Abstract
Objectives. We examined the effect of current patterns of smoking rates on future radon-related lung cancer.
Methods. We combined the model developed by the National Academy of Science's Committee on Health Risks of Exposure to Radon (the BEIR VI committee) for radon risk assessment with a forecasting model of US adult smoking prevalence to estimate proportional decline in radon-related deaths during the present century with and without mitigation of high-radon houses.
Results. By 2025, the reduction in radon mortality from smoking reduction (15 percentage points) will surpass the maximum expected reduction from remediation (12 percentage points).
Conclusions. Although still a genuine source of public health concern, radon-induced lung cancer is likely to decline substantially, driven by reductions in smoking rates. Smoking decline will reduce radon deaths more that remediation of high-radon houses, a fact that policymakers should consider as they contemplate the future of cancer control.
The Environmental Protection Agency (EPA) estimates that radon in the home is responsible for over 21 000 lung cancer deaths annually among Americans, making radon the major cause of lung cancer after tobacco use. The agency considers radon a major public health problem and, since 1986, has mounted an aggressive campaign urging the public to test their homes for radon and take remedial actions when airborne concentrations of radon exceed 4 picocuries per liter of air (4 pCi/L).1
For its most current risk assessment, the EPA employed the BEIR VI model, developed by the Committee on Health Risks of Exposure to Radon (the BEIR VI committee) of the National Academy of Sciences (NAS).2 The BEIR VI model's calculation of radon-related risk (as was the case for its predecessor, BEIR IV) was estimated from data on miners, who are subject to much higher levels of radon than is the average population and have shown a significant correlation between lung cancer risk and radon exposure. Although the extrapolation of the results from miners to the much less exposed general public initially caused controversy, the BEIR VI implications of risk have been validated by recent case–control studies at the population level.3–5 The BEIR VI model is thus broadly accepted as a valid predictor of the radon-related risk for typical individuals.
The available data suggest a strong interaction effect between radon exposure and smoking status in the determination of lung cancer risk, which means that smokers are at a much higher risk of dying from radon-induced lung cancer than are nonsmokers. This interaction is recognized in the BEIR VI model, which postulates a superadditive (but less than multiplicative) interaction between smoking and radon. To appreciate the magnitude of this interaction, consider the fact that the background lung cancer risk ratio between ever and never smokers is 13 to 1.6 A multiplicative interaction between radon and smoking would imply that, at the same level of radon exposure, the ratio of radon-induced excess risk between ever and never smokers would be the same as the ratio of background lung cancer risks between those 2 groups (i.e., 13 to 1). On the other hand, an additive relationship between radon and smoking would imply that radon would add the same extra risk to ever and never smokers exposed to the same dosage, making the excess risks ratio between the 2 groups equal 1 to 1. Using the BEIR VI model, the EPA calculates that, at a radon level of 4 pCi/L, the lifetime risk of radon-induced lung cancer death is 62 per 1000 for ever smokers and 7 per 1000 for never smokers, yielding an excess risk ratio of 8.86 to 1 between the 2 groups.1 As 8.86 falls between 1 and 13, the BEIR VI model implies that radon adds more risk to ever smokers than to never smokers, but that excess risk is less than proportional to the lung cancer background risk of those 2 groups, suggesting a submultiplicative (but superadditive) relationship between smoking and radon. The BEIR VI model does not distinguish between current and former smokers.
Given this implied superadditive interaction, the number of future radon deaths will heavily depend on population smoking rates. As smoking rates in the United States have been falling for several decades and are expected to continue declining, the overall magnitude of the radon death toll is likely to decline as well. The question we try to address is what is the magnitude of this expected decline?
We extend the EPA's analysis by examining the sensitivity of radon-related lung cancer in the United States to future smoking rates. We estimate the proportional decline in the number of lung cancer deaths caused by radon for the period 2006 through 2100, assuming a likely scenario for smoking rates. We do not forecast specific numbers of radon-induced lung cancer deaths because these numbers will depend on many factors likely to change over such a long period of time. Instead, we concentrate on the relative impact of the smoking decline on the overall radon death toll and also examine the benefits of remediating houses with high radon levels given the results of our analysis. Following the EPA's approach, in our computations, we employ the BEIR VI model, thereby assuming a submultiplicative relationship between smoking and radon. In the remaining sections of the report, we discuss the assumptions, models, and data employed in our analysis, our findings, and the implications of the results for both the magnitude of radon-related risk to the population and the effectiveness of housing remediation in reducing such risk.
