Abstract
Synaptic terminals often contain metabotropic receptors that act as autoreceptors to control neurotransmitter release. Less appreciated is the heterosynaptic crossover of glutamate receptors to control GABA release and vice versa GABA receptors which control glutamate release. In the brainstem, activation of solitary tract (ST) afferents releases glutamate onto second-order neurons within the solitary tract nucleus (NTS). Multiple metabotropic receptors are expressed in NTS for glutamate (mGluRs) and for GABA (GABAB). The present report identifies mGluR regulation of glutamate release at second and higher order sensory neurons in NTS slices. We found strong inhibition of glutamate release to Group II and III mGluR activation on mechanically isolated NTS neurons. However, the same mGluR-selective antagonists paradoxically decreased glutamate release (miniature, mEPSCs) at identified second-order NTS neurons. Unaltered amplitudes were consistent with selective presynaptic mGluR actions. GABAB blockade in slices resolved the paradoxical differences and revealed a Group II/III mGluR negative feedback of mEPSC frequency similar to isolated neurons. Thus, the balance of glutamate control is tipped by mGluR receptors on GABA terminals resulting in predominating heterosynaptic GABAB inhibition of glutamate release. Regulation by mGluR or GABAB was not consistently evident in EPSCs in higher-order NTS neurons demonstrating metabotropic receptor distinctions in processing at different NTS pathway stages. These cellular localizations may figure importantly in understanding interventions such as brain-penetrant compounds or microinjections. We conclude that afferent glutamate release in NTS produces a coordinate presynaptic activation of co-localized mGluR and GABAB feedback on cranial afferent terminals to regulate glutamate release.
Keywords: presynaptic modulation, nucleus tractus solitarius, autonomic, visceral afferent, brainstem, metabotropic, glutamate
Introduction
The balance between excitatory and inhibitory transmission dominates communication between central nervous system cells, chiefly through glutamate release and γ-amino butyric acid (GABA), respectively. As part of this transmission process, metabotropic receptors located on synaptic terminals control neurotransmitter release, often as autoreceptors. Less appreciated is the potential influence of crossover or heterosynaptic signals in which similar metabotropic receptors control both glutamate and GABA release onto single neurons. Thus, metabotropic glutamate receptors (mGluRs) regulate GABA release and release of GABA activates metabotropic GABA receptors (GABAB) to inhibit glutamate release. Visceral primary cranial afferents release glutamate onto second-order neurons within the solitary tract nucleus (NTS) (Pilowsky and Goodchild, 2002; Andresen et al., 2004; Travagli et al., 2006). GABAergic NTS neurons are overwhelmingly second-order neurons themselves (Bailey et al., 2008) and often provide di-synaptic delivery of GABA to other second-order neurons including baroreceptive NTS neurons (Andresen and Yang, 1995; Bailey et al., 2008). These GABA mechanisms in NTS inhibit reflex responses including the baroreceptor reflex (Bousquet et al., 1982; Catelli and Sved, 1988). Thus, afferent glutamate weighs on both sides of the excitatory and inhibitory balance and this balance determines the integrative outcome of afferent information within NTS. Afferent glutamate spills over onto local GABAergic terminals and this heterosynaptic crosstalk strongly influences the magnitude of inhibition at the second-order NTS neurons through activation of multiple mGluR subtypes on GABA terminals (Jin et al., 2004a).
Heterogeneous subtypes of mGluRs (mGluR1-8) couple to second-messengers that often act presynaptically to either increase or decrease neurotransmitter release (Conn and Pin, 1997; Cartmell and Schoepp, 2000; Schoepp, 2001). In NTS and nodose ganglion (location of many ST afferent cell bodies), immunohistochemical and mRNA suggests the potential for all mGluR subtypes (Hay et al., 1999; Hoang and Hay, 2001; Pamidimukkala et al., 2002). Less is known about their functional presence, subcellular location (e.g. presynaptic and postsynaptic), or coupling at NTS neuron subclasses (second-order vs. higher order). Extracellular and intracellular recordings suggested highly varied responses within and across neurons (Glaum and Miller, 1993; Glaum and Brooks, 1996; Liu et al., 1998; Foley et al., 1999) or with NTS microinjections of mGluR-directed compounds (Foley et al., 1999; Matsumura et al., 1999; Viard and Sapru, 2002). Underlying heterogeneity may give rise to these varied experimental results.
Activation of ST afferent axons elicits large excitatory postsynaptic currents (EPSCs) (Doyle and Andresen, 2001) onto second-order NTS neurons and, many of these neurons also receive later arriving, ST-linked GABAergic IPSCs (Andresen and Yang, 1990). Afferent synaptic transmission is depressed in a frequency-dependent manner at moderate stimulation frequencies (Andresen and Yang, 1995) and mGluR blockade reduced this depression (Liu et al., 1998; Chen et al., 2002). The wide range and differential distribution of various receptor subtypes and opposite coupling (negative or positive) suggest a potentially complicated variety of configurations and feedback arrangements for interaction of glutamate and GABA – a potential that seems realized in the variation in results reported (Feldman and Felder, 1991). Such variation might originate across cell types but also may represent interactions between glutamate and GABA receptors that regulate neurotransmitter release.
The present studies used two strategies to separate and identify key sources of glutamate-GABA interaction within NTS. We used mechanically dispersed medial NTS neurons to physically isolate individual NTS neurons with attached nerve terminals (Jin et al., 2004a; Jin et al., 2004b; Jin et al., 2010). In isolated neurons, drugs had access to only two key component sites of action – presynaptic neurotransmitter release apparatus and postsynaptic neurotransmission mechanisms – without interconnecting neurons or local circuits present. A second level of experimental complexity used recordings of EPSCs in NTS slices. The slice approach also allowed us to identify two classes of neurons - second or higher order neurons - using ST-evoked synaptic response characteristics (Doyle and Andresen, 2001; Doyle et al., 2004; Bailey et al., 2008; McDougall et al., 2009). We isolated EPSC generation using TTX (mEPSCs) to limit our assays to local mechanisms of glutamate release (the pre- and postsynaptic elements) without activity-driven or local circuit components. The results suggest that mGluRs regulating GABA release dominate the pharmacological response profile of mGluR control of glutamate release through a heterosynaptic crossover of altering GABA release which acts at GABAB receptors co-localized at glutamatergic terminals. Thus, even at single terminals, release status reflects a balance of ongoing metabotropic GABA and glutamate receptor mechanisms.
Experimental Procedures
NTS slices
Horizontal hindbrain slices of male Sprague-Dawley rats (2–8 wks old, Charles River Laboratories, Inc., Wilmington, MA) were prepared as previously described for studies of the caudal NTS (Doyle et al., 2004). Slices from the youngest rats (2–3 wks) were selected for dissociating medial NTS neurons as previously described (Jin et al., 2004b). All animal procedures were conducted with the approval of the University Animal Care and Use Committee in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Guide). The hindbrain was placed in ice cold artificial cerebrospinal fluid (ACSF) composed of (mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 D-glucose, 2 CaCl2, and bubbled with 95%O2 / 5%CO2. Extracellular CaCl2 is elevated above normal to enhance synaptic transmission but this does not qualitatively alter ST-glutamate release (Bailey et al., 2006b; Andresen and Peters, 2008; Peters et al., 2008; Peters et al., 2010; Shoudai et al., 2010). The medulla was trimmed to a 1 cm block (rostral-caudal) centered on the obex. An angled wedge of tissue was removed from the ventral surface to realign the brainstem so that a substantial length of the ST was intact within the same plane as the caudal NTS (Doyle et al., 2004) when mounted in a vibrating microtome (Leica VT-1000S; Nussloch, Germany). This procedure yields a single, slightly off-horizontal slice that contains several mm of the ST together with the caudal NTS and the caudal end of the 4th ventricle (250 µm thick for slice recording and 150–170 µm thick for dissociation). These slices were cut with sapphire blades in cold normal ACSF (Delaware Diamond Knives, Wilmington, DE). Our protocols focused our studies on neurons within the medial subnucleus of the NTS that contains the highest concentration of arterial baroreceptor terminal puncta (Mendelowitz et al., 1992; Doyle et al., 2004). We use the visible ST and the caudal most end of the 4th ventricle to direct our recording pipettes in search of neurons (Andresen and Peters, 2008; McDougall et al., 2009).
