Abstract
It is unclear how haptic touch with a stable surface reduces postural sway. We hypothesized that haptic input enhances postural stability due to alterations in axial postural tone. We measured the influence of heavy and light touch of the hands on a stable bar on axial postural tone and postural sway during stance in 14 healthy adults. A unique “Twister” device measured hip torque by fixing the upper body in space while oscillating the surface in yaw ±10 deg at 1deg/s. Subjects were tested while: 1) standing quietly with their arms at their sides, 2) lightly touching a rigid bar in front of them and 3) firmly gripping the bar. Horizontal and vertical sway was not restricted by the device s yaw fixation, therefore, the subjects remained in a state of active postural control during the three touch conditions. Haptic touch significantly increased hip postural tone by 44% during light touch, from 2.5 ± 0.9 to 3.6 ± 1.0 Nm (P=0.005), and by 40% during firm grip to 3.5 ± 0.8 Nm (P=0.005). Increases in hip postural tone were associated with a reduction in postural sway (r=−0.55, P =0.001). This is the first study showing that axial postural tone can be modified by remote somatosensory input and provides a potential explanation for how light touch improves postural stability. Changes in subjects perception from trunk to surface rotation when changing from no touch to haptic touch, suggests that the central nervous system changes from using a global, to a local, trunk reference frame for control of posture during touch. The increase of hip postural tone during touching and gripping can be explained as a suppression of hip muscle shortening reactions that normally assist axial rotation.
Keywords: Postural tone, postural stability, stance, center of pressure, light touch
The relationship between postural stability and axial muscle tone is largely unexplored. Postural tone has been defined as the background tonic muscle activation that supports the body against gravity (Horak and Macpherson, 1996). Unlike resting muscle tone, postural tone is not fixed in amplitude, it is continuously modulated during equilibrium control for stance posture (Sherrington, 1909) and in preparation for potentially destabilizing movements (Massion, 1994). Although axial postural tone is thought to be important for postural stability, very few studies of axial tone exist due to the difficulty in quantifying axial tone (Nagumo and Hirayama, 1993, 1996, Gurfinkel et al., 2006, Mak et al., 2007, Wright et al., 2007b).
Recently, our laboratory developed a device to measure the distribution of axial tone in actively standing individuals. Our “Twister” device is uniquely designed to measure axial torque in a natural, active state, while restricting movement only in axial torsion during yaw twisting. When standing in this device, subjects must use their axial and proximal musculature to counteract gravity and to maintain equilibrium, thereby activating their postural tone. We showed that healthy adults axial tone is highest in the trunk, lowest in the neck, and intermediate in the hips and that axial tone was reduced during torsional body motion by active lengthening and shortening reactions of trunk and leg muscles (Gurfinkel et al., 2006). Lengthening reactions manifested as a decrease in muscle activity during muscle lengthening and the shortening reactions as an increase in muscle activity during muscle shortening (Sherrington, 1909). We also showed that increased postural tone due to rigidity of Parkinson s disease or stiffness of back pain is associated with loss of these normal lengthening and shortening reactions that assist the imposed axial twisting motions (Wright et al., 2007b, Franzén et al., 2009, Cacciatore et al., Submitted).
Tonic muscle activity for stance posture is supported, if not generated, by the resting discharge of sensory afferents from all over the body (Meyer and Bullock, 1977, Burke et al., 1992, Wright et al., 2007b). However, it is unclear how sensory inputs such as haptic and proprioceptive inputs influence postural tone. This study is the first to focus on how postural tone in the axial and proximal hip musculature is influenced by haptic and proprioceptive input from the hands.
Earlier studies have shown that contact cues from the fingertip and proprioceptive information from the arm can provide information that reduces postural sway, even when the applied forces are physically inadequate to stabilize the body (Jeka and Lackner, 1994, 1995, Jeka, 1997, Clapp and Wing, 1999, Dickstein et al., 2001). Light touch with both index fingers enhances postural stability more than one index finger (Dickstein, 2005) and the surface touched must be stable to reduce postural sway (Lackner et al., 2001). The existing theory is that sensory information about sway from the contact cues from the fingertip in conjunction with proprioceptive signals about arm configuration reduces the threshold for detecting sway to initiate earlier corrective actions (Jeka and Lackner, 1994, 1995, Jeka, 1997, Clapp and Wing, 1999).
Exactly how this sensory input improves balance remains unclear. Correlations among body sway, fingertip forces and postural muscle activity suggest that feedforward activation of postural muscles is involved in stabilizing postural sway during light touch (Jeka and Lackner, 1995). However, this theory only explains changes in phasic postural muscle activity and not any potential changes in tonic activity. During stance, postural tone and postural sway may be altered by light touch because touching a stable contact with an arm provides an additional, reference system of the trunk for postural control that can have a strong influence on perception of self- and world-motion (Gurfinkel and Levik, 1993, Lackner et al., 1999).
Based on our previous studies of axial tone (Gurfinkel et al., 2006, Wright et al., 2007b, Franzén et al., 2009) and studies showing reduced phasic postural activity during light touch, we hypothesized a decrease in tonic postural activity associated with touch during stance. Jeka (1997) found that phasic postural muscle activity not only correlated with finger touch shear forces but that it also decreased during light and heavy touch compared to quiet stance. The magnitude of automatic, postural responses to surface perturbations are also significantly reduced by light or heavy touch (Nardone et al., 1990, Chong et al., 1999). It is unknown whether tonic postural activity would also decrease with haptic touch similar to phasic postural activity or, instead, increase to stabilize posture via increased body stiffness.
