Abstract
Current evidence suggests that exercise and glial cell line-derived neurotrophic factor (GDNF) independently cause significant morphological changes in the neuromuscular system. The aim of the current study was to determine if increased physical activity regulates GDNF protein content in rat skeletal muscle. Extensor Digitorum Longus (EDL) and Soleus (SOL) hindlimb skeletal muscles were analyzed following 2 weeks of involuntary exercise and 4 hours of field stimulation or stretch in muscle bath preparations. GDNF protein content was measured via ELISA. Two weeks of exercise increased GDNF protein content in SOL as compared to sedentary controls (4.4 ±0.3 pg GDNF/mg tissue and 3.1 ±0.6 pg GDNF/mg tissue, respectively) and decreased GDNF protein content in EDL as compared to controls (1.0 ±0.1 pg GDNF/mg tissue and 2.3 ±0.7 pg GDNF/mg tissue, respectively). GDNF protein content in the EDL decreased following both field stimulation (56% ±18% decrease from controls) and stretch (66% ±10% decrease from controls). SOL responded to field stimulation with a 38% ±7% increase from controls in GDNF protein content, but showed no change following stretch. Pre-treatment with α-bungarotoxin abolished the effects of field stimulation in both muscles and blocked the effect of stretch in EDL. α-bungarotoxin pre-treatment and stretch increased GDNF protein content to 240% ±10% of controls in the SOL. Exposure to carbamylcholine decreased GDNF protein content to 51% ±28% of controls in the EDL but not SOL. These results suggest that GDNF protein content in skeletal muscle may be controlled by stretch, where it may increase GDNF protein content, and membrane depolarization/ACh which acts to decrease GDNF protein content.
Keywords: Neuromuscular Junction, Exercise, Stretch Stimulation, Field Stimulation
1.1 Effect of exercise on the peripheral nervous system
Increased physical activity has been shown to alter the structure and function of the neuromuscular junction (NMJ). Exercise increases the size and degree of branching of motor nerve terminals at the NMJ (Andonian and Fahim, 1987), increases total area of both pre- and postsynaptic elements (Deschenes et al., 1993), and increases quantal content of acetylcholine (ACh) release (Dorlochter et al., 1991). The dispersion of acetylcholine receptors (AChRs), including both endplate perimeter length and area, are increased following resistance training (Deschenes et al., 2000). Endplate staining with α-bungarotoxin increases by 20% in skeletal muscles of various fiber types following exercise (Desaulniers et al., 1998). Endurance exercise of young animals results in hypertrophy of nerve terminals and an increase of neurotransmitter release (Fahim, 1997).
Exercise has been found to have beneficial effects in individuals with neurodegenerative diseases and nerve injury. Increased physical activity extends the lifespan of mouse mutants with SOD1-associated motor neuron disease (Kaspar et al., 2005; Kirkinezos et al., 2003), spinal muscular atrophy (Grondard et al., 2005), and progressive motor neuronopathy (Ferrer-Alcon et al., 2008). Animals with spinal cord injury show an increase in axonal growth and sprouting, synapse formation and a decrease of muscle atrophy following 4 weeks of exercise (Goldshmit et al., 2008). Increased exercise training has also been shown to have affects on neurotrophic factor expression in rat skeletal muscle.
1.2 Effect of GDNF in the peripheral nervous system
Glial cell line-derived neurotrophic factor (GDNF) was first discovered in glial cells and supports dopaminergic neurons of the central nervous system (Lin et al., 1993). Since its discovery, expression of GDNF has been found in a variety of tissues outside of the central nervous system, including skeletal muscle and Schwann cells (Henderson et al., 1994; Suter-Crazzolara and Unsicker, 1994; Nguyen et al., 1998; Springer et al., 1995; Suzuki et al., 1998). GDNF is one of the most potent survival factors for motor neurons (MNs) during the period of developmental cell death (Henderson et al., 1994). GDNF promotes the survival of somatic MNs from programmed cell death, rescues MNs from axotomy-induced cell death (Oppenheim et al., 1995), and slows the loss of MNs in mice exhibiting progressive motor neuropathy (Sagot et al., 1996). Alterations in GDNF expression have been observed in skeletal muscle from humans with amyotrophic lateral sclerosis (Yamamoto et al., 1996), polymyositis, and Duchenne type muscular dystrophy (Suzuki et al., 1998). GDNF has also been found to help protect MNs from chronic degeneration (Corse et al., 1999), suggesting an important role for GDNF in motor nervous system recovery.
1.3 GDNF and exercise have similar effects on the NMJ
Levels of expression of brain derived neurotrophic factor, neurotrophin 3, neurotrophin 4, and GDNF in skeletal muscle have been shown to be elevated following both involuntary and voluntary exercise (Dupont-Versteegden et al., 2004; Gomez-Pinilla et al., 2001; Funakoshi et al., 1995; Wehrwein et al., 2002). Our laboratory has previously shown that GDNF protein content increases with involuntary exercise in skeletal muscle but decreases with hindlimb suspension (Wehrwein et al., 2002). Over-expression of GDNF in skeletal muscle leads to hyperinnervation at the NMJ (Nguyen et al., 1998) and maintains multiple innervated endplates past the period of normal synapse elimination (Zwick et al., 2001). Exogenous GDNF application increases spontaneous transmitter release from motor nerve terminals in skeletal muscles of both neonatal mice (Ribchester et al., 1998) and from nerve-muscle co-cultures (Wang et al., 2002). Treatment with exogenous GDNF causes continuous synaptic remodeling and axonal branching at the NMJ (Keller-Peck et al., 2001). Expression of GDNF in skeletal muscle decreases with age, but is continuously maintained at the NMJ through adulthood (Nagano and Suzuki, 2003).