Mechanical dissociation
Slices were incubated for 1–3 hrs) in well-bubbled ACSF at 31 °C before mechanical dispersion. The brain stem slices were transferred to 35mm petri dishes (Falcon 1008; Becton Dickinson, Franklin Lakes, NJ) containing standard external solution (mM), 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES and 10 glucose (pH was adjusted to 7.4 with Tris-base) and individual neurons dissociated. Using a custom-made vibrator held by a micromanipulator (Jin et al., 2004b), the oscillating pipette tip (polished to 100–120 µm tip outer diameter) was lowered to the slice surface under visual guidance. The pipette oscillated at 30 Hz horizontally with excursions of 100–300 µm. The vibrating tip was slowly moved to circumscribe the area of the sub nucleus from which we record in slice work - generally oriented by the landmark of the caudal end of the fourth ventricle and collected cells rostro-caudally for ±250 µm and bordered medially from the ST to within 50 µm of the edge of the 4th ventricle. Neurons were dissociated from the upper 100 µm from the dorsal surface of the slices. The slice was removed and 20 min were allowed for dispersed neurons to settle and adhere to the bottom of the dish. Neurons dispersed in this manner generally have visible synaptic boutons that release glutamate that have cranial visceral afferent characteristics (Jin et al., 2004b).
Slice recordings
The horizontal brainstem slice is optimized for electrophysiological identification of 2nd order neurons (Doyle et al., 2004). Neurons were visualized within NTS using infrared illumination and differential interference contrast (IR-DIC) optics (40× water immersion lens) on an Axioskop FS2 microscope (Carl Zeiss, Thornwood, NJ) coupled to an infrared sensitive camera Axiocam (Zeiss). We selected neurons for recording from the same region that neurons were dispersed from – the medial NTS bounded up to 500 µm medially to the ST. Recording electrodes (2.5 to 3.5 MΩ) were filled with an intracellular solution containing (in mM): 10 NaCl, 40 KCl, 90 K Gluconate, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 2mM Na2ATP and 0.2mM Na2GTP; pH 7.3; 295 mOsm. Voltage clamp was performed in the open, whole cell configuration (Axopatch 200A, Axon Instruments, Foster City, CA). Data were filtered at 3–5 kHz and sampled at 50–100 kHz using a Digidata 1322A for acquisition and pClamp8 software (Axon Instruments, Foster City, CA). For ST activation, a concentric bipolar electrode (200 µm diameter, Frederick Haer Co., Bowdoinham, ME) was placed within the stripe of ST as far from the recording region as possible (1–3 mm) so as to isolate stimuli to afferent axons.
Drugs
Agonist/antagonist concentrations were chosen based on preliminary studies but agonists generally were at 2× their IC50 and antagonists were 10× IC50. I, II and III designated the respective mGluR Group subtypes in association with agonist and antagonist drugs as outlined below. The orders of presentation of drugs were varied and the averaged results presented in summaries combine test results regardless of order delivery. Drugs (Sigma; St. Louis, MO, USA) included: L-AP4 (Group III mGluR agonist, 10 µM), bicuculline methiodide (GABAAR antagonist, 100 µM), gabazine (SR95531, GABAAR antagonist, 3 µM), CGP 35348 (GABABR antagonist), DCG4 (Group II mGluR agonist, 10 µM), DHPG (Group I mGluR agonist, 10 µM), LY341495 (Group II mGluR antagonist, 10–20 nM), LY367385 (Group I mGluR antagonist, 160 µM), and MSOP (Group III mGluR antagonist, 200 µM). L-AP4 and LY341495 were dissolved in 0.1N NaOH at 10 mM before diluting with external solution. In isolated neurons, all drugs were applied via a rapid application Y-tube system that provided complete solution changes surrounding the recorded neurons within 20 msec (Murase et al., 1989). Note that as gabazine and bicuculline have similar actions attributed to GABAA block in these NTS neurons (Doyle and Andresen, 2001; McDougall et al., 2008), there is apparently no concern regarding the potential for additional actions of bicuculline on calcium-dependent potassium channels(Khawaled et al., 1999). In slices, drugs were applied by bath perfusion in total bath volume 0.7 ml that flowed at a rate of ≥2 ml/min.
Slice recorded NTS neurons were continuously perfused with 32–34°C ACSF (TC2BIP with HPRE2 and TH-10Km bath probe, Cell MicroControls, Norfolk, VA). Neurons were classified as directly connected to ST afferents (i.e. 2nd order neurons) or indirectly coupled to ST afferents (higher order neurons) judging from the characteristics of ST-evoked EPSCs established previously (Doyle and Andresen, 2001; Doyle et al., 2004; Andresen and Peters, 2008). Graded ST intensity recruitment curves were essential to this process(McDougall et al., 2009). 2nd order neurons had minimal latency variation (SD of latency = jitter that was <200 µsec) near zero synaptic failure rates for the first EPSC in a train of five ST shocks. Bursts of 5 ST shocks every 6 s (i.e. 0.83 Hz, shock duration 0.1 ms) were generated at burst frequencies of 50 Hz with a Master-8 isolated programmable stimulator (AMPI. Jerusalem, Israel). Synaptic latencies were measured as the time between the onset of the stimulus artifact and the onset of the synaptic current. Following identification of neurons with 2nd order synaptic characteristics, GABAA receptors were blocked with gabazine. This condition of complete GABAA blockade isolated EPSCs but permitted subsequent ST stimulation to release afferent Glu and, therefore, producing evoked EPSCs. Note that ST-evoked responses were used to classify neurons in slices as second or higher order but then subjected to pharmacological blockers including TTX to study miniature EPSCs.
For voltage clamp recording in dissociated cells, neurons were visualized using a phase-contrast microscope (Nikon TE2000S, Japan) with a 60× objective and 10× ocular lens. Voltage clamp recordings were made using an Axopatch 2B and pClamp 8 software. Electrical measurements utilized nystatin-perforated patch recordings at room temperature. Recording electrodes were filled with a solution composed of (mM): 50 KCl, 100 K gluconate, 10 HEPES and the pH of this solution was adjusted to 7.2 with Tris-OH. The final concentration of nystatin was 450 µg/ml. Neurons dispersed in this manner have intact presynaptic boutons as indicated by the presence of spontaneous synaptic events: IPSCs and EPSCs (Jin et al., 2004b). Neurons were voltage clamped to −60 mV and currents sampled every 20 µsec and saved to computer. Data were analyzed offline using pClamp8 software and Mini Analysis Program (Synaptosoft Inc., Decatur, GA).
