SUMMARY
The startle response, a simple defensive response to a sudden stimulus signaling proximal threat, has been well studied in rodents and humans, but has been rarely examined in monkeys. The first goal of the present studies was to develop a minimally immobilizing startle measurement paradigm and validate its usefulness by testing two core features of the startle response (habituation and graded responsivity) in squirrel monkey subjects. Two different types of startle stimuli were used: standard broad-band noise bursts, and species-specific alarm vocalizations (“yaps”) which are elicited in response to threat in both wild and captive animals. The second goal of the present studies was to test whether yaps produce enhanced startle responsivity due to their increased biological salience compared to simple, non-biologically relevant noise bursts. The third goal of the present studies was to evaluate the hypothalamic pituitary-adrenal (HPA) axis response to startle stimuli, as little is known about the stress-activating role of startle stimuli in any species. These experiments determined that the whole-body startle response in relatively unrestrained squirrel monkeys habituates across repeated stimulus presentations and is proportional to stimulus intensity. In addition, differential habituation was observed across biologically salient vs. standard acoustic startle stimuli. Responses to “yaps” were larger initially but attenuated more rapidly over trials. Responses to “yaps” were also larger in the early subepochs of the response window but then achieved a lower level than responses to noise bursts in the later subepochs. Finally, adrenocorticotropic hormone and cortisol concentrations were significantly elevated above baseline after startle stimuli presentation, though monkeys did not exhibit differential HPA axis responses to the two types of startle stimuli. The development of monkey startle methodology may further enhance the utility of this paradigm in translational studies of human stress-related psychiatric disorders.
Keywords: ACTH, acoustic startle, alarm call, biological salience, cortisol, HPA axis, primate models, squirrel monkey
INTRODUCTION
The startle response is a simple defensive response to a sudden acoustic, tactile, or visual stimulus signaling proximal threat (Landis and Hunt, 1939). Startle responses habituate rapidly and response magnitude is a monotonic function of stimulus intensity (Davis and File, 1984; Pilz and Schnitzler, 1996; Pilz, Schnitzler, and Menne, 1987). The neural circuitry of the startle response and its primary modulating inputs have been described in detail (Davis, Gendelman, Tischler, and Gendelman, 1982; Davis, Walker, and Lee, 1997). In animals, the startle response is typically measured by the magnitude of whole body movement. In humans, the most common response channel has been contraction of the orbicularis oculi muscle, though cardiac acceleration and scalp electroencephalographic potential are also used (Blumenthal, Cuthbert, Filion, Hackley, Lipp, and van Boxtel, 2005). Across species, the startle response can be potentiated or attenuated by a variety of factors (Bradley, Codispoti, and Lang, 2006; Lang and Davis, 2006), in particular, fear and stress (Brown, Kalish, and Farber, 1951; Davis, 1984). Startle is enhanced in people with anxiety disorders (Grillon and Baas, 2003; Stam, 2007), and can be attenuated by anxiolytic medications (Bitsios, Philpott, Langley, Bradshaw, and Szabadi, 1999). For these reasons, the startle response is a leading tool in translational research into human psychopathology.
There have been few attempts to develop startle paradigms in monkeys with a few important exceptions (Davis, Antoniadis, Amaral, and Winslow, 2008; Linn and Javitt, 2001; Winslow, Parr, and Davis, 2002). Monkey models are important because corticolimbic brain substrates involved in complex cognition and emotion regulation differ significantly in rats and mice compared to human and non-human primates (Ongur and Price, 2000; Preuss, 1995). Because functional abnormalities in these brain regions are thought to underlie stress-related psychiatric disorders characterized by enhanced startle responsivity, and neurobiological assessments can be made readily in monkeys but not in humans, monkey models bridge a critical gap between existing rodent and human research paradigms.
The first goal of the present studies was to develop an acoustic startle paradigm for use in squirrel monkeys that was minimally immobilizing and therefore did not require extensive acclimation prior to experimental initiation. The specific details of this startle paradigm are described below. Our studies sought to evaluate two core features of the startle response: habituation to repeated stimulus presentations and monotonically increasing response magnitudes to stimuli of increasing intensity.
The second goal of these studies was to examine whether the biological salience of acoustic stimuli alters the two core features of the startle response evaluated in these experiments. In view of the fact that broadband noise bursts are rare under free-living conditions in nature, we chose to employ a second type of stimulus, a biologically salient one, which could be expected to elicit abrupt imperative interruptions of on-going activity. Squirrel monkeys utilize a relatively large corpus of species-specific vocalizations (Jurgens, 1998). Among them, “yap” alarm vocalizations are typically elicited in response to threats (e.g., terrestrial carnivores), and serve to orient other troop members to them (Newman, 1985). It is noteworthy that “yaps” are otherwise physically divergent from classic broad band noise burst startle stimuli especially in having long rise-times (see Figure 1). Because yaps are typically elicited in response to threatening circumstances (Newman, 1985), we hypothesized that yaps would produce enhanced startle responsivity due to their increased biological salience compared to simple, non-biologically relevant noise bursts.
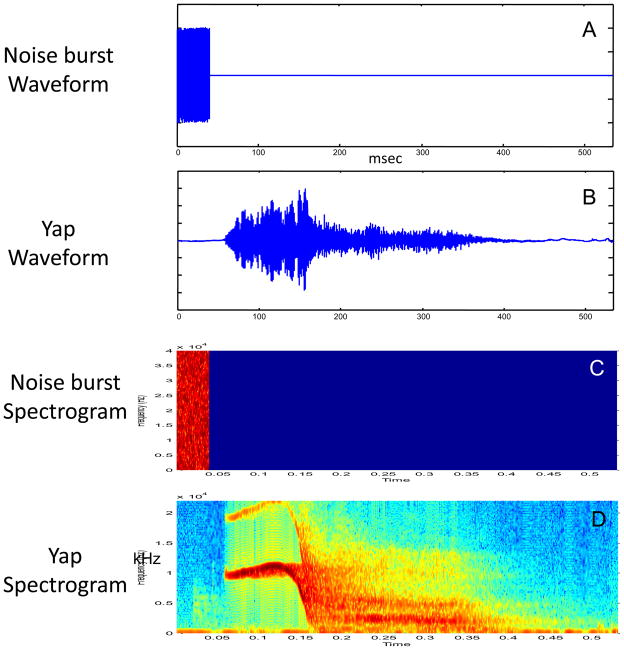
Figure 1.