Independent studies have found that GDNF and exercise elicit similar responses at the NMJ, however it is unknown if the two are linked. Thus, we propose two specific aims for this study. 1) To determine whether 2 weeks of involuntary exercise can alter GDNF protein content in rat hindlimb skeletal muscle. 2) To determine whether electrical activity or stretch are important regulators of GDNF protein content in hindlimb skeletal muscle. We hypothesize that positive effects of exercise on the motor nervous system structure and function are driven in part by changes in GDNF. In the present study, rats were exercised for 2 weeks and GDNF levels in skeletal muscle were analyzed via ELISA. Muscle bath studies examining effects of electrical stimulation and stretch were performed to examine regulation of GDNF levels in vitro. Muscles were pre-treated with α-bungarotoxin to examine a possible role for nAChRs on GDNF levels. Findings from these studies reveal that increased physical activity alters GDNF protein content in skeletal muscle and that activation of nAChRs appears to be involved in regulating this process.
2. EXPERIMENTAL PROCEDURES
2.1 Subjects
All animal experiments were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council) and protocols were approved by the Institutional Animal Care and Use Committee at Western Michigan University. Four-week-old male Sprague Dawley rats (Charles River, Kalamazoo, MI) were utilized for in vitro muscle incubations. This age was chosen as the small muscle size helps to minimize development of anoxic core in the skeletal muscle preparations. Seventeen-week-old male Fischer 344 rats (Charles River, Portage, MI) were utilized for in vivo exercise studies. This age was selected because it has previously been demonstrated that animals at this age have a relatively stable distribution of skeletal muscle fiber types (Maltin et al., 1989). All animals were given access to food and water ad libitum and were maintained on a 12h light/dark cycle. Rats were euthanized via CO2 asphyxiation followed by thoracotomy.
2.2 Training Protocol
Rats were randomly divided into two groups. One group underwent involuntary exercise (n = 4), and the second group was kept as sedentary age-matched controls (n = 8). Exercised animals had 2 weeks of training in individual forced running wheels (Lafayette Instruments, Lafayette, IN) before initiation of the involuntary exercise protocol. At the end of the training session, animals were exercised for 5 consecutive days at 10 m/min for 45 min, followed by two consecutive days of rest. All exercised animals followed this protocol for a total of 2 weeks. Rats were euthanized 72 hours after the last bout of exercise.
2.3 Muscle Bath Studies
Bilateral nerve-muscle preparations of the sciatic nerve-soleus (SOL) muscle and the sciatic nerve-extensor digitorum longus (EDL) muscle were removed and placed in a tissue bath containing Krebs-Ringer bicarbonate solution (in mM): 120.2 NaCl, 25.1 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2, and 5 D-glucose (Gissel and Clausen, 1999). Experiments were performed at room temperature (25°C) to increase the physiological stability of the tissues for the duration of the experiment (Segal and Faulkner, 1985; Gissel and Clausen, 1999). The bath was continuously bubbled with O2/CO2 (95%/5%) and was maintained at a working pH of 7.4. Sutures (4-0 silk) were tied to tendons at each end of the tissue and muscles were mounted between a fixed glass hook and a force transducer in the bath. All bath experiments were performed with contralateral tissues as controls.
2.4 Stimulation Protocol
SOL (n = 6) and EDL (n = 7) muscles were suspended between a fixed glass hook and a force transducer (Grass Technologies, FT03D, West Warwick, RI) using 4-0 silk sutures. Muscles were placed between two zig-zag electrodes (ADInstruments, Colorado Springs, CO) and were stimulated (Grass Technologies, S88) to determine optimum length. Contractile force was measured and recorded on a pen recorder (Grass Technologies, 7D Polygraph). After optimum length of the tissues were determined, tissues were electrically stimulated at 0.1Hz (1.0-ms pulses) for 4 hours with supramaximal voltage (Gissel and Clausen, 1999).
2.5 Stretch Protocol
Resting length was measured in situ and SOL (n = 6) and EDL (n = 6) muscles were placed in baths with the distal ends attached to a glass hook and the proximal ends were attached to a fabricated cam. The motor (Globe Motors, 409A582) was fixed above the tissue bath and the cam was offset by 3 mm from the center of the motor's shaft. The design allowed for tissues to be stretched ±3 mm from resting length per revolution which resulted in a ±14% and 15% stretch for the SOL and EDL muscles, respectively. This is within the physiological range of stretch (10-15%) as determined for skeletal muscle (Chen and Grinnell, 1997). Tissues were stretched at a rate of 2 revolutions in 0.5 second at 0.1 Hz with a 15 second delay between revolutions for an overall duration of 4 hours. This protocol mimicked the contractile activity of the stimulation protocol used above.
2.6 Blockade of Acetylcholine Receptors
Acetylcholine (Sigma) was delivered to the bath at a concentration of 88.1μM. This concentration was determined to be more than adequate to obtain maximal force production for the tissues. Baths were rinsed with fresh Ringer's solution between applications and incubated for 30 min with α-bungarotoxin (Biotium) at a concentration of 0.25mM for EDL muscle (n = 15) and 0.38mM for SOL muscle (n = 12). Tissues were washed with fresh Ringer's for 30 min to remove any unbound toxin. ACh was then applied at double the original concentration which elicited no response (Harborne et al., 1978).
2.7 Carbamylcholine (carbachol) Exposure
SOL (n = 6) and EDL (n = 7) muscles were removed and optimum length was established. Carbachol was then added to the bath so the final concentration was 10μM. Tissues were exposed to carbachol for 4 hours and contralateral control tissues were left in normal Ringer's solution for the duration of the experiment.
2.8 Tissue Processing
At the conclusion of all experiments, SOL and EDL muscles were removed and frozen on dry ice. To determine GDNF protein content, samples were subsequently dipped in liquid nitrogen and smashed into a fine powder. Sample processing buffer (0.55 M NaCl, 0.02 M NaH2PO4, 0.08 M Na2HPO4, 2 mM EDTA, 0.1 mM benzethonium chloride, 2 mM benzamidine, 20 KIU/ml aprotinin, 0.5% BSA, and 0.05% Tween-20) was added and the mixture was homogenized on ice. Samples were centrifuged for 30 min at 4°C and supernatant was collected and stored at -80°C. GDNF protein content was determined using an enzyme-linked immunosorbant assay (ELISA).