To further explore the role of haptic and proprioceptive sensory feedback in postural control, our study was designed to evaluate the relationship between changes in postural sway and changes in axial postural tone. Specifically, the aim of this study was to investigate how axial tone is influenced by haptic touch of a stable surface to obtain insights into how sensory inputs related to touch stabilize postural sway. We theorize that the reduction of postural sway during haptic touch is due to changes in axial postural tone related to adding a new earth-stable reference for postural control.
EXPERIMENTAL PROCEDURES
Subjects
A total of 13 healthy human subjects (ages 20–55; 9 females, 4 males), in addition to a single 85-year old male subject, provided informed consent in accordance with the Oregon Health & Science University Institutional Review Board regulations for human subjects studies and the Helsinki Declaration.
Protocol
Three experimental conditions were investigated: no touch (NT), heavy touch (HT) and light touch (LT) as illustrated in Fig. 1A. In all three conditions, the subjects stood with eyes closed while the platform on the support surface rotated their feet and legs clockwise and counterclockwise for 3–5 cycles. In the NT conditions, the subjects (n=14) were instructed to stand with their hands hanging relaxed. During the HT condition, the subjects (n=14) were instructed to grasp the stabilizing bar with both hands using a palmar grip and comfortably hold on without resisting rotation. During the LT condition, the subjects (n=11) were instructed to lightly touch the rigid bar with both index fingers without using the stabilizing bar to provide support. After every complete set of cycles, the subjects were disconnected and encouraged either to sit or to walk about to prevent fatigue or stiffness.
Fig. 1.
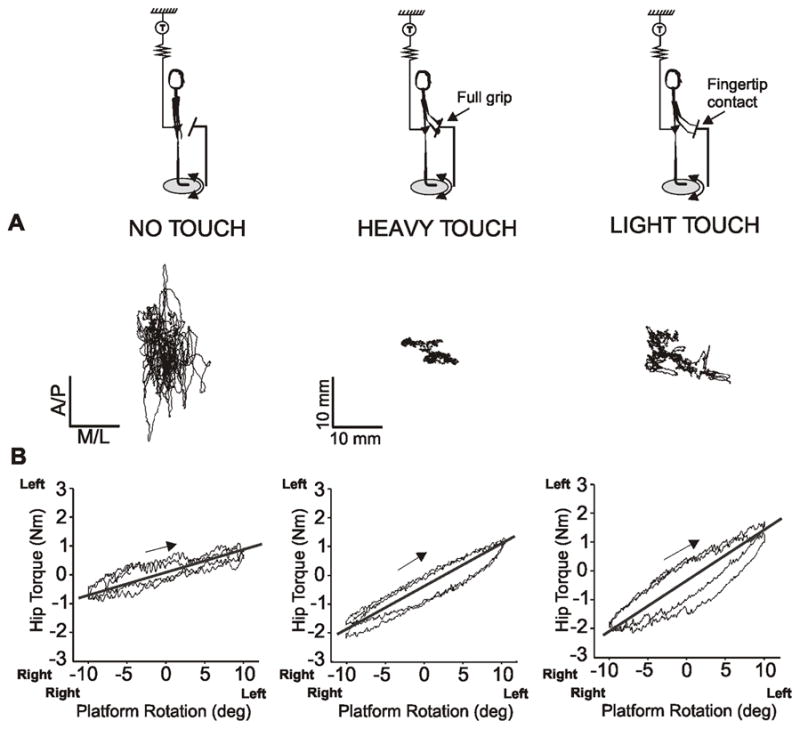
Changes in center of pressure and the torque-angle relation during no touch, heavy touch and light touch. A) xy-plot of the displacement of the center of pressure in anterior/posterior (A/P) and medial/lateral (M/L) that illustrates the changes in the center of pressure during heavy and light touch compared to no touch in a representative, healthy subject. B) Shows the relation between the hip torque and platform rotation angle during 2 cycles of rotation (±10º, 1º/s). A flatter slope of the torque- angle relationship indicates relatively less, resistance to axial rotation.
The HT and LT conditions were verified by measuring the unloading (decrease) of vertical force registered by the AMTI force plate on which the subjects were standing. The average decrease in body vertical force was 3.4 ± 1.0 % of body weight during HT (23 ± 8 N, range =11–40 N) and 0.41 ± 0.4 % during LT (2.7 ± 2.5 N, range =0.5–7 N).
Each subject stood on Twister , a rotating platform enclosed by a rigid, steel frame, custom-built to twist the axis of the body (Gurfinkel et al., 2006, Wright et al., 2007b, Franzén et al., 2009). External attachments to a double-hinged suspension apparatus prevented selected parts of the axial body from twisting, while not allowing any stability or earth reference in the horizontal or vertical directions (Gurfinkel et al., 2006). These attachments can be configured to fixate the body axis at different levels allowing isolated axial segments to be rotated torsionally. In this protocol, torsonal strain was measured about the hips yaw axis with a torque sensor (Futek TFF400, Irvine, CA) located between the frame and the suspension apparatus, which was attached to a belt on the pelvis that did not allow yaw rotation. A torque motor rotated the platform and the feet at ± 10 deg at 1 deg/s. A potentiometer measured the angular position of the platform (linearity=0.25%). Pelvis fixation allowed the legs to be passively rotated relative to the pelvis in order to measure hip muscle tone during active stance.