METHODS
The BEIR VI model, published in 1999 by the NAS, remains the most current and widely accepted method for evaluating lung cancer risk caused by radon exposure. The BEIR VI committee of the NAS developed 2 alternative models: the “exposure-age-concentration” model and the “exposure-age-duration” model. Both models express the relative risk of lung cancer in the following form:
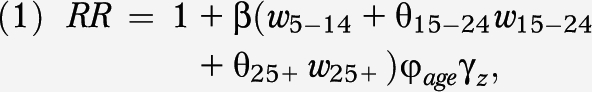 |
where β is the exposure-response parameter; w5–14, w15–24 and w25+ represent total radon exposure attained in the intervals 5 to 14, 15 to 24, and 25 or more (25+) years before current age, respectively; θ represents the relative contribution of the windows of exposure; and ϕage and γz represent, respectively, multiple categories of attained age and either exposure rate or exposure duration (depending on the model). All model parameters were specified and are available in the committee's report.2
The parameters specified in equation 1 apply to the combined population of ever and never smokers. To apply the model differentially according to smoking status, the BEIR VI committee identified a synergistic relationship between radon and smoking. From the analysis of data on miners used to parameterize the models, the BEIR VI committee concluded that the interaction effect between radon and smoking was more than additive but less than multiplicative. The committee published guidelines on how to assess the submultiplicative relationship (but stated that they could not rule out a multiplicative structure).2 Specifically, when the radon risk for never smokers is calculated, the β coefficient of equation 1 is multiplied by a factor of 2, and by a factor of 0.9 when ever smokers are considered.
The EPA combined the 2 BEIR VI models into 1 that yields results midway between the original models. EPA analysts then used this model, employing gender- and age-specific smoking prevalence estimates, to compute the number of annual radon-induced deaths. On the basis of this analysis, the EPA estimated that out of a total 146 400 lung cancer deaths nationally in 1995, 21 100 were radon related, with a 90% uncertainty range of 8000 to 45 000.6 Out of these radon-induced deaths, the EPA estimated that 18 200 (86%) were among ever smokers, who accounted for 48% of the adult population in 1995.
In extending the EPA analysis to account for reductions in ever-smoking prevalence, we first applied the BEIR VI model to the US population in 2006, the most recent year for which we have prevalence data on ever smokers by age, using a slightly different approach than the EPA's, and obtained a baseline value of 22 416 radon-related lung cancer deaths. This minor discrepancy (our estimate being 6% higher than the EPA's) stems from slight differences in the way we and the EPA implemented the BEIR VI model. Specifically, the EPA calculated summary lifetime etiologic fractions and applied them to observed number of lung cancer deaths in 1995 to calculate the number of radon-related deaths, whereas we computed those deaths directly from mortality tables, applying the BEIR VI model. Additionally, the EPA smoothed the step function specified in BEIR VI for the dependence of relative risk on age, whereas we did not. The approach and computations performed by the EPA are described in detail elsewhere.6
The specific model constructs and dynamics used in our calculations are shown in a technical appendix (available as a supplement to the online version of this article at http://www.ajph.org). In summary, we used a dynamic model of smoking prevalence that we have developed and discussed elsewhere, and combined it with the BEIR VI model to estimate future lung cancer deaths attributable to radon exposure.7,8 Parameters and inputs to the model included lung cancer mortality rates, obtained from the Surveillance, Epidemiology and End Results (SEER) Cancer Statistics Review, 1975–20069; population estimates, obtained from the US Census Bureau10; smoking prevalence rates, obtained from the National Health Interview Survey of 200611; and all-cause probability of death for men and women (smokers and nonsmokers), estimated by Mendez and Warner.12
The model started by estimating the total expected radon-related lung cancer deaths for the 2006 population, assuming an average radon exposure of 1.25 pCi/L, the average US residential radon level.13 We then followed each male and female cohort from that population to forecast prevalence of ever smokers as well as radon-related lung cancer deaths as predicted by the BEIR VI model. Smoking initiation was assumed to take place at age 18 years at the 2006 rate (23.6%). We then followed the new male and female birth cohorts introduced over the next 94 years and estimated their ever-smoking prevalence and radon lung cancer deaths. The size of the population was held constant throughout the forecasting horizon at the 2006 level to concentrate our analysis on the changes on radon-related deaths resulting from the decline in smoking rates rather than by changes in population size. The dynamics of smoking prevalence were tracked independently and forecasted as accurately as possible, as explained in the technical appendix.
