantly older than HIV-negative women; age is both a known and important predictor of childbearing intentions and is associated with a number of other covariates (e.g., parity, education status). In an attempt to address this difference in age, we conducted a subanalysis of childbearing intentions restricted to women aged younger than 30 years. We found no differences in the variables that predicted childbearing intentions or in the magnitude of the associations. The results of the subanalysis suggest that our overall findings are robust, despite differences in age at baseline.
Finally, a quantitative analysis such as this fails to capture the salient influence of cultural dynamics on childbearing decision making. Cultural beliefs and practices have often and consistently been discussed as critical determinants of childbearing intentions.53,54 HIV status and HAART use alone are unlikely to be the sole or even primary drivers of reproductive decision making.23 Indeed, qualitative studies have highlighted that the desire for motherhood, opinions of partners and health care providers, religious values, and the perceived capacity to successfully parent emerge as critical factors influencing the childbearing decision making of HIV-positive women.4,51
Conclusions
Our findings suggest important associations between childbearing intentions and HIV status, largely irrespective of HAART use. The childbearing intention profile of HIV-positive women in Soweto demands that integrated HAART, HIV care, and reproductive health services be made available to support the rights of HIV-positive women to safely achieve their childbearing goals, while minimizing risks of vertical and horizontal transmission. The substantial childbearing intentions of HIV-negative women in this HIV hyperendemic region are of great importance and demand consideration of targeted reproductive health services to minimize their risks of HIV acquisition through realization of their reproductive goals.
Acknowledgments
The project described was supported by the University of California, San Diego Center for AIDS Research (grant P30AI036214 from the National Institute of Allergy and Infectious Diseases, the Canadian Foundation for AIDS Research, and the Social Sciences and Humanities Research Council of Canada. A. Kaida is supported by the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research.
We gratefully acknowledge the research and clinical staff and clients at the Perinatal HIV Research Unit for their contributions to this study.
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Human Participant Protection
All participants provided informed consent and all procedures were approved by the Human Research Ethics Committee of the University of the Witwatersrand, the University of British Columbia Health Research Ethics Board, the Simon Fraser University Office of Research Ethics, and the University of California, San Diego institutional review board. Information letters and consent forms were available in English and in 1 of 2 local languages (Zulu and Sesotho) to ensure comprehensive understanding of the study objectives, potential risks, and benefits.
References
- 1.UNAIDS Report on the Global HIV/AIDS Epidemic 2008. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2008 [Google Scholar]
- 2.National HIV and Syphilis Prevalence Survey, South Africa 2006. Pretoria: Dept of Health, South Africa; 2007 [Google Scholar]
- 3.South Africa Demographic and Health Survey 2003. Pretoria: Dept of Health, South Africa; 2004 [Google Scholar]
- 4.Cooper D, Harries J, Myer L, Orner P, Bracken H, Zweigenthal V. “Life is still going on”: reproductive intentions among HIV-positive women and men in South Africa. Soc Sci Med. 2007;65(2):274–283 [DOI] [PubMed] [Google Scholar]