2.9 Enzyme-Linked Immunosorbant Assay
Briefly, 96-well plates were incubated overnight at room temperature using a monoclonal antibody raised against GDNF (R&D Systems). Remaining sites on the plates were blocked with PBS containing 1% BSA and 5% sucrose. Plates were rinsed 3 times in wash buffer and tissue supernatant or GDNF standards were added to each well. These plates were incubated at room temperature for at least 2 hours. Wells were then washed 3 times and an anti-GDNF antibody conjugated to biotin (R&D Systems) was added and incubated for 2 hours at room temperature. Plates were washed again and horseradish peroxidase conjugated to streptavidin (Pierce) was added for 20 min at room temperature. Plates had a final wash (3 times), and the tetramethylbenzidine color reagent (Sigma) was added according to manufacturer's specifications. The reaction was stopped with 0.1 M phosphoric acid and absorbance was measured at 450 nm.
2.10 Immunohistochemistry
The EDL and SOL muscles were selected as they represent muscles used for locomotion, consisting primarily of fast (Ariano et al., 1973) and slow oxidative fibers (Armstrong and Phelps, 1984), respectively. Tissues were fixed in Zamboni's fixative (Stefanini et al., 1967) for 15 min, were washed three times in phosphate-buffered saline (PBS: 0.225 M NaCl, 0.02 M NaH2PO4, and 0.08 M Na2HPO4) for 5 min each wash, and flash frozen in 2-methylbutane chilled on dry ice. The middle sections of the SOL and EDL were used for endplate analysis. Tissues were embedded in medium (O.C.T. Compound) and cut into transverse sections (60μm) on a Leica cryotome. Slides were incubated with primary antibodies that were diluted 1:100 in PBS containing 1% bovine serum albumin, 0.1% triton X-100 for 2 hours at room temperature. Rabbit anti-GDNF (Santa Cruz Biotechnology) was used to visualize GDNF, and mouse anti-neurofilament (Millipore) was used to visualize nerve fibers. Slides were rinsed 3 times, 5 minutes each, in PBS. Donkey anti-mouse conjugated to Alexafluor 647 (Molecular Probes) and goat anti-rabbit conjugated to Alexafluor 568 (Molecular Probes) secondary antibodies were diluted 1:1000 in PBS containing 1% bovine serum albumin, 0.1% triton X-100 and, were incubated on slides for 30 minutes at room temperature. To visualize nAChRs, α-bungarotoxin directly conjugated to Alexafluor 488 (Molecular Probes) was diluted 1:1000 in PBS containing 1% bovine serum albumin, 0.1% triton X-100 for 2 hours at room temperature. Slides were washed several times in PBS and mounted in PBS:Glycerol (1:1). Images were viewed with a Zeiss Axiovert 100M confocal microscope.
2.11 Endplate Analysis
Endplate areas stained with α-bungarotoxin were examined in both the EDL and SOL muscle sections from sedentary control animals and were compared to those from exercised animals. Ten representative endplates in the horizontal plane were selected from each muscle to determine endplate area. Total area of the endplate perimeter was measured which included stained receptor clusters along with non-stained regions interspersed within those clusters (Deschenes et al., 2006). The endplate areas were calculated using the Zeiss LSM 5 Image Examiner program.
2.12 Statistical Analysis
Data are displayed as mean ± the standard error of the mean (S.E.M.) for all variables. For the in vitro experiments, protein values of the experimental group were normalized to the control group values and reported as a percent change from control ± S.E.M. In order to determine significance, a student's t-test was performed, except when variance was not homogeneous; in those instances Wilcoxon rank-sum comparisons were used. Comparisons between the in vivo muscles were determined by a student's t-test for direct comparison of two groups. For all tests, significance was set to P ≤ 0.05.
3. RESULTS
3.1 GDNF protein content increases in SOL and decreases in EDL following 2 weeks of exercise
We took into consideration that there may be differences between Fischer 344 and Sprague-Dawley animals with regards to GDNF protein content of skeletal muscle. Control animals aged 12 weeks were euthanized from the two strains and GDNF protein content was measured from both the EDL and SOL hindlimb muscles. GDNF protein content of the EDL muscle was not significantly different in the Fischer 344 rats compared to that in muscle from Sprague-Dawley rats (1.1 ± 0.3 pg GDNF/mg tissue and 0.9 ± 0.2 pg GDNF/mg tissue, respectively). GDNF protein content of the SOL muscle from the Fischer 344 rats was not significantly different from that in the Sprague-Dawley rats (0.5 ± 0.2 pg GDNF/mg tissue and 0.9 ± 0.4 pg GDNF/mg tissue, respectively).
GDNF protein content from the 4-week-old Sprague-Dawley rats was significantly higher in the SOL muscle (96.5 ± 11.6 pg GDNF/mg tissue) than in the EDL muscle (37.2 ± 1.7 pg GDNF/mg tissue). However, for the 17-week-old control animals, there was no difference between the SOL muscle (3.1 ± 0.6 pg GDNF/mg tissue) and the EDL muscle (2.3 ± 0.7 pg GDNF/mg tissue).
Following 2 weeks of involuntary exercise of the 17-week-old Fischer 344 rats, there was a significant decrease in GDNF protein content of the EDL muscle (1.0 ± 0.1 pg GDNF/mg tissue) as compared to age-matched sedentary controls (2.3 ± 0.7 pg GDNF/mg tissue; Figure 1a). GDNF protein content of the exercised SOL muscles were significantly increased (4.4 ± 0.3 pg GDNF/mg tissue) as compared to age-matched sedentary controls (3.1 ± 0.6 pg GDNF/mg tissue; Figure 1b). Furthermore, to determine if changes in GDNF protein content were due to skeletal muscle hypertrophy, we measured the ratio of skeletal muscle weight to body weight and found that the ratio was not altered in SOL or EDL following exercise (data not shown).
Figure 1.