The total number of estimated radon lung cancer deaths for each year was computed by adding the estimated radon lung cancer deaths from each male and female cohort living during that year. We then repeated the same exercise to forecast the impact of immediate remediation of all houses with radon levels above 4 pCi/L. To compute the average postmitigation level, we performed a Monte Carlo simulation employing the national distribution of radon levels estimated by the EPA (lognormal, with a mean of 1.25, median of 0.67, and geometric standard deviation of 3.11)13 and assumed that all radon readings above 4 pCi/L were reduced to 2 pCi/L, the lowest radon level the EPA asserts can be reliably achieved by remediation.1 Taking the average of the samples from the Monte Carlo simulation, we estimated the average postmitigation radon level to be 0.99 pCi/L.
RESULTS
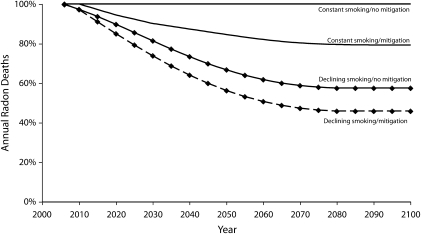
Figure 1 depicts the estimated proportional reduction in the number of radon-induced deaths among the US population throughout the 21st century under different assumptions. The top 2 lines correspond to the scenario that ever-smoking prevalence will remain constant at the 2006 level (41%), a figure that reflects smoking initiation rates among earlier cohorts that were considerably higher than that of recent cohorts. The bottom 2 lines (our base case scenario) reflect the conservative assumption that the smoking initiation rate will remain constant throughout the century at the 2006 level (24%). As a consequence, ever-smoking prevalence will continue to decline for most of the 21st century.
FIGURE 1.
Estimated reduction in annual radon deaths among the US population, factoring in smoking prevalence and housing remediation, from 2006 to 2100.
For each of those 2 sets of lines, the top line shows the projected decline in annual radon deaths in the absence of remediation efforts, whereas the bottom line assumes complete and instantaneous remediation of all houses above the recommended action level of 4 pCi/L. Table 1 shows the same information as Figure 1, highlighting specific years.
TABLE 1.
Estimated Relative Percentages of Annual Radon Deaths Among the US Population, Factoring in Smoking Prevalence and Housing Remediation, From 2006 to 2100
| With Constant Ever-Smoking Prevalence, % |
With Declining Ever-Smoking Prevalence (24% Initiation Rate),a % |
|||
| Year | No Remediation | Instantaneous Remediation in 2006 | No Remediation | Instantaneous Remediation in 2006 |
| 2006 | 100b | 100 | 100 | 100 |
| 2025 | 100 | 92 | 85 | 79 |
| 2050 | 100 | 85 | 67 | 57 |
| 2075 | 100 | 80 | 58 | 46 |
| 2100 | 100 | 79 | 58 | 46 |
The 2006 adult smoking initiation rate of 24% remained constant throughout the period of analysis.
Total number of radon deaths in 2006 was estimated to be 22 416.
Our results showed that, assuming that ever-smoking levels will remain constant at the 2006 level, the most benefit to be expected from complete compliance with current government recommendations is a reduction of 21 percentage points in the annual radon mortality toll by the end of the 21st century. If, on the other hand, we considered the more plausible scenario that ever-smoking prevalence will continue to decline, driven by present and expected levels of the smoking initiation rate, then the benefits from mitigation will be substantially less. We found that the most benefit expected from mitigation under this scenario is a reduction of 12 percentage points (from 58% to 46%) in the annual radon death toll by the year 2100.
The results also suggest that, absent any remediation and driven only by the decline in ever-smoking rates, annual radon deaths will continue to decline until well after midcentury. Under our base case scenario, we estimate that annual radon deaths will drop by 42% by the year 2075. Again, under our base case scenario, if instantaneous and complete compliance with the government's remediation guidelines had been achieved in 2006, we find that by the end of the century, the United States could have decreased the number of radon deaths per year by 54% (from 100% to 46%), but only 12 of those percentage points (from 58% to 46%) could be attributed to the effects of remediation and the rest to the decline in rates of ever smoking. In fact, we find that by the year 2025, the benefits from smoking reduction on reducing radon mortality (15 percentage points) will surpass the maximum benefit we could ever expect from remediation (12 percentage points).