Normalized data are presented as percentage of control and averages ± standard errors (SEM). Statistical comparisons between two groups were made using unpaired Student’s t-test. To evaluate differences for more than three groups, parameter values were compared by a repeated measures analysis of variance (ANOVA) or one-way ANOVA (as appropriate) for the effects of treatments or groups. Post hoc comparisons of means used Bonferroni's correction for multiple comparisons where appropriate (Statview 4.57, Abacus Concepts, SAS Campus Drive, Cary, NC). P-values <0.05 indicated significant differences.
Results
Group II and III mGluR depress spontaneous glutamate release in isolated NTS neurons
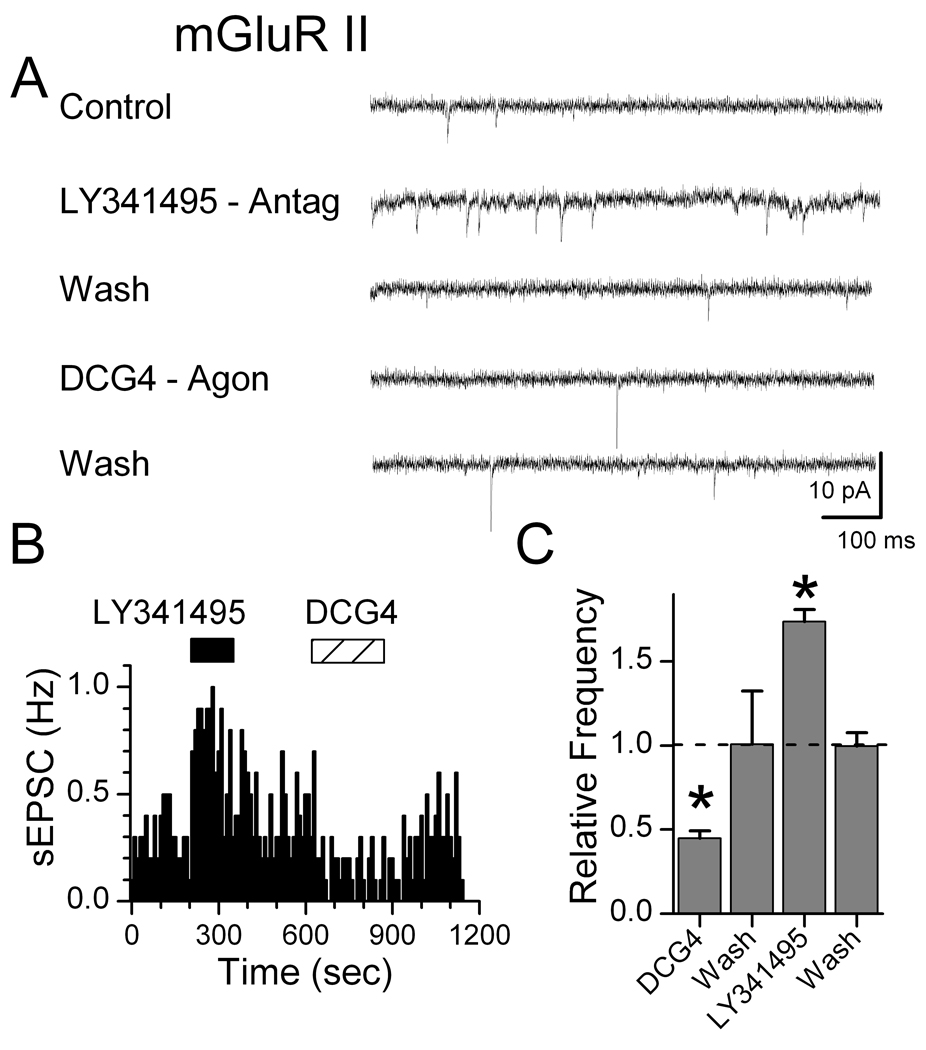
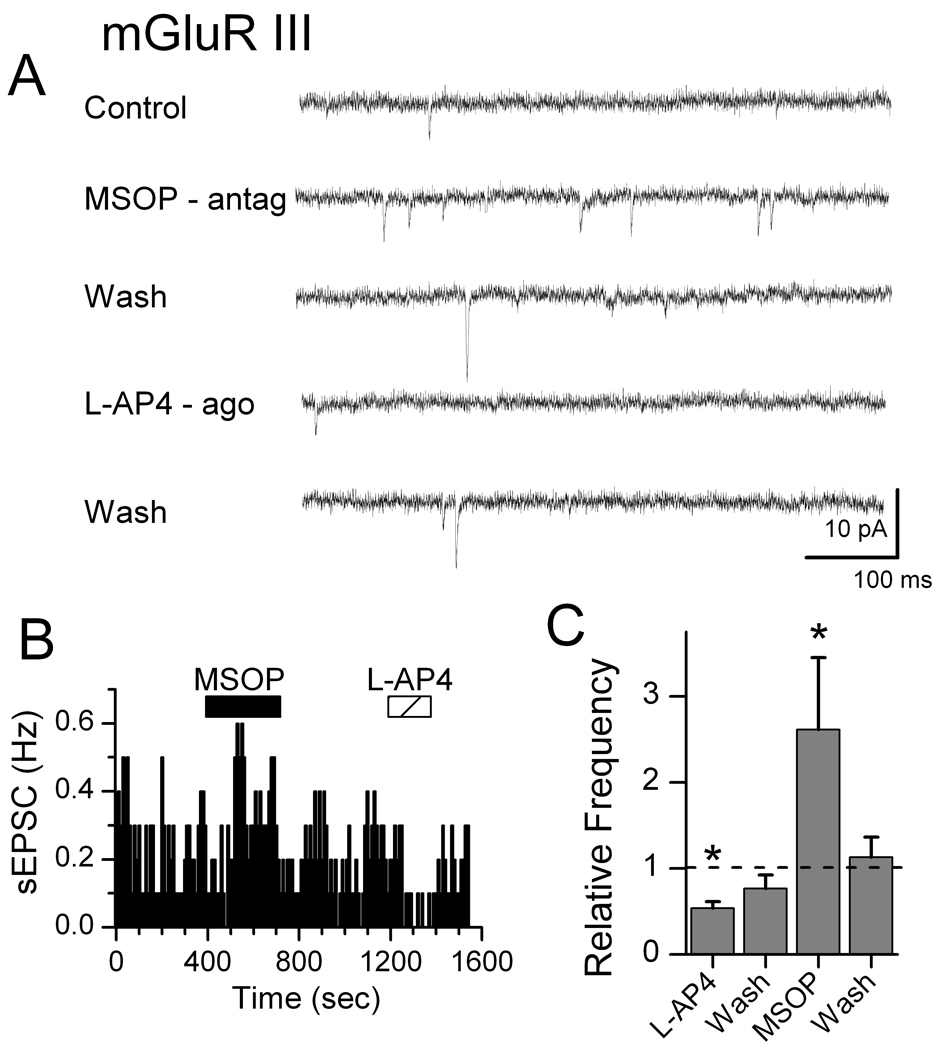
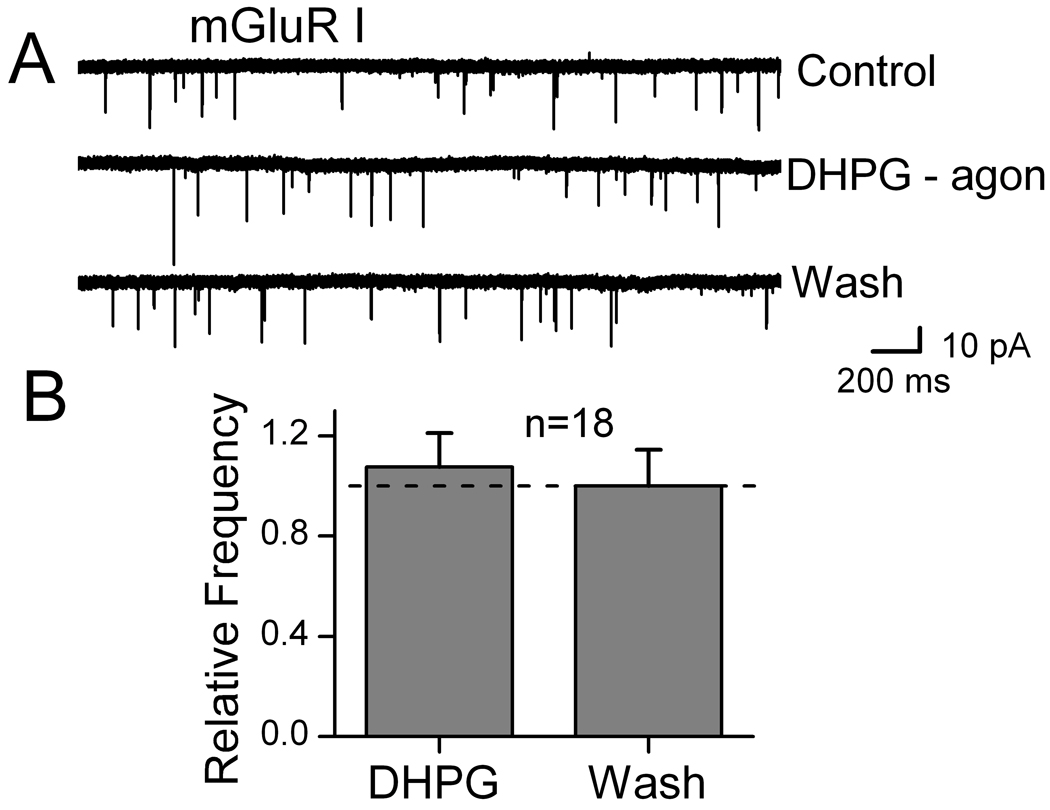
Gentle mechanical dispersion without enzymes yielded isolated individual NTS neurons that retained functioning glutamatergic and GABAergic synaptic boutons (Jin et al., 2004a; Jin et al., 2004b; Jin et al., 2010). In order to study glutamatergic EPSCs, all solutions for isolated cells contained 100 µM bicuculline to block IPSCs. Blockade of mGluR II using LY341495 (10 nM) rapidly increased the rate of sEPSCs, an effect that readily reversed during washing with ACSF (Figure 1). The mGluR II agonist DCG4 (10 µM) reduced the rate of spontaneous glutamate release. Qualitatively similar responses were observed with mGluR III specific agents (Figure 2). In contrast, sEPSCs recorded in isolated NTS neurons were unaffected by mGluR I activation (10 µM, DHPG, Figure 3). Since frequency but not amplitudes of these EPSCs were altered by mGluR II/III agents, our results are consistent with a presynaptic negative feedback mechanism that inhibits glutamate release. Our results here on mEPSCs on second-order neurons contrast with our earlier work (Jin et al., 2004a) in similar NTS neurons in which we found that Group I mGluR strongly increased and Group II/III decreased GABA release in response to ST-evoked glutamate.
Figure 1.
Group II mGluR receptors negatively feedback on glutamate release in mechanically dissociated, medial NTS neurons. In a representative neuron (A), mGluR II antagonist LY341495 (10 nM) rapidly increased the rate of sEPSCs, an effect reversed by Wash in drug-free ACSF in our rapid flow y-tube system. The mGluR II agonist DCG4 (10 µM) rapidly decreased sEPSC frequency. Diary plot of sEPSC events (B) shows the rapid onset and reversal time course of each drug. On average across neurons (C), mean sEPSC frequency significantly decreased during mGluR II activation and increased during antagonist. (* indicates significant differences from control, p<0.01; n=7). All responses were normalized against their pre-drug controls in which EPSCs were isolated by the GABAa antagonist bicuculline (100 µM) that was present throughout. Amplitudes of sEPSCs were not changed (not shown).