Comparative time-domain and spectrographic representations of the standard noise burst (panels A and C) and biologically salient yap (panels B and D) startle stimuli. It is evident that these are highly contrastive physical stimuli. The noise burst offsets precede the onsets of the energetic portion of the yaps. Yap onsets are relatively graded in comparison to noise bursts. Yaps are also harmonically complex (possess distinct formants), segment, and extend in time.
The third goal of these studies was to examine the hypothalamic-pituitary-adrenal (HPA) axis response to startle stimuli in monkeys. There is considerable evidence that the HPA axis impacts startle responsivity in rodents and humans. Pharmacological pretreatment with drugs that either increase HPA axis drive [e.g., corticotropin-releasing hormone (CRH) agonists or metyrapone] or block negative feedback (e.g., glucocortiocoid receptor antagonists) enhance startle response amplitude (Korte, Korte-Bouws, Koob, De Kloet, and Bohus, 1996; Liang, Melia, Miserendino, Falls, Campeau, and Davis, 1992; Roemer, Nees, Richter, Blumenthal, and Schachinger, 2009; Swerdlow, Geyer, Vale, and Koob, 1986). In contrast, pretreatment with CRH antagonists or glucocorticoids attenuates the startle response (Buchanan, Brechtel, Sollers, and Lovallo, 2001; Liang et al., 1992; Sandi, Venero, and Guaza, 1996; Swerdlow, Britton, and Koob, 1989; Swerdlow et al., 1986). Far less researched is the stress-activating role of startle paradigms on HPA axis responsivity in any species. For example, corticosterone is elevated after startle exposure in rats (Engelmann, Thrivikraman, Su, Nemeroff, Montkowski, Landgraf, Holsboer, and Plotsky, 1996; Glowa, Geyer, Gold, and Sternberg, 1992) and mice (Anisman, Hayley, Kelly, Borowski, and Merali, 2001). However, no known studies have examined the HPA axis response to startle stimuli in monkeys. We therefore examined whether adrenocorticotropic hormone (ACTH) and cortisol concentrations were elevated following startle stimuli presentation, and whether monkeys exhibited differential HPA axis responses to the two types of startle stimuli employed.
GENERAL METHODS
Subjects
Twelve (N=6 females, N=6 males) Guyanese squirrel monkeys (Saimiri sciureus) born and raised at the AAALAC-accredited Stanford University Research Animal Facility served as subjects in each experiment. With the exception of one male monkey that was unavailable for testing when experiment 2 was being conducted, the same monkeys were used in both experiments detailed below. A total of 13 monkeys (N=6 females and N=7 males) therefore served as subjects in these experiments. All monkeys wore number tags on necklaces to facilitate identification. Monkeys were 1.6 to 3.2 years of age, a range which spans the late juvenile period in this species (Brady, 2000). Age-matched subjects were housed in social groups comprised of 3–6 same sex individuals. Groups were housed indoors in 1.8 × 1.2 × 1.8-m wire-mesh cages that were cleaned daily. Housing and testing occurred in climate-controlled rooms with an ambient temperature of 26° C. Light/dark cycles were 12:12 hours with lights on at 0700 hours. All monkeys were provided unrestricted access to fresh drinking water and commercial monkey chow with daily fruit and vegetable supplements. Various toys, swinging perches, and simulated foraging activities were provided for environmental enrichment. To facilitate husbandry-related activities and experimental manipulations, monkeys were trained using vocal commands to quickly leave the home cage through a small sliding door connected to a stainless steel wire-mesh transport box used for capture and transportation. All procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care and carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Startle Response Measurement
Figure 2 depicts the custom-built device used in this study. In addition to the wide-band (10 Hz to 35 kHz) auditory stimulus presentation subsystem described below, its principal features are 1) the absence of any direct restraint or positional restriction of the animal beyond that imposed by the small enclosure, and 2) a relatively compliant (sub mg) movement-transducing mechanism. The testing chamber measured 21 × 21 × 26.5 (h) cm. Animals moved freely during testing sessions and adopted a wide range of body positions. The floor of the chamber was hinged at the rear with a preload provided by a steel spring. A single-axis Silicon Designs 2010 2g accelerometer was glued to the underside of the measurement floor. Power for the accelerometer was supplied by a Condor 675-MLL12-0.25A power supply. Mildly adherent rubber foam sheeting sandwiched within the hinge mechanism provided locational stability and mild damping. Additional damping was provided by two miniature hydraulic shock absorbers (Ace Controls; Farmington Hills, MI) positioned between the measurement floor and the base of the device. The net effect of the damping components combined with the monkey’s mass limited large reverberant oscillations of the floor. Because the magnitude of floor deflections induced by monkey movement is, in this design, partially determined by the linear distance of the monkey from the hinge, valid measurement requires that distance to be a random variate with respect to groups and conditions. The small size of the enclosure militates against large variations in this parameter. Stimulus presentation and data collection were controlled via Matlab. The voltage output of the accelerometer was digitized at 600 Hz at 12-bit precision via a Measurement Computing LS1028 data acquisition system.