- 5.Homsy J, Bunnell R, Moore D, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS One. 2009;4(1):e4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier M, Andia I, Emenyonu N, et al. Antiretroviral therapy is associated with increased fertility desire, but not pregnancy or live birth, among HIV+ women in an early HIV treatment program in rural Uganda. AIDS Behav. 2009;13(suppl 1):28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21(4):278–285 [DOI] [PubMed] [Google Scholar]
- 8.Nakayiwa S, Abang B, Packel L, et al. Desire for children and pregnancy risk behavior among HIV-infected men and women in Uganda. AIDS Behav. 2006;10(suppl 4):S95–S104 [DOI] [PubMed] [Google Scholar]
- 9.Desgrees-Du-Lou A, Msellati P, Viho I, et al. Contraceptive use, protected sexual intercourse and incidence of pregnancies among African HIV-infected women. DITRAME ANRS 049 Project, Abidjan 1995–2000. Int J STD AIDS. 2002;13(7):462–468 [DOI] [PubMed] [Google Scholar]
- 10.Gray GE, McIntyre JA. HIV and pregnancy. BMJ. 2007;334(7600):950–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollerbach PE. Fertility Decision-Making Processes: A Critical Essay. New York, NY: Population Council; 1982 [Google Scholar]
- 12.Thornton AC, Romanelli F, Collins JD. Reproduction decision making for couples affected by HIV: a review of the literature. Top HIV Med. 2004;12(2):61–67 [PubMed] [Google Scholar]
- 13.Delvaux T, Nostlinger C. Reproductive choice for women and men living with HIV: contraception, abortion and fertility. Reprod Health Matters. 2007;15(suppl 29):46–66 [DOI] [PubMed] [Google Scholar]
- 14.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409 [DOI] [PubMed] [Google Scholar]
- 15.HIV-infected pregnant women and vertical transmission in Europe since 1986 European collaborative study. AIDS. 2001;15(6):761–770 [PubMed] [Google Scholar]
- 16.Mofenson LM. Mother–child HIV-1 transmission: timing and determinants. Obstet Gynecol Clin North Am. 1997;24(4):759–784 [DOI] [PubMed] [Google Scholar]
- 17.Nunn AJ, Mulder DW, Kamali A, Ruberantwari A, Kengeya-Kayondo JF, Whitworth J. Mortality associated with HIV-1 infection over five years in a rural Ugandan population: cohort study. BMJ. 1997;315(7111):767–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makumbi FE, Gray RH, Serwadda D, et al. The incidence and prevalence of orphanhood associated with parental HIV infection: a population-based study in Rakai, Uganda. AIDS. 2005;19(15):1669–1676 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34(48):721–726, 731–732 [PubMed] [Google Scholar]
- 20.Myer L, Morroni C, Cooper D. Community attitudes towards sexual activity and childbearing by HIV-positive people in South Africa. AIDS Care. 2006;18(7):772–776 [DOI] [PubMed] [Google Scholar]
- 21.Dyer SJ. The value of children in African countries: insights from studies on infertility. J Psychosom Obstet Gynaecol. 2007;28(2):69–77 [DOI] [PubMed] [Google Scholar]
- 22.Dyer SJ, Abrahams N, Hoffman M, van der Spuy ZM. “Men leave me as I cannot have children”: women's experiences with involuntary childlessness. Hum Reprod. 2002;17(6):1663–1668 [DOI] [PubMed] [Google Scholar]
- 23.Kaida A, Andia I, Maier M, et al. The potential impact of antiretroviral therapy on fertility in sub-Saharan Africa. Curr HIV/AIDS Rep. 2006;3(4):187–194 [DOI] [PubMed] [Google Scholar]
- 24.Hogg RS, O'Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349(9061):1294. [DOI] [PubMed] [Google Scholar]
- 25.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860 [DOI] [PubMed] [Google Scholar]
- 26.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–454 [DOI] [PubMed] [Google Scholar]
- 27.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286(20):2568–2577 [DOI] [PubMed] [Google Scholar]
- 28.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29(5):484–494 [DOI] [PubMed] [Google Scholar]
- 29.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40(1):96–101 [DOI] [PubMed] [Google Scholar]
- 30.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190(5):879–885 [DOI] [PubMed] [Google Scholar]
- 31.Berer M. HIV/AIDS, sexual and reproductive health: intersections and implications for national programmes. Health Policy Plan. 2004;19(suppl 1):i62–i70 [DOI] [PubMed] [Google Scholar]