Figure 2.
Group III mGluR receptors inhibit spontaneous EPSCs in dissociated medial NTS neurons. In a representative neuron (A), mGluR III antagonist MSOP (200 µM) rapidly increased the rate of sEPSCs. Activation of mGluR III with the agonist L-AP4 (10 µM) nearly eliminated sEPSCs. Diary plot of sEPSC events (B) from the neuron in A shows that mGluR III drug actions were rapid and readily reversed by washing. Across neurons (C), relative mean sEPSC frequency decreased significantly during mGluR III activation and increased during antagonism (* indicates significant differences from control, p<0.01; n=8). Amplitudes of sEPSCs were not changed (not shown). Bicuculline (100 µM) was present throughout to block IPSCs.
Figure 3.
Group I mGluR receptors failed to alter spontaneous EPSCs in dissociated medial NTS neurons. The mGluR Group I agonist DHPG (10 µM) was ineffectual on sEPSCs. Note that this concentration was maximally effective on sIPSCs in similar medial NTS neurons (Jin et al., 2004a). On average across 18 neurons (B), mean frequency of sEPSCs was unchanged by mGluR I activation. Data were not different from control values (p=0.58; n=18). Bicuculline (100 µM) was present continuously to block IPSCs.
Group II / III mGluRs paradoxically inhibit glutamate release in brainstem slices
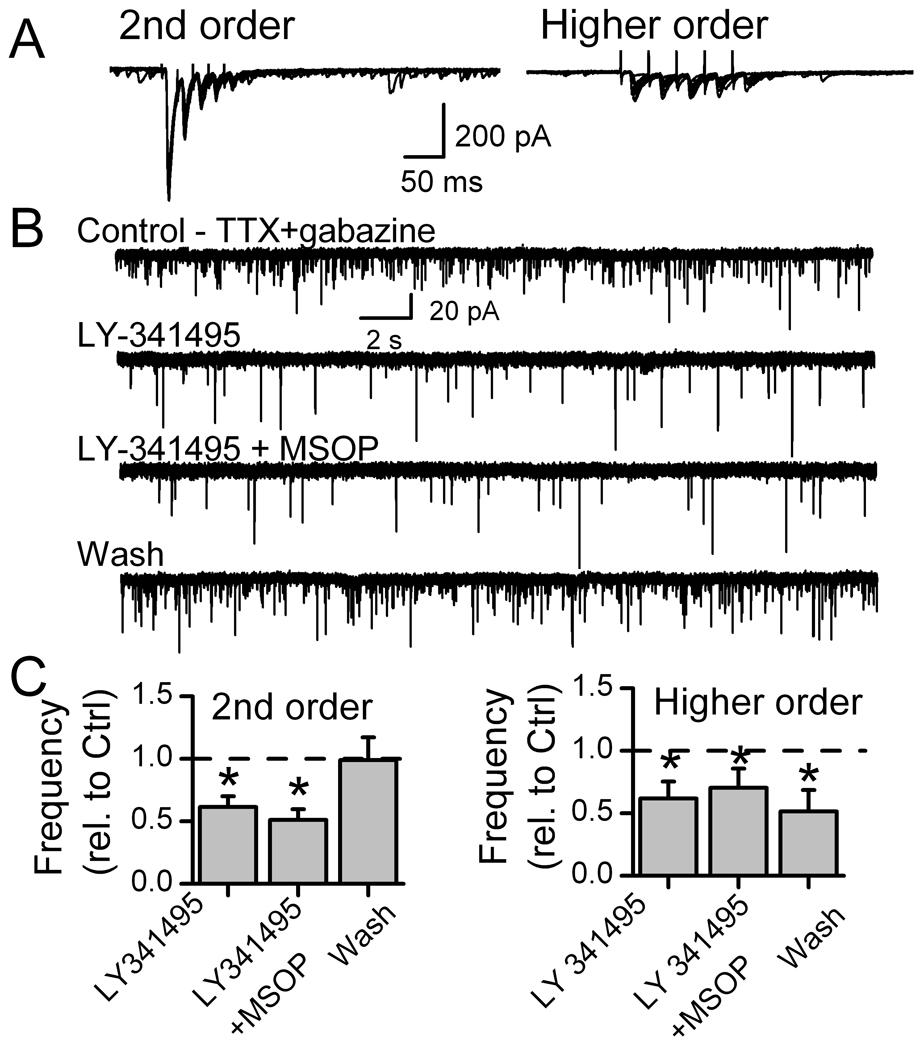
Isolated neurons are one of the structurally simplest assays of neural transmission but these cells lack distal synaptic terminals or neighboring cells that are part of their normal functioning environment. In order to identify the synaptic role (i.e. order) of the recorded neurons, we examined mGluR modulation of glutamate release in slices in which we could activate the ST afferent pathway. In slices (Figure 4), we activated afferent axons with ST shocks and classified the neurons as second-order neurons or higher order neurons based largely on our jitter cutoff of 200 µs and recent refinements (Doyle and Andresen, 2001; McDougall et al., 2009). Note that not only do higher order neurons respond with higher jitter, but failures were present in response to the first shock (EPSC1 failures) and frequency dependent depression was minimal in higher order neurons.