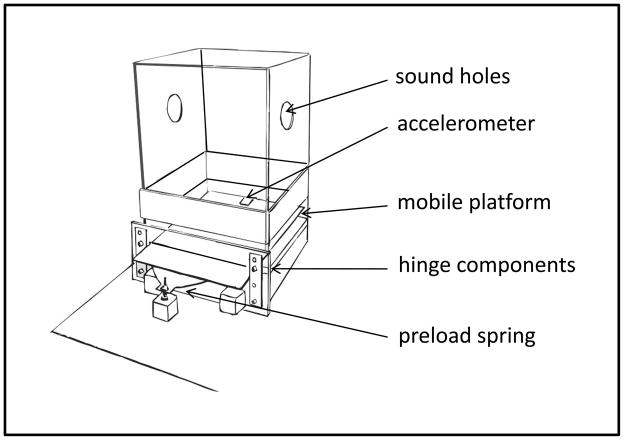
Figure 2.
The custom-built whole-body acoustic startle device is depicted in this drawing. The upper chamber is removed from the base and the animal transferred from below, after which a false floor is inserted. The chamber is then slowly righted and set onto the base. As the false floor is removed the animal steps down onto the instrumented platform. The platform, itself, is cantilevered from a low-friction hinge. An adjustable flat steel spring provides preload; a pair of small shock-absorbers (not shown) damp post-movement oscillations; the accelerometer transduces platform movements. Wide-band headphones (not shown) supply acoustic stimuli through large holes cut into the sidewalls of the upper chamber and guarded by acoustically transparent metal screens. Safety features include tape strips indicating the otherwise transparent enclosure walls and a padded ceiling. Further details are supplied in the text.
Stimuli
Stimuli were broad-band noise bursts (hereafter noise bursts) and species-specific alarm vocalizations called “yaps” (hereafter yaps). The noise bursts were based upon a single random-number series generated in Matlab with length equivalent to 40 msec at 80 kHz sample rate. Forty msec was chosen because it is a standard duration for broadband startle stimuli across human and animal literatures, and therefore would facilitate comparison of our data with those of other studies. Yaps were based upon a single digitized (44.1 kHz) token 534 msec in duration (graciously provided to us in digital form by Dr. Claudia Fichtel from the Department of Behavioral Ecology and Sociobiology at the German Primate Center). The duration of the yap stimulus was based on our hypothesis that yaps carry much of their species-specific signal value in their temporal structure, and therefore a complete yap stimulus is required to elicit a species-typical response.
Stimulus intensities were calibrated using a Bruel & Kjaer Model 2209 impulse precision sound level meter using a #4134 microphone providing a relatively flat (“C-weighted”) response curve extending to approximately 50 kHz. The “impulse-hold” setting of the Model 2209 was used in order to equilibrate the short-term integrated sound pressure of the noise bursts and yaps at 80, 90, 100, 110 and 120 dB. Observing a common standard in the human startle literature, noise bursts and yaps were presented over a continuous 76 dB broad-band background noise (Blumenthal et al., 2005).
Auditory stimuli were output through a Creative Labs Audigy 2ZS sound card at line level, then amplified by a Xenos 3HA headphone amplifier and presented by a pair of Audio-Techinica ATH-700 headphones positioned over two-inch diameter orifices in the testing chamber at approximate head height. The headphones were guarded by microphone screens. The total system bandwidth of the audio presentation subsystem was 10 Hz to 35 kHz, and thus met or exceeded the hearing range of the squirrel monkey, thought to be 0.3 to 32 kHz (Pelleg-Toiba and Wollberg, 1989; Wienicke, Hausler, and Jurgens, 2001). Efforts were made to match this range because high-frequency components of sounds carry rise-time information which, in turn, is a determinant of the human startle response (Blumenthal et al., 2005).
Data Reduction
Raw accelerometer voltages were determined to have near-zero (~1 × 10−9) net slope over the test sessions, with mean level weakly associated with animal weight (p = 0.10). Lack of a stronger association could reflect session-to-session variability in typical linear distance maintained by the monkeys from the fulcrum as well as aggregate non-linearity in the damping components of the system. There was also no linear relationship between animal weight and magnitude of accelerometer output as quantified by the median of a running standard deviation (per 0.5 second) of the five-minute initial baseline period or the median of all responses quantified as described below. Hence, no adjustment of response magnitudes contingent on animal weight was justified.
Testing procedures
Experimental testing occurred in a dedicated procedure room adjacent to the monkeys’ home cage colony rooms. Subjects were transferred from the home cage to the procedure room in a transport box. Monkeys were placed into the startle device and were allowed to acclimate for a short period of time before testing began as described in detail below. Ambient light levels were approximately 10 lux.
Blood sampling and hormone quantification
Blood samples were collected between 1430 and 1530 hours from all monkeys an average of 4 weeks before the beginning of the experiment and an average of 4 weeks after completion of the repeated test sessions to establish a summary measure of baseline ACTH and cortisol levels in undisturbed home cage conditions. Blood samples were also collected immediately after each test session to examine the adrenocortical response to different types of acoustic stimuli (i.e., noise bursts vs. yaps). Post-test blood samples were collected between 1430 and 1810 hours. ACTH and cortisol levels during this late afternoon period are relatively stable compared to a similar period during post-wake morning hours. Our sampling period was therefore selected to help control for fluctuations in the diurnal HPA axis rhythm (Zeitzer, Buckmaster, Parker, Hauck, Lyons, and Mignot, 2003), which also may be associated with variability in startle amplitude (Miller and Gronfier, 2006).
Blood samples were collected from manually restrained monkeys while blood (1 ml) was drawn from the femoral vein with a sterile single-use syringe containing 20 μL of ethylenediamine tetraacetic acid (EDTA). Blood samples were then centrifuged at 4°C and the plasma fraction was transferred to chilled polypropylene tubes and frozen on dry ice. Most blood samples were collected within three minutes of being removed from either the home cage (i.e., the undisturbed baseline sample collections) or from the startle box (i.e., the post-test session sample collections). Median latency to sample collection was 130 seconds (range: 44 – 477 seconds). All blood samples were stored at −80°C prior to quantification.
ACTH and cortisol were both measured in duplicate using commercially prepared radioimmunoassay kits (ACTH: Diasorin Inc, Stillwater, MN; cortisol: Diagnostic Products Corporation, Los Angeles, CA). Complete sample subsets from each condition and gender were included in every assay run. Intra- and inter-assay coefficients of variation were below 10% for both hormone assays. Sensitivity of the ACTH assay is 7 pg/ml and the sensitivity of the cortisol assay is 3 μg/dl.