The rate of rotation was constant to minimize the effects of inertia, except during directional changes, when rotation followed a parabolic trajectory to reduce acceleration to <12 deg/s2. In 9 of the 13 subjects, an AMTI AccuSwayplus force platform (50 x 50 cm) (Advanced Mechanical Technology, Inc., Watertown, MA) measured 3-dimensional forces (Fx, Fy, Fz) and 3-dimentional moments (Mx, My, Mz) to provide the location of the center of pressure (CoP), as well as vertical force. For the touch trials, a 40 cm horizontal rigid metal bar was integrated into the external frame and instrumented with a second, 1-dimensional torque sensor. This stabilizing bar was positioned at waist height in front of the subject at a comfortable distance (20–25 cm) from the body midline, and the integrated sensor registered the horizontal torque applied by the subject s hands. The resistive torque of the hips, horizonatal torque from the hands, and force platform data were recorded at 100 Hz using Spike2 software (Cambridge Electronic Devices, Cambridge, UK) and analyzed offline using Matlab software (Mathworks, Natick, MA).
Previous studies have shown that, during very slow surface rotations with the eyes closed, subjects generally perceive no surface motion and interpret pressure changes on the pelvis as passive rotation of the trunk and pelvis with respect to a stable surface under the feet (Gurfinkel et al., 2006, Wright et al., 2007b, Franzén et al., 2009). Since touch of the hands on a surface fixed in space may alter this perception, we asked subjects about their perception of upper and lower body motion after trials with each of the 3 touch conditions.
Data analysis
The primary outcome measure for the HT, LT and NT conditions was the peak-to-peak amplitude of hip torque opposite to the direction of surface rotation. The symmetry of hip torsional resistance was defined as the difference in average peak torque during clockwise and counterclockwise rotations (Wright et al., 2007b). Standard deviation of the torque was measured during a 100-second period in the middle of the trial of each condition. A 5th order Butterworth IIR, high-pass filter, with a cut-off frequency of 0.05 Hz was used to remove the stimulus signal (rotation).
Postural sway was measured from the displacement of the CoP during a 100-s period in the middle of a trial for each condition as illustrated in Fig. 1A. Motion of the CoP was quantified separately in the anterior/posterior (A/P) and medial/lateral (M/L) directions in terms the root mean square (RMS) location, mean velocity, and frequency at 95% of the total power (Maurer and Peterka, 2005, Rocchi et al., 2006). Mean vertical force on the support surface was also analyzed during this 100-s period to validate the difference between the light touch and heavy touch conditions. We assumed that any reduction in vertical force under the feet during HT and LT compared to NT resulted from vertical unloading by the hands on the stabilizing bar. The torque measurement from the stabilizing bar was also analyzed in terms of the peak-to-peak amplitude. An evaluation of in-phase and counter-phase patterns between the bar torque and the hip torque was performed by visual inspection.
Muscle activity was measured from surface electromyographic (EMG) signals from the biceps femoris muscles of both legs in a subset of 8 out of 13 subjects: biceps femoris (external leg rotator. EMG activity was recorded using bipolar, surface, Ag-AgCl electrodes placed over the muscle bellies parallel to the muscle fibers. The reference electrode was located over the patella. On-line, the raw EMG signal was amplified (1,000 times) and digitized (2,000 samples/s). Offline, the EMG activity was band-pass filtered (50–500 Hz) and full-wave rectified. Modulation of EMG during surface rotation was distinct enough to allow accurate visual analysis of in-phase versus out-of-phase activation.
Statistical analysis
A one-way analysis of variance (ANOVA) using linear mixed modeling methods was used to evaluate the effects of different touch conditions on hip torque and CoP measures, since linear mixed modeling allows for partial sets of outcome values without the need to fill in missing values. Planned comparisons were used to analyze significant differences between conditions. Changes in vertical force between the conditions were analyzed with a one-way repeated measurement ANOVA. Pearson product moment correlation was used to describe the relationship between hip tone and postural stability. The significance level was set at p ≤ 0.05.
RESULTS
Hip postural tone
During stance without touching the stabilizing bar (NT), the hip torque showed a relatively flat (horizontal) phase-plot of the hip torque-angle relation (Fig. 1B). A slope near horizontal indicates relatively little resistance to axial rotation, potentially due to assistance from active muscle contraction (Gurfinkel et al., 2006). In contrast, during the HT and LT conditions, hip torque was significantly higher and less modulated, as shown by steeper torque-angle relationships (HT - P=0.0001 and LT - P=0.003) in Fig. 1B. During NT, the slope of the torque-angle relation was 0.13 ± 0.05 Nm/deg compared to 0.18 ± 0.04 Nm/deg for HT and 0.18 ± 0.05 Nm/deg for LT. The elderly subject behaved similarly to the younger subjects and did not affect statistical results.
Hip resistance to axial rotation of the legs (±10º, 1º/s) during NT, HT and LT conditions is illustrated in Fig. 2 for a representative subject. Torque resistance in the hips increased during both HT and LT (grey area) compared to NT (white area, middle row). On average, hip torque increased 54% during HT and 48% during LT. There was a significant main effect of touch condition on hip torque (F(2,36)=6.18, P=0.005), in which both HT and LT were larger than NT (P=0.005). However, hip torque did not differ between the HT and LT touch conditions (P=0.879); mean hip torque was 2.5 ± 0.9 Nm during NT, 3.5 ± 0.8 Nm during HT and 3.6 ± 1.0 Nm during LT (Fig. 3A). All subjects but one showed increased hip torque during HT (range 2.4 – 4.9 Nm), and all but one showed increased hip torque during LT (range 2.2 – 4.8 Nm) compared to NT (range 1.5–4.2 Nm), although the exception in each case was a different subject. These results indicate that, in comparison to the NT condition, hip axial tone increased in healthy subjects with either sensory (LT) or mechanical (HT) haptic inputs. Moreover, the SD of the axial torque was significantly higher during HT compared to NT (P=0.003).