Table 2 shows the sensitivity of our results to changes in smoking initiation rates. Again assuming that full remediation efforts occurred instantaneously in 2006, we estimated the impact on annual radon deaths under the scenario that the country will move linearly from current adult initiation levels, taken as the smoking prevalence among persons aged 18 years (24% in 2006) to 20%, 15%, and 10% by the year 2020. The results (Table 2) showed that, assuming that initiation steadily drops to 10% by 2020, without any remediation, the number of annual radon deaths will drop by 61% by the end of the century. In this scenario, remediation could only have averted an additional 8 percentage points of the total reduction in deaths from the 2006 levels by the year 2100.
TABLE 2.
Relative Percentages of Annual Deaths From Radon Among the US Population, Factoring in Changes in Smoking Initiation Rates, From 2006 to 2100
| Initiation Rate Drops to 20% by 2020, % |
Initiation Rate Drops to 15% by 2020, % |
Initiation Rate Drops to 10% by 2020, % |
||||
| Year | No Housing Remediation | Instantaneous Remediation in 2006 | No Housing Remediation | Instantaneous Remediation in 2006 | No Housing Remediation | Instantaneous Remediation in 2006 |
| 2006 | 100a | 100 | 100 | 100 | 100 | 100 |
| 2025 | 85 | 79 | 85 | 79 | 85 | 79 |
| 2050 | 67 | 57 | 67 | 57 | 67 | 57 |
| 2075 | 56 | 45 | 53 | 42 | 49 | 39 |
| 2100 | 53 | 42 | 46 | 37 | 39 | 31 |
Total number of radon deaths in 2006 was estimated to be 22 416.
DISCUSSION
If we accept the risks implied by the BEIR VI model, radon then poses a significant public health problem. However, we found that, because of the decline in smoking rates, the population-level risk of radon-induced lung cancer will continuously decline over the foreseeable future without any change in population radon exposure or current smoking behavior. By exploring the risk relationship between radon and smoking stipulated by the BEIR VI model, our results showed that smoking rates have a profound impact on the radon death toll. We found that if the level of radon exposure remains constant and current smoking initiation patterns persist, the radon death toll will decline as much as 42% from its current level by the end of the century. This decline will occur because radon risk is postulated to be much higher for ever smokers than for never smokers, and the proportion of ever smokers in the population is declining and will continue to decline for many years to come, as cohorts with high prevalence of current and former smokers die off.
Current ever-smoking prevalence (41% in 2006) contains population cohorts that joined the smoking population at a much higher rate than younger cohorts. For example, in 1965, the smoking prevalence among those aged 20 years was 45.5%. Those individuals were 64 years old in 2009 and are still very much part of the population. Smoking rates among young adults have never been that high since 1965. In fact, initiation rates have declined ever since and have remained around 24% since 2003. If current initiation rates persist, the proportion of ever smokers will eventually be 24%, almost half of what it is now, which will drive down the adverse consequences of radon.
Our results have significant policy implications. We found that, in the best-case scenario, the government's recommendations to remediate homes with radon levels above 4 pCi/L will produce a fraction of the benefits obtained by smoking reduction. In fact, given our assumptions, instantaneous remediation of all houses with levels above 4 pCi/L in 2006 will eventually produce a maximum benefit of 2700 averted deaths per year, a value that will not be achieved until around 2075 and that represents only a 12% decline in radon deaths from current levels. The assumption of instantaneous remediation of all houses exaggerates the benefit that a realistic mitigation program would achieve.
As smoking causes 450 000 deaths per year in the United States, the country continues its efforts to reduce tobacco use. The goal of reducing smoking is embedded in the Healthy People 2010 targets, the government's decennial public health goals for the nation.14 At a 24% initiation rate—the rate in the mid-2000s—almost a quarter of young adults were becoming regular smokers every year. Most likely, this initiation rate has fallen since then, and the country will make efforts to drive this figure down even further. Such an outcome, in turn, will make the radon problem less salient. In fact, our calculations showed that if nobody smoked, the annual number of radon deaths would be one fourth of their current number, and only a quarter of those deaths could be averted by radon remediation.
Our results should not be taken as forecasts, but rather as a measure of the relative impact of smoking on the overall population radon risk. Many variables can affect our specific results and are likely to change in the span of over 90 years. Population size, a constant in our model, will very likely increase, driving up the total number of radon deaths; lung cancer and overall mortality rates will undoubtedly change during this century, also affecting the number of radon-related deaths. Additionally, as the housing stock is replaced, new housing construction may have provisions to deter radon. On the other hand, if new homes become more energy efficient (and airtight), they could potentially trap radon gas inside the house, elevating its concentration.