EXPERIMENT 1: Assessing habituation of whole-body startle responses to repeated stimuli presentations within test sessions
Stimuli
Experiment 1 consisted of two test sessions per subject, with test sessions spaced an average of 30 days a part (range = 11 – 49 days). Noise burst and yap habituation stimulus series each contained 10 120 dB stimuli with 60-second inter-stimulus intervals. Sessions began with a 330 second initial baseline period and ended with a 210 second baseline period during which the background noise continued. Time spent in the testing chamber was approximately 17.5 minutes. Order of test session administration for noise burst and yap stimuli was randomized across subjects (i.e., half of the monkeys received noise burst first, half received yap first) and balanced across genders.
Data Reduction
Accelerometer voltage outputs were first detrended and rectified. Responses to stimuli were quantified by calculating the sample standard deviation of the detrended, rectified accelerometer voltage output per one-half second beginning with stimulus onset and continuing for three seconds yielding six contiguous movement estimates. These were divided by the sample standard deviation of the two-second epoch immediately prior to stimulus onset.
Statistics
Behavioral and neuroendocrine data were examined using separate univariate repeated measures ANOVAs (Systat 11.0, Richmond, CA; and SPSS 17.0, Chicago, IL). GENDER (male vs. female) and stimulus ORDER (i.e., order of test session administration of noise burst vs. yap stimuli) were the two between group factors. Stimulus TYPE (noise burst vs. yap), stimulus TRIAL (stimulus presentations 1 – 10), and response BIN (the 6 half-second post-stimulus response subepochs) were the three within group factors for the behavioral data. Blood sample time point (baseline hormone levels or hormone levels following noise burst or yap stimulus presentation) was the within group factor for neuroendocrine data analyses. The Huynh-Feldt Epsilon correction was used to adjust for multiple comparisons across the repeated test-block factor, and Bonferroni corrections were applied for all post hoc test comparisons. Hormone values were log-transformed to stabilize the variance across groups and to satisfy the equal variance assumptions of parametric statistical tests. For all analyses, test statistics were evaluated with two-tail probabilities (P < 0.05) and descriptive statistics are presented as mean ± S.E.M.
Behavioral Results
No effects of GENDER or ORDER or their interaction were observed. Similarly, neither GENDER nor ORDER nor their interaction interacted, in turn, with any within–subjects factor. Whole body startle responses exhibited a main effect of TRIAL (F(9,72) = 3.99, p < 0.023 H-F) consistent with habituation. The TYPE by TRIAL interaction was significant (F(9,72) = 4.15, p < 0.007 H-F) reflecting the differential habituation trajectories of responses to noise bursts and yaps. As is apparent in Figure 3, responses to yaps were initially larger than those to noise bursts but then habituated more rapidly, ultimately achieving a lower steady-state. In contrast, responses to noise bursts were initially smaller, habituated less rapidly, and ultimately achieved a higher and less stable level over later trials. There was no main effect of stimulus TYPE (F(1,8) = 0.94, n.s.).
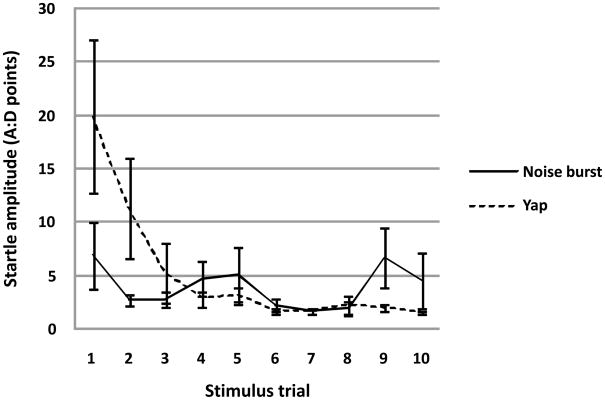
Figure 3.
Monkey subjects (N=12) differentially habituated to repeated presentation of biologically salient yap vs. standard noise burst startle stimuli [TYPE by TRIAL interaction: (F(9,72) = 4.15, p < 0.007 H-F]. Figure 3 depicts mean ± SEM whole body startle responses to 10 repeated stimulus trials collapsed across bins for both the noise burst and yap test sessions.
The effect of BIN was significant (F(5,40) = 6.29, p < 0.009 H-F) simply reflecting the “front-loading” of responses within the three-second post-stimulus response measurement epoch. The TYPE by BIN interaction approached significance (F(5,40) = 2.96, p = 0.071 H-F). It is notable that this interaction was formally reminiscent of the TYPE by TRIAL interaction, in that responses to yaps were larger in the early epochs of the response window but achieved a lower level than responses to noise bursts in the last 1500 milliseconds (See Figure 4). The TRIAL by BIN interaction was significant (F(45,360) = 4.70, p < 0.007 H-F) which reflected the fact that later responses were smaller and so effectively more homogeneous over the three-second response measurement epoch. A TYPE by TRIAL by BIN interaction (F(45,360) = 3.80, p < 0.004 H-F) also emerged as a function of the extended response epoch.
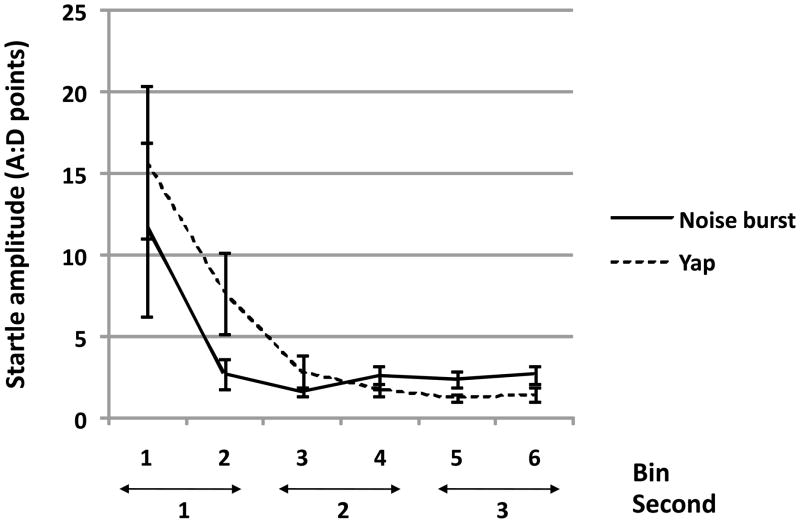
Figure 4.