Fig. 2.
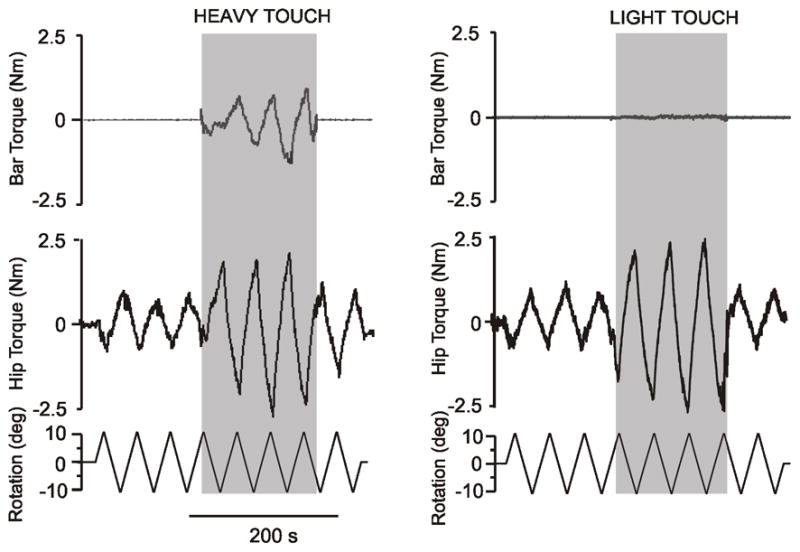
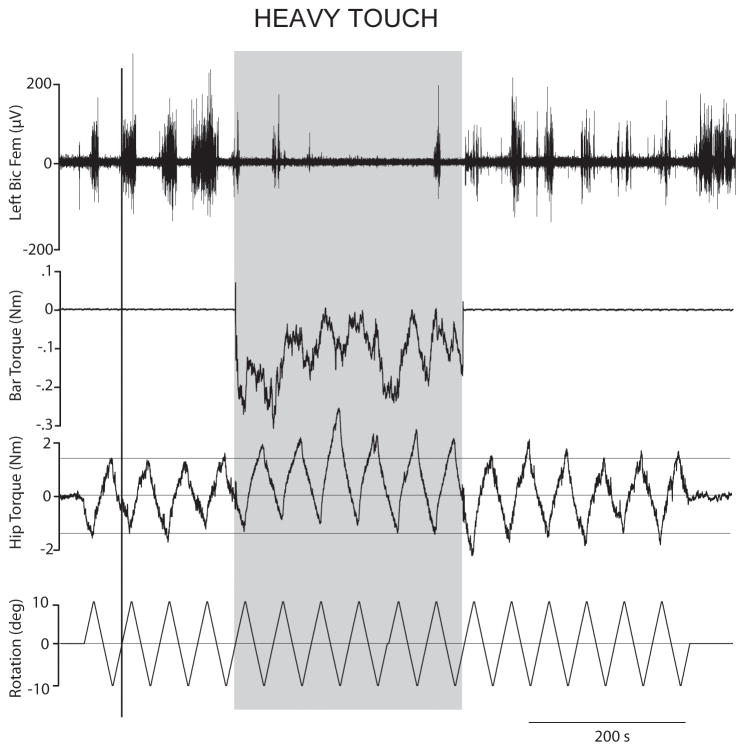
Raw data of the resistance to twisting the feet and legs from the torque sensor at the hip (middle trace), sensor on the bar (top trace), and as well as the signal from the rotating plate, ±10º, 1º/s (bottom trace) in one representative subject. Fig. 1A shows traces from a subject standing with the arms hanging (no touch) for three cycles, thereafter the subjects is holding onto the bar (heavy touch in grey area) for approximately 3 cycles, which is then followed by standing with the arms hanging again. Fig. 1B shows the same traces as in A except that the subjects are lightly touching the bar with both index fingers (light touch in grey area) instead of heavy touch
Fig. 3.
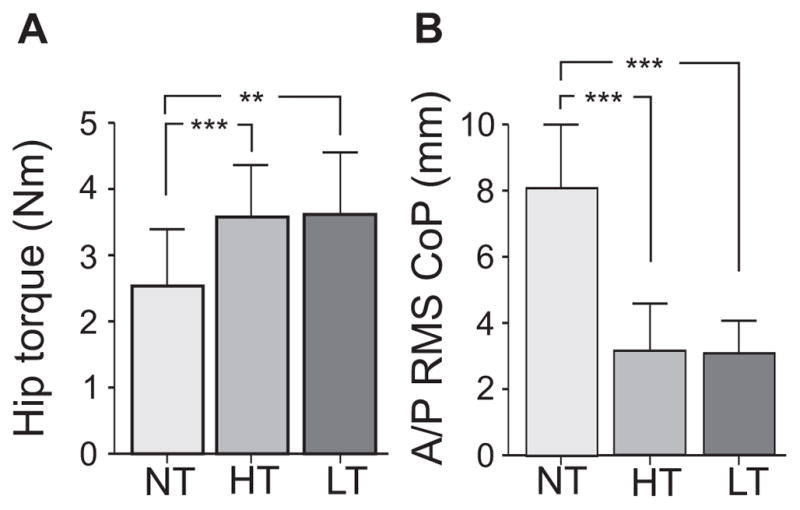
A) Group mean and SD of the hip resistance to twisting the feet and legs ±10º at 1º/s and B) the mean and SD of the root mean square (RMS) of the center of pressure (CoP) in the anterior /posterior (A/P) direction during no touch (NT), heavy touch (HT) and light touch (LT). ***denotes p<0.001 and ** denotes p<0.01
No significant asymmetries in hip resistance to torsional rotation were observed (difference in average peak torque during clockwise and counterclockwise rotations; 0.16 ± 0.34 Nm) during NT (consistent with healthy controls in earlier studies but not subjects with PD, Wright et al., 2007b) as well as during HT and LT (P=0.707).