GDNF protein content increases in SOL and decreases in EDL following 2 weeks of involuntary exercise. The EDL and SOL muscles were removed from exercised and age-matched sedentary control animals. Tissues were processed for GDNF protein content using an ELISA. (A) A significant decrease in GDNF protein content of the EDL was detected in animals that had undergone 2 weeks of involuntary exercise as compared to sedentary control animals. (B) A significant increase in GDNF protein content was detected in SOL from animals that had undergone 2 weeks of involuntary exercise as compared to sedentary control animals. Values are displayed as the mean ± SEM. Asterisk (*) indicates significance (p ≤ 0.05).
We were able to visualize positive staining for GDNF around the endplate region for both the EDL and SOL muscles. GDNF appears to be concentrated around the NMJ and follows the same outline as the NMJ for both muscles. There was no observable difference in GDNF staining between the EDL and the SOL muscles, or between exercise and control muscles. Figure 2 shows staining for GDNF protein in control EDL muscle, displaying positive GDNF staining at the NMJ.
Figure 2.
Staining for GDNF appears to be localized to the region of the NMJ. The EDL muscle was stained with anti-GDNF antibodies (red), anti-neurofilament (neuronal fibers, blue) and α-bungarotoxin directly conjugated to AlexaFluor 488 (AChRs, green). Scale bar represents 20μm.
3.2 Total endplate area increases in SOL and decreases in EDL following 2 weeks of exercise
To determine changes in postsynaptic size of the NMJ following 2 weeks of involuntary training in the SOL and EDL muscles, tissue sections were stained with α-bungarotoxin directly conjugated to AlexaFluor488 to label nAChRs. The area of the endplates was measured with the Zeiss LSM 5 Image Examiner. The average total area of endplate staining in the EDL muscle of exercised animals was significantly decreased (918.5 ± 36.8 μm2) as compared to endplates of sedentary control animals (1095.9 ± 30.3 μm2; Figure 3a). The dispersion of AChRs was measured by dividing the stained area by the total area of the endplates and multiplying by 100 (Deschenes et al., 2006). There was no difference in the percent dispersion of control and exercised EDL endplates, (71.0% ± 3.1% and 76.6% ± 3.5%, respectively). The average total area of endplate staining in the SOL muscle from exercised animals was significantly increased (1205.7 ± 29.8 μm2) as compared to sedentary control animals (958.7 ± 57.4 μm2; Figure 3b). The dispersion of AChRs in the control SOL was not different from the exercised SOL endplates (77.2% ± 4.8% and 82.1% ± 3.3%, respectively).
Figure 3.
Total endplate area increases in SOL and decreases in EDL following 2 weeks of exercise. The EDL and SOL muscles were removed from exercised and control rats and cut transversely in 60μm sections. Tissues were stained with α-bungarotoxin directly conjugated to AlexaFluor 488. (A) The average total area of EDL endplates were significantly decreased in exercised animals as compared to sedentary control animals. Inset panel represents an exercised EDL endplate used to measure area. (B) The average total area of SOL endplates were significantly increased in exercised animals as compared to sedentary control animals. Inset panel represents an exercised SOL endplate used to measure area. Values are displayed as the mean ± SEM. Asterisk (*) indicates significance (p ≤ 0.05).
3.3 Pre-treatment with α-bungarotoxin eliminates both the field stimulation-induced decrease in GDNF protein content in EDL and increase in GDNF protein content in SOL
To determine the effects of field stimulation on skeletal muscle GDNF protein content, EDL and SOL muscles were placed in isolated tissue baths and electrically stimulated. After 4 hours of field stimulation, tissues were removed and processed for analysis of GDNF protein content. Field stimulation significantly decreased GDNF protein content of the EDL muscle to 56% ± 18% of unstimulated, contralateral control tissues. Stimulation of the SOL muscle however, significantly increased GDNF protein content to 138% ± 7% of control tissues (Figure 4a).
Figure 4.
Pre-treatment with α-bungarotoxin eliminates both the field stimulation-induced decrease in GDNF protein content in EDL and increase in GDNF protein content in SOL. The EDL and SOL muscles were removed and placed in a tissue bath. Muscles were placed between zig-zag electrodes and were stimulated at 0.1Hz (1.0-ms pulses) for 4 hours with supramaximal voltage. (A) Four hours of field stimulation of the EDL caused a significant decrease in GDNF protein content to 56% ± 18% (n = 7) of unstimulated control tissues. Field stimulation of the SOL caused a significant increase in GDNF protein content to 138% ± 7% (n = 6) of unstimulated control tissues. (B) Prior to stimulation, muscles were incubated for 30 minutes with α-bungarotoxin to block effects of ACh. Following exposure to α-bungarotoxin, 4 hours of field stimulation had no significant effect on GDNF protein content of the EDL (n = 9) or SOL (n = 6) when compared to unstimulated controls. Asterisk (*) indicates significance (p ≤ 0.05).
To determine whether ACh release from nerve terminals was affecting GDNF protein content, muscles were pre-treated with α-bungarotoxin and subsequently stimulated. Pre-treatment with α-bungarotoxin abolished both the significant decrease in GDNF protein content in the EDL muscle to 125% ± 31% of control EDL tissues and the significant increase in GDNF protein content in SOL muscle to 140% ± 16% of control SOL tissues (Figure 4b). Treatment with α-bungarotoxin alone had no significant effect on GDNF protein content in either EDL muscles (105% ± 9% of control tissues) or SOL muscles (74% ± 16% of control tissues).