Figure 4.
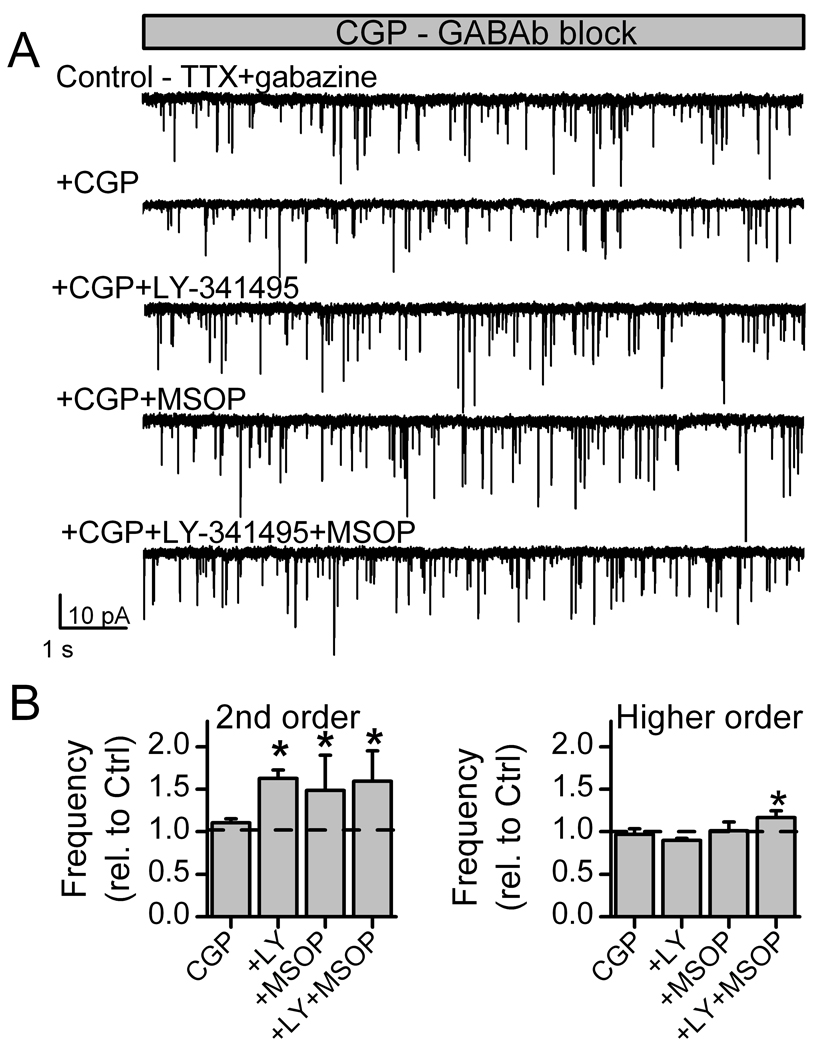
Group II or III mGluR blockade decreases autonomous or miniature glutamate release (mEPSC) in medial NTS neurons within brainstem slices. Recorded neurons were initially characterized by their solitary tract (ST) evoked EPSCs as second-order neurons with low (<200 µs) jitter (invariant latency), or as higher order (ST-EPSC jitter >200 µs). Panel A shows responses to ten successive ST shock bursts (5 shocks at 50 Hz each 6 s) in representative second-order neuron and higher order neurons. ST-EPSCs rapidly depressed in second-order NTS neurons (left, ten traces) but had high jitter, lower amplitude, little depression and synaptic failures of EPSC1 typical of higher order neurons (right, ten traces). In 1 µM TTX (B, TTX+gabazine), the second-order neuron of part A had substantial mEPSC activity. Gabazine (SR95531) was 3 µM. The mGluR II antagonist LY341495 (20 nM) substantially decreased mEPSC frequency and addition of mGluR III antagonist MSOP (200 µM) had little additional effect. On average (C), mGluR II or combined Group II and III antagonism decreased mEPSC rates in second-order NTS neurons (left, n=8) by almost half. On average in higher-order mNTS neurons (right, n=7), mGluR II and III antagonism also decreased mEPSC rates. (* is significantly different from control, p=0.01).
Following neuron class identification (Figure 4), action potentials were blocked by TTX so that mEPSCs could be measured and local circuit contributions minimized before antagonists were tested. In light of the preceding isolated neuron work, Group II, Group III and combined Group II with III mGluR antagonists were tested but surprisingly inhibited the frequency of mEPSCs rather than facilitated afferent glutamate release (Figure 4). Amplitudes were unaltered, an observation consistent with a presynaptic effect – just as in the isolated neurons. Both second-order and higher order NTS neurons showed similar decreases in mEPSC frequency. Basal mEPSC rates (TTX and gabazine) were similar (p=0.63) in second-order (4.5±1.0 Hz, n=6) and higher order (3.8±1.0 Hz, n=7) NTS neurons. Antagonist responses were generally readily reversed by washing, but some higher order neurons showed delayed recoveries for unknown reasons (Figure 4). Given these unexpected decreases in mEPSC rates rather than the increases predicted by the isolated neuron work, we considered the possibility that our drugs were acting at additional mGluR sites. Antagonism of mGluR I failed to alter mEPSC rate in identified second-order NTS neurons (160 µM LY367385, 0.98±0.02 % of Control, n=3). In light of previous work on GABA release (Jin et al., 2004a), we immediately suspected an indirect, complicating factor – an action of mGluRs on GABA release that might modify glutamate release.