Postural stability during haptic touch
The RMS amplitude and mean velocity of the A/P CoP excursion decreased with both LT and HT conditions as illustrated by the example in Fig 1A. Lightly touching the bar resulted in similar changes in CoP excursion as to heavy touch, (see Table 1). Fig. 3B shows that the A/P RMS amplitude of the CoP excursion decreased during HT and LT compared to NT.
Table 1.
Mean (SD) and p-values of the center of pressure measures in the anterior/posterior and medial/lateral direction.
| Center of pressure measures | NT | HT | LT | NT vs. HT | NT vs. LT | HT vs. LT |
|---|---|---|---|---|---|---|
| Anterior/posterior | ||||||
| Root mean square (mm) | 8.0 (2.0) | 3.1 (1.5) | 3.0 (1.1) | 0.000 | 0.000 | 0.916 |
| Mean velocity (mm/s) | 11.0 (2.8) | 4.8 (1.5) | 7.2 (4.2) | 0.000 | 0.006 | 0.070 |
| F95 (Hz) | 0.6 (0.1) | 1.9 (1.8) | 0.6 (0.2) | 0.004 | 0.909 | 0.009 |
| Medial /lateral | ||||||
| Root mean square (mm) | 5.7 (2.2) | 3.6( 1.2) | 4.2 (1.5) | 0.000 | 0.056 | 0.034 |
| Mean velocity (mm/s) | 8.6 (2.8) | 3.7 (1.3) | 6.3 (2.0) | 0.000 | 0.022 | 0.009 |
| F95 (Hz) | 0.6 (0.1) | 1.5 (0.9) | 1.0 (0.5) | 0.000 | 0.126 | 0.042 |
F95=95% power frequency, Bold indicates p>0.05
As shown in a previous study (Rabin et al., 1999), lightly touching a stable reference in front of oneself stabilizes A/P postural sway, but not M/L sway. In the present study, M/L sway decreased during HT compared to NT, but not during LT (Table 1).
Relationship between hip tone and postural stability
The relationship between hip tone (torque) and postural stability (CoP excursion) was examined by measuring the correlation between the RMS amplitude of the A/P CoP and the magnitude of hip resistance across all conditions (see Fig. 4). Postural sway in the A/P direction showed a significant inverse relation with hip torque in all three conditions (total r= −0.55, P=0.001 and individually NT: r= −0.15, HT: r=−0.28 and LT: r=−0.68). However, the relationship between the M/L postural sway and hip torque was not significant (r= 0.23, N.S.).
Fig. 4.
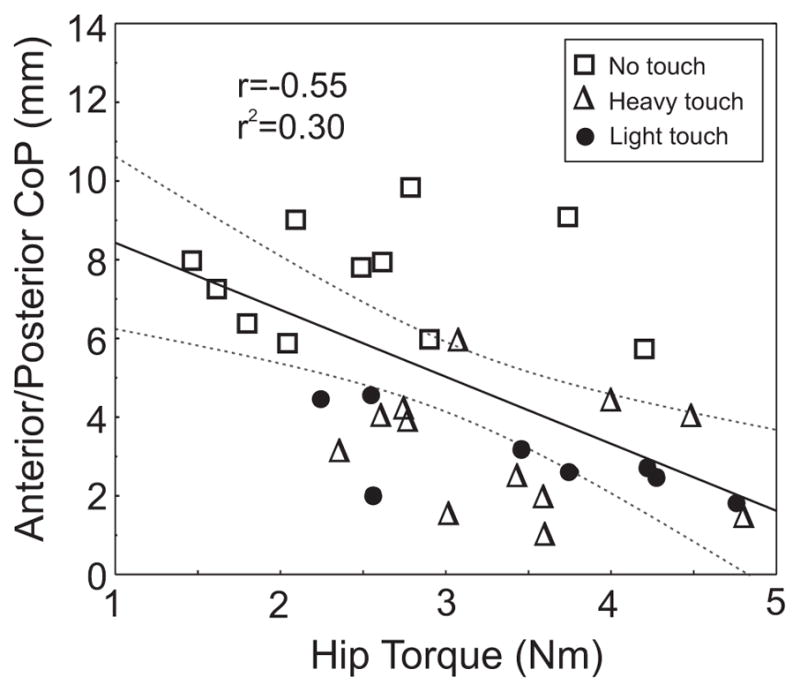
Relation between the hip torque and the center of pressure (CoP) in the anterior/posterior direction during no touch, light touch and heavy touch conditions. The peak-to-peak torque magnitude is inversely related to the root mean square (RMS) of the CoP in the anterior/posterior direction in healthy adults across all three conditions. Regression line (solid line) and 95% confidence interval (dashed line) are displayed.