- 32.Gruskin S, Ferguson L, O'Malley J. Ensuring sexual and reproductive health for people living with HIV: an overview of key human rights, policy and health systems issues. Reprod Health Matters. 2007;15(suppl 29):4–26 [DOI] [PubMed] [Google Scholar]
- 33.Pritchett LH. Desired fertility and the impact of population policies. Popul Dev Rev. 1994;20(1):1–55 [Google Scholar]
- 34.National Antiretroviral Treatment Guidelines Pretoria: Dept of Health, South Africa; 2004 [Google Scholar]
- 35.Scaling Up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach. 2003 Revisions Geneva, Switzerland: World Health Organization; 2004 [Google Scholar]
- 36.Becker S, Sutradhar SC. Fertility intentions: are the undecided more like those who want more or want no more children? J Biosoc Sci. 2007;39(1):137–145 [DOI] [PubMed] [Google Scholar]
- 37.Pagano M, Gauvreau K. Principles of Biostatistics. 2nd ed. Pacific Grove, CA: Duxbury; 2000 [Google Scholar]
- 38.Van Ness PH, Allore HG. Using SAS to investigate effect modification. Paper 195-31. : Proceedings of the Thirty-First Annual SAS® Users Group International Conference. Cary, NC: SAS Institute Inc; 2007. Available at: http://www2.sas.com/proceedings/sugi31/195-31.pdf. Accessed October 10, 2007 [Google Scholar]
- 39.Stanwood NL, Cohn SE, Heiser JR, Pugliese M. Contraception and fertility plans in a cohort of HIV-positive women in care. Contraception. 2007;75(4):294–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogilvie GS, Palepu A, Remple VP, et al. Fertility intentions of women of reproductive age living with HIV in British Columbia, Canada. AIDS. 2007;21(suppl 1):S83–S88 [DOI] [PubMed] [Google Scholar]
- 41.Kapp C. South Africa unveils new 5-year HIV/AIDS plan. Lancet. 2007;369(9573):1589–1590 [DOI] [PubMed] [Google Scholar]
- 42.Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector: Progress Report 2008. Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 43.Chen JL, Philips KA, Kanouse DE, Collins RL, Miu A. Fertility desires and intentions of HIV-positive men and women. Fam Plann Perspect. 2001;33(4):144–152, 165 [PubMed] [Google Scholar]
- 44.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS. 2008;22(14):1815–1820 [DOI] [PubMed] [Google Scholar]
- 45.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–536 [DOI] [PubMed] [Google Scholar]
- 46.Ahluwalia IB, DeVellis RF, Thomas JC. Reproductive decisions of women at risk for acquiring HIV infection. AIDS Educ Prev. 1998;10(1):90–97 [PubMed] [Google Scholar]
- 47.Pivnick A. Loss and regeneration: influences on the reproductive decisions of HIV positive, drug-using women. Med Anthropol. 1994;16(1):39–62 [DOI] [PubMed] [Google Scholar]
- 48.Selwyn PA, Schoenbaum EE, Davenny K, et al. Prospective study of human immunodeficiency virus infection and pregnancy outcomes in intravenous drug users. JAMA. 1989;261(9):1289–1294 [PubMed] [Google Scholar]
- 49.Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counseling and testing in a Black township in Cape Town, South Africa. Sex Transm Infect. 2003;79(6):442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinsasa O, Simbayi LC. Nelson Mandela/HSRC Study of HIV/AIDS: South African National HIV Prevalence, Behavioral Risks and Mass Media, Household Survey. Cape Town, South Africa: Human Sciences Research Council; 2002 [Google Scholar]
- 51.Kirshenbaum SB, Hirky AE, Correale J, et al. “Throwing the dice”: pregnancy decision-making among HIV-positive women in four US cities. Perspect Sex Reprod Health. 2004;36(3):106–113 [DOI] [PubMed] [Google Scholar]
- 52.Taulo F, Berry M, Tsui A, et al. Fertility intentions of HIV-1 infected and uninfected women in Malawi: a longitudinal study. AIDS Behav. 2009;13(suppl 1):20–27 [DOI] [PubMed] [Google Scholar]
- 53.Sonko S. Fertility and culture in sub-Saharan Africa: a review. Int Soc Sci J. 1994;46:397–411 [Google Scholar]
- 54.Caldwell JC, Caldwell P. The cultural context of high fertility in sub-Saharan Africa. Popul Dev Rev. 1987;13:409–437 [Google Scholar]