In a pattern formally similar to habituation over trials, responses to yaps tended to be larger in the early subepochs of the response window but achieved a lower level than responses to noise bursts in the later subepochs [TYPE by BIN interaction approached significance: (F(5,40) = 2.96, p = 0.071 H-F]. Figure 4 depicts mean ± SEM whole body startle responses for the 6 subepochs, collapsed across all 10 trials for both the noise burst and yap test sessions (N=12 monkey subjects).
Neuroendocrine Results
No effects of GENDER or ORDER or their interaction on ACTH or cortisol levels were observed. A significant within subjects effect of blood sample time point was observed for both ACTH (F=(2,16)=34.457, p<0.0001 H-F) and cortisol (F=(2,16)=11.057, p=0.001 H-F) (See Table 1). Monkeys exhibited a significant rise from baseline ACTH levels following completion of both yap (p<0.0001) and noise burst (p<0.0001) stimuli presentations. Yap and noise burst stimuli elicited similar pituitary responses, as post-test ACTH levels did not differ significantly between yap and noise burst stimuli presentations (p=1.00). Cortisol levels were likewise significantly elevated above baseline following completion of both yap (p=0.015) and noise burst (p=0.027) stimuli presentations. Similar to ACTH, post-test cortisol levels did not differ significantly between yap and noise burst stimuli presentations (p=1.00).
Table 1.
Neuroendocrine response to biologically salient (yap) and standard (noise burst) acoustic startle stimuli
| Study 1: Habituation of the startle response to repeated stimuli (test session duration = 17.5 min test) | |||||
|---|---|---|---|---|---|
| Baseline (± SEM) | Post-yap (± SEM) | Change from baseline | Post-noise burst (± SEM) | Change from baseline | |
| Plasma ACTH (pg/ml) | 56.52 ± 4.29 a | 164.43 ± 17.87 b | 191% | 176.91 ± 19.50 b | 213% |
| Plasma cortisol (μg/dl) | 47.55 ± 10.02 a | 76.73 ± 5.64 b | 61% | 71.19 ± 7.19 b | 50% |
|
Study 2: Relationship of the startle response to stimulus intensity (test session duration = 33.5 min test) | |||||
|---|---|---|---|---|---|
| Baseline (± SEM) | Post-yap (± SEM) | Change from baseline | Post-noise burst (± SEM) | Change from baseline | |
| Plasma ACTH (pg/ml) | 83.43 ± 10.89 a | 146.32 ± 15.67 b | 75% | 132.04 ± 13.45 b | 58% |
| Plasma cortisol (μg/dl) | 126.39 ± 21.07 a | 267.37 ± 31.03 b | 112% | 231.33 ± 14.63 b | 83% |
Superscripts a, b = groups with no shared letters differ significantly (p < 0.05).
EXPERIMENT 2: Assessing the relationship between acoustic startle stimulus intensities and whole-body startle response amplitudes
Stimuli
Experiments 1 and 2 for the 11 monkeys common to both studies were conducted an average of 573 ± 5.377 days a part. Experiment 2 consisted of two test sessions per subject, with test sessions spaced an average of 10.25 days a part (range = 3 – 28 days). Both intensity stimulus series contained 34 stimuli with 40-second inter-stimulus intervals. Sessions began with a 150 second initial baseline period during which only the background noise was presented. Time spent in the testing chamber was approximately 33.5 minutes. To remove the expected, large, inter-subject variance in initial startle responses from analyses of the relatively small intensity-dependent effects, subjects were pre-habituated. Based upon the results of Experiment 1, four 120 dB habituation stimuli were presented and associated responses discarded. The remaining 30 stimuli contained equal numbers of tokens at each of three intensity levels, 100, 110 and 120 dB and were presented in three pseudo-random orders that were randomized across subjects and balanced across genders.
Data Reduction
Accelerometer voltage outputs were treated as in Experiment 1 except that only the first 500 msec post-stimulus were quantified.
Statistics
Behavioral and neuroendocrine data were examined using separate univariate repeated measures ANOVAs similar to experiment 1. Briefly, GENDER (male vs. female) and stimulus ORDER (i.e., order of test session administration for noise burst vs. yap stimuli) were the two between group factors. Stimulus TYPE (noise burst vs. yap) and stimulus INTENSITY (i.e., intensities of 100, 110 and 120 dB) were the two within group factors for the behavioral data. Blood sample time point (baseline hormone levels or hormone levels following noise burst or yap stimulus presentation) was the within group factor for the neuroendocrine statistical analyses. Hormone values were again log-transformed to stabilize the variance across groups and to satisfy the equal variance assumptions of parametric statistical tests.