Cross-talk between glutamatergic and GABAergic terminals at second-order NTS neurons
GABAB receptors are known to be important in NTS (Catelli and Sved, 1988). To test whether mGluR antagonists might be altering GABA release from local inhibitory terminals, we re-tested these mGluR agents in the presence of the selective GABAB antagonist CGP 35348 (5 µM). With GABAB blocked, Group II, the Group III or the combined Group II with III mGluR antagonists increased mEPSC rates without altering event amplitudes (Figure 5). We conclude from this result that the inhibitory mGluR responses on mEPSCs (Figure 4) were indirect responses mediated by increases in GABA release triggered by the removal of an inhibitory brake at inhibitory terminals populated with Group II and Group III mGluRs. As a corollary, the combined results indicate that the GABAB control of glutamate release was more powerful than the direct mGluR control of glutamate release since decreases were observed when mGluRs were challenged and free to act on both glutamate and GABA terminals. Together, the results demonstrate a powerful heterosynaptic crosstalk between the metabotropic receptors on excitatory and inhibitory terminals with glutamate release acting on mGluRs simultaneously to alter the mix of glutamate and GABA released from their respective terminals.
Figure 5.
Blockade of GABAB receptors revealed ongoing mGluR-mediated negative feedback of glutamate release in second-order NTS neurons within brainstem slices. Second and higher order NTS neurons were identified as in Figure 4. In the presence of CGP 35348 (5 µM), a selective GABAB antagonist, mEPSCs were recorded in TTX and gabazine. In a representative second-order neuron (A), the mGluR II antagonist LY341495 (20 nM), mGluR III antagonist MSOP (200 µM) or the combination of both drugs increased mEPSC frequency. On average (B), the mean frequency increased with mGluR Group II and III antagonism in second-order neurons (n=6, left) but had little effect on higher order neuron responses (n=5, right). * indicates a significant difference from the control (p<0.01).
Discussion
Central neurons receive inhibitory and excitatory inputs and the net, integrated combination of these influences determines their functional contributions within reflex pathways. Here we report on the presynaptic interactions of glutamatergic and GABAergic signaling at two classes of medial NTS neurons. ST afferents activated second-order sensory NTS neurons directly via ionotropic glutamate receptors and indirectly via serial polysynaptic pathways to both second-and higher order NTS neurons via pathways that use glutamatergic NTS interneurons. We isolated mEPSCs at these NTS neurons using dissociated neurons and/or TTX to remove contributions from action potential-driven local circuits. Our results suggest that mGluRs provide classic negative feedback to inhibit glutamate release at second-order neurons but interestingly not at higher order, glutamatergic synapses. This indicates an important fundamental difference between ST afferent transmission and controls over glutamate release at non-ST synapses. Unlike mGluRs, presynaptic GABAB receptors were present at both ST and non-ST glutamate synapses. Revealing these mGluR differences required blockade of the GABAB mechanism on ST terminals. The results suggest a different arrangement of GABAB and mGluR receptors on synaptic terminals at second and higher order NTS neurons (Figure 6). Together, our results suggest that presynaptic GABA actions at GABAB receptors dominate control of glutamate release on to second-order neurons compared with mGluR presynaptic control. The neuron class-specific (second-order vs. higher order) and heterosynaptic glutamate-GABA interactions have strong implications for understanding afferent processing within NTS and signal differentiation across NTS local circuits.
Figure 6.
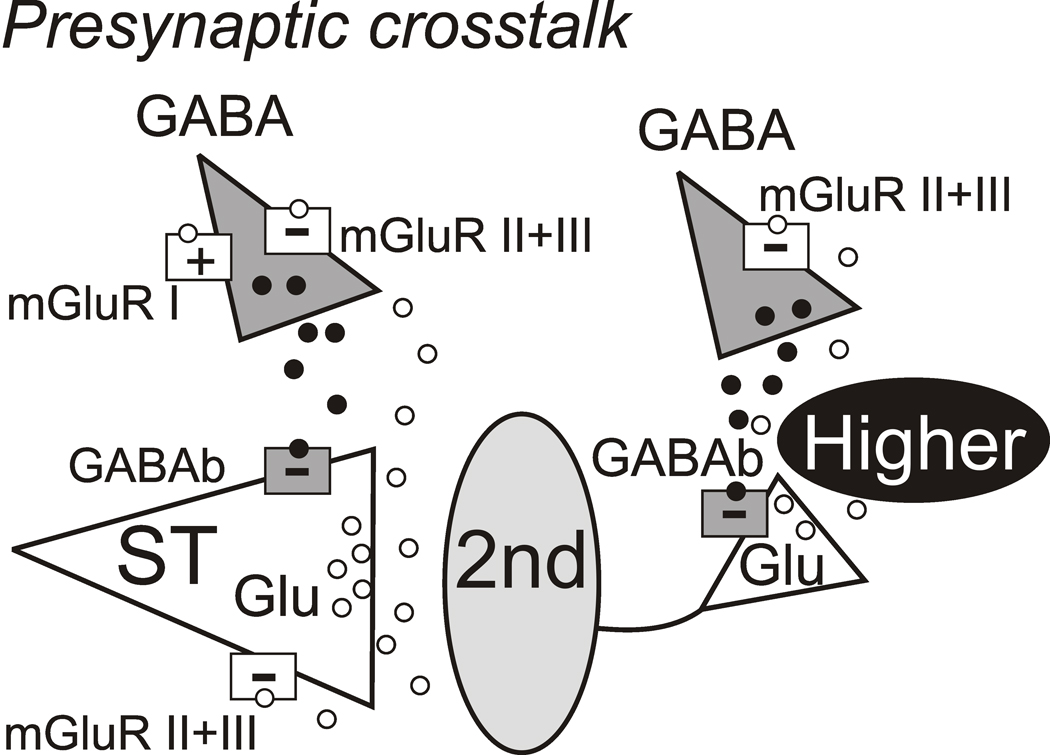
Summary of functioning presynaptic distributions of mGluR subtypes and GABAB on synaptic terminals in second-order and higher order NTS neurons. Glutamate (Glu) mechanisms are white with triangles representing synapses and GABA mechanisms are gray. Released glutamate (unfilled circles) and GABA vesicles (filled circles) are within diffusion distance of glutamate and GABA terminals. Afferent activity activates Glu release from solitary tract (ST) axons. NTS excitatory interneurons including second-order neurons (2nd) also release Glu to excite higher order NTS neurons (gray oval) that appear as polysynaptic EPSCs upon ST activation. Presynaptic mGluRs (unfilled squares) are located on both ST Glu terminals and GABA terminals. Both Group II and III subtypes (white squares, −) and GABAB receptors (gray squares, −) are negatively coupled and inhibit transmitter release. Afferent glutamate reaches mGluR located on GABA releasing terminals that either promote (+, Group I) or inhibit (−, Group II and/or III) but the two were never mixed (I with II/III) (Jin et al., 2004a). GABAB receptors on ST afferent terminals strongly inhibit afferent glutamate release. Note that positively coupled Group I mGluRs populate some GABA terminals and are segregated to different second-order NTS neurons than Group II/III (Jin et al., 2004a). Thus, the GABA terminal on the left serves to depict both cases although both never occur together. Afferent Glu likely never reaches terminals on higher order neurons but these mGluRs on GABA terminals rather responds to locally released Glu (e.g. interneuronal).