3.4 Pre-treatment with α-bungarotoxin blocks stretch-induced decrease in GDNF protein content in EDL and uncovers stretch-induced increase in GDNF protein in SOL
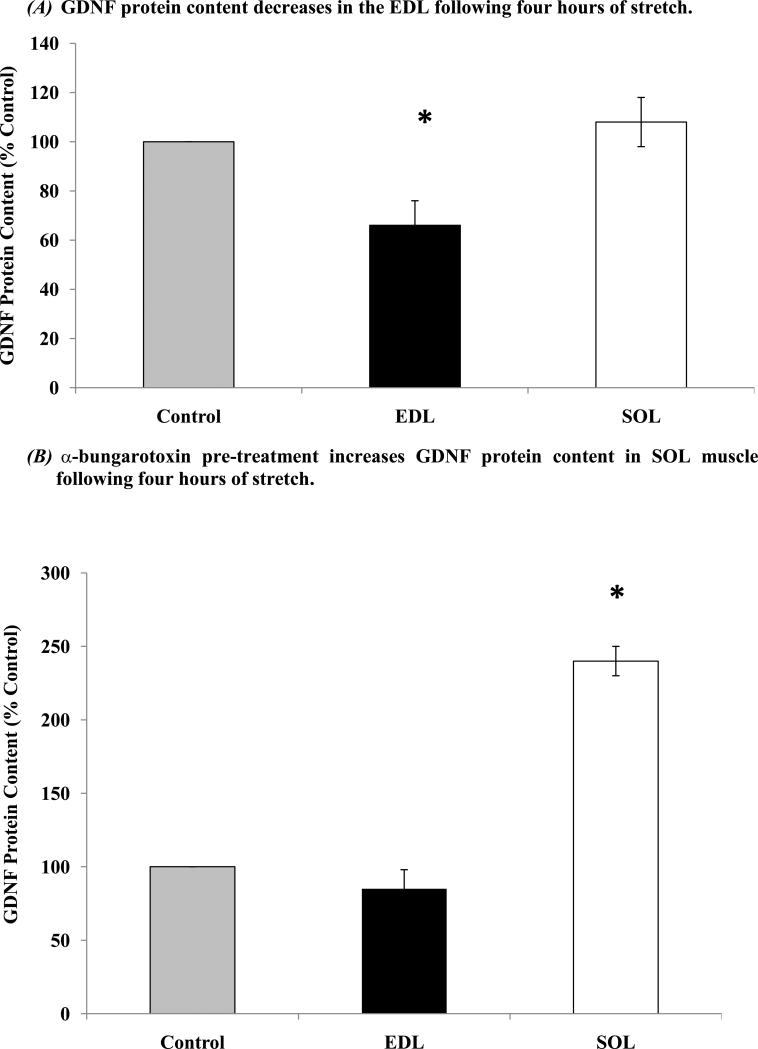
In order to determine the effects of passive stretching on GDNF protein content of skeletal muscle, EDL and SOL muscles were removed and placed in an isolated tissue bath. Tissues were cyclically stretched for 4 hours. Tissues were removed and processed for GDNF protein content analysis. Following 4 hours of passive stretching, the EDL muscle displayed a significant decrease in GDNF protein content to 66% ± 10% of the unstimulated control tissues. There was no significant effect for GDNF protein content in the SOL muscle (108% ± 10% of control tissues) following 4 hours of passive stretching (Figure 5a).
Figure 5.
Pre-treatment with α-bungarotoxin blocks stretch-induced decrease in GDNF protein content in EDL and uncovers stretch-induced increase in GDNF protein in SOL. EDL and SOL muscles were removed and placed in a tissue bath. Muscles were placed in baths with the distal ends attached to a glass hook, while the proximal ends were attached to a motor/cam assembly that was fixed above the tissue bath. (A) Four hours of cyclical, passive stretching of the EDL muscle caused a significant decrease in GDNF protein content to 66% ± 10% (n = 6) of the unstretched control tissues. There was no significant effect of passive stretching on GDNF protein content in the SOL (n = 6) when compared to unstretched controls. (B) Prior to stretching, muscles were incubated for 30 minutes with α-bungarotoxin to block effects of ACh. There was no significant effect of 4 hours of cyclical passive stretching after 30 minutes of α-bungarotoxin treatment on GDNF protein content in the EDL (n = 6) as compared to the unstretched, α-bungarotoxin treated control tissues. Passive stretching after α-bungarotoxin treatment of the SOL caused a significant increase in GDNF protein content to 240% ± 10% (n = 6) of the unstretched, α-bungarotoxin treated control tissues. Asterisk (*) indicates significance (p ≤ 0.05).
To determine the role of the nAChRs in this response, tissues were pretreated with α-bungarotoxin and subsequently stretched. Pre-treatment with α-bungarotoxin in the EDL muscle blocked the effect of passive stretching on GDNF protein content (85% ± 13% of the unstretched, α-bungarotoxin treated control muscles). With the SOL muscle, pre-treatment with α-bungarotoxin and subsequent passive stretching caused a significant increase in GDNF protein content to 240% ± 10% of the α-bungarotoxin treated, unstretched controls (Figure 5b).
3.5 Carbachol treatment decreases GDNF protein content in EDL
To further characterize the role of the nAChRs in regulation of GDNF protein content in skeletal muscle, EDL and SOL muscles were exposed to 10μM of the nAChR agonist carbachol for 4 hours in a muscle bath. Carbachol caused a significant decrease in GDNF protein content for the EDL muscle to 49% ± 28% of the control tissues. Carbachol treatment had no effect on GDNF protein content in the SOL muscle (126% ± 22% of control tissues; Figure 6). To determine whether GDNF protein content was being released from skeletal muscle into the muscle bath, studies were run in which GDNF protein content was measured in the bathing medium. However, the results showed that GDNF protein content of the bathing medium was below the detection limit of the ELISA.
Figure 6.
Carbachol decreases GDNF protein content in EDL. The EDL and SOL muscles were removed and placed in a tissue bath. Four hours of carbachol (10μM) treatment caused a significant decrease in GDNF protein content to 49% ± 28% of the untreated control EDL tissues (n = 7). Carbachol treatment had no significant effect on GDNF protein content in the SOL (n = 6). Asterisk (*) indicates significance (p ≤ 0.05).
4. DISCUSSION
The aim of the current study was two-fold in determining changes in GDNF protein content of skeletal muscle after exercise. First, we wanted to determine if 2 weeks of exercise would alter GDNF protein content in rat skeletal muscle. Second, to determine whether electrical activity or stretch are important regulators of GDNF protein content in skeletal muscle. We have now demonstrated that as little as 2 weeks of involuntary exercise, or 4 hours of field stimulation or stretch alters GDNF protein content in skeletal muscle. It has been previously shown that neurotrophic factor protein levels are increased with voluntary exercise in skeletal muscle, such as brain derived neurotrophic factor (Gomez-Pinilla et al., 2002) and GDNF protein levels following 4 weeks of voluntary exercise (Wehrwein et al., 2002). To our knowledge, this is the first report that shows 2 weeks of involuntary exercise and 4 hours of direct electrical stimulation and stretch alters GDNF protein content in skeletal muscle. We found changes in endplate size that were in the same direction as the changes in GDNF protein content. Some of the effects from field stimulation and stretch were blocked following α-bungarotoxin treatment, suggesting a role for AChR activation in regulating GDNF protein content in skeletal muscle.