Behavioral Results
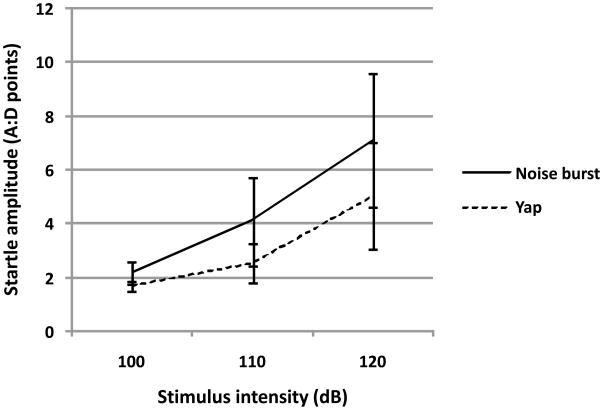
No effects of ORDER, GENDER or their interaction were observed. Likewise, no between-group effects interacted with any within-group effect. The sole within-group effect to achieve statistical significance was that of INTENSITY (F(2,16) = 7.05, p < 0.014 H-F), indicating that the whole body startle response is monotonically proportional to the intensity of the stimulus (See Figure 5). Individual levels of the intensity variable were not significantly different from one another (100 vs. 110, p = 0.87, 110 vs. 120, p = 0.58, 100 vs. 120, p = 0.26). There was no effect of TYPE (F(1,16) = 0.69, n.s) and no TYPE by INTENSITY interaction (F(2,16) = 0.35, n.s).
Figure 5.
Monkey subjects (N=12) exhibited whole body startle responses monotonically related to stimulus intensity for both biologically salient yap and standard noise burst startle stimuli [F(2,16) = 7.05, p < 0.014 H-F]. Figure 5 depicts mean ± SEM whole body startle responses to three different stimulus intensities for both the noise burst and yap test sessions.
Neuroendocrine Results
No effects of GENDER or ORDER or their interaction on ACTH or cortisol levels were observed. As observed in experiment 1, a significant within subjects effect of blood sample time point was observed for both ACTH (F=(2,16)=22.224, p<0.0001 H-F) and cortisol (F=(2,16)=48.866, p<0.0001 H-F) (See Table 1). Monkeys exhibited a significant rise from baseline ACTH levels following completion of both yap (p=0.002) and noise burst (p=0.004) stimulus presentations. Yap and noise burst stimuli elicited similar pituitary responses, as post-test ACTH levels did not differ significantly between yap and noise burst stimuli presentations (p=0.399). Cortisol levels were likewise significantly elevated above baseline following completion of both yap (p<0.0001) and noise burst (p<0.0001) stimulus presentations. Similar to ACTH, post-test cortisol levels did not differ significantly between yap and noise burst stimuli presentations (p=0.368).
DISCUSSION
These results indicate that a minimally immobilizing acoustic startle paradigm produces whole-body startle responses in squirrel monkeys that habituate across repeated stimulus presentations and are directly proportional to stimulus intensity. These data are similar to findings from humans and rodents (Lang and Davis, 2006), and replicate and extend recent findings in rhesus monkeys (Davis et al., 2008) and capuchins (Linn and Javitt, 2001). Our paradigm is unique among monkey startle paradigms in that it is characterized by the absence of any direct restraint or positional restriction of the test subject beyond that imposed by the testing chamber. Because immobilization restraint alters parameters that acoustic startle likewise probes (e.g., emotionality), use of restraint in startle paradigms necessitates extensive acclimation prior to experimental initiation to avoid such confounds. Our low-restraint paradigm therefore provides an expeditious alternative to more time-consuming immobilization-based startle paradigms.
These experiments also examined whether standard broad-band noise bursts and species-specific alarm vocalizations (“yaps”) produce differential startle responses. Yaps are typically elicited in response to threatening circumstances (Newman, 1985), leading us to hypothesize that yaps might produce enhanced responsivity due to their increased biological salience as compared to simple, non-biologically relevant noise bursts. Although monkey subjects exhibited habituation and graded responses to both types of acoustic startle stimuli, we indeed observed significant effects of stimulus type. Yap stimuli elicited larger initial whole-body startle responses which subsequently habituated more quickly than standard noise burst stimuli. Yaps did so despite violating the requirement that startle stimuli have near-instantaneous rise-times. It is tempting to speculate that the efficacy of yaps in this context reflects their specific biological salience. An inductive test of this hypothesis could examine the impact of variations in yap stimulus parameters on startle-like responses. It would also be of interest to determine whether startle-like squirrel monkey responses to yaps, like those to noise bursts, are inhibited or facilitated by prepulses, attenuated by anxiolytics, and so represent a useful alternative probe of fear system function. Similar studies might be possible in macaques employing their “shrill bark” alarm calls (cf. Romanski, Averbeck, and Diltz, 2005). It would also be of interest to determine whether previously neutral stimuli diverging from classical startle stimuli in rise-time, intensity, and bandwidth might, through conditioning, come to produce startle-like responses in monkeys.
There is considerable pharmacological evidence indicating that activation of the HPA axis increases startle responsivity as reviewed above, but few previous rodent (Anisman et al., 2001; Engelmann et al., 1996; Glowa et al., 1992), and no known primate, studies have examined the stress-activating role of startle stimuli on the HPA axis. In the present studies, ACTH and cortisol concentrations were significantly elevated above baseline levels after startle stimuli presentation in squirrel monkeys. Following the 17.5 min habituation to repeated stimuli experiment (experiment 1), monkeys exhibited an average percentage increase of 202% and 56% above baseline levels, respectively, for ACTH and cortisol. Monkeys likewise exhibited an average percentage increase of 67% and 97% above baseline levels for ACTH and cortisol, respectively, following the 33.5 min experiment examining the relationship between stimulus intensity and response amplitude (experiment 2). The differences in percentage increases between ACTH and cortisol activation within each study (i.e., percentage increase is higher for ACTH vs. cortisol in experiment 1 whereas the reverse was observed in experiment 2) likely reflect the temporal dynamics of the HPA axis. Because the adrenal response to stress temporally follows that of the pituitary, it is likely that the 17.5 min period was better suited for capturing maximal pituitary activation, whereas the 33.5 min period was better suited for detecting the onset of adrenal activation (Parker, Buckmaster, Sundlass, Schatzberg, and Lyons, 2006).