We chose to keep all those variables constant in our analysis to illustrate the impact of smoking rates on the radon problem. We have shown that, other things being equal, future smoking rates will have a major effect on the total risk derived from radon and that smoking rates are a substantially more important determinant of radon population risk than is the direct remediation of high-radon houses. The EPA's approach to lowering the population's lung cancer risk caused by radon exposure has been to motivate the public to reduce the level of radon in their homes if these levels exceed 4 pCi/L, regardless of the occupants' smoking status. Even though full compliance with the EPA policy entails a considerable expenditure of resources, the EPA's analysis concluded that the cost per life-year saved from radon mitigation efforts was well below the lower end for the value of a statistical life.15
However, by extending the EPA's analysis in light of the expected future decline in smoking rates, we showed that the current policy oriented toward reducing the public's level of radon exposure will gradually become less effective over time, simply because those at the highest risk from radon exposure—smokers—are decreasing.
We are not implying that remediation of high-radon homes should be abandoned as an option to reduce the risk of radon exposure in the population. Even if smoking decline reduces radon risk by a factor of 2, ten thousand deaths per year still represents a serious public health problem. Essentially, we concluded that as health policy planners contemplate the future of cancer control, they need to recognize that radon-induced lung cancer is becoming less of a public health problem, a fact to be taken into account when formulating policies to abate population radon risk. That this fact is the result of decreasing smoking rates, undertaken independently of concerns about radon, merely emphasizes the value of tobacco control as a public health strategy with broad ramifications for health.
Acknowledgments
This work was supported by a grant from the National Cancer Institute (no. NIH R01 CA120126-01A1).
Human Participant Protection
No human participants were involved in this study.
References
- 1.A Citizen's Guide to Radon: The Guide to Protecting Yourself and Your Family From Radon. Washington, DC: Environmental Protection Agency; 2009. EPA report 402-K-09-001 [Google Scholar]
- 2.Committee on Health Risks of Exposure to Radon (BEIR VI) Health Effects of Exposure to Radon, BEIR VI. Washington, DC: National Academy Press; 1999 [Google Scholar]
- 3.Pavia M, Bianco A, Pileggi C, Angelillo IF. Meta-analysis of residential exposure to radon gas and lung cancer. Bull World Health Organ. 2003;81(10):732–738 [PMC free article] [PubMed] [Google Scholar]
- 4.Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case–control studies. Epidemiology. 2005;16(2):137–145 [DOI] [PubMed] [Google Scholar]
- 5.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case–control studies. BMJ. 2005;330(7485):223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EPA Assessment of Risks From Radon in Homes. Washington, DC: US Environmental Protection Agency, Office of Radiation and Indoor Air; June 2003 [Google Scholar]
- 7.Mendez D, Warner KE, Courant PN. Has smoking cessation ceased? Expected trends in the prevalence of smoking in the United States. Am J Epidemiol. 1998;148(3):249–258 [DOI] [PubMed] [Google Scholar]
- 8.Mendez D, Warner KE. Adult cigarette smoking prevalence: declining as expected (not as desired). Am J Public Health. 2004;94(2):251–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horner MJ, Ries LAG, Krapcho M, et al. ,. eds SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Available at: http://seer.cancer.gov/csr/1975_2006. Accessed November 30, 2010
- 10.Population Division, US Census Bureau Annual monthly population estimates by age, sex, race and Hispanic origin for the United States: April 1, 2000 to July 1, 2009. Available at: http://www.census.gov/popest/national/asrh/NC-EST2009-asrh.html. Accessed December 2, 2010
- 11.Pleis JR, Lethbridge-Çejku M. Summary health statistics for US adults: National Health Interview Survey, 2006. National Center for Health Statistics. Vital Health Stat 10. 2007;No. 235 [PubMed] [Google Scholar]
- 12.Mendez D, Warner KE. The relative risk of death for former smokers: the influence of age and years-quit. 2001. Available at: http://www.umich.edu/∼dmendez/tobacco/RRiskmonograph.doc. Accessed December 1, 2010
- 13.The National Residential Radon Survey. Washington, DC: US Environmental Protection Agency; 1993 [Google Scholar]
- 14.Healthy People 2010: Understanding and Improving Health. Washington, DC: US Dept of Health and Human Services, Public Health Service; 2000 [Google Scholar]
- 15.Technical Support Document for the 1992 Citizen's Guide to Radon. Washington, DC: US Environmental Protection Agency; 1992. Publication EPA 400-R-92-011 [Google Scholar]