Muscle activity
Increased hip torque during touch was not likely due to increased hip muscle activity. During stance on an unmoving surface, biceps femoris muscle activity was quiet in all subjects. However, during torsional rotation, the biceps femoris EMG showed large bursts of activity during the shortening phase in all subjects, despite the low speed of rotation (1 deg/sec). For example, as the platform turned to the left from the neutral, middle position, external rotation of the left foot evoked bursts of muscle activity in the shortened left biceps femoris (Fig. 5). However, the bursts of muscle activity in biceps femoris ceased as soon as each subject touched the stabilizing bar with their fingers or grasped it with their hands, and bursting started again when contact with the bar was terminated (Fig. 5). Thus lack of an active shortening response during surface rotations resulted in increased hip torque during light touch.
Fig. 5.
Example of modulated and unmodulated EMG responses in the left biceps femoris (top panel) during no touch and heavy touch (grey area) when axially rotating the legs, ±10º, 1º/s (bottom panel). As indicated by the vertical line, muscle activation occurred during the shortening phase as the platform turned to the left (positive) and the left foot rotated externally and the right internally. These phasic muscle bursts (shortening responses) stopped as soon as the subject touched the bar (second panel from above) and started again when heavy touch contact with the bar was terminated
Contact moments from grip
When the subject s feet were being rotated about the hips and the subject grasped the stabilizing bar, the hands applied a rotary moment in the horizontal plane (Fig. 2). This moment was insignificant in the light finger contact condition but reached appreciable values with firm grip (LT=0.15 Nm ± 0.1 and HT=0.96 Nm ± 0.8, P=0.021). The direction of bar moment was opposite to direction of the hip torque for 6 subjects but in the same direction of the hip torque for 2 subjects. The development of rotary moments from gripping the stabilizing bar was delayed by 3–5 s, unlike hip torque, which increased as soon as the hand contacted the stabilizing bar.
Perception
When subjects were standing on the rotating surface with their eyes closed and arms at their sides, they all reported perceiving no support surface rotation, rather interpreting pressure changes on the pelvis as due to passive rotation of the upper body. In contrast, during the LT or HT conditions, 9 out of 10 subjects reported a sensation of support surface rotation with a stable upper body.
DISCUSSION
The results of this study did not support our hypothesis that stabilization of postural sway by haptic touch would be accompanied by a decrease in axial tone. Both light and heavy haptic touch from the hands in contact with a stable reference during support surface rotation in yaw, significantly increased (~ 40%) resistance of the hips during stance. This increase in hip axial tone was comparable for both light and heavy touch, and it occurred without any change in direct somatosensory inputs to the twisting segments around the hips. In all conditions, external stabilization of the pelvis in yaw resulted in the same lack of rotation of the upper body in space while the legs slowly rotated. Thus, changes in somatosensory inputs in the hands resulted in significant changes in axial tone in the hips.
We propose that the observed increase in axial postural tone during haptic touch on a stable contact during active postural sway is responsible for increased postural stability. Despite the hips being prevented from rotating in the yaw direction by the Twister apparatus, anterior-posterior and left-right postural sway was unencumbered, which allowed us to use the CoP excursion to analyze the effect of haptic touch on postural sway. Consistent with the literature, we found a significant decrease in postural sway with light fingertip touch on a stable reference (Jeka and Lackner, 1994, 1995, Jeka, 1997, Clapp and Wing, 1999, Dickstein et al., 2001, Albertsen et al., 2010). Light touch does not provide a significant biomechanical support, so changes in postural sway have been attributed to central neural integration of somatosensory information providing an additional spatial reference frame for postural control (Gurfinkel and Levik, 1993, Massion, 1994, Lackner et al., 2001). Another interpretation is that the central nervous system used shear forces at the finger tips, in contact with a stable support, in combination with whole arm proprioception is used in a feedforward manner to reduce motion of whole body center of mass during stance (Jeka and Lackner, 1994, 1995, Lackner et al., 2001). However, it is likely that the powerful effect of light touch in reducing postural sway is due to more than just feedforward activation of postural muscles, for this effect persists even when light touch involves the legs, back or neck (Rogers et al., 2001, Krishnamoorthy et al., 2002), during vibration of the contacting hand muscles (Rabin et al., 2008); when tactile sensation from the fingertip is removed (Kouzaki and Masani, 2008), and when hand mobility is restricted (Rabin et al., 2008).
Active processes are involved in controlling muscle activity during postural sway (Peterka, 2002). Since trunk posture is more stabilized in space during hand contact, we hypothesized a decrease in axial tone during light touch. Consistent with our hypothesis, previous studies have shown that phasic postural responses to surface dorsiflexions and translations were significantly reduced during light touch (Cordo and Nashner, 1982, Nardone et al., 1990, Chong et al., 1999). Nevertheless, in our study, we found a significant increase in axial tone with haptic touch under the same conditions that would result in decreased phasic responses to postural disequilibrium. We propose that the increase in axial tone during light touch in our study reflects reduced active, postural shortening reactions that assisted the twisting during the NT condition (Gurfinkel et al., 2006). A change from NT to either LT or HT conditions was associated with a change from the activation of the leg external rotator, biceps femoris, during shortening into lack of activation during shortening. An earlier study by our group found that axial tone was smaller in subjects who showed active lengthening and shortening reactions during torsional rotation of the axis compared to subjects who did not show phasic modulation of leg and trunk muscle activity (Gurfinkel et al., 2006). Thus, the increase in postural tone during the stabilizing touch conditions may be the result of unmodulated axial muscle activity because posture is more stable during the touch conditions, and therefore there is a decreased need for the axial muscles to regulate postural stability via shortening and lengthening reactions.