It should be noted that while our experimental goal was to compare baseline and post-test cortisol levels within experiments, it is evident from Table 1 that there are pronounced differences in baseline as well as post-test cortisol values between experiments. This observed difference in cortisol levels between studies is likely due to circannual changes in circulating “total” (bound + unbound) cortisol (Schiml, Mendoza, Saltzman, Lyons, and Mason, 1999). Our cortisol assay measures “total” cortisol levels, and as experiments 1 and 2 were conducted during different times of the year, this fact likely accounts for the observed differences in “total” cortisol levels between experiments 1 and 2. In should be noted that basal and post-test blood samples were collected during tight time periods within experiments, and therefore cortisol measurements within experiments are unlikely to be confounded with circannual cortisol rhythms. Moreover, stress responses are relatively stable across the year, with cortisol levels post-stress generally related to baseline values (Coe and Levine, 1995), indicating minimal circannual influences on the magnitude of HPA axis activation.
In these experiments, monkeys did not exhibit differential neuroendocrine responses to standard acoustic startle vs. biologically salient stimuli. This is in contrast to the somatic findings reviewed above. It is possible that HPA axis activity was not temporally sensitive enough to manifest such differences, unlike those afforded by other relatively rapid biological measurement techniques such as electrocardiography. Several studies have shown differential cardiac responses to biologically salient vs. non-biologically salient acoustic stimuli when presented to great apes, dolphins, and birds (Berntson and Boysen, 1989; Miksis, Grund, Nowacek, Solow, Connor, and Tyack, 2001; Ryden, 1980). In other studies, heart rate has been used to differentiate between startle, defensive, and orienting responses to standard acoustic startle stimuli (Berntson and Boysen, 1984; Graham, 1979). Future studies using electrocardiography combining these two approaches would be valuable to examine whether monkeys exhibit differential cardiac response signatures to biologically salient vs. non-biologically salient acoustic startle stimuli.
This study has several limitations. The monkeys studied in these experiments were juvenile animals, so we do not know whether these results generalize across lifespan development, or whether gender differences emerge following pubertal changes in circulating gonadal steroids as has been reported for rodents and humans (Aasen, Kolli, and Kumari, 2005; Lehmann, Pryce, and Feldon, 1999; Toufexis, Myers, and Davis, 2006). A second limitation of these studies is that we cannot determine from the available data the extent to which post-test HPA axis responses are due to exposure to the startle stimuli vs. exposure to the startle apparatus. Group separation and subsequent placement in a novel environment have been shown to activate the HPA axis in squirrel monkeys (Coe, Franklin, Smith, and Levine, 1982). Follow up studies will require inclusion of an additional experimental condition to determine the extent to which exposure to the startle apparatus per se induces HPA axis activation to address this unanswered question. Finally, our studies did not validate pre-pulse inhibition or fear conditioning aspects of the startle response, as have other monkey startle paradigms (Linn and Javitt, 2001; Winslow et al., 2002). Investigation of fear conditioning and extinction is a particularly attractive direction for future monkey research as abnormal fear memory responses are thought to be a core characteristic of anxiety disorders. Though a goal of our investigation was to evaluate an expeditious startle assessment paradigm that could be more easily embedded in multi-element testing sequences, we cannot conclusively state that these monkeys exhibited lower HPA axis-indexed stress responses than if they had been head-restrained. A direct test of this possibility may be warranted. Measurement-related stress is often ignored in human studies despite the potential for interactions with trait fear and anxiety (Eatough, Shirtcliff, Hanson, and Pollak, 2009). It is self-evident that reducing uncontrolled measurement-related stress responses should be a goal of translational studies in this area.
Development of monkey models which examine differences in fear reactivity and fear recovery, as has been done in rodents (Bush, Sotres-Bayon, and LeDoux, 2007; Imanaka, Morinobu, Toki, and Yamawaki, 2006), will provide tractable means by which to model and manipulate fear memory formation and extinction, two core features of stress vulnerability and resilience (Yehuda, Flory, Southwick, and Charney, 2006). We are well-positioned to examine these two core features as they pertain to resilience, having recently developed a squirrel monkey model of early life stress inoculation-induced resilience. In our laboratory, monkeys exposed to early life stress inoculation protocols subsequently exhibit diminished anxiety, attenuated stress-induced HPA axis activation, greater prefrontal inhibition of behavior, and larger ventromedial prefrontal cortical volumes compared to non-inoculated control monkeys (Katz, Liu, Schaer, Parker, Ottet, Epps, Buckmaster, Bammer, Moseley, Schatzberg, Eliez, and Lyons, 2009; Levine and Mody, 2003; Lyons, Martel, Levine, Risch, and Schatzberg, 1999; Parker, Buckmaster, Justus, Schatzberg, and Lyons, 2005; Parker, Buckmaster, Schatzberg, and Lyons, 2004; Parker et al., 2006). Future studies will examine whether stress inoculated versus non-inoculated monkeys exhibit differential startle responses, and higher resistance to form, and faster rates to extinguish, fear memories.
Acknowledgments
This research was supported by funding from Stanford University (KJP, SMT) and grants MH066537 (KJP), MH077884 (DML), and MH47573 (AFS) from the National Institutes of Health. We gratefully acknowledge Blair Brewster for technical assistance with Figure 2, Dr. Eric Knudsen for use of his sound meter, and Elias Godoy and Cesar Veloz for excellent care of our animals.