Both pre- and/or postsynaptic mGluRs effectively dampen glutamatergic excitation including NTS (Glaum et al., 1993). Across subtypes, Group I mGluRs are often predominantly expressed in cell bodies where they decrease post-synaptic neuronal excitability by regulating the activity of ion channels whereas Group II and III mGluRs often presynaptically regulate neurotransmitter release (Cartmell and Schoepp, 2000; Schoepp and Conn, 2002). From the ST-NTS perspective, ion channels and receptors present at nodose neuron cell bodies may predict mechanisms present at central terminals (Mendelowitz and Kunze, 1992; Mendelowitz et al., 1995). However, varied evidence supports Group I mGluR expression in cell bodies of both nodose and NTS neurons (Hoang and Hay, 2001; Chen et al., 2002; Page et al., 2005; Peeters et al., 2006). Certainly mGluRs including Group I function in peripheral portions of primary cranial afferent neurons (Hay and Kunze, 1994; Chen et al., 2002; Young et al., 2007). For example, activation of mGluRs inhibits calcium currents including N-type in nodose sensory neurons (Hay and Kunze, 1994). Group I mGluRs have been visualized at light level in NTS (Austgen et al., 2009) and, for example, facilitate postsynaptic calcium currents in unidentified NTS neurons (Endoh, 2004). If Group I mGluRs were expressed and functioning at central synaptic terminals of nodose afferents, then we would expect to observe presynaptic effects on ST transmission and glutamate release. However, in our second-order NTS neurons, Group II and III mGluRs strongly inhibited mEPSCs but Group I mGluR activation or blockade was ineffective, observations consistent with others (Chen et al., 2002). Thus, the evidence suggests that Group I mGluRs are either not present or not functional on the afferent synaptic terminals of NTS neurons. This absence of Group I mGluRs directly on glutamate terminals contrasts to robust positive coupling of Group I mGluRs to GABA release during ST activation (Jin et al., 2004a).
A likely target for presynaptic inhibition by mGluRs and GABAB is calcium channels that mediate synchronous glutamate release from ST terminals. The N-type voltage-activated calcium channel predominates ST afferent synaptic transmission in NTS (Mendelowitz et al., 1995; Peters et al., 2010) and single ST afferents branch to make on average 20 terminations on single second-order neurons (Bailey et al., 2006b; Andresen and Peters, 2008; Peters et al., 2008). This very high release probability produces substantial use-dependent depression. Much less is known about the glutamate release machinery from synaptic terminals of NTS interneurons. In our studies, ST-evoked polysynaptic EPSCs had distinctly high failure rates and often low amplitudes (Andresen and Yang, 1995; Bailey et al., 2006a; Appleyard et al., 2007; McDougall et al., 2009). To date, evidence of synaptic performance of glutamatergic transmission to higher order NTS neuron suggests that NTS interneurons employ a lower fidelity release process with fewer, weaker, lower release probability synaptic contacts (Allen and Stevens, 1994). Our present results suggest that this interneuronal glutamatergic mechanism lacks mGluR autoreceptor-mediated negative feedback on glutamate release (e.g. no Group II/III mGluRs, Figure 6). Thus, depression predominates at ST synapses but not at glutamatergic transmission originating from NTS glutamatergic neurons.
Subtypes of mGluRs are similarly deployed at discrete synaptic connections between NTS and a major output pathway, the dorsal motor nucleus of the vagus (DMV) (Browning and Travagli, 2007). The non-uniform mGluR representations within NTS include both different subcellular targets as well as different distributions on second and higher order NTS neurons. Thus, discrete expression mGluRs within different local and projection circuits may fine tune afferent information transfer to output targets like DMV. However, the differential representation of mGluRs within NTS – especially ST afferent terminals and GABA terminals – complicates interpretation of drug interventions that affect both sites (Jones et al., 1998; Foley et al., 1999). On the basis of our synaptic analysis, we propose the following organization of mGluR and GABAB receptors within medial NTS (Figure 6). The excitation conveyed by glutamate released from ST afferents is inhibited both by mGluRs (Group II and III) but this ST glutamate additionally triggered GABA release from nearby inhibitory terminals (Jin et al., 2004a). Note that NTS GABA-releasing neurons are overwhelmingly second-order neurons themselves (Bailey et al., 2008) and this released GABA activates GABAB receptors on ST terminals. The relationship between ST triggered glutamate and GABA release is itself non-uniform since many of these inhibitory terminals are controlled by Group II/III mGluRs that inhibit GABA release (Jin et al., 2004a) and thus this finding anticipates particular circuits expressing one or the other GABA.
Importantly, in our pharmacologic approach, our drug interventions likely act simultaneously on all like receptors and this is quite different than the discrete circuit-driven activation of selective groups of terminals. Nonetheless, our survey indicates possible mGluR mechanisms and their net effect and prevalence rather than the manner in which these mechanisms are engaged in the intact system. The degree of diversity supported by our experiments suggests a dominance of Group II/III mGluR and GABAB at ST terminals. Also our approach does not identify the source of glutamate activating these mGluRs in more intact circumstances. Clearly there are substantial numbers of non-ST glutamatergic synapses within NTS including at second-order neurons (Bailey et al., 2006a; Bailey et al., 2008; McDougall et al., 2009). One could speculate that some mGluR groups might be activated by glutamatergic projections reaching NTS that affect ST transmission by presynaptic action and thus not an autoreceptor function. ST afferent driven pathways through NTS arise most often from relatively sparse inputs and often are dominated by individual afferent modalities (Donoghue et al., 1981; Donoghue et al., 1985; Andresen et al., 2004). Our results cannot predict individual pathway performance but point to future studies using additional approaches in which neuroanatomical tracers might discriminate pathways or cells types and then determine the expression and functional impact of mGluRs/GABAB in those pathways (Bailey et al., 2006a; Bailey et al., 2007). Clearly in the natural circumstance, the activity levels (e.g. myelinated vs. unmyelinated, baroreceptor vs. gastrointestinal afferent) will differently drive these potential mechanisms that may result in a specific balance. Released GABA also could arrive from sources outside of NTS such as the interesting example of the central nucleus of the amygdala which sends GABAergic processes into medial and other portions of caudal NTS and inhibits the baroreceptor reflex and blood pressure regulation as part of a stress response pathway (Saha et al., 2000; Saha, 2005).
GABAB-mGluR interactions in NTS demonstrate a complex interplay and have important implications for the understanding activity-dependent excitation and inhibition (Figure 6). Ample evidence including the present studies indicates that afferent glutamate reduces further glutamate release through Group II/III mGluRs (Glaum and Miller, 1993; Chen et al., 2002). Afferent glutamate can diffuse to additional Group II/III mGluRs on GABA terminals on second-order NTS neurons and these mGluRs were segregated to individual neurons that showed either positive or negative coupling but never mixed (i.e. mGluR I with II/III) suggesting specific patterning (Jin et al., 2004a). Our GABAB results suggest more universal expression at both ST afferent as well as non-ST terminals. Our higher order NTS neuron results suggest a very different arrangement of these receptors (Figure 6). Higher order neurons receive non-ST glutamatergic inputs that are devoid of mGluR receptors but that possess GABAB receptors that suppress glutamate release derived from NTS excitatory interneurons. This scheme is of course an oversimplification and is afferent centric as were our experiments. We have not included for example ionotropic GABA receptors which will also contribute to circuit inhibition by reducing action potential generation. Afferent activity is uneven (lower in unmyelinated afferents than in myelinated afferents) and physiologically contextual – different peripheral circumstances will activate different afferents and may recruit in parallel, di-synaptic inhibition that arrives out of sync to afferent excitatory volleys and thus will depend on the sustained activity of afferents. This afferent-driven behavior of NTS pathways is poorly understood at this time. In our organizational scheme, afferent drive may predominate by promoting this GABA release that evokes powerful GABAB actions. This notion is consistent with at least some in vivo results for example indicating that the level of baroreceptor afferent activation was associated with GABA-dependent blood pressure and sympathetic responses in the reflex system (Zubcevic and Potts, 2010).