4.1 GDNF protein content may be dependent on muscle fiber type
Our observation of increased GDNF protein content in the SOL muscle and decreased GDNF protein content in the EDL muscle following physical activity may be in part due to the exercise protocols chosen for the study. Our protocols are a low-intensity exercise protocol, likely activating the slow-twitch muscle fibers to a greater extent than fast-twitch muscle fibers. There may be an activity-dependent relationship that determines the amount of GDNF protein levels found in the different types of skeletal muscle. This concept that neurotrophic factor expression is activity-dependent has been reported for neurotrophic factor-4 (NT-4) expression in skeletal muscle (Funakoshi et al., 1995; Wang and Poo, 1997). A greater expression of NT-4 was observed in the slow, fibers of the SOL compared to the fast fibers of the gastrocnemius (Funakoshi et al., 1995). The EDL muscle is composed primarily of fast-twitch muscle fibers with published reports of 98% of the overall composition being of the fast-twitch phenotype. The SOL is composed primarily of slow-twitch muscle fibers which account for approximately 89% of the overall muscle fiber composition (Alnaqeeb and Goldspink, 1987). Nagano and Suzuki (2003) demonstrated a developmental difference in GDNF protein content between muscles with differing fiber type composition (SOL [slow] and gastrocnemius [fast]) and suggested that inherent differences in muscle fiber composition might contribute to the GDNF protein content differences observed. Our data also lends support to this idea of differential GDNF protein content between muscles of different fiber type compositions. We observed that the SOL muscle from the 4-week-old animals had significantly higher GDNF protein content than the EDL muscle. The EDL muscle responded to direct electrical stimulation, passive stretch and low-intensity involuntary exercise with a decrease in GDNF protein content. The SOL muscle displayed an increase in GDNF protein content following involuntary exercise, direct electrical stimulation, and an even larger increase in GDNF protein content following passive stretch in the presence of α-bungarotoxin. For the EDL muscle, changes in GDNF protein content observed following direct electrical stimulation and stretch appear to be linked to activation of nAChR. For the SOL muscle, changes in GDNF protein content in response to stimulation appear to be only slightly affected by nAChR activity.
4.2 ACh may inhibit GDNF protein content
A number of cellular changes elicited by membrane depolarization and muscle contraction may impact GDNF protein expression in skeletal muscle. Release of ACh from nerve terminals, increased intracellular calcium as well as mechanical events associated with muscle contraction could influence GDNF protein expression. Moreover, the GDNF measured in the processed whole muscle could be derived from a number of sources, including skeletal muscle, Schwann cells, neurons and smooth muscle in blood vessels.
Neurotransmitters have been shown to affect neurotrophic factor expression in other tissues. In vascular and bladder smooth muscle cells in culture, neurotransmitters from sympathetic neurons alter the level of secretion of nerve growth factor by these cells (Clemow et al., 1999; Spitsbergen et al., 1995). We found that α-bungarotoxin pre-treatment altered the effects of electrical and mechanical stimulation on GDNF protein content in skeletal muscle in vitro, suggesting that ACh is acting on nAChRs to regulate GDNF protein content in these isolated muscle preparations. The observation that treatment of isolated skeletal muscle with a cholinergic agonist caused a decrease in GDNF protein content also suggests that nAChR activation inhibits GDNF protein content in skeletal muscle. Tissues treated with carbachol lacked the coordinated mechanical activity associated with whole muscle contraction, or cyclical passive stretching, yet still had significant decreases in GDNF protein content, possibly suggesting that cholinergic agonists exert effects that are not linked to muscle contraction or stretch.
An inhibitory effect of ACh on GDNF expression would help to explain why GDNF mRNA levels are elevated in denervated skeletal muscle (Lie and Weis, 1998), and why blockade of nAChR on skeletal muscle enhanced the survival of developing MNs (Oppenheim et al., 2000). However, this effect cannot explain an increase in GDNF protein content following exercise (Wehrwein et al., 2002). We found that following blockade of nAChRs, GDNF protein content was increased in the SOL muscle exposed to passive stretch in a muscle bath. This was the largest change in GDNF protein content observed with any of the in vitro manipulations tested. These results suggest that mechanical activity, mediated through passive stretch, may act as a strong signal to increase GDNF protein content in skeletal muscle. An effect of stretch on neurotrophic factor expression has also been demonstrated in smooth muscle, where rhythmic stretching of vascular and bladder smooth muscle cells in culture caused an increase in nerve growth factor secretion (Clemow et al., 2000).
Our findings show that low intensity exercise training decreases nerve terminal area of the EDL muscle, which is consistent with results of previous studies (Andonian and Fahim, 1987). Low frequency electrostimulation of the EDL muscle for 30 days results in smaller endplates with larger synaptic grooves, suggesting that the synaptic contact is reduced and the terminal withdraws from the junction (Mussini and Marchioro, 1991). The quantal release of ACh with low frequency stimulation is higher in the EDL muscle than the SOL muscle. This has a net result of a larger release of ACh per unit of terminal area for the EDL muscle (Wood and Slater, 1997; Reid et al., 1999). It has been suggested that the SOL muscle, in response to low-intensity training, has a greater amount of stored ACh in the nerve terminal which requires a larger endplate area (Deschenes et al., 1993; Dorlochter et al., 1991). Chen and Grinnell (1997) have demonstrated that there is a direct, mechanical modulation of the release of neurotransmitter with stretch. If low intensity exercise and/or stretch elicit an augmented release of ACh in the EDL muscle, then this may help to explain our observation of a decrease in GDNF protein content following low-intensity stimulation in vitro and a decrease in GDNF protein content and endplate size following exercise in vivo.