Footnotes
All authors declare that no conflict of interest, financial or otherwise, exists with regard to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. J Psychopharmacol. 2005;19(1):39–45. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci. 2001;115(2):443–54. [PubMed] [Google Scholar]
- Berntson GG, Boysen ST. Cardiac startle and orienting responses in the great apes. Behav Neurosci. 1984;98(5):914–8. doi: 10.1037//0735-7044.98.5.914. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Boysen ST. Specificity of the cardiac response to conspecific vocalization in chimpanzees. Behav Neurosci. 1989;103(2):235–45. doi: 10.1037//0735-7044.103.2.235. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E. Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. J Psychopharmacol. 1999;13(3):226–34. doi: 10.1177/026988119901300303. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43(5):486–97. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Brady AG. Research techniques for the squirrel monkey (Saimiri sp.) Ilar J. 2000;41(1):10–8. doi: 10.1093/ilar.41.1.10. [DOI] [PubMed] [Google Scholar]
- Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol. 1951;41(5):317–28. doi: 10.1037/h0060166. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav. 2001;68(2):203–10. doi: 10.1016/s0091-3057(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20(4):413–22. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Coe C, Levine S. Diurnal and Annual Variation in Adrenocortical Activity in the Squirrel Monkey. American Journal of Primatology. 1995;35:283–292. doi: 10.1002/ajp.1350350404. [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol Behav. 1982;29(6):1051–7. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Davis M, editor. The mammalian startle response. Plenum; New York: 1984. [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT. Acoustic startle reflex in rhesus monkeys: a review. Reviews in the Neurosciences. 2008;19(2–3):171–85. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Davis M, File SE, editors. Intrinsic and extrinsic mechanisms of habituation and sensitization: Implications for the design and analysis of experiments. Academic Press; New York, NY: 1984. [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352(1362):1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34(8):1242–6. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Thrivikraman KV, Su Y, Nemeroff CB, Montkowski A, Landgraf R, Holsboer F, Plotsky PM. Endocrine and behavioral effects of airpuff-startle in rats. Psychoneuroendocrinology. 1996;21(4):391–400. doi: 10.1016/0306-4530(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Geyer MA, Gold PW, Sternberg EM. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology. 1992;56(5):719–23. doi: 10.1159/000126298. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defensive, and startle reflexes. In: Kimmel HD, van Olst EH, Orlebeke JF, editors. The orienting reflex in humans. Erlbaum; Hillsdale, NJ: 1979. [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114(9):1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Imanaka A, Morinobu S, Toki S, Yamawaki S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav Brain Res. 2006;173(1):129–37. doi: 10.1016/j.bbr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neuronal control of mammalian vocalization, with special reference to the squirrel monkey. Naturwissenschaften. 1998;85(8):376–88. doi: 10.1007/s001140050519. [DOI] [PubMed] [Google Scholar]
- Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, Buckmaster CL, Bammer R, Moseley ME, Schatzberg AF, Eliez S, Lyons DM. Prefrontal plasticity and stress inoculation-induced resilience. Dev Neurosci. 2009;31:293–299. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Koob GF, De Kloet ER, Bohus B. Mineralocorticoid and glucocorticoid receptor antagonists in animal models of anxiety. Pharmacol Biochem Behav. 1996;54(1):261–7. doi: 10.1016/0091-3057(95)02172-8. [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA. The Startle Pattern. Farrar and Rinehart; New York, NY: 1939. [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res. 1999;104(1–2):113–7. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27(1–2):83–9. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12(6):2303–12. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn GS, Javitt DC. Phencyclidine (PCP)-induced deficits of prepulse inhibition in monkeys. Neuroreport. 2001;12(1):117–20. doi: 10.1097/00001756-200101220-00031. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm Behav. 1999;36(3):266–75. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- Miksis JL, Grund MD, Nowacek DP, Solow AR, Connor RC, Tyack PL. Cardiac responses to acoustic playback experiments in the captive bottlenose dolphin (Tursiops truncatus) J Comp Psychol. 2001;115(3):227–32. doi: 10.1037/0735-7036.115.3.227. [DOI] [PubMed] [Google Scholar]
- Miller MW, Gronfier C. Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology. 2006;43(3):297–301. doi: 10.1111/j.1469-8986.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. Squirrel monkey communication. In: Rosenblum LA, Coe CL, editors. Handbook of Squirrel Monkey Research. Plenum Press; New York, NY: 1985. pp. 99–126. [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57(8):848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61(9):933–41. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A. 2006;103(8):3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleg-Toiba R, Wollberg Z. Tuning properties of auditory cortex cells in the awake squirrel monkey. Exp Brain Res. 1989;74(2):353–64. doi: 10.1007/BF00248869. [DOI] [PubMed] [Google Scholar]
- Pilz PK, Schnitzler HU. Habituation and sensitization of the acoustic startle response in rats: amplitude, threshold, and latency measures. Neurobiol Learn Mem. 1996;66(1):67–79. doi: 10.1006/nlme.1996.0044. [DOI] [PubMed] [Google Scholar]
- Pilz PK, Schnitzler HU, Menne D. Acoustic startle threshold of the albino rat (Rattus norvegicus) J Comp Psychol. 1987;101(1):67–72. [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Roemer S, Nees F, Richter S, Blumenthal TD, Schachinger H. Endogenous cortisol suppression with metyrapone enhances acoustic startle in healthy subjects. Horm Behav. 2009;55(2):314–8. doi: 10.1016/j.yhbeh.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93(2):734–47. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Ryden OO. Heart rate response in great tit nestlings (Parus major) to an alarm call. J Comp Physiol Psychol. 1980;94(3):426–35. doi: 10.1037/h0077680. [DOI] [PubMed] [Google Scholar]
- Sandi C, Venero C, Guaza C. Nitric oxide synthesis inhibitors prevent rapid behavioral effects of corticosterone in rats. Neuroendocrinology. 1996;63(5):446–53. doi: 10.1159/000127070. [DOI] [PubMed] [Google Scholar]
- Schiml PA, Mendoza SP, Saltzman W, Lyons DM, Mason WA. Annual physiological changes in individually housed squirrel monkeys (Saimiri sciureus) Am J Primatol. 1999;47(2):93–103. doi: 10.1002/(SICI)1098-2345(1999)47:2<93::AID-AJP1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body Part 1: human studies. Neurosci Biobehav Rev. 2007;31(4):530–57. doi: 10.1016/j.neubiorev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41) Neuropsychopharmacology. 1989;2(4):285–92. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88(2):147–52. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50(4):539–49. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Wienicke A, Hausler U, Jurgens U. Auditory frequency discrimination in the squirrel monkey. J Comp Physiol [A] 2001;187(3):189–95. doi: 10.1007/s003590100189. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol Psychiatry. 2002;51(11):859–66. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–96. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]