Increases in axial tone during light touch of the stabilizing bar may be due to a change in the postural reference frame. Without hand contact with the bar, subjects interpreted relative motion between the legs and trunk as rotation of the trunk over a stable base of support, even though the trunk did not rotate during surface rotations (Mergner et al., 1983, Gurfinkel and Levik, 1993). In fact, we previously showed that, when subjects are asked to point straight ahead during slow surface yaw rotations in Twister, they point in the direction their feet are pointing, because they perceive their stable upper bodies to be rotating in the opposite direction of their feet, which are perceived not to be moving in space (Wright et al., 2007a). When our subjects touched the stabilizing bar, however, their interpretations of body rotation changed immediately to the correct interpretation of surface and leg rotation, which was associated with a significant increase in hip axial tone. Many studies have shown that standing on an unstable surface increases reliance on vestibulospinal control of posture and decreases proprioceptive control, but it is not clear if such a change in sensory weighting is the basis for the change in hip axial tone following hand contact with a stable object (Peterka and Benolken, 1995, Mergner et al., 2003).
The similarity of effects of both light touch and heavy touch in reducing postural sway and increasing postural tone supports the hypothesis that both light and heavy haptic contact have similar influences on postural control, despite differences in physical characteristics and in the types of sensory receptors activated by hand contact. A limitation of this study is that we did not directly record vertical forces under the fingers and hands and we did not constrain light touch to be under 1 N using biofeedback because of the considerable attentional resources required for this task (Vuillerme et al., 2006). It seems unlikely that the torque exerted by the hands on the stabilizing bar, however, was responsible for postural stability during haptic contact since there were no moments during light touch. Also, there are no multiarticular trunk muscles that could provide the transfer of muscular moment from the trunk to the hands. The only muscle that could potentially provide this function, the psoas, acts as a flexor, and not a rotator, of the trunk in erect stance (Marre-Brunenghi et al., 2008). In fact, development of moment in the hands lagged the onset of moment in the hips by 3–5 seconds and was primarily counter-phase to trunk moments.
We further hypothesize that the modulation of the moment produced by the hands on the stabilizing bar, despite lack of any trunk motion, reflect the activation of propriospinal influences evoked by hip rotation and transmitted to the muscles of the arms. During normal walking, pelvis and shoulder girdle muscles are reciprocally activated in counter-phase. Although the arms did not swing in response to hip twisting in the no touch condition, moments from the hands on the grab bar were counter-phase to hip twisting moments. It is possible that hand contact facilitated mechanisms of inter-limb coordination subserving locomotor synergies (Zehr and Duysens, 2004, Dietz and Michel, 2009). It is also possible that arm moments evoked under our experimental conditions reflect the action of an automatic mechanism to stabilize an illusion of externally-imposed trunk rotation.
In conclusion, touch contact of the hands to a stable surface appears to decrease postural sway during stance because of an associated increase in axial muscle tone. This increase of axial postural tone may be due to a change from a foot-in-space to a trunk-in-space stable reference system for control of postural orientation.
Research highlights.
Axial postural tone can be modified by remote somatosensory input
Axial hip tone increases in healthy adults during haptic touch
This increase might be a consequence of a suppression of muscle shortening reactions
A local reference frame for control of posture is used during haptic touch
Acknowledgments
Supported by the NIH R01 NS061304, R37-AG006457, Swedish Brain Foundation and the Center for Health Care Sciences at Karolinska Institutet. The authors thank Edward King for his assistance with graphic illustration.
List of abbreviations
- ANOVA
Analysis of variance
- AMTI
Advanced Mechanical Technology, Inc
- A/P
Anterior/Posterior
- CoP
Center of Pressure
- EMG
Electromyography
- HT
Heavy Touch
- LT
Light Touch
- NT
No Touch
- M/L
Medial/Lateral
- RMS
Root Mean Square
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertsen IM, Temprado JJ, Berton E. Effect of haptic supplementation on postural stabilization: A comparison of fixed and mobile support conditions. Hum Mov Sci. 2010 doi: 10.1016/j.humov.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of afferents to propriospinal-like neurones in man during voluntary contractions. J Physiol. 1992;449:673–687. doi: 10.1113/jphysiol.1992.sp019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciatore TW, Gurfinkel VS, Horak FB, Cordo PJ, Ames KE. Increased dynamic regulation of postural tone through training. J Applied Physiol. doi: 10.1016/j.humov.2010.10.002. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RK, Jones CL, Horak FB. Postural set for balance control is normal in Alzheimer's but not in Parkinson's disease. J Gerontol A Biol Sci Med Sci. 1999;54:M129–135. doi: 10.1093/gerona/54.3.m129. [DOI] [PubMed] [Google Scholar]
- Clapp S, Wing A. Light touch contribution to balance in normal bipedal stance. Exp Brain Res. 1999;125:521–524. doi: 10.1007/s002210050711. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Dickstein R. Stance stability with unilateral and bilateral light touch of an external stationary object. Somatosens Mot Res. 2005;22:319–325. doi: 10.1080/08990220500420640. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Shupert CL, Horak FB. Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture. 2001;14:238–247. doi: 10.1016/s0966-6362(01)00161-8. [DOI] [PubMed] [Google Scholar]