Research Highlights
The presence of mGluR II/III on both glutamate and GABA terminals at solitary tract neurons (NTS) obscured mGluR responses by triggered GABA release via GABAb receptors controlling glutamate.
GABAb receptors are present on glutamate terminals at both second- and higher order NTS neurons but mGluRII/III are present only at second-order neurons.
Solitary tract glutamate release is differently controlled than other glutamate release in NTS.
No functioning presynaptic mGlurR I was found.
Acknowledgements
The work supported by grants from the National Institutes of Health HL-41119 (MCA), HL-83115 (MCA) and a CAPES International Fellowship (LGF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Doyle MW, Bailey TW, Jin Y-H. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz J Med Biol Res. 2004;37:549–558. doi: 10.1590/s0100-879x2004000400012. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2008;295:H2032–H2042. doi: 10.1152/ajpheart.00568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus tractus solitarius. J Neurophysiol. 1995;74:1518–1528. doi: 10.1152/jn.1995.74.4.1518. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of Group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience. 2009;159:701–716. doi: 10.1016/j.neuroscience.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Aicher SA, Andresen MC. Target-specific, dynamic pathway tuning by A-type potassium channels in solitary tract nucleus: cranial visceral afferent pathways to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol. 2007;582:613–628. doi: 10.1113/jphysiol.2007.132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: Target-specific synaptic reliability and convergence patterns. J Neurosci. 2006a;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin Y-H, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci. 2006b;26:6131–6142. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet P, Feldman J, Bloch R, Schwartz J. Evidence for a neuromodulatory role of GABA at the first synapse of the baroreceptor reflex pathway. Effects of GABA derivatives injected into the NTS. Naunyn-Schmiedeberg's Arch Pharmacol. 1982;319:168–171. doi: 10.1007/BF00503932. [DOI] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci. 2007;27:8979–8988. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol. 1988;151:243–248. doi: 10.1016/0014-2999(88)90804-7. [DOI] [PubMed] [Google Scholar]
- Chen CY, Ling Eh EH, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol. 1985;360:261–273. doi: 10.1113/jphysiol.1985.sp015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S, Fox RE, Kidd C, Koley BN. The distribution in the cat brain stem of neurones activated by vagal non-myelinated fibres from the heart and lungs. Q J Exp Physiol Cogn Med Sci. 1981;66:391–404. doi: 10.1113/expphysiol.1981.sp002582. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;37:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Endoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 2004;1024:212–224. doi: 10.1016/j.brainres.2004.07.074. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Felder RB. Effects of gamma-aminobutyric acid and glycine on synaptic excitability of neurones in the solitary tract nucleus. Neuropharmacology. 1991;30:225–236. doi: 10.1016/0028-3908(91)90149-6. [DOI] [PubMed] [Google Scholar]
- Foley CM, Vogl HW, Mueller PJ, Hay M, Hasser EM. Cardiovascular response to group I metabotropic glutamate receptor activation in NTS. Am J Physiol. 1999;276:R1469–R1478. doi: 10.1152/ajpregu.1999.276.5.R1469. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Brooks PA. Tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat medulla: Evidence for a presynaptic locus. J Neurophysiol. 1996;76:30–38. doi: 10.1152/jn.1996.76.1.30. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Sunter DC, Udvarhelyi PM, Watkins JC, Miller RJ. The actions of phenylglycine derived metabotropic glutamate receptor antagonists on multiple (1S,3R)-ACPD responses in the rat nucleus of the tractus solitarius. Neuropharmacology. 1993;32:1419–1425. doi: 10.1016/0028-3908(93)90039-6. [DOI] [PubMed] [Google Scholar]
- Hay M, Kunze DL. Glutamate metabotropic receptor inhibition of voltage-gated calcium currents in visceral sensory neurons. J Neurophysiol. 1994;72:421–430. doi: 10.1152/jn.1994.72.1.421. [DOI] [PubMed] [Google Scholar]
- Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- Hoang CJ, Hay M. Expression of metabotropic glutamate receptors in nodose ganglia and the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2001;281:H457–H462. doi: 10.1152/ajpheart.2001.281.1.H457. [DOI] [PubMed] [Google Scholar]
- Jin YH, Cahill EA, Fernandes LG, Wang X, Chen W, Smith SM, Andresen MC. Optical tracking of phenotypically diverse individual synapses on solitary tract nucleus neurons. Brain Res. 2010;1312:54–66. doi: 10.1016/j.brainres.2009.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second order neurons via distinctly segregated mGluRs. J Neurosci. 2004a;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J Neurosci. 2004b;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NM, Lawrence AJ, Beart PM. In vivo microdialysis reveals facilitatory metabotropic glutamate receptors regulating excitatory amino acid release in rat nucleus tractus solitarius. Neurochem Int. 1998;32:31–38. doi: 10.1016/s0197-0186(97)00050-8. [DOI] [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Metabotropic glutamate receptors depress vagal and aortic baroreceptor signal transmission in the NTS. Am J Physiol. 1998;275:H1682–H1694. doi: 10.1152/ajpheart.1998.275.5.H1682. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Kagiyama S, Abe I, Fujishima M. Subtypes of metabotropic glutamate receptors in the nucleus of the solitary tract of rats. Brain Res. 1999;842:461–468. doi: 10.1016/s0006-8993(99)01889-2. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Bailey TW, Mendelowitz D, Andresen MC. Propofol enhances both tonic and phasic inhibitory currents in second-order neurons of the solitary tract nucleus (NTS) Neuropharmacology. 2008;54:552–563. doi: 10.1016/j.neuropharm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci. 2009;29:12886–12895. doi: 10.1523/JNEUROSCI.3491-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Characterization of calcium currents in aortic baroreceptor neurons. J Neurophysiol. 1992;68:509–517. doi: 10.1152/jn.1992.68.2.509. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Reynolds PJ, Andresen MC. Heterogeneous functional expression of calcium channels at sensory and synaptic regions in nodose neurons. J Neurophysiol. 1995;73:872–875. doi: 10.1152/jn.1995.73.2.872. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Hoang CJ, Hay M. Expression of metabotropic glutamate receptor 8 in autonomic cell groups of the medulla oblongata of the rat. Brain Res. 2002;957:162–173. doi: 10.1016/s0006-8993(02)03619-3. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Aerssens J, de HR, Stanisz A, Gohlmann HW, Hillsley K, Meulemans A, Grundy D, Stead RH, Coulie B. Molecular profiling of murine sensory neurons in the nodose and dorsal root ganglia labeled from the peritoneal cavity. Physiol Genomics. 2006;24:252–263. doi: 10.1152/physiolgenomics.00169.2005. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol. 2005;32:450–456. doi: 10.1111/j.1440-1681.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience. 2000;99:613–626. doi: 10.1016/s0306-4522(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoepp DD, Conn PJ. Metabotropic glutamate receptors. Pharmacol Biochem Behav. 2002;74:255–256. doi: 10.1016/s0091-3057(02)00953-x. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard E, Sapru HN. Cardiovascular responses to activation of metabotropic glutamate receptors in the nTS of the rat. Brain Res. 2002;952:308. doi: 10.1016/s0006-8993(02)03260-2. [DOI] [PubMed] [Google Scholar]
- Young RL, Page AJ, O'Donnell TA, Cooper NJ, Blackshaw LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol. 2007;292:G501–G511. doi: 10.1152/ajpgi.00353.2006. [DOI] [PubMed] [Google Scholar]
- Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol. 2010;95:909–918. doi: 10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]