Positive staining for GDNF was identified around the NMJ, however our staining does not show which cells are expressing GDNF. Others have shown that there is positive GDNF immunoreactivity near the NMJ in human skeletal muscles (Suzuki et al., 1998). Both Schwann cells and skeletal muscle are known to produce GDNF (Henderson et al., 1994; Springer et al., 1995), and Schwann cells and motor neurons produce the primary GDNF receptor, GFR α-1 (Trupp et al. 1997; Hase et al., 1999). Thus the GDNF immunoreactivity near the NMJ in our images could be GDNF that is produced by muscle or Schwann cells or GDNF that is bound to GFR α-1 receptors on Schwann cells or the motor nerve terminal. While our results are inconclusive as to which cells are producing GDNF, our images show that GDNF is concentrated around the NMJ, suggesting that it is readily available for the nervous system.
4.3 GDNF protein content may decrease with aging
We observed that 4-week-old animals had high levels of GDNF protein content in the SOL muscle (96.5 ± 11.6 pg GDNF/mg Tissue) and EDL muscle (37.2 ± 1.7 pg GDNF/mg Tissue) as compared to the SOL and EDL of the 17-week-old animals (3.1 ± 0.6 pg GDNF/mg Tissue and 2.3 ± 0.7 pg GDNF/mg Tissue, respectively). These findings suggest that GDNF levels decrease with age. It has previously been shown that GDNF protein content in the SOL muscle was significantly decreased in 3-month-old animals as compared to 1-month-old animals (Nagano and Suzuki, 2003). Some of the main changes that are associated with aging are the loss of MNs (Jacob, 1998) and loss of inputs to motor nerve cell bodies (Kullberg et al., 1998). One possibility for this loss of MN innervation could be due to decreased expression of GDNF with age (Bergman et al., 1999). Since GDNF has been recognized as one of the most potent survival factors for MNs (Henderson et al., 1994; Oppenheim et al., 1995), decreased GDNF content in skeletal muscle could negatively impact MN structure and function with aging. We found that low-intensity physical activity was able to reverse GDNF protein content loss in some of the skeletal muscles examined. This suggests that one way to counteract the loss of GDNF found with aging could be with increased physical activity.
In conclusion, the present study has demonstrated that levels of GDNF protein content in skeletal muscle may be controlled by stretch, such that increased mechanical activity leads to an increase in GDNF protein content and ACh leads to a decrease in GDNF protein content. Thus, the increased mechanical activity occurring with walk-training could lead to the elevated GDNF protein expression observed by Wehrwein et al. (2002) while with hindlimb suspension there would be a decrease in mechanical activity with continued ACh effects (Alford et al., 1987; Blewett and Elder, 1993), leading to a decrease in GDNF protein expression. If activity of skeletal muscle is an important regulator of GDNF protein expression, then this could have significant consequences for aging individuals and those with limited mobility, such as with injury or neurodegenerative diseases. A positive effect of mechanical activity on GDNF protein expression may also help to explain beneficial effects of physical therapy (Feys et al., 2004) or weight bearing activities (Matsuura et al., 2001) for recovery from nervous system injury.
Acknowledgements
Preliminary results from these studies were presented at the Experimental Biology conference in 2008. The authors would like to thank Micah Dawati for his contribution of sample collection and analysis and to Dr. Omar Akhtar for expert assistance on the project.
Grants
This work was supported by was supported by NIH grant 1 R15 AG022908-01A2, Western Michigan University, and Michigan State University –Kalamazoo Center for Medical Studies.
Abbreviations
- GDNF
Glial cell line-derived neurotrophic factor
- ACh
Acetylcholine
- EDL
Extensor digitorum longus
- SOL
Soleus
- NMJ
Neuromuscular Junction
- ELISA
Enzyme-linked immunosorbant assay
- AChR
Acetylcholine receptor
- nAChR
Nicotinic acetylcholine receptor
- MN
Motor neuron
- NT-4
Neurotrophic factor-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp Neurol. 1987;96(3):635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Goldspink G. Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat. 1987;153:31–45. [PMC free article] [PubMed] [Google Scholar]
- Andonian MH, Fahim MA. Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Neurocytol. 1987;16:589–599. doi: 10.1007/BF01637652. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochm Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Bergman E, Kullberg S, Ming Y, Ulfhake B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J Neurosci Res. 1999;57(2):153–165. doi: 10.1002/(SICI)1097-4547(19990715)57:2<153::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Blewett C, Elder GC. Quantitative EMG analysis in soleus and plantaris during hindlimb suspension and recovery. J Appl Physiol. 1993;74(5):2057–2066. doi: 10.1152/jappl.1993.74.5.2057. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Kinetics, Ca2+ dependence, and biophysical properties of integrin-mediated mechanical modulation of transmitter release from frog motor nerve terminals. J Neurosci. 1997;17(3):904–916. doi: 10.1523/JNEUROSCI.17-03-00904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemow DB, Spitsbergen JM, McCarty R, Steers WD, Tuttle JB. Altered NGF regulation may link a genetic predisposition for hypertension with hyperactive voiding. J Urol. 1999;161(4):1372–1377. [PubMed] [Google Scholar]
- Clemow DB, Steers WD, Tuttle JB. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J Cell Physiol. 2000;183:289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol of Disease. 1999;6:335–346. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- Desaulniers P, Lavoie PA, Gardiner PF. Endurance training increases acetylcholine receptor quantity at neuromuscular junctions of adult rat skeletal muscle. Neuroreport. 1998;9:3549–3552. doi: 10.1097/00001756-199811160-00001. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Judelson DA, Kraemer WJ, Meskaitis VJ, Volek JS, Nindl BC, Harman FS, Deaver DR. Effects of resistance training on neuromuscular junction morphology. Muscle Nerve. 2000;23:1576–1581. doi: 10.1002/1097-4598(200010)23:10<1576::aid-mus15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Maresh CM, Crivello JF, Armstrong LE, Kraemer WJ, Covault J. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol. 1993;22:603–615. doi: 10.1007/BF01181487. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Tenny KA, Wilson MH. Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neurosci. 2006;137:1277–1283. doi: 10.1016/j.neuroscience.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Dorlochter M, Irintchev A, Brinkers M, Wernig A. Effects of enhanced activity on synaptic transmission in mouse extensor digitorum longus muscle. J Physiol. 1991;436:283–292. doi: 10.1113/jphysiol.1991.sp018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houle JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- Ferrer-Alcon M, Winkler-Hirt C, Madani R, Perrin FE, Kato AC. Low intensity exercise attenuates disease progression and stimulates cell proliferation in the spinal cord of a mouse modle with progressive motor neuronopathy. Neurosci. 2008;152:291–295. doi: 10.1016/j.neuroscience.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Feys H, De Weerdt W, Verbeke G, Steck GC, Capiau C, Kiekens C, Dejaeger E, Van Hoydonck G, Vermeersch G, Cras P. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke. 2004;35:924–929. doi: 10.1161/01.STR.0000121645.44752.f7. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am J Physiol. 1999;276(2 Pt 2):R331–R339. doi: 10.1152/ajpregu.1999.276.2.R331. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25:449–466. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13(6):1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Grondard C, Biondi O, Armand AS, Lecolle S, Gaspera BD, Pariset C, Li H, Gallien CL, Vidal PP, Chanoine C, Charbonnier F. Regular exercise prolongs survival in a Type 2 spinal muscular atrophy mouse model. J Neurosci. 2005;25(33):7615–7622. doi: 10.1523/JNEUROSCI.1245-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne AJ, Smith ME, Jones R. The effect of hydrolytic enzymes on the acetylcholine sensitivity of the skeletal muscle cell membrane. Pflügers Arch. 1978;377:147–153. doi: 10.1007/BF00582845. [DOI] [PubMed] [Google Scholar]
- Hase A, Suzuki H, Arahata K, Akazawa C. Expression of human GFRα-1 (GDNF receptor) at the neuromuscular junction and myelinated nerves. Neurosci Lett. 1999;269:55–57. doi: 10.1016/s0304-3940(99)00419-x. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Jacob JM. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev. 1998;106:205–216. doi: 10.1016/s0047-6374(98)00117-1. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- Kullberg S, Ramirez-Leon V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998;53:B369–B379. doi: 10.1093/gerona/53a.5.b369. [DOI] [PubMed] [Google Scholar]
- Lie DC, Weis J. GDNF expression is increased in denervated human skeletal muscle. Neurosci Lett. 1998;250(2):87–90. doi: 10.1016/s0304-3940(98)00434-0. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Maltin CA, Delday MI, Baillie AG, Grubb DA, Garlick PJ. Fiber-type composition of nine rat muscles. I. Changes during the first year of life. Am J Physiol. 1989;257:E823–E827. doi: 10.1152/ajpendo.1989.257.6.E823. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Ikata T, Takata S, Kashiwaguchi S, Niwa M, Sogabe T, Koga K. Effect of weight bearing on recovery from nerve injury in skeletal muscle. J Appl Physiol. 2001;91:2334–2341. doi: 10.1152/jappl.2001.91.5.2334. [DOI] [PubMed] [Google Scholar]
- Mussini I, Marchioro L. Low frequency nerve stimulation of rat EDL muscle: Morphology of myofibers and neuromuscular junctions. BAM. 1991;1(1):71–81. [Google Scholar]
- Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci Res. 2003;45:391–399. doi: 10.1016/s0168-0102(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279(5357):1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373(6512):344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette D, D'Costa A, Wang S, Houenou LJ, McIntosh JM. Reduction of neuromuscular activity is required for the rescue of motoneurons from naturally occurring cell death by nicotinic-blocking agents. J Neurosci. 2000;20:6117–6124. doi: 10.1523/JNEUROSCI.20-16-06117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Slater CR, Bewick GS. Synaptic vesicle dynamics in rat fast and slow motor nerve terminals. J Neurosci. 1999;19:2511–2521. doi: 10.1523/JNEUROSCI.19-07-02511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR, Thomson D, Haddow LJ, Ushkaryov YA. Enhancement of spontaneous transmitter release at neonatal mouse neuromuscular junctions by the glial cell line-derived neurotrophic factor (GDNF). J Physiol. 1998;512(3):635–641. doi: 10.1111/j.1469-7793.1998.635bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Tan SA, Hammang JP, Aebischer P, Kato AC. GDNF slows loss of motoneurons but not axonal degeneration or premature death of pmn/pmn mice. J Neurosci. 1996;16:2335–2341. doi: 10.1523/JNEUROSCI.16-07-02335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Stewart JS, Tuttle JB. Altered regulation of nerve growth factor secretion by cultured VSMCs from hypertensive rats. Am J Physiol. 1995;269:H621–628. doi: 10.1152/ajpheart.1995.269.2.H621. [DOI] [PubMed] [Google Scholar]
- Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP, Bergman LW. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurobiol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Suter-Crazzolara C, Unsicker K. GDNF is expressed in two forms in many tissues outside the CNS. Neuroreport. 1994;5(18):2486–2488. doi: 10.1097/00001756-199412000-00020. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Miyata Y, Arahata K, Akazawa C. Prominent expression of glial cell line-derived neurotrophic factor in human skeletal muscle. J Comp Neurol. 1998;402:303–312. [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-a indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17(10):3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Poo MM. Potentiation of developing synapses by postsynaptic release of Neurotrophin-4. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- Wang CY, Yang F, He XP, Je HS, Zhou JZ, Eckermann K, Kawamura D, Feng L, Shen L, Lu B. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. J Biol Chem. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26(2):206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor of neuromuscular transmission in rat fast- and slow-twitch muscles. J Physiol. 1997;500(Pt 1):165–176. doi: 10.1113/jphysiol.1997.sp022007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of glial cell line-derived growth factor mRNA in the spinal cord and muscle in amyotrophic lateral sclerosis. Neurosci Lett. 1996;204:117–120. doi: 10.1016/0304-3940(96)12342-9. [DOI] [PubMed] [Google Scholar]
- Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]