- Dietz V, Michel J. Human Bipeds Use Quadrupedal Coordination during Locomotion. Annals of the New York Academy of Sciences. 2009;1164:97–103. doi: 10.1111/j.1749-6632.2008.03710.x. [DOI] [PubMed] [Google Scholar]
- Franzén E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol. 2009;219:430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96:2678–2687. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Levik YS. The suppression of cervico-ocular response by the haptokinetic information about the contact with a rigid, immobile object. Exp Brain Res. 1993;95:359–364. doi: 10.1007/BF00229794. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Smith JL, editor. Handbook of Physiology: Section 12: Exercise: Regulation and Integration of Multiple Systems. Vol. 12. New York: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- Jeka JJ. Light touch contact as a balance aid. Phys Ther. 1997;77:476–487. doi: 10.1093/ptj/77.5.476. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Exp Brain Res. 1994;100:495–502. doi: 10.1007/BF02738408. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Lackner JR. The role of haptic cues from rough and slippery surfaces in human postural control. Exp Brain Res. 1995;103:267–276. doi: 10.1007/BF00231713. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Masani K. Reduced postural sway during quiet standing by light touch is due to finger tactile feedback but not mechanical support. Exp Brain Res. 2008;188:153–158. doi: 10.1007/s00221-008-1426-5. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Slijper H, Latash ML. Effects of different types of light touch on postural sway. Exp Brain Res. 2002;147:71–79. doi: 10.1007/s00221-002-1206-6. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P, Jeka J, Horak F, Krebs D, Rabin E. Precision contact of the fingertip reduces postural sway of individuals with bilateral vestibular loss. Exp Brain Res. 1999;126:459–466. doi: 10.1007/s002210050753. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Rabin E, DiZio P. Stabilization of posture by precision touch of the index finger with rigid and flexible filaments. Exp Brain Res. 2001;139:454–464. doi: 10.1007/s002210100775. [DOI] [PubMed] [Google Scholar]
- Mak MK, Wong EC, Hui-Chan CW. Quantitative measurement of trunk rigidity in parkinsonian patients. J Neurol. 2007;254:202–209. doi: 10.1007/s00415-006-0327-4. [DOI] [PubMed] [Google Scholar]
- Marre-Brunenghi G, Camoriano R, Valle M, Boero S. The psoas muscle as cause of low back pain in infantile cerebral palsy. J Orthop Traumatol. 2008;9:43–47. doi: 10.1007/s10195-008-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Postural control system. Current Opinion in Neurobiology. 1994;4:877–887. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol. 2005;93:189–200. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- Mergner T, Maurer C, Peterka RJ. A multisensory posture control model of human upright stance. Prog Brain Res. 2003;142:189–201. doi: 10.1016/S0079-6123(03)42014-1. [DOI] [PubMed] [Google Scholar]
- Mergner T, Nardi GL, Becker W, Deecke L. The role of canal-neck interaction for the perception of horizontal trunk and head rotation. Experimental Brain Research (Berlin) 1983;49:198–208. doi: 10.1007/BF00238580. [DOI] [PubMed] [Google Scholar]
- Meyer DL, Bullock TH. The hypothesis of sense–organ-dependent tonus mechanisms: history of a concept. Ann N Y Acad Sci. 1977;290:3–17. doi: 10.1111/j.1749-6632.1977.tb39712.x. [DOI] [PubMed] [Google Scholar]
- Nagumo K, Hirayama K. A study on truncal rigidity in parkinsonism--evaluation of diagnostic test and electrophysiological study. Rinsho Shinkeigaku. 1993;33:27–35. [PubMed] [Google Scholar]
- Nagumo K, Hirayama K. Axial (neck and trunk) rigidity in Parkinson's disease, striatonigral degeneration and progressive supranuclear palsy. Rinsho Shinkeigaku. 1996;36:1129–1135. [PubMed] [Google Scholar]
- Nardone A, Giordano A, Corra T, Schieppati M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain. 1990;113 ( Pt 1):65–84. doi: 10.1093/brain/113.1.65. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Experimental Brain Research (Berlin) 1995;105:101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- Rabin E, Bortolami SB, DiZio P, Lackner JR. Haptic stabilization of posture: changes in arm proprioception and cutaneous feedback for different arm orientations. J Neurophysiol. 1999;82:3541–3549. doi: 10.1152/jn.1999.82.6.3541. [DOI] [PubMed] [Google Scholar]
- Rabin E, DiZio P, Ventura J, Lackner JR. Influences of arm proprioception and degrees of freedom on postural control with light touch feedback. J Neurophysiol. 2008;99:595–604. doi: 10.1152/jn.00504.2007. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Cappello A, Horak FB. Identification of distinct characteristics of postural sway in Parkinson's disease: a feature selection procedure based on principal component analysis. Neurosci Lett. 2006;394:140–145. doi: 10.1016/j.neulet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Wardman DL, Lord SR, Fitzpatrick RC. Passive tactile sensory input improves stability during standing. Exp Brain Res. 2001;136:514–522. doi: 10.1007/s002210000615. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. A mammalian spinal preparation. J Physiol. 1909;38:375–383. doi: 10.1113/jphysiol.1909.sp001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel V, King L, Horak F. Parkinson's disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett. 2007a;417:10–15. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol. 2007b;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillerme N, Isableu B, Nougier V. Attentional demands associated with the use of a light fingertip touch for postural control during quiet standing. Exp Brain Res. 2006;169:232–236. doi: 10.1007/s00221-005-0142-7. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Duysens J. Regulation of arm and leg movement during human locomotion. Neuroscientist. 2004;10:347–361. doi: 10.1177/1073858404264680. [DOI] [PubMed] [Google